Insulation Materials Susceptibility to Biological Degradation Agents: Molds and Subterranean Termites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fungal Growth Tests
2.3. Termite Attack Tests
3. Results
3.1. Resistance to Fungal Attacks
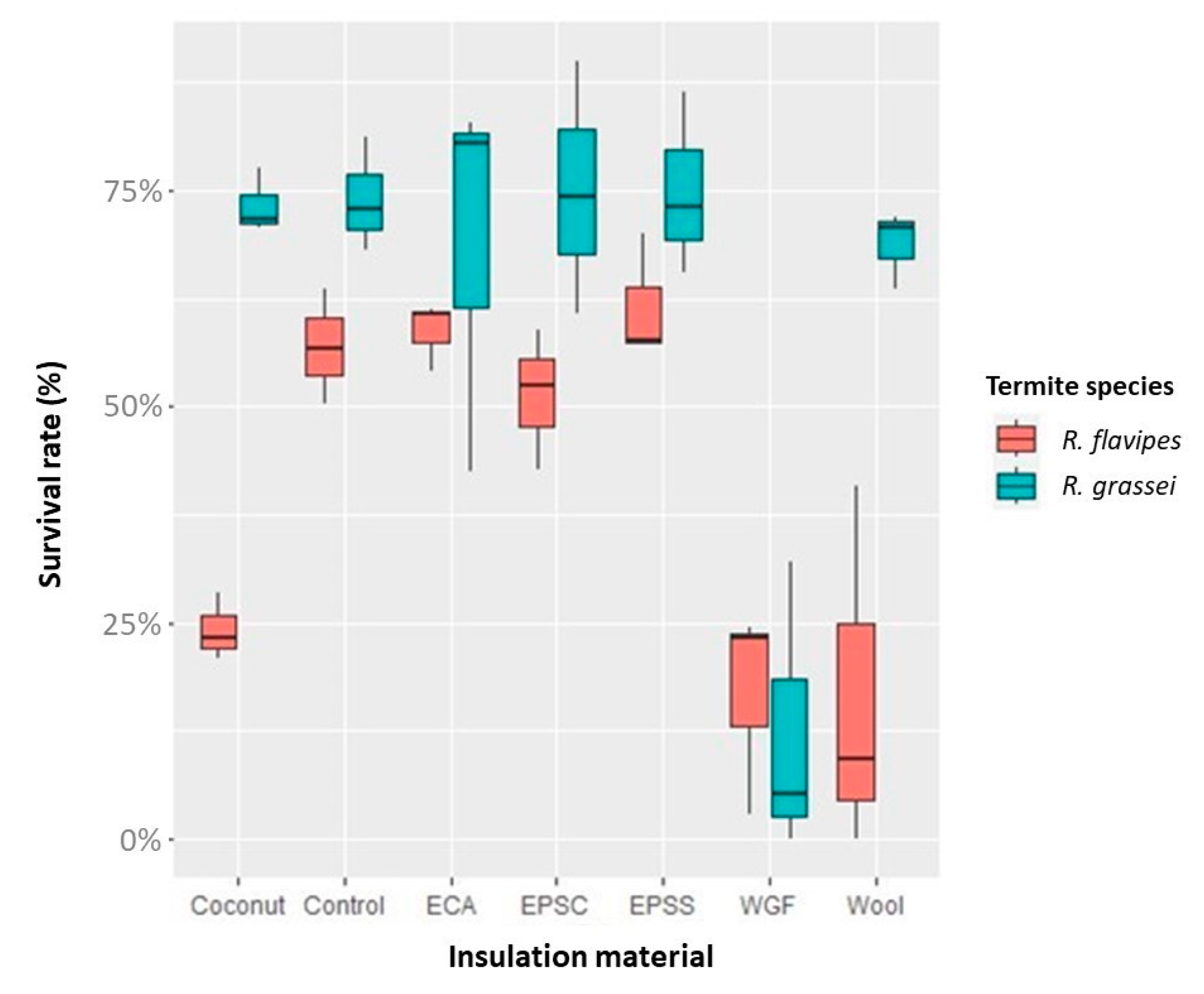
3.2. Termite Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Environment Programme (UNEP). Annual Report of the United Nations Environment Programme; United Nations Environment Programme: Nairobi, Kenya, 2021; 24p. [Google Scholar]
- Petersdorff, C.; Boermans, T.; Harnisch, J. Mitigation of CO2 emissions from the EU-15 building stock—Beyond the EU directive on the energy performance of buildings. Environ. Sci. Pollut. Control 2006, 13, 350–358. [Google Scholar] [CrossRef]
- Kumar, A.; Suman, B.M. Experimental evaluation of insulation materials for walls and roofs and their impact on indoor thermal comfort under composite climate. Build. Environ. 2013, 59, 635–643. [Google Scholar] [CrossRef]
- Nguyen, D.M.; Grillet, A.-C.; Diep, T.M.H.; Bui, Q.B.; Woloszyn, M. Influence of thermo-pressing conditions on insulation materials from bamboo fibers and proteins based bone glue. Ind. Crop Prod. 2018, 111, 834–845. [Google Scholar] [CrossRef]
- Pavlu, T.; Fortova, K.; Divis, J.; Hajek, P. The utilization of recycled masonry aggregate and recycled EPS for concrete blocks for mortarless masonry. Materials 2019, 12, 1923. [Google Scholar] [CrossRef] [PubMed]
- Odgaard, T.; Bjarlov, S.P.; Rode, C. Interior insulation—Characterisation of the historic, solid masonry building segment and analysis of the heat saving potential by 1d, 2d and 3d simulation. Energy Build. 2018, 162, 1–11. [Google Scholar] [CrossRef]
- Vereecken, E.; Roels, S. A comparison of the hygric performance of interior insulation systems: A hot box-cold box experiment. Energy Build. 2014, 80, 37–44. [Google Scholar] [CrossRef]
- Vereecken, E.; Van Gelder, L.; Janssen, H.; Roels, S. Interior insulation for wall retrofitting—A probabilistic analysis of the energy savings and hygrothermal risks. Energy Build. 2015, 89, 231–244. [Google Scholar] [CrossRef]
- Guizzardi, M.; Carmeliet, J.; Derome, D. Risk analysis of biodeterioration of wooden beams embedded in internally insulated masonry walls. Construct. Build. Mater. 2015, 99, 159–168. [Google Scholar] [CrossRef]
- Energy Saving Trust. Good Practice Guide 268—Energy Efficient Ventilation in Dwellings—A Guide for Specifiers; Energy Saving Trust: London, UK, 2006. [Google Scholar]
- Schmidt, O. Wood and Tree Fungi: Biology, Damage, Protection, and Use; Springer: Berlin/Heidelberg, Germany, 2006; 336p. [Google Scholar]
- Johansoon, P.; Ekstrand-Tobin, A.; Svensson, T.; Bok, G. Laboratory study to determine the critical moisture level for mould growth on building materials. Int. Biodeterior. Biodegr. 2012, 73, 23–32. [Google Scholar] [CrossRef]
- Hoang, C.P.; Kinney, K.A.; Corsi, R.L.; Szaniszlo, P.J. Resistance of green building materials to fungal growth. Int. Biodeterior. Biodegr. 2010, 64, 104–113. [Google Scholar] [CrossRef]
- Abu-Jdayil, B.; Mourad, A.-H.; Hittini, W.; Hassan, W.; Hameedi, S. Traditional, state-of-the-art and renewable thermal building insulation materials: An overview. Construct. Build. Mater. 2019, 214, 709–735. [Google Scholar] [CrossRef]
- Viel, M.; Collet, F.; Lecieux, Y.; François, M.L.M.; Colson, V.; Lanos, C.; Hussain, A.; Lawrence, M. Resistance to mold development assessment of bio-based building materials. Composites Part B 2019, 158, 406–418. [Google Scholar] [CrossRef]
- Verdier, T.; Coutand, M.; Bertron, A.; Roques, C. A review of indoor microbial growth across building materials and sampling and analysis methods. Build. Environ. 2014, 80, 136–149. [Google Scholar] [CrossRef]
- Horn, W.; Jann, O.; Wilke, O. Suitability of small environmental chambers to test the emission of biocides from treated materials into the air. Atmos. Environ. 2003, 37, 5477–5483. [Google Scholar] [CrossRef]
- Gaylarde, C.C.; Morton, L.H.G.; Loh, K.; Shirakawa, M.A. Biodeterioration of architectural paint films—A review. Int. Biodeterior. Biodegr. 2011, 65, 1189–1198. [Google Scholar] [CrossRef]
- Hyvärinen, A.; Meklin, T.; Vepsäläinen, A.; Nevalainen, A. Fungi and actinobacteria in moisture-damaged building materials—Concentrations and diversity. Int. Biodeterior. Biodegr. 2002, 49, 27–37. [Google Scholar] [CrossRef]
- Klamer, M.; Morsing, E.; Husemoen, T. Fungal growth on different insulation materials exposed to different moisture regimes. Int. Biodeterior. Biodegr. 2004, 54, 277–282. [Google Scholar] [CrossRef]
- Tobon, A.M.; Andres, Y.; Locoge, N. Impacts of test methods on the assessment of insulation materials’ resistance against moulds. Build. Environ. 2020, 179, 106963. [Google Scholar] [CrossRef]
- Parracha, J.; Borsoi, G.; Flores-Colen, I.; Veiga, R.; Nunes, L.; Dionísio, A.; Gomes, M.G.; Faria, P. Performance parameters of ETICS: Correlating water resistance, bio-susceptibility and surface properties. Constr. Build. Mater. 2021, 272, 121956. [Google Scholar] [CrossRef]
- Su, N.; Ban, P.; Scheffrahn, R.H. Resistance of insecticide-treated foam board insulation against the eastern subterranean termite and the Formosan subterranean termite (Isoptera: Rhinotermitidae). J. Econ. Entomol. 2003, 96, 1526–1529. [Google Scholar] [CrossRef]
- Tucker, C.L.; Koehler, P.G.; Pereira, R.M. Development of a method to evaluate the effects of eastern subterranean termite damage to the thermal properties of building construction materials (Isoptera: Rhinotermitidae). Sociobiology 2008, 51, 589–600. [Google Scholar]
- Ardente, F.; Beccali, M.; Cellura, M.; Mistretta, M. Building energy performance: A LCA case study of kenaf-fibres insulation board. Energy Build. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- ASTM D5590-00:2010; Standard Test Method for Determining the Resistance of Paint Films and Related Coatings to Fungal Defacement by Accelerated Four-Week Agar Plate Assay. ASTM International: West Conshohocken, PA, USA, 2010.
- EN ISO 846:1997; Plastics—Evaluation of the Action of Microorganisms. British Standards Institute: London, UK, 1997.
- Nobre, T.; Nunes, L.; Eggleton, P.; Bignell, D.E. Distribution and genetic variation of Reticulitermes (Isoptera: Rhinotermitidae) in Portugal. Heredity 2006, 96, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, J.G.N.; Nobre, T.; Duarte, S.; Jones, D.; Esteves, B.; Nunes, L. Proof-of-principle that cellular automata can be used to predict infestation risk by Reticulitermes grassei (Blattodea: Isoptera). Forests 2022, 13, 227. [Google Scholar] [CrossRef]
- EN 118:2013; Wood Preservatives. Determination of Preventive Action against Reticulitermes species (European termites) (Laboratory Method). European Committee for Standardization: Brussels, Belgium, 2013.
- Parracha, J.; Cortay, A.; Borsoi, G.; Veiga, R.; Nunes, L. Evaluation of ETICS characteristics that affect surface mould development. In Proceedings of the XV International Conference on Durability of Building Materials and Components (DBMC 2020), Barcelona, Spain, 20–23 October 2020. [Google Scholar] [CrossRef]
- Hu, J.; Chang, S.; Peng, K.; Hu, K.; Thévenon, M.-F. Bio-susceptibility of shells of Camellia oleifera Abel. fruits to fungi and termites. Int. Biodeterior. Biodegr. 2015, 104, 219–223. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Medrano, M.; Martorell, I.; Pérez, G.; Fernández, I. Experimental study on the performance of insulation materials in Mediterranean construction. Energy Build. 2010, 42, 630–636. [Google Scholar] [CrossRef]
- Knapic, S.; Oliveira, V.; Machado, J.S.; Pereira, H. Cork as a building material: A review. Eur. J. Wood Prod. 2016, 74, 775–791. [Google Scholar] [CrossRef]
- Pereira, C.S.; Soares, G.; Oliveira, A.C.; Rosa, M.E.; Pereira, H.; Moreno, N.; Romão, M.V.S. Effect of fungal colonization on mechanical performance of cork. Int. Biodeterior. Biodegr. 2006, 57, 244–250. [Google Scholar] [CrossRef]
- Pereira, H. Chemical composition and variability of cork from Quercus suber L. Wood Sci. Technol. 1998, 22, 211–218. [Google Scholar] [CrossRef]
- Miranda, I.; Gominho, J.; Pereira, H. Cellular structure and chemical composition of cork from the Chinese cork oak (Quercus variabilis). J. Wood Sci. 2013, 59, 1–9. [Google Scholar] [CrossRef]
- Fabbri, K.; Tronchin, L.; Barbieri, F. Coconut fibre insulators: The hygrothermal behaviour in the case of green roofs. Constr. Build. Mater. 2021, 266, 121026. [Google Scholar] [CrossRef]
- Kumar, N.S.; Buddi, T.; Lakshmi, A.A.; Durga Rajesh, K.V. Synthesis and evaluation of mechanical properties for coconut fiber composites—A review. Mater. Today Proc. 2021, 44, 2482–2487. [Google Scholar] [CrossRef]
- Dab, M.; Wong, A.H.H.; Unger, W. Shells of coconut and their durability against termite attack. In Proceedings of the International Research Group on Wood Protection 46th Annual Meeting, IRG/WP 15-10853, Viña del Mar, Chile, 10–14 May 2015. [Google Scholar]
- Nami Kartal, S.; Terzi, E.; Muin, M.; Hassanin, A.H.; Hamuoda, T.; Kilic, A.; Candan, Z. Hybrid green composites manufactured with glass fiber and jute fabric skin by VARTM process: Fungal, mold, and termite resistance tests. In Proceedings of the International Research Group on Wood Protection 48th Annual Meeting, IRG/WP 17-40780, Ghent, Belgium, 10–14 May 2017. [Google Scholar]
- Meklin, T.; Husman, T.; Vepsalainen, A.; Vahteristo, M.; Koivisto, J.; Halla-Aho, J.; HyvFarinen, A.; Moschandreas, D.; Nevalainen, A. Indoor air microbes and respiratory symptoms of children in moisture damaged and reference schools. Indoor Air 2002, 12, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Jerábková, E.; Tesarová, D. Resistance of various materials and coatings used in wood constructions to growth of microorganisms. Wood Res. 2018, 63, 993–1002. [Google Scholar]
- Nuryawan, A.; Hutauruk, N.O.; Purba, E.Y.S.; Masruchin, N.; Batubara, R.; Risnasari, I.; Satrio, F.K.; Rahmawaty; Basyuni, M.; McKay, D. Properties of wood composite plastics made from predominant Low Density Polyethylene (LDPE) plastics and their degradability in nature. PLoS ONE 2020, 15, e0236406. [Google Scholar] [CrossRef]
- Elsamahy, T.; Sun, J.; Elsilk, S.E.; Ali, S.S. Biodegradation of low-density polyethylene plastic waste by a constructed tri-culture yeast consortium from wood-feeding termite: Degradation mechanism and pathway. J. Hazard. Mater. 2023, 448, 130944. [Google Scholar] [CrossRef]
- Berlanga, M.; Guerrero, R. The holobiont concept: The case of xylophagous termites and cockroaches. Symbiosis 2016, 68, 49–60. [Google Scholar] [CrossRef]
- Duarte, S.; Nunes, L.; Borges, P.A.V.; Fossdal, C.G.; Nobre, T.T. Living inside termites: An overview of symbiotic interactions, with emphasis on flagellate protists. Arquipelago. Life Mar. Sci. 2017, 34, 21–43. [Google Scholar]
- Zaragoza-Benzal, A.; Ferrández, D.; Atanes-Sánchez, E.; Saíz, P. Dissolved recycled expanded polystyrene as partial replacement in plaster composites. J. Build. Eng. 2023, 65, 105697. [Google Scholar] [CrossRef]
- Curling, S.F.; Stefanowski, B.K.; Mansour, E.; Ormondroyd, G.A. Applicability of wood durability testing methods to bio-based building materials. In Proceedings of the International Research Group on Wood Protection 46th Annual Meeting, IRG/WP 15-20561, Viña del Mar, Chile, 10–14 May 2015. [Google Scholar]



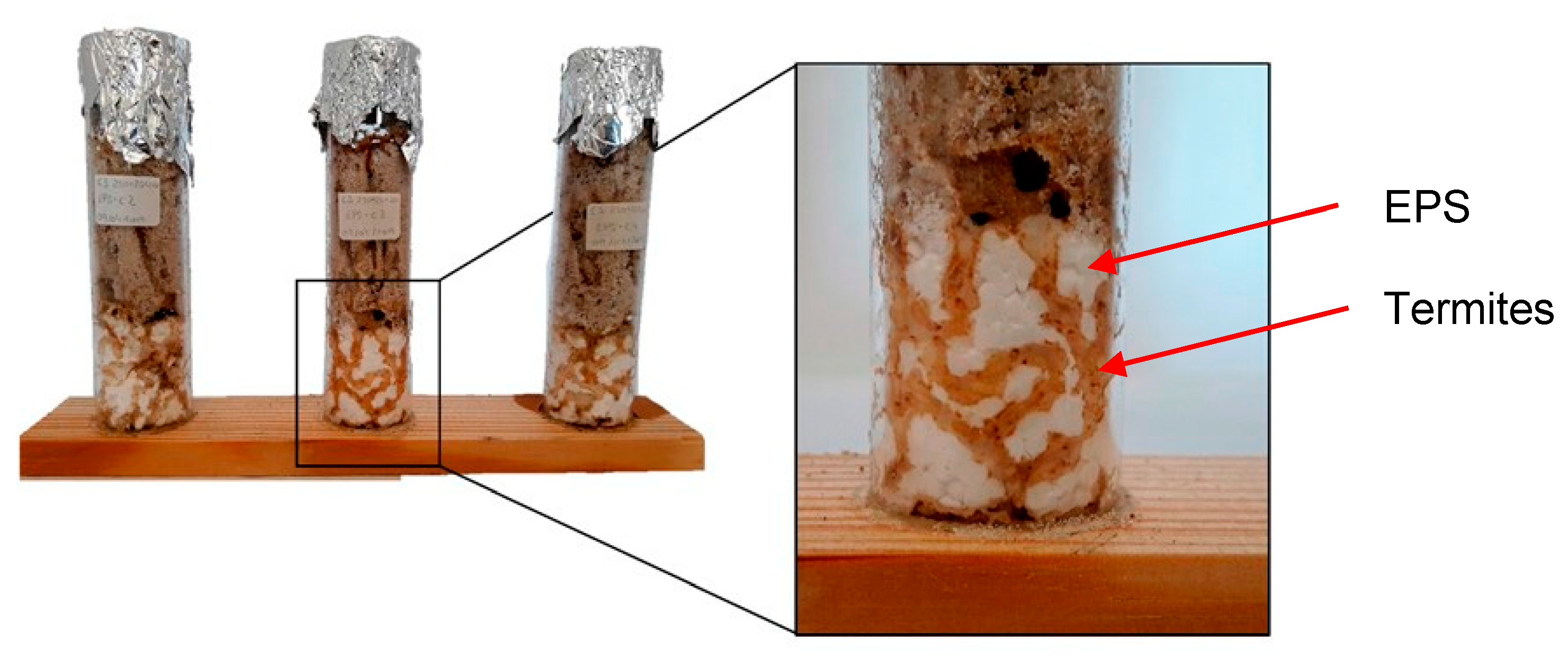
| Intensity of Growth | Evaluation | Covering of the Sample’s Surface |
|---|---|---|
| 0 | No growth apparent under the microscope | 0% |
| 1 | Traces of growth | <10% |
| 2 | Light growth | 10–30% |
| 3 | Moderate growth | 30–60% |
| 4 | Heavy growth (to complete surface coverage) | 60–100% |
| Natural Indoor Inoculation | Artificial Inoculation with: | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. niger and P. funiculosum | A. pullulans | |||||||||||
| Week 1 | Week 2 | Week 3 | Week 4 | Week 1 | Week 2 | Week 3 | Week 4 | Week 1 | Week 2 | Week 3 | Week 4 | |
| Control | 4.0 | 4.0 | 4.0 | 4.0 | 3.7 ± 0.6 | 3.7 ± 0.6 | 3.7 ± 0.6 | 4.0 | 3.0 | 4.0 | 4.0 | 4.0 |
| EPS-C | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| EPS-S | 0.0 | 0.7 ± 0.6 | 0.7 ± 0.6 | 0.7 ± 0.6 | 0.0 | 0.3 ± 0.6 | 0.3 ± 0.6 | 0.3 ± 0.6 | 0.0 | 0.3 ± 0.6 | 0.3 ± 0.6 | 0.3 ± 0.6 |
| MW | 0.0 | 0.0 | 0.0 | 0.3 ± 0.6 | 0.3 ± 0.6 | 0.3 ± 0.6 | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Co | 2.0 | 2.3 ± 0.6 | 3.7 ± 0.6 | 3.7 ± 0.6 | 1.7 ± 0.6 | 2.3 ± 0.6 | 2.7 ± 0.6 | 2.7 ± 0.6 | 1.0 | 1.3 ± 0.6 | 2.0 | 2.0 |
| Reticulitermes grassei | Reticulitermes flavipes | |||
|---|---|---|---|---|
| Survival Rate (%) | Attack Degree | Survival Rate (%) | Attack Degree | |
| Control | 74.0 ± 6.7 a | 4.0 ± 0.0 | 56.9 ± 6.6 a | 4.0 ± 0.0 |
| Coconut fiber | 73.3 ± 4.5 a | 4.0 ± 0.0 | 24.1 ± 3.9 bc | 4.0 ± 0.0 |
| Co | 68.5 ± 22.7 a | 4.0 ± 0.0 | 58.7 ± 4.0 a | 4.0 ± 0.0 |
| EPS-C | 75.1 ± 14.6 a | 4.0 ± 0.0 | 51.3 ± 8.0 ac | 4.0 ± 0.0 |
| EPS-S | 75.1 ± 10.5 a | 4.0 ± 0.0 | 61.6 ± 7.2 a | 4.0 ± 0.0 |
| WGF | 12.4 ± 17.2 b | 4.0 ± 0.0 | 16.8 ± 12.1 bc | 3.0 ± 1.0 |
| MW | 68.8 ± 4.5 a | 4.0 ± 0.0 | 16.7 ± 21.4 b | 4.0 ± 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, L.; Duarte, S.; Parracha, J.L.; Jones, D.; Paulmier, I.; Kutnik, M. Insulation Materials Susceptibility to Biological Degradation Agents: Molds and Subterranean Termites. Appl. Sci. 2023, 13, 11311. https://doi.org/10.3390/app132011311
Nunes L, Duarte S, Parracha JL, Jones D, Paulmier I, Kutnik M. Insulation Materials Susceptibility to Biological Degradation Agents: Molds and Subterranean Termites. Applied Sciences. 2023; 13(20):11311. https://doi.org/10.3390/app132011311
Chicago/Turabian StyleNunes, Lina, Sónia Duarte, João L. Parracha, Dennis Jones, Ivan Paulmier, and Magdalena Kutnik. 2023. "Insulation Materials Susceptibility to Biological Degradation Agents: Molds and Subterranean Termites" Applied Sciences 13, no. 20: 11311. https://doi.org/10.3390/app132011311
APA StyleNunes, L., Duarte, S., Parracha, J. L., Jones, D., Paulmier, I., & Kutnik, M. (2023). Insulation Materials Susceptibility to Biological Degradation Agents: Molds and Subterranean Termites. Applied Sciences, 13(20), 11311. https://doi.org/10.3390/app132011311








