Linking Thermal Indices, Productivity, Phenotypic Traits, and Stressors for Assessing the Health of Centennial Traditional Olive Trees
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Olive Trees Selection
2.2. Productivity Metrics of Olive Trees
2.3. Phenotypic Metrics of Olive Trees
2.4. Assessment of Biotic Stressors—Olive Leaf Spot
2.5. Assessment of Abiotic Stressors—Crop Water Stress Index
2.6. Assesment of Olive Trees’ Health State
2.7. Statistical Analysis
3. Results
3.1. Centennial Olive Trees’ Traits and Productivity
3.2. Olive Trees’ Trunk Humidity and Thermal Pattern Variations
3.3. Interrelationships among Productivity, Phenotypic Traits, Thermal and Humidity Indices, and CWSI
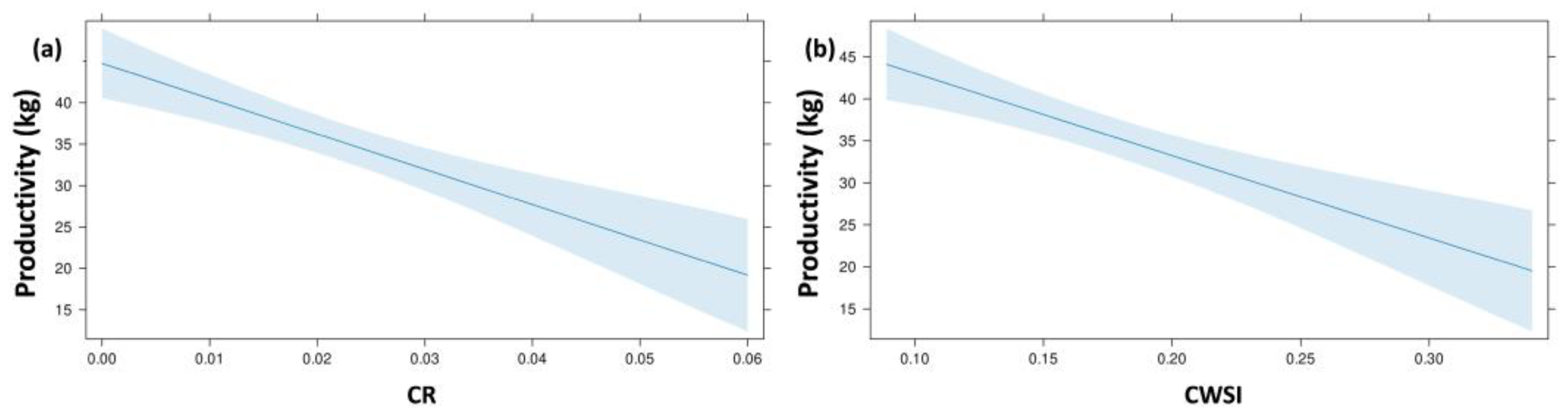
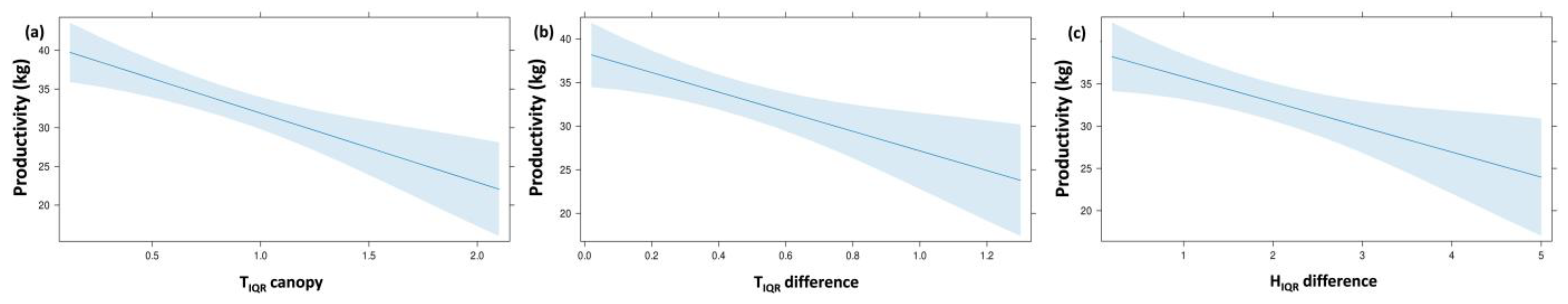
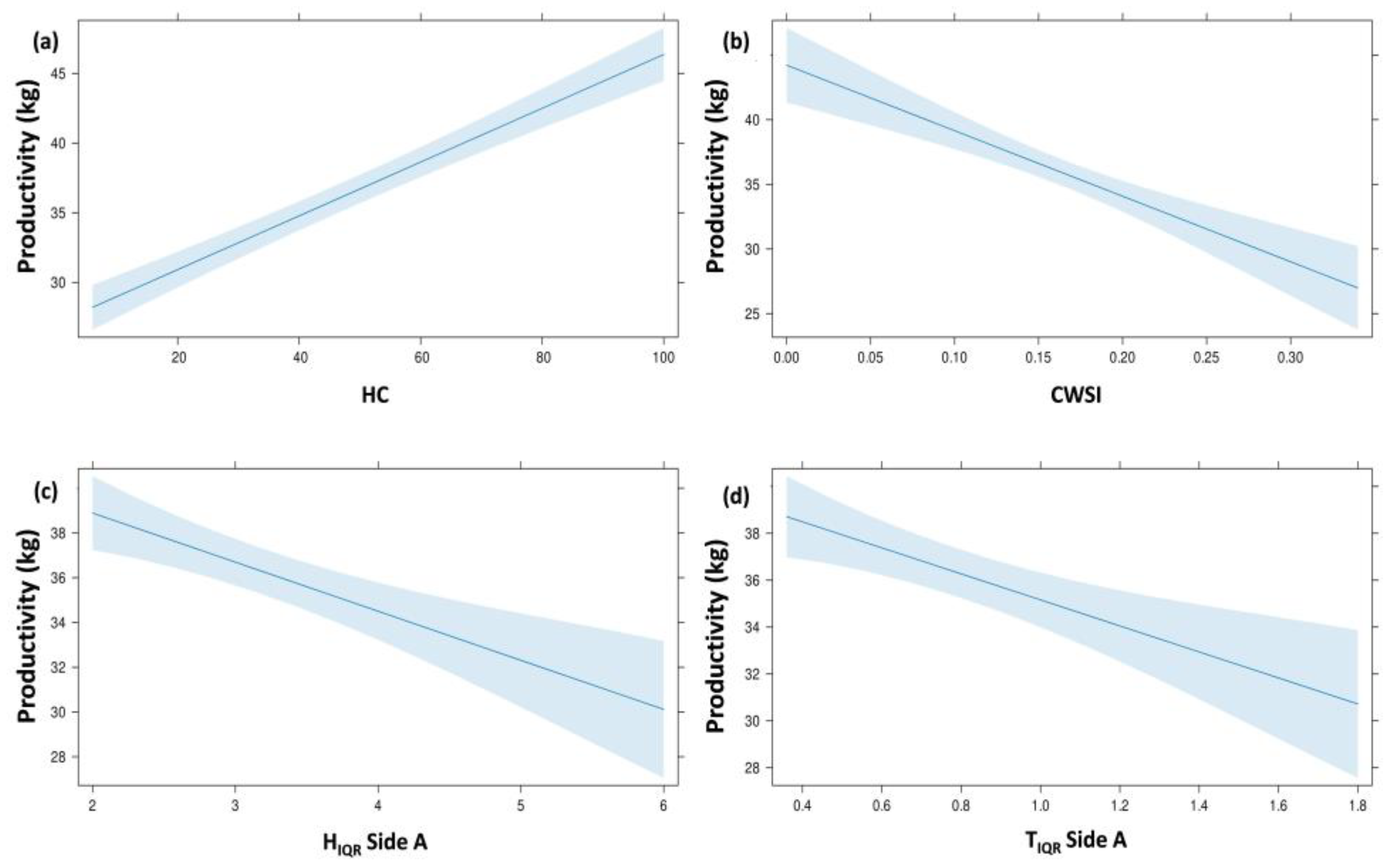
3.4. Influence of Phenotypic Traits, Thermal, and Humidity Indices on Productivity and CWSI
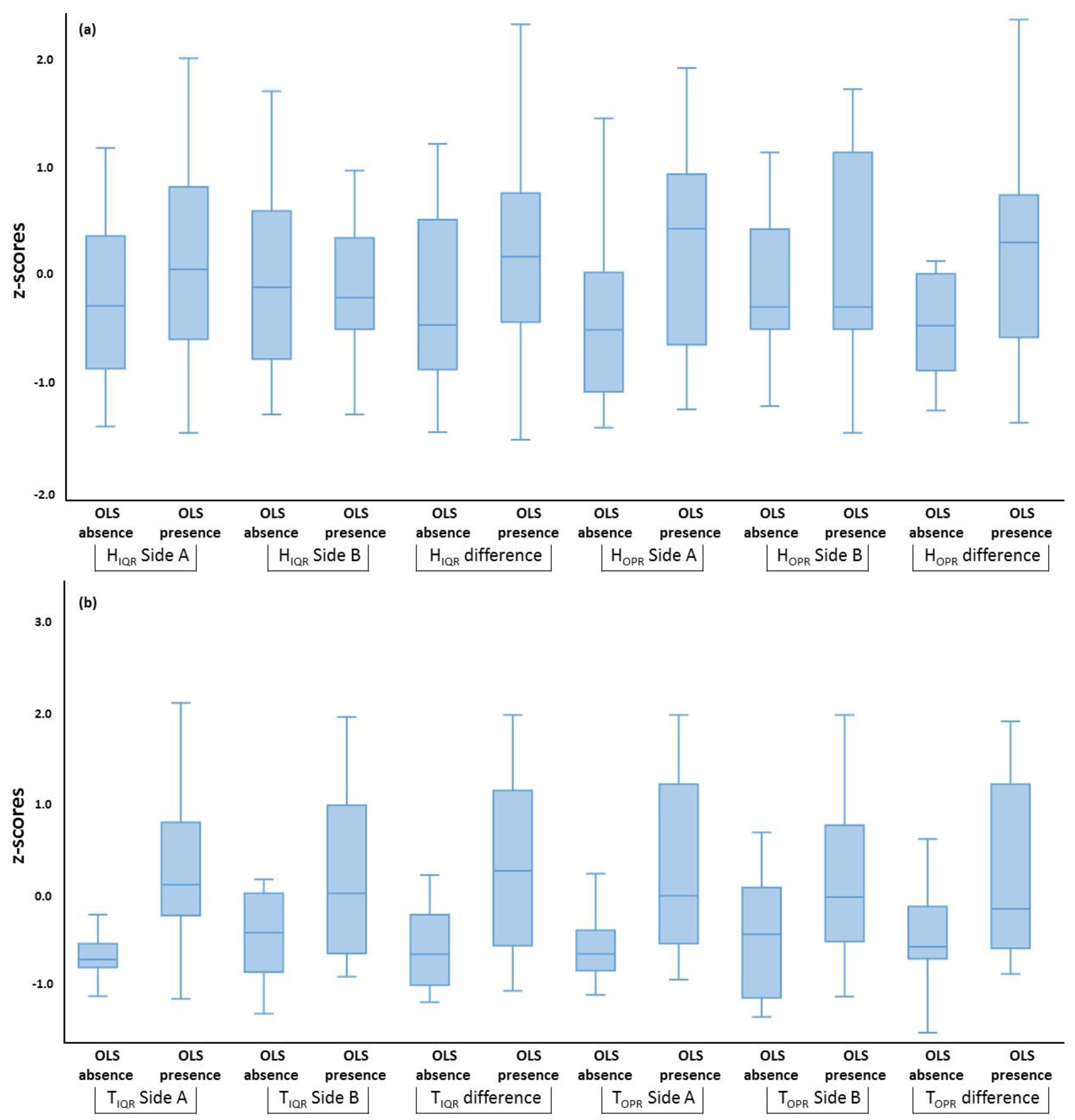
3.5. Assessing the Occurrence of OLS
3.6. Differences between Infected and Non-Infected Trees
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variables Set | Response Variable | Predictor Variables | B | SE B | β | t | p-Value | R2 adj. | F |
|---|---|---|---|---|---|---|---|---|---|
| Phenotypic traits | P 2017 | (constant) | 47.67 | 9.45 | 5.04 | 0.000 | 0.477 | 14.087 | |
| CA | −0.20 | 0.08 | −0.39 | −2.28 | 0.028 | ||||
| CR | −469.53 | 214.6 | −0.37 | −2.18 | 0.035 | ||||
| HC | 0.32 | 0.09 | 0.67 | 3.54 | 0.001 | ||||
| Thermal and humidity indices | P 2017 | (constant) | 61.71 | 4.30 | 14.33 | 0.001 | 0.513 | 19.543 | |
| TIQR difference | −5.55 | 1.88 | −0.33 | −2.94 | 0.005 | ||||
| HIQR difference | −30.10 | 6.43 | −0.53 | −4.67 | 0.001 | ||||
| Combined model | P 2017 | (constant) | 53.34 | 6.77 | 7.87 | 0.001 | 0.613 | 23.716 | |
| HIQR Side A | −19.74 | 6.01 | −0.35 | −3.28 | 0.002 | ||||
| TIQR difference | −4.43 | 1.43 | −0.32 | −3.08 | 0.004 | ||||
| HC | 0.17 | 0.05 | 0.36 | 3.31 | 0.002 |
| Variables Set | Response Variable | Predictor Variables | B | SE B | β | t | p-Value | R2 adj. | F |
|---|---|---|---|---|---|---|---|---|---|
| Phenotypic traits | P 2018 | (constant) | 35.25 | 6.55 | 5.38 | 0.001 | 0.295 | 9.995 | |
| HC | 0.11 | 0.05 | 0.32 | 2.34 | 0.024 | ||||
| CWSI | −80.10 | 28.56 | −0.38 | −2.80 | 0.008 | ||||
| Thermal and humidity indices | P 2018 | (constant) | 44.11 | 3.32 | 13.26 | 0.001 | 0.465 | 19.652 | |
| TIQR difference | −4.30 | 1.46 | −0.33 | −2.95 | 0.005 | ||||
| HIQR difference | −23.33 | 4.97 | −0.53 | −4.69 | 0.001 | ||||
| Combined model | P 2018 | (constant) | 30.87 | 4.96 | 6.22 | 0.001 | 0.586 | 13.164 | |
| TIQR difference | −28.76 | 8.86 | −0.66 | −3.24 | 0.002 | ||||
| TIQR canopy | −9.22 | 3.54 | −0.36 | −2.60 | 0.013 | ||||
| TIQR Side A | 36.97 | 9.78 | 1.06 | 3.77 | 0.001 | ||||
| TOPR Side A | −6.43 | 1.66 | −0.75 | −3.86 | 0.001 | ||||
| HC | 0.086 | 0.04 | 0.23 | 2.06 | 0.046 |
| Variables Set | Response Variable | Predictor Variables | B | SE B | β | t | p-Value | R2 adj. | F |
|---|---|---|---|---|---|---|---|---|---|
| Phenotypic traits | P 2019 | (constant) | 62.17 | 7.65 | 8.12 | 0.001 | 0.474 | 20.406 | |
| HC | 0.18 | 0.06 | 0.36 | 3.09 | 0.003 | ||||
| CWSI | −141.26 | 33.37 | −0.49 | −4.23 | 0.001 | ||||
| Thermal and humidity indices | P 2019 | (constant) | 65.96 | 5.22 | 12.64 | 0.001 | 0.296 | 9.442 | |
| HIQR difference | −4.74 | 2.40 | −0.275 | −1.97 | 0.05 | ||||
| TIQR canopy | −13.85 | 4.87 | −0.397 | −2.84 | 0.007 | ||||
| Combined model | P 2019 | (constant) | 73.74 | 9.12 | 8.07 | 0.001 | 0.511 | 12.246 | |
| HOPR Side A | −1.85 | 0.82 | −0.619 | −2.25 | 0.030 | ||||
| HOPR difference | 1.50 | 0.74 | 0.530 | 2.02 | 0.050 | ||||
| HC | 0.17 | 0.06 | 0.346 | 3.00 | 0.005 | ||||
| CWSI | −119.14 | 35.9 | −0.419 | −3.31 | 0.002 |
| Variables Set | Response Variable | Predictor Variables | B | SE B | β | t | p-Value | R2 adj. | F |
|---|---|---|---|---|---|---|---|---|---|
| Phenotypic traits | P 2020 | (constant) | 29.34 | 5.75 | 5.09 | 0.001 | 0.422 | 16.671 | |
| HC | 0.186 | 0.044 | 0.51 | 4.21 | 0.001 | ||||
| CWSI | −58.08 | 25.10 | −0.28 | −2.31 | 0.026 | ||||
| Thermal and humidity indices | P 2020 | (constant) | 60.46 | 6.43 | 9.40 | 0.001 | 0.374 | 9.563 | |
| HOPR Side A | −2.09 | 0.63 | −0.97 | −3.29 | 0.002 | ||||
| HOPR difference | 1.253 | 0.60 | 0.61 | 2.06 | 0.046 | ||||
| TIQR Side A | −13.36 | 4.17 | −0.39 | −3.20 | 0.003 | ||||
| Combined model | P 2020 | (constant) | 29.72 | 5.42 | 5.48 | 0.001 | 0.437 | 17.660 | |
| HOPR Side A | −0.66 | 0.26 | −0.311 | −2.56 | 0.014 | ||||
| HC | 0.18 | 0.04 | 0.511 | 4.22 | 0.001 |
References
- Myers, N.; Mittermeler, R.A.; Mittermeler, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Blondel, J. The “design” of Mediterranean landscapes: A millennial story of humans and ecological systems during the historic period. Hum. Ecol. 2006, 34, 713–729. [Google Scholar] [CrossRef]
- Stroosnijder, L.; Mansinho, M.I.; Palese, A.M. OLIVERO: The project analysing the future of olive production systems on sloping land in the Mediterranean basin. J. Environ. Manag. 2008, 89, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, D.I.; Brown, A.H.; Cuong, P.H.; Collado-Panduro, L.; Latournerie-Moreno, L.; Gyawali, S. 2008 A global perspective of the richness and evenness. Proc. Natl. Acad. Sci. USA 2008, 105, 5326–5331. [Google Scholar] [CrossRef]
- Achtak, H.; Ater, M.; Oukabli, A.; Santoni, S.; Kjellberg, F.; Khadari, B. Traditional agroecosystems as conservatories and incubators of cultivated plant varietal diversity: The case of fig (Ficus carica L.) in Morocco. BMC Plant Biol. 2010, 10, 28. [Google Scholar] [CrossRef]
- Altieri, M.A. The ecological role of biodiversity in agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 19–31. [Google Scholar] [CrossRef]
- Loumou, A.; Giourga, C. Olive groves: “The life and identity of the Mediterranean". Agric. Hum. Values 2003, 20, 87–95. [Google Scholar] [CrossRef]
- Kizos, T.; Dalaka, A.; Petanidou, T. Farmers’ attitudes and landscape change: Evidence from the abandonment of terraced cultivations on Lesvos, Greece. Agric. Hum. Values 2010, 27, 199–212. [Google Scholar] [CrossRef]
- Solomou, A.; Sfougaris, A. Comparing conventional and organic olive groves in central Greece: Plant and bird diversity and abundance. Renew. Agric. Food Syst. 2011, 26, 297–316. [Google Scholar] [CrossRef]
- Keenleyside, C.; Beaufoy, G.; Tucker, G.; Jones, G. High Nature Value Farming Throughout EU-27 and Its Financial Support under the CAP. 2014; Report Prepared for DG Environment, Contract No ENV B.1/ETU//0035; Institute for European Environmental Policy: London, UK, 2014. [Google Scholar]
- Benton, T.G.; Vickery, J.A.; Wilson, J.D. Farmland biodiversity: Is habitat heterogeneity the key? Trends Ecol. Evol. 2003, 18, 182–188. [Google Scholar] [CrossRef]
- Fleskens, L. Conservation Scenarios for Olive Farming on Sloping Land in the Mediterranean; Wageningen University and Research: Wageningen, The Netherlands, 2007; ISBN 9789085047179. [Google Scholar]
- Morgado, R.; Santana, J.; Porto, M.; Sánchez-Oliver, J.S.; Reino, L.; Herrera, J.M.; Rego, F.; Beja, P.; Moreira, F. A Mediterranean silent spring? The effects of olive farming intensification on breeding bird communities. Agric. Ecosyst. Environ. 2020, 288, 106694. [Google Scholar] [CrossRef]
- Ben Abdallah, S.; Parra-López, C.; Elfkih, S.; Suárez-Rey, E.M.; Romero-Gámez, M. Sustainability assessment of traditional, intensive and highly-intensive olive growing systems in Tunisia by integrating Life Cycle and Multicriteria Decision analyses. Sustain. Prod. Consum. 2022, 33, 73–87. [Google Scholar] [CrossRef]
- Caraveli, H. A comparative analysis on intensification and extensification in mediterranean agriculture: Dilemmas for LFAs policy. J. Rural Stud. 2000, 16, 231–242. [Google Scholar] [CrossRef]
- Pienkowski, M.; Beaufoy, G. The Environmental Impact of Olive Oil Production in the European Union: Practical Options for Improving the Environmental Impact; European Forum on Nature Conservation and Pastoralism (EFNCP): Peterborough, UK, 2002; pp. 1–73. [Google Scholar]
- Donald, P.F.; Green, R.E.; Heath, M.F. Agricultural intensification and the collapse of Europe’s farmland bird populations. Proc. R. Soc. B Biol. Sci. 2001, 268, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity—Ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Rescia, A.J.; Willaarts, B.A.; Schmitz, M.F.; Aguilera, P.A. Changes in land uses and management in two Nature Reserves in Spain: Evaluating the social-ecological resilience of cultural landscapes. Landsc. Urban Plan. 2010, 98, 26–35. [Google Scholar] [CrossRef]
- Sokos, C.K.; Mamolos, A.P.; Kalburtji, K.L.; Birtsas, P.K. Farming and wildlife in Mediterranean agroecosystems. J. Nat. Conserv. 2013, 21, 81–92. [Google Scholar] [CrossRef]
- Modica, G.; Praticò, S.; Di Fazio, S. Abandonment of traditional terraced landscape: A change detection approach (a case study in Costa Viola, Calabria, Italy). L. Degrad. Dev. 2017, 28, 2608–2622. [Google Scholar] [CrossRef]
- Tarifa, R.; Martínez-Núñez, C.; Valera, F.; González-Varo, J.P.; Salido, T.; Rey, P.J. Agricultural intensification erodes taxonomic and functional diversity in Mediterranean olive groves by filtering out rare species. J. Appl. Ecol. 2021, 58, 2266–2276. [Google Scholar] [CrossRef]
- Schicchi, R.; Speciale, C.; Amato, F.; Bazan, G.; Di Noto, G.; Marino, P.; Ricciardo, P.; Geraci, A. The Monumental Olive Trees as Biocultural Heritage of Mediterranean Landscapes: The Case Study of Sicily. Sustainability 2021, 13, 6767. [Google Scholar] [CrossRef]
- Pointereau, P.; Paracchini, M.L.; Terres, J.-M.; Jiguet, F.; Bas, Y.; Biala, K. Identification of High Nature Value Farmland in France through Statistical Information and Farm Practice Surveys; Report EUR 22786 EN.; Publications Office of the EU: Luxembourg, 2007; ISBN 9789279064753. [Google Scholar]
- Beaufoy, G.; Cooper, T. The Application of the High Nature Value Impact Indicator. 2009. Available online: https://www.men-d.de/uploads/media/guidance_document_application_HNV_impact_indicator_annex.pdf (accessed on 26 September 2023).
- Duarte, F.; Jones, N.; Fleskens, L. Traditional olive orchards on sloping land: Sustainability or abandonment? J. Environ. Manag. 2008, 89, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Brunori, E.; Salvati, L.; Antogiovanni, A.; Biasi, R. Worrying about “vertical landscapes”: Terraced olive groves and ecosystem services in marginal land in central Italy. Sustainability 2018, 10, 1164. [Google Scholar] [CrossRef]
- Solomou, A.D.; Sfougaris, A.I.; Vavoulidou, E.M.; Csuzdi, C. Species Richness and Density of Earthworms in Relation to Soil Factors in Olive Orchard Production Systems in Central Greece. Commun. Soil Sci. Plant Anal. 2013, 44, 301–311. [Google Scholar] [CrossRef]
- Camarsa, G.; Gardner, S.; Jones, W.; Eldridge, J.; Hudson, T.; Thorpe, E.; O’Hara, E. LIFE among the Olives—Good Practice in Improving Environmental Performance in the Olive Oil Sector; European Commission, Environment Directorate-General. European Union: Brussels, Belgium, 2010; ISBN 9789279141546. [Google Scholar]
- Pellegrini, G.; Ingrao, C.; Camposeo, S.; Tricase, C.; Contò, F.; Huisingh, D. Application of water footprint to olive growing systems in the Apulia region: A comparative assessment. J. Clean. Prod. 2016, 112, 2407–2418. [Google Scholar] [CrossRef]
- Mairech, H.; López-Bernal, Á.; Moriondo, M.; Dibari, C.; Regni, L.; Proietti, P.; Villalobos, F.J.; Testi, L. Is new olive farming sustainable? A spatial comparison of productive and environmental performances between traditional and new olive orchards with the model OliveCan. Agric. Syst. 2020, 181, 102816. [Google Scholar] [CrossRef]
- Mairech, H.; López-Bernal, Á.; Moriondo, M.; Dibari, C.; Regni, L.; Proietti, P.; Villalobos, F.J.; Testi, L. Sustainability of olive growing in the Mediterranean area under future climate scenarios: Exploring the effects of intensification and deficit irrigation. Eur. J. Agron. 2021, 129, 126319. [Google Scholar] [CrossRef]
- Davy, C.M.; Russo, D.; Fenton, M.B. Use of native woodlands and traditional olive groves by foraging bats on a Mediterranean island: Consequences for conservation. J. Zool. 2007, 273, 397–405. [Google Scholar] [CrossRef]
- Fleskens, L.; Duarte, F.; Eicher, I. A conceptual framework for the assessment of multiple functions of agro-ecosystems: A case study of Trás-os-Montes olive groves. J. Rural Stud. 2009, 25, 141–155. [Google Scholar] [CrossRef]
- Bernabei, M. The age of the olive trees in the Garden of Gethsemane. J. Archaeol. Sci. 2015, 53, 43–48. [Google Scholar] [CrossRef]
- Gomes, T.; Pereira, J.A.; Moya-Laraño, J.; Poveda, J.; Lino-Neto, T.; Baptista, P. Deciphering plant health status: The link between secondary metabolites, fungal community and disease incidence in olive tree. Front. Plant Sci. 2023, 14, 1048762. [Google Scholar] [CrossRef]
- Shigo, A.L.; Marx, H.G. Compartmentalization of Decay in Trees; Compartmentalization of Decay in Trees (No. 405); Department of Agriculture, Forest Service: Washington, DC, USA, 1977. [Google Scholar]
- Boddy, L. Fungal Community Ecology and Wood Decomposition Processes in Angiosperms: From Standing Tree to Complete Decay of Coarse Woody Debris. Ecol. Bull. 2001, 49, 43–56. [Google Scholar]
- Rose, C.L.; Marcot, B.G.; Mellen, T.K.; Ohmann, J.L.; Waddell, K.L.; Lindley, D.L.; Schreiber, B. Decaying wood in Pacific Northwest forests: Concepts and tools for habitat management. In Wildlife-Habitat Relationships in Oregon and Washington; Oregon State University Press: Corvallis, OR, USA, 2001; pp. 580–623. [Google Scholar]
- Carpio, A.J.; Castro, J.; Mingo, V.; Tortosa, F.S. Herbaceous cover enhances the squamate reptile community in woody crops. J. Nat. Conserv. 2017, 37, 31–38. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Laurance, W.F. The ecology, distribution, conservation and management of large old trees. Biol. Rev. 2017, 92, 1434–1458. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.; Berg, C.; Landa, B.B.; Auerbach, A.; Moissl-Eichinger, C.; Berg, G. Plant genotype-specific archaeal and bacterial endophytes but similar Bacillus antagonists colonize Mediterranean olive trees. Front. Microbiol. 2015, 6, 138. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, P.; Lindenmayer, D. Tree Hollows and Wildlife Conservation in Australia; CSIRO Publishing: Collingwood, Austria, 2002. [Google Scholar]
- Johnstone, D.; Moore, G.; Tausz, M.; Nicolas, M. The Measurement of Wood Decay in Landscape Trees. Arboric. Urban For. 2010, 36, 121–127. [Google Scholar] [CrossRef]
- Soge, A.O.; Popoola, O.I.; Adetoyinbo, A.A. A four-point electrical resistivity method for detecting wood decay and hollows in living trees. Eur. J. Wood Wood Prod. 2019, 77, 465–474. [Google Scholar] [CrossRef]
- Zevgolis, Y.G.; Kamatsos, E.; Akriotis, T.; Dimitrakopoulos, P.G.; Troumbis, A.Y. Estimating Productivity, Detecting Biotic Disturbances, and Assessing the Health State of Traditional Olive Groves, Using Nondestructive Phenotypic Techniques. Sustainability 2021, 14, 391. [Google Scholar] [CrossRef]
- Van Hees, A.F.M. Growth and morphology of pedunculate oak (Quercus robur L) and beech (Fagus sylvatica L.) seedlings in relation to shading and drought. Ann. For. Sci. 1997, 54, 9–18. [Google Scholar] [CrossRef]
- López-Bernal, Á.; Alcántara, E.; Testi, L.; Villalobos, F.J. Spatial sap flow and xylem anatomical characteristics in olive trees under different irrigation regimes. Tree Physiol. 2010, 30, 1536–1544. [Google Scholar] [CrossRef]
- Johnson, D.M.; Wortemann, R.; McCulloh, K.A.; Jordan-Meille, L.; Ward, E.; Warren, J.M.; Palmroth, S.; Domec, J.C. A test of the hydraulic vulnerability segmentation hypothesis in angiosperm and conifer tree species. Tree Physiol. 2016, 36, 983–993. [Google Scholar] [CrossRef]
- Heilmann-Clausen, J.; Boddy, L. Chapter 14 Distribution patterns of wood-decay basidiomycetes at the landscape to global scale. In British Mycological Society Symposia Series; Academic Press: Cambridge, MA, USA, 2008; Volume 28, pp. 263–275. [Google Scholar]
- LaMontagne, J.M.; Kilgour, R.J.; Anderson, E.C.; Magle, S. Tree cavity availability across forest, park, and residential habitats in a highly urban area. Urban Ecosyst. 2015, 18, 151–167. [Google Scholar] [CrossRef]
- International Olive Council. Production Techniques in Olive Growing; IOC: Madrid, Spain, 2007; ISBN 9788493166366. [Google Scholar]
- Salman, M. Biological control of Spilocaea oleagina, the causal agent of olive leaf spot disease, using antagonistic bacteria. J. Plant Pathol. 2017, 99, 741–744. [Google Scholar] [CrossRef]
- López-Doncel, L.M.; Viruega Puente, J.R.; Trapero Casas, A. Respuesta del olivo a la inoculación con Spilocaea oleagina, agente del repilo. Bol. Sanid. Veg. Plagas 2000, 26, 349–364. [Google Scholar]
- Sanei, S.J.; Razavi, S.E. Survey of Spilocaea oleagina, causal agent of olive leaf spot, in North of Iran. J. Yeast. Fungal. Res. 2011, 2, 33–38. [Google Scholar] [CrossRef]
- Obanor, F.O.; Walter, M.; Jones, E.E.; Jaspers, M.V. Efficacy of systemic acquired resistance inducers in olive leaf spot management. Australas. Plant Pathol. 2013, 42, 163–168. [Google Scholar] [CrossRef]
- Tognetti, R.; Andria, R.D.; Sacchi, R.; Lavini, A.; Morelli, G.; Alvino, A. Deficit irrigation affects seasonal changes in leaf physiology and oil quality of Olea europaea (cultivars Frantoio and Leccino). Ann. Appl. Biol. 2007, 150, 169–186. [Google Scholar] [CrossRef]
- Connor, D.J.; Gómez-del-Campo, M.; Rousseaux, M.C.; Searles, P.S. Structure, management and productivity of hedgerow olive orchards: A review. Sci. Hortic. 2014, 169, 71–93. [Google Scholar] [CrossRef]
- Silveira, C.; Almeida, A.; Ribeiro, A.C. Technological Innovation in the Traditional Olive Orchard Management: Advances and Opportunities to the Northeastern Region of Portugal. Water 2022, 14, 4081. [Google Scholar] [CrossRef]
- Bacelar, E.A.; Correia, C.M.; Moutinho-Pereira, J.M.; Goncalves, B.C.; Lopes, J.I.; Torres-Pereira, J.M.G. Sclerophylly and leaf anatomical traits of five field-grown olive cultivars growing under drought conditions. Tree Physiol. 2004, 24, 233–239. [Google Scholar] [CrossRef]
- Sofo, A.; Manfreda, S.; Fiorentino, M.; Dichio, B.; Xiloyannis, C. The olive tree: A paradigm for drought tolerance in Mediterranean climates. Hydrol. Earth Syst. Sci. 2008, 12, 293–301. [Google Scholar] [CrossRef]
- Palese, A.M.; Nuzzo, V.; Favati, F.; Pietrafesa, A.; Celano, G.; Xiloyannis, C. Effects of water deficit on the vegetative response, yield and oil quality of olive trees (Olea europaea L., cv Coratina) grown under intensive cultivation. Sci. Hortic. 2010, 125, 222–229. [Google Scholar] [CrossRef]
- Maccherini, S.; Santi, E.; Bonini, I.; Amici, V.; Pruscini, S.; Palazzo, D.; Selva, F.C. The impact of land abandonment on the plant diversity of olive groves. Biodivers. Conserv. 2013, 22, 3067–3083. [Google Scholar] [CrossRef]
- Motilva, M.J.; Tovar, M.J.; Romero, M.P.; Alegre, S.; Girona, J. Influence of regulated deficit irrigation strategies applied to olive trees (Arbequina cultivar) on oil yield and oil composition during the fruit ripening period. J. Sci. Food Agric. 2000, 80, 2037–2043. [Google Scholar] [CrossRef]
- Kozlowski, T.T.; Kramer, P.J.; Pallardy, S.G. The Physiological Ecology of Woody Plants; Academic Press Inc.: Cambridge, MA, USA, 2012. [Google Scholar]
- Goh, C.L.; Abdul Rahim, R.; Fazalul Rahiman, M.H.; Mohamad Talib, M.T.; Tee, Z.C. Sensing wood decay in standing trees: A review. Sens. Actuators A Phys. 2018, 269, 276–282. [Google Scholar] [CrossRef]
- Varo, A.; Moral, J.; Lozano-Tóvar, M.D.; Trapero, A. Development and validation of an inoculation method to assess the efficacy of biological treatments against Verticillium wilt in olive trees. BioControl 2016, 61, 283–292. [Google Scholar] [CrossRef]
- Al Bashish, D.; Braik, M.; Bani-Ahmad, S. Detection and Classification of Leaf Diseases using K-means-based Segmentation and Neural-networks-based Classification. Inf. Technol. J. 2011, 10, 267–275. [Google Scholar] [CrossRef]
- Fang, Y.; Ramasamy, R. Current and Prospective Methods for Plant Disease Detection. Biosensors 2015, 5, 537–561. [Google Scholar] [CrossRef]
- Khaled, A.Y.; Abd Aziz, S.; Bejo, S.K.; Nawi, N.M.; Seman, I.A.; Onwude, D.I. Early detection of diseases in plant tissue using spectroscopy—Applications and limitations. Appl. Spectrosc. Rev. 2018, 53, 36–64. [Google Scholar] [CrossRef]
- Al Hagrey, S.A. Geophysical imaging of root-zone, trunk, and moisture heterogeneity. J. Exp. Bot. 2007, 58, 839–854. [Google Scholar] [CrossRef]
- Blekos, K.; Tsakas, A.; Xouris, C.; Evdokidis, I.; Alexandropoulos, D.; Alexakos, C.; Katakis, S.; Makedonas, A.; Theoharatos, C.; Lalos, A. Analysis, modeling and multi-spectral sensing for the predictive management of verticillium wilt in olive groves. J. Sens. Actuator Netw. 2021, 10, 15. [Google Scholar] [CrossRef]
- Buja, I.; Sabella, E.; Monteduro, A.G.; Chiriacò, M.S.; De Bellis, L.; Luvisi, A.; Maruccio, G. Advances in Plant Disease Detection and Monitoring: From Traditional Assays to In-Field Diagnostics. Sensors 2021, 21, 2129. [Google Scholar] [CrossRef]
- Fernández, J.E.; Torres-Ruiz, J.M.; Diaz-Espejo, A.; Montero, A.; Álvarez, R.; Jiménez, M.D.; Cuerva, J.; Cuevas, M.V. Use of maximum trunk diameter measurements to detect water stress in mature ‘Arbequina’ olive trees under deficit irrigation. Agric. Water Manag. 2011, 98, 1813–1821. [Google Scholar] [CrossRef]
- Aissaoui, F.; Chehab, H.; Bader, B.; Salem, B.A.; M’barki, N.; Laamari, S.; Chihaoui, B.; Mahjoub, Z.; Boujnah, D. Early water stress detection on olive trees (Olea europaea L. cvs ‘chemlali’ and ‘Chetoui’) using the leaf patch clamp pressure probe. Comput. Electron. Agric. 2016, 131, 20–28. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, Z.; Li, D.; Liu, X. Phenotypic techniques and applications in fruit trees: A review. Plant Methods 2020, 16, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Catena, A.; Catena, G.; Lugaresi, D.; Gasperoni, R.L. Termografia rivela la presenza di danni anche nell’apparato radicale degli alberi. Agric. Ric. 2002, 81–100. [Google Scholar]
- Bellett-Travers, M.; Morris, S. The relationship between surface temperature and radial wood thickness of twelve trees harvested in nottinghamshire. Arboric. J. 2010, 33, 15–26. [Google Scholar] [CrossRef]
- Humplík, J.F.; Lazár, D.; Husičková, A.; Spíchal, L. Automated phenotyping of plant shoots using imaging methods for analysis of plant stress responses—A review. Plant Methods 2015, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Al-doski, J.; Shattri, B.M.; Helmi-Zulhai, B.M.-S. Thermal Imaging for Pests Detecting—A Review. Int. J. Agric. For. Plant. 2016, 2, 10–30. [Google Scholar]
- Asner, G.P.; Martin, R.E.; Keith, L.M.; Heller, W.P.; Hughes, M.A.; Vaughn, N.R.; Hughes, R.F.; Balzotti, C. A spectral mapping signature for the Rapid Ohia Death (ROD) pathogen in Hawaiian forests. Remote Sens. 2018, 10, 404. [Google Scholar] [CrossRef]
- Catena, A.; Catena, G. Overview of thermal imaging for tree assessment. Arboric. J. 2008, 30, 259–270. [Google Scholar] [CrossRef]
- Zevgolis, Y.G.; Akriotis, T.; Dimitrakopoulos, P.G.; Troumbis, A.Y. Integrating Thermal Indices and Phenotypic Traits for Assessing Tree Health: A Comprehensive Framework for Conservation and Monitoring of Urban, Agricultural, and Forest Ecosystems. Appl. Sci. 2023, 13, 9493. [Google Scholar] [CrossRef]
- Sepulcre-Cantó, G.; Zarco-Tejada, P.J.; Jiménez-Muñoz, J.C.; Sobrino, J.A.; De Miguel, E.; Villalobos, F.J. Detection of water stress in an olive orchard with thermal remote sensing imagery. Agric. For. Meteorol. 2006, 136, 31–44. [Google Scholar] [CrossRef]
- Ben-Gal, A.; Agam, N.; Alchanatis, V.; Cohen, Y.; Yermiyahu, U.; Zipori, I.; Presnov, E.; Sprintsin, M.; Dag, A. Evaluating water stress in irrigated olives: Correlation of soil water status, tree water status, and thermal imagery. Irrig. Sci. 2009, 27, 367–376. [Google Scholar] [CrossRef]
- Agam, N.; Cohen, Y.; Berni, J.A.J.; Alchanatis, V.; Kool, D.; Dag, A.; Yermiyahu, U.; Ben-Gal, A. An insight to the performance of crop water stress index for olive trees. Agric. Water Manag. 2013, 118, 79–86. [Google Scholar] [CrossRef]
- Egea, G.; Padilla-Díaz, C.M.; Martinez-Guanter, J.; Fernández, J.E.; Pérez-Ruiz, M. Assessing a crop water stress index derived from aerial thermal imaging and infrared thermometry in super-high density olive orchards. Agric. Water Manag. 2017, 187, 210–221. [Google Scholar] [CrossRef]
- Terho, M. An assessment of decay among urban Tilia, Betula, and Acer trees felled as hazardous. Urban For. Urban Green. 2009, 8, 77–85. [Google Scholar] [CrossRef]
- Zevgolis, Y.G.; Alsamail, M.Z.; Akriotis, T.; Dimitrakopoulos, P.G.; Troumbis, A.Y. Detecting, quantifying, and mapping urban trees’ structural defects using infrared thermography: Implications for tree risk assessment and management. Urban For. Urban Green. 2022, 75, 127691. [Google Scholar] [CrossRef]
- Hunt, J.F.; Gu, H.; Lebow, P.K. Theoretical thermal conductivity equation for uniform density wood cells. Wood Fiber Sci. 2008, 40, 167–180. [Google Scholar]
- Pitarma, R.; Crisóstomo, J.; Ferreira, M.E. Contribution to trees health assessment using infrared thermography. Agriculture 2019, 9, 171. [Google Scholar] [CrossRef]
- Ishimwe, R.; Abutaleb, K.; Ahmed, F. Applications of Thermal Imaging in Agriculture—A Review. Adv. Remote Sens. 2014, 3, 128–140. [Google Scholar] [CrossRef]
- Still, C.; Powell, R.; Aubrecht, D.; Kim, Y.; Helliker, B.; Roberts, D.; Richardson, A.D.; Goulden, M. Thermal imaging in plant and ecosystem ecology: Applications and challenges. Ecosphere 2019, 10, e02768. [Google Scholar] [CrossRef]
- Lapidot, O.; Ignat, T.; Rud, R.; Rog, I.; Alchanatis, V.; Klein, T. Use of thermal imaging to detect evaporative cooling in coniferous and broadleaved tree species of the Mediterranean maquis. Agric. For. Meteorol. 2019, 271, 285–294. [Google Scholar] [CrossRef]
- HNMS Climatic Data for Selected Stations in Greece, Hellenic National Meteorological Service Climatic Data for Selected Stations in Greece. Available online: http://www.emy.gr/emy/en/climatology/climatology_month (accessed on 31 October 2022).
- Kostelenos, G.; Kiritsakis, A. Olive Tree History and Evolution. In Olives and Olive Oil as Functional Foods; Wiley: Hoboken, NJ, USA, 2017; pp. 1–12. ISBN 9781119135340. [Google Scholar]
- Bazakos, C.; Alexiou, K.G.; Ramos-Onsins, S.; Koubouris, G.; Tourvas, N.; Xanthopoulou, A.; Mellidou, I.; Moysiadis, T.; Vourlaki, I.; Metzidakis, I.; et al. Whole genome scanning of a Mediterranean basin hotspot collection provides new insights into olive tree biodiversity and biology. Plant J. 2023, 116, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.; Casal, S.; Pinho, T.; Peres, A.M.; Bento, A.; Baptista, P.; Pereira, J.A. Ancient olive trees as a source of olive oils rich in phenolic compounds. Food Chem. 2019, 276, 231–239. [Google Scholar] [CrossRef]
- Arnan, X.; López, B.C.; Martínez-Vilalta, J.; Estorach, M.; Poyatos, R. The age of monumental olive trees (Olea europaea) in northeastern Spain. Dendrochronologia 2012, 30, 11–14. [Google Scholar] [CrossRef]
- Fabbri, A.; Bartolini, G.; Lambardi, M.; Kailis, S.G. Olive Propagation Manual; CSIRO Publishing: Collingwood, Australia, 2004; ISBN 0-643-06676-4. [Google Scholar]
- Plasquy, E.; Sola-Guiraldo, R.R.; Florido, M.d.C.; García, J.M.; Blanco-Roldán, G. Evaluation of a manual olive fruit harvester for small producers. Res. Agric. Eng. 2019, 65, 105–111. [Google Scholar] [CrossRef]
- Zevgolis, Y.G.; Christopoulos, A. Entrapped in Olive-Harvesting Nets: A Case of a Grass Snake Natrix natrix from an Olive-Growing Greek Aegean Island. Diversity 2023, 15, 452. [Google Scholar] [CrossRef]
- Obanor, F.O.; Jaspers, M.V.; Jones, E.E.; Walter, M. Greenhouse and field evaluation of fungicides for control of olive leaf spot in New Zealand. Crop Prot. 2008, 27, 1335–1342. [Google Scholar] [CrossRef]
- Jackson, R.D.; Idso, S.B.; Reginato, R.J.; Pinter, P.J. Canopy temperature as a crop water stress indicator. Water Resour. Res. 1981, 17, 1133–1138. [Google Scholar] [CrossRef]
- Jackson, R.D. Canopy Temperature and Crop Water Stress; Elsevier: Amsterdam, The Netherlands, 1982; Volume 1, pp. 43–85. [Google Scholar]
- Idso, S.B.; Reginato, R.J.; Jackson, R.D.; Pinter, P.J. Measuring yield-reducing plant water potential depressions in wheat by infrared thermometry. Irrig. Sci. 1981, 2, 205–212. [Google Scholar] [CrossRef]
- Jackson, R.D.; Kustas, W.P.; Choudhury, B.J. A reexamination of the crop water stress index. Irrig. Sci. 1988, 9, 309–317. [Google Scholar] [CrossRef]
- Jones, H.G. Use of infrared thermometry for estimation of stomatal conductance as a possible aid to irrigation scheduling. Agric. For. Meteorol. 1999, 95, 139–149. [Google Scholar] [CrossRef]
- Minkina, W.; Dudzik, S. Algorithm of Infrared Camera Measurement Processing Path. In Infrared Thermography; John Wiley & Sons, Ltd: Chichester, UK, 2009; pp. 41–60. ISBN 9780470747186. [Google Scholar]
- Faye, E.; Dangles, O.; Pincebourde, S. Distance makes the difference in thermography for ecological studies. J. Therm. Biol. 2016, 56, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Irmak, S.; Haman, D.Z.; Bastug, R. Determination of Crop Water Stress Index for Irrigation Timing and Yield Estimation of Corn. Agron. J. 2000, 92, 1221–1227. [Google Scholar] [CrossRef]
- Moller, M.; Alchanatis, V.; Cohen, Y.; Meron, M.; Tsipris, J.; Naor, A.; Ostrovsky, V.; Sprintsin, M.; Cohen, S. Use of thermal and visible imagery for estimating crop water status of irrigated grapevine. J. Exp. Bot. 2006, 58, 827–838. [Google Scholar] [CrossRef]
- Cohen, Y.; Alchanatis, V.; Meron, M.; Saranga, Y.; Tsipris, J. Estimation of leaf water potential by thermal imagery and spatial analysis*. J. Exp. Bot. 2005, 56, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Grant, O.M.; Tronina, Ł.; Jones, H.G.; Chaves, M.M. Exploring thermal imaging variables for the detection of stress responses in grapevine under different irrigation regimes. J. Exp. Bot. 2007, 58, 815–825. [Google Scholar] [CrossRef]
- Briscoe, N.J.; Handasyde, K.A.; Griffiths, S.R.; Porter, W.P.; Krockenberger, A.; Kearney, M.R. Tree-hugging koalas demonstrate a novel thermoregulatory mechanism for arboreal mammals. Biol. Lett. 2014, 10, 20140235. [Google Scholar] [CrossRef]
- Lavee, S. Biennial bearing in olive (Olea europaea). Annales 2007, 17, 102–112. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Asase, A.; Tetteh, D.A. Tree diversity, carbon stocks, and soil nutrients in cocoa-dominated and mixed food crops agroforestry systems compared to natural forest in southeast Ghana. Agroecol. Sustain. Food Syst. 2016, 40, 96–113. [Google Scholar] [CrossRef]
- Torralba, M.; Fagerholm, N.; Burgess, P.J.; Moreno, G.; Plieninger, T. Do European agroforestry systems enhance biodiversity and ecosystem services? A meta-analysis. Agric. Ecosyst. Environ. 2016, 230, 150–161. [Google Scholar] [CrossRef]
- Henle, K.; Alard, D.; Clitherow, J.; Cobb, P.; Firbank, L.; Kull, T.; McCracken, D.; Moritz, R.F.A.; Niemelä, J.; Rebane, M.; et al. Identifying and managing the conflicts between agriculture and biodiversity conservation in Europe-A review. Agric. Ecosyst. Environ. 2008, 124, 60–71. [Google Scholar] [CrossRef]
- Plieninger, T.; Bieling, C. Resilience-based perspectives to guiding high-nature-value farmland through socioeconomic change. Ecol. Soc. 2013, 18, 15. [Google Scholar] [CrossRef]
- Fekete, R.; Vincze, O.; Süveges, K.; Bak, H.; Malkócs, T.; Löki, V.; Urgyán, R.; Molnár, V.A. The role of olive groves in the conservation of Mediterranean orchids. Glob. Ecol. Conserv. 2023, 44, e02490. [Google Scholar] [CrossRef]
- Vidal, D.; Pitarma, R. Infrared thermography applied to tree health assessment: A review. Agriculture 2019, 9, 156. [Google Scholar] [CrossRef]
- Zevgolis, Y.G.; Sazeides, C.I.; Zannetos, S.P.; Grammenou, V.; Fyllas, N.M.; Akriotis, T.; Dimitrakopoulos, P.G.; Troumbis, A.Y. Investigating the effect of resin collection and detecting fungal infection in resin-tapped and non-tapped pine trees, using minimally invasive and non-invasive diagnostics. For. Ecol. Manag. 2022, 524, 120498. [Google Scholar] [CrossRef]
- Catena, G. A new application of thermography. Atti Della Fond. Giorgio Ronchi 1990, 45, 947–952. [Google Scholar]
- Nunak, T.; Nunak, N.; Tipsuwanporn, V.; Suesut, T. Surrounding effects on temperature and emissivity measurement of equipment in electrical distribution system. Lect. Notes Eng. Comput. Sci. 2015, 2219, 292–296. [Google Scholar]
- Pineda, M.; Barón, M.; Pérez-Bueno, M.-L. Thermal Imaging for Plant Stress Detection and Phenotyping. Remote Sens. 2020, 13, 68. [Google Scholar] [CrossRef]
- Ibarra-Castanedo, C.; Bendada, A.; Maldague, X.P. Infrared vision applications for the nondestructive testing of materials. In Proceedings of the 5th Pan American Conference for NDT, Cancun, Mexico, 2–6 October 2011. [Google Scholar]
- Usamentiaga, R.; Venegas, P.; Guerediaga, J.; Vega, L.; Molleda, J.; Bulnes, F.G. Infrared thermography for temperature measurement and non-destructive testing. Sensors 2014, 14, 12305–12348. [Google Scholar] [CrossRef] [PubMed]
- Omran, E.S.E. Early sensing of peanut leaf spot using spectroscopy and thermal imaging. Arch. Agron. Soil Sci. 2017, 63, 883–896. [Google Scholar] [CrossRef]
- Domec, J.C.; Lachenbruch, B.; Pruyn, M.L.; Spicer, R. Effects of age-related increases in sapwood area, leaf area, and xylem conductivity on height-related hydraulic costs in two contrasting coniferous species. Ann. For. Sci. 2012, 69, 17–27. [Google Scholar] [CrossRef]
- Burcham, D.C.; Leong, E.C.; Fong, Y.K.; Tan, P.Y. An evaluation of internal defects and their effect on trunk surface temperature in Casuarina equisetifolia L. (Casuarinaceae). Arboric. Urban For. 2012, 38, 277–286. [Google Scholar] [CrossRef]
- Leong, E.C.; Burcham, D.C.; Fong, Y.K. A purposeful classification of tree decay detection tools. Arboric. J. 2012, 34, 91–115. [Google Scholar] [CrossRef]
- Kim, Y.; Rahardjo, H.; Tsen-Tieng, D.L. Stability analysis of laterally loaded trees based on tree-root-soil interaction. Urban For. Urban Green. 2020, 49, 126639. [Google Scholar] [CrossRef]
- Kim, Y.; Rahardjo, H.; Tsen-Tieng, D.L. Mechanical behavior of trees with structural defects under lateral load: A numerical modeling approach. Urban For. Urban Green. 2021, 59, 126987. [Google Scholar] [CrossRef]
- Paoletti, A.; Rosati, A.; Famiani, F. Effects of cultivar, fruit presence and tree age on whole-plant dry matter partitioning in young olive trees. Heliyon 2021, 7, e06949. [Google Scholar] [CrossRef]
- Fernandez-Escobar, R.; de la Rosa, R.; Leon, L.; Gomez, J.A.; Testi, L.; Orgaz, F.; Gil-Ribes, J.A.; Quesada-Moraga, E.; Trapero, A.; Msallem, M. Evolution and sustainability of the olive production systems. Options Méditerranéennes. Séries A Mediterr. Semin. 2013, 106, 11–41. [Google Scholar]
- Paoletti, A.; Cinosi, N.; Lodolini, E.M.; Famiani, F.; Rosati, A. Effects of root constriction on vegetative growth and yield efficiency in young trees of a low- and a high-vigor olive cultivar. Trees 2023, 37, 1179–1187. [Google Scholar] [CrossRef]
- Bustan, A.; Dag, A.; Yermiyahu, U.; Erel, R.; Presnov, E.; Agam, N.; Kool, D.; Iwema, J.; Zipori, I.; Ben-Gal, A. Fruit load governs transpiration of olive trees. Tree Physiol. 2016, 36, 380–391. [Google Scholar] [CrossRef]
- Robles, H.; Ciudad, C.; Matthysen, E. Tree-cavity occurrence, cavity occupation and reproductive performance of secondary cavity-nesting birds in oak forests: The role of traditional management practices. For. Ecol. Manag. 2011, 261, 1428–1435. [Google Scholar] [CrossRef]
- Grüebler, M.U.; Schaller, S.; Keil, H.; Naef-Daenzer, B. The occurrence of cavities in fruit trees: Effects of tree age and management on biodiversity in traditional European orchards. Biodivers. Conserv. 2013, 22, 3233–3246. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, D.; Turgeon, R.; Chen, J.; Lin, T.; Huang, J.; Luo, J.; Zhu, Y.; Zhang, C.; Lv, Z. Physiological and proteomic responses of mulberry trees (Morus alba. L.) to combined salt and drought stress. Int. J. Mol. Sci. 2019, 20, 2486. [Google Scholar] [CrossRef] [PubMed]
- Pallardy, S.G. The Woody Plant Body. In Physiology of Woody Plants, 3rd ed.; Pallardy, S.G., Ed.; Academic Press: Cambridge, MA, USA, 2008; pp. 9–38. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, Z.; Zeng, Y.; Ouyang, S.; Chen, L.; Lei, P.; Deng, X.; Zhao, Z.; Fang, X.; Xiang, W. Tree-level stomatal regulation is more closely related to xylem hydraulic traits than to leaf photosynthetic traits across diverse tree species. Agric. For. Meteorol. 2023, 329, 109291. [Google Scholar] [CrossRef]
- Giourga, C.; Loumou, A.; Tsevreni, I.; Vergou, A. Assessing the sustainability factors of traditional olive groves on Lesvos Island, Greece (Sustainability and traditional cultivation). GeoJournal 2008, 73, 149–159. [Google Scholar] [CrossRef]
- Stateras, D.; Kalivas, D. Assessment of olive tree canopy characteristics and yield forecast model using high resolution uav imagery. Agric. 2020, 10, 385. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Moutinho-Pereira, J.; Correia, C.M. Drought Stress Effects and Olive Tree Acclimation under a Changing Climate. Plants 2019, 8, 232. [Google Scholar] [CrossRef]
- Fernández, J.E. Plant-based sensing to monitor water stress: Applicability to commercial orchards. Agric. Water Manag. 2014, 142, 99–109. [Google Scholar] [CrossRef]
- Torres-Ruiz, J.M.; Diaz-Espejo, A.; Morales-Sillero, A.; Martín-Palomo, M.J.; Mayr, S.; Beikircher, B.; Fernández, J.E. Shoot hydraulic characteristics, plant water status and stomatal response in olive trees under different soil water conditions. Plant Soil 2013, 373, 77–87. [Google Scholar] [CrossRef]
- Torres-Ruiz, J.M.; Diaz-Espejo, A.; Perez-Martin, A.; Hernandez-Santana, V. Role of hydraulic and chemical signals in leaves, stems and roots in the stomatal behaviour of olive trees under water stress and recovery conditions. Tree Physiol. 2015, 35, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Santana, V.; Rodriguez-Dominguez, C.M.; Fernández, J.E.; Diaz-Espejo, A. Role of leaf hydraulic conductance in the regulation of stomatal conductance in almond and olive in response to water stress. Tree Physiol. 2016, 36, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Akkuzu, E.; Kaya, Ü.; Çamoğlu, G.; Mengü, G.P.; Aşik, Ş. Determination of Crop Water Stress Index and Irrigation Timing on Olive Trees Using a Handheld Infrared Thermometer. J. Irrig. Drain. Eng. 2013, 139, 728–737. [Google Scholar] [CrossRef]
- Buonaurio, R.; Almadi, L.; Famiani, F.; Moretti, C.; Agosteo, G.E.; Schena, L. Olive leaf spot caused by Venturia oleaginea: An updated review. Front. Plant Sci. 2023, 13, 1061136. [Google Scholar] [CrossRef] [PubMed]

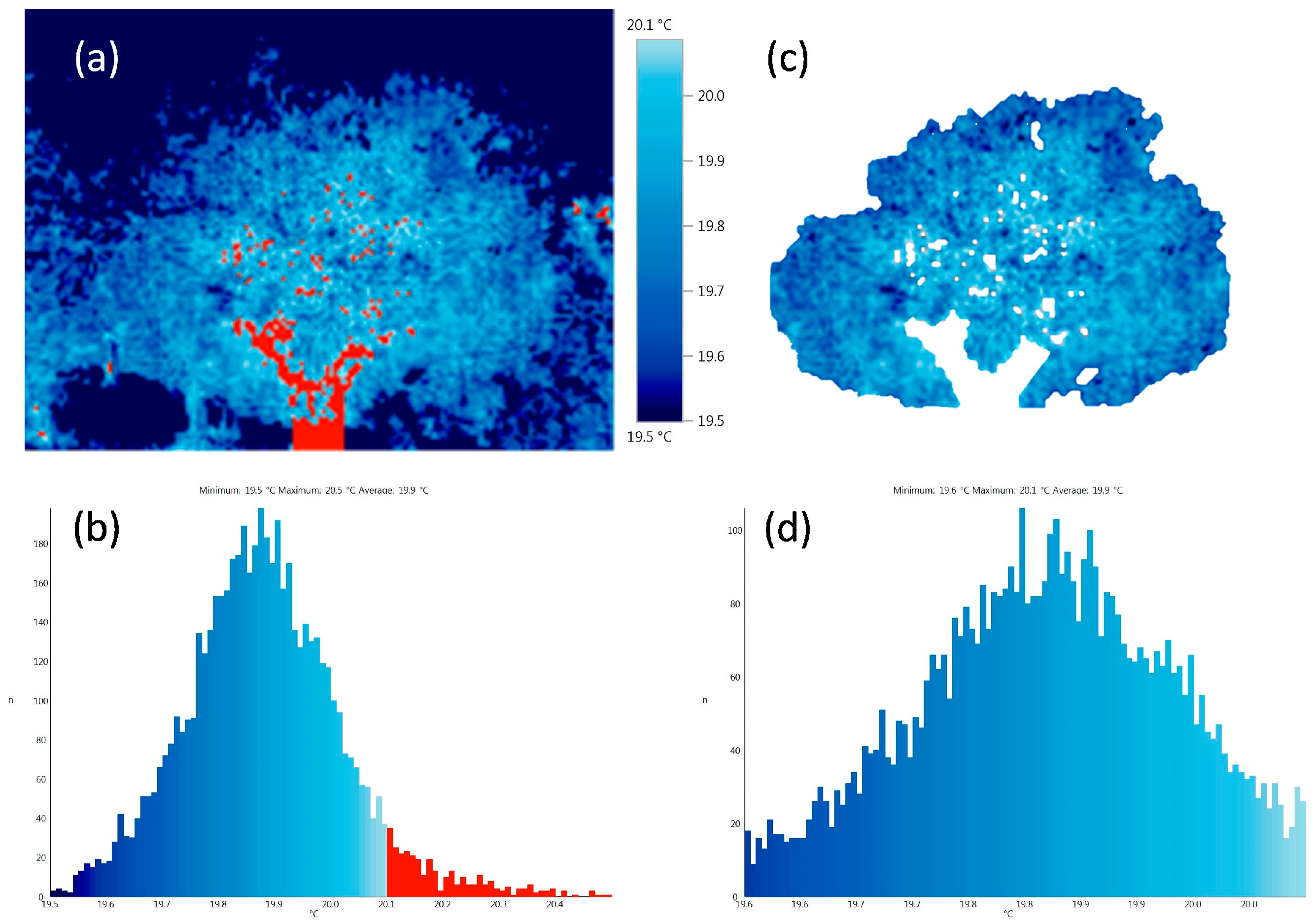
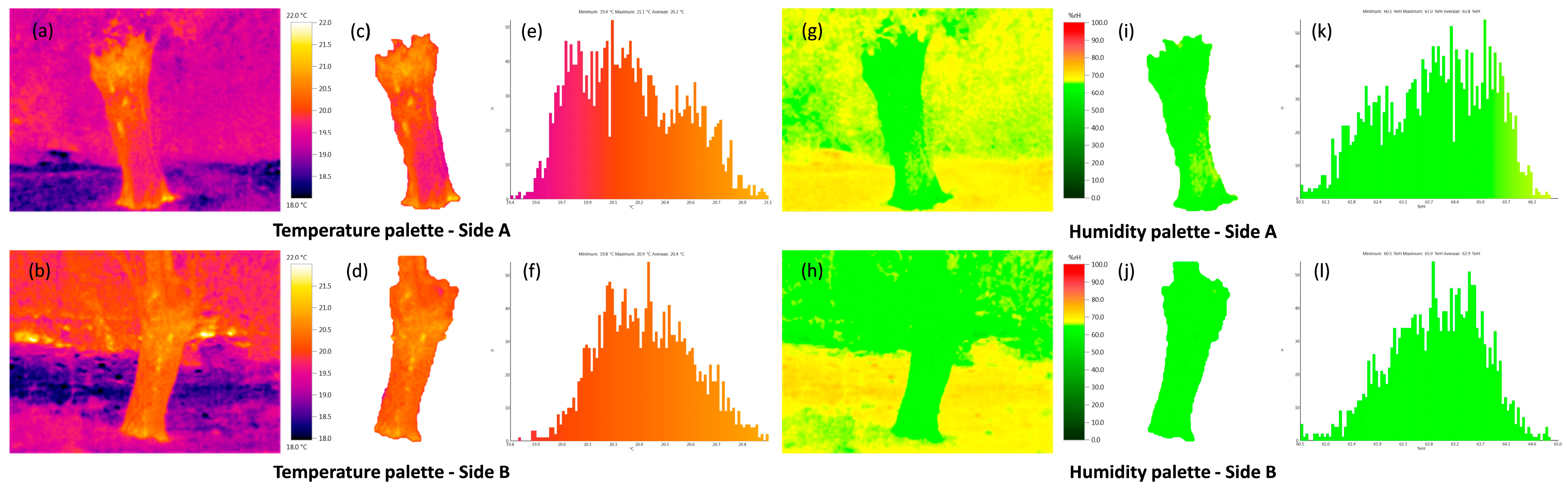
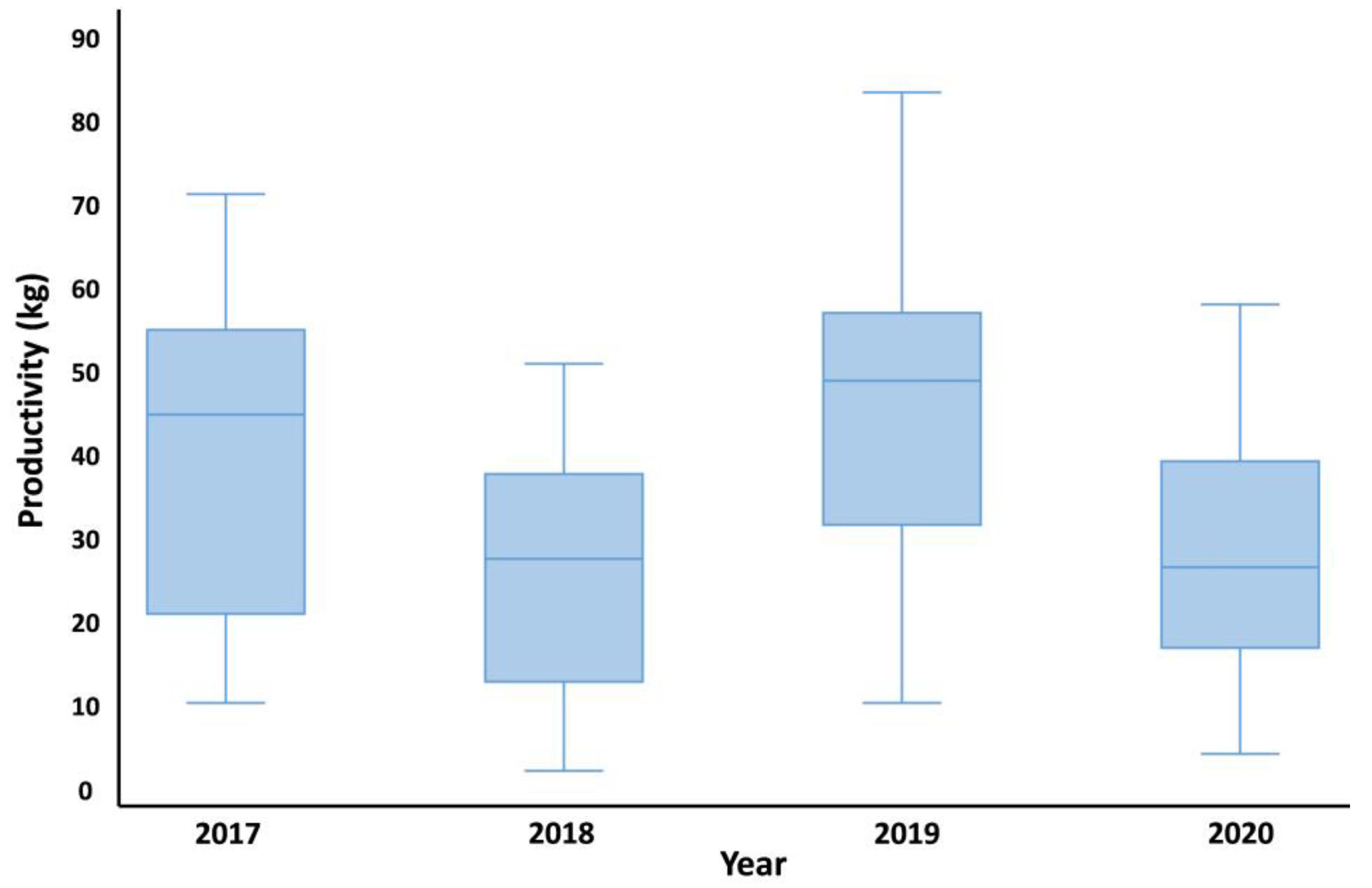
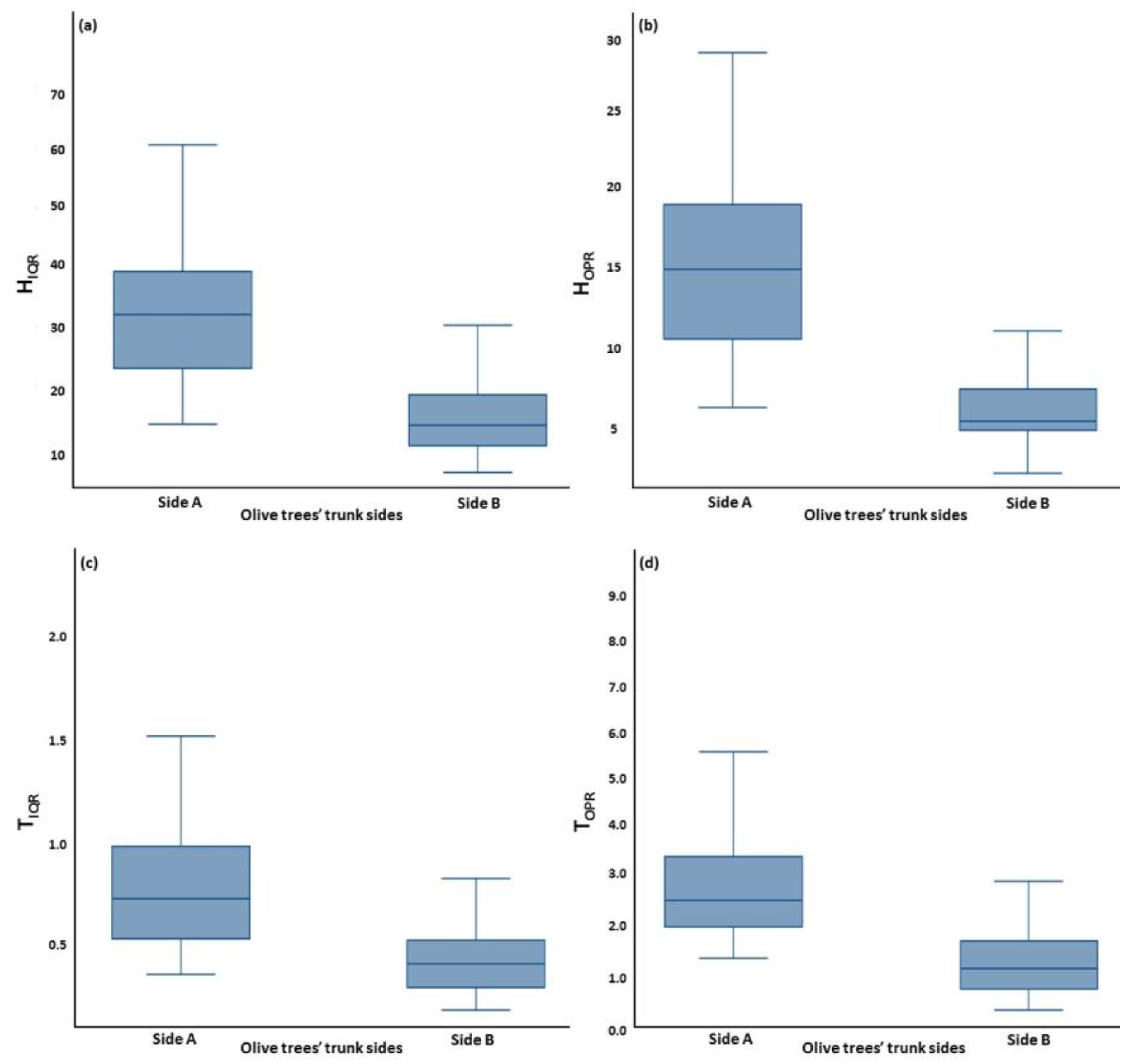
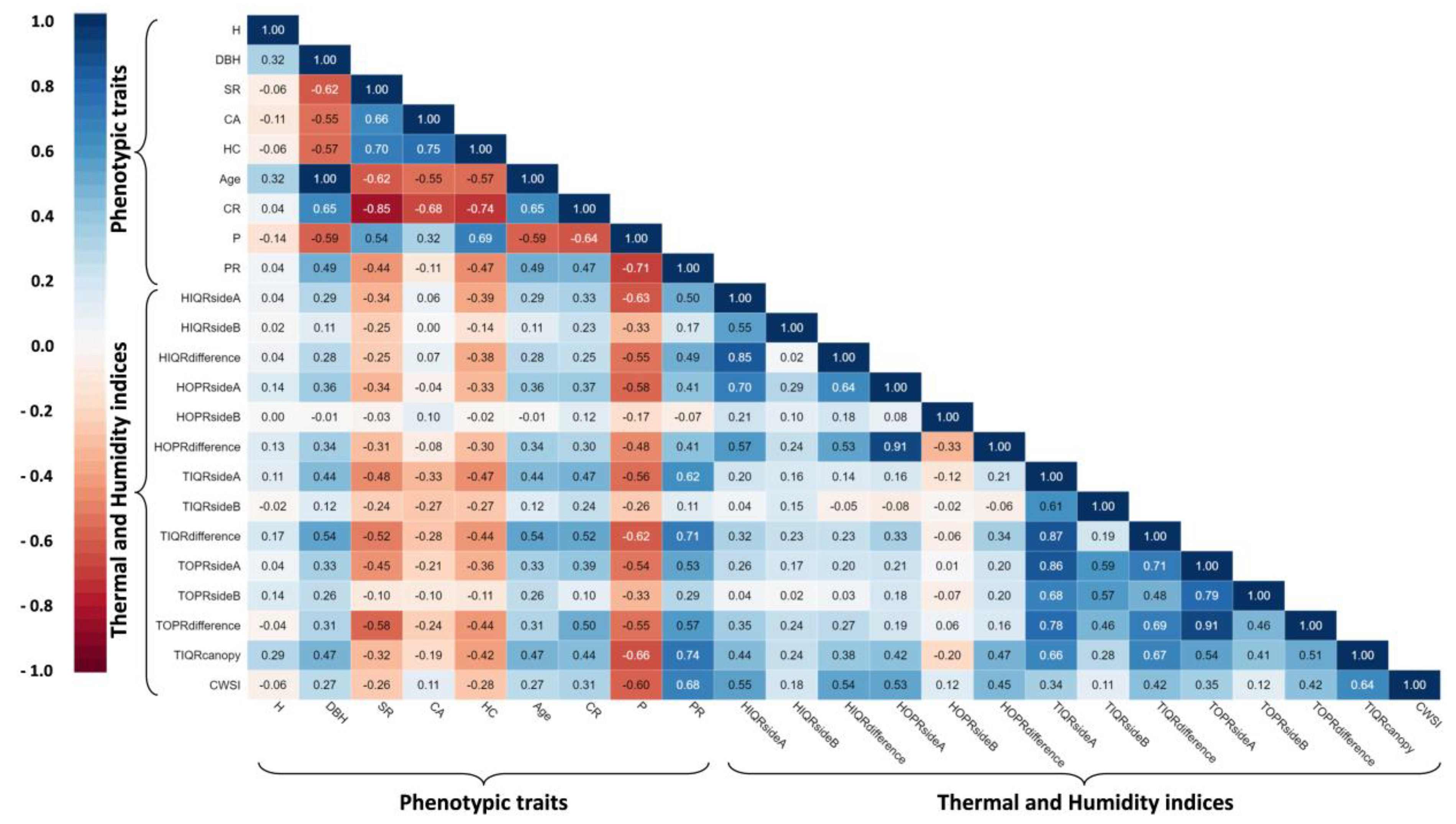
| Phenotypic Traits | Mean | SD | S.E | Min | Max |
|---|---|---|---|---|---|
| Height (m) | 6.98 | 1.75 | 1.16 | 4.85 | 13.31 |
| Diameter (cm) | 68.03 | 24.39 | 3.67 | 43.52 | 156.69 |
| Productive shoots | 10.45 | 6.59 | 0.99 | 1.00 | 23.00 |
| Unproductive shoots | 3.70 | 2.35 | 0.35 | 1.00 | 9.00 |
| Shoots ratio | 0.67 | 0.24 | 0.03 | 0.11 | 0.96 |
| Crown area (m2) | 69.29 | 35.59 | 5.36 | 16.23 | 140.43 |
| Healthy crown (m2) | 49.89 | 37.64 | 5.67 | 5.94 | 140.43 |
| Age (years) | 190.27 | 51.46 | 7.75 | 121.18 | 314.04 |
| Cavities | 4.52 | 4.04 | 0.60 | 0.00 | 18.00 |
| Cavities ratio | 0.02 | 0.01 | 0.00 | 0.00 | 0.06 |
| Variables Set | Response Variable | Predictor Variables | B | SE B | β | t | p-Value | R2 adj. | F |
|---|---|---|---|---|---|---|---|---|---|
| Phenotypic traits | P | (constant) | 59.79 | 3.88 | 15.38 | 0.001 | 0.545 | 26.799 | |
| CR | −434.06 | 102.9 | −0.47 | −4.21 | 0.001 | ||||
| CWSI | −86.067 | 22.14 | −0.43 | −3.88 | 0.001 | ||||
| Thermal and humidity indices | P | (constant) | 54.28 | 2.86 | 18.96 | 0.001 | 0.570 | 19.983 | |
| TIQR canopy | −7.13 | 3.45 | −0.29 | −2.06 | 0.046 | ||||
| TIQR difference | −14.00 | 5.52 | −0.34 | −2.53 | 0.015 | ||||
| HIQR difference | −4.27 | 1.30 | −0.35 | −3.28 | 0.002 | ||||
| Combined model | P | (constant) | 54.39 | 5.29 | 10.27 | 0.001 | 0.711 | 27.384 | |
| HC | 0.129 | 0.03 | 0.37 | 3.74 | 0.001 | ||||
| CWSI | −49.01 | 20.12 | −0.24 | −2.43 | 0.020 | ||||
| HIQR Side A | −3.04 | 1.02 | −0.30 | −2.98 | 0.005 | ||||
| TIQR Side A | −8.19 | 3.12 | −0.24 | −2.62 | 0.012 |
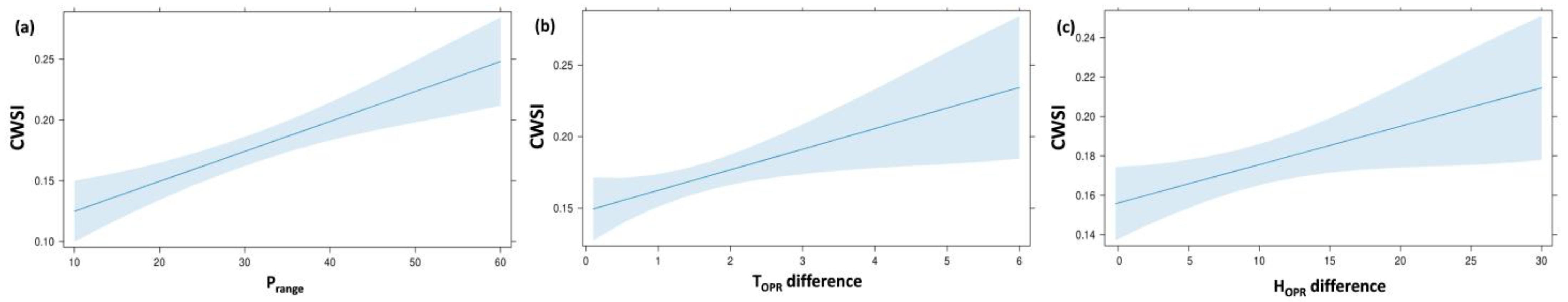
| Variables Set | Response Variable | Predictor Variables | B | SE B | β | t | p-Value | R2 adj. | F |
|---|---|---|---|---|---|---|---|---|---|
| Combined model | CWSI | (constant) | 0.18 | 0.02 | 6.95 | 0.001 | 0.475 | 13.948 | |
| Prange | −0.00 | 0.00 | −0.45 | −3.99 | 0.001 | ||||
| HOPR difference | 0.00 | 0.00 | 0.29 | 2.61 | 0.011 | ||||
| TOPR difference | 0.01 | 0.00 | 0.29 | 2.59 | 0.011 |
| Variable Set | Predictor | Β | S.E. | Wald’s χ2 | df | p-Value |
|---|---|---|---|---|---|---|
| Phenotypic traits | Productivity | −0.29 | 0.09 | 9.68 | 1 | 0.002 |
| Constant | 11.76 | 3.84 | 9.39 | 1 | 0.002 | |
| Thermal and humidity indices | TIQR Side A | 7.61 | 2.51 | 9.21 | 1 | 0.002 |
| Constant | −4.95 | 1.67 | 8.81 | 1 | 0.003 | |
| Combined model | CR | −128.90 | 77.29 | 2.78 | 1 | 0.045 |
| Productivity | −0.21 | 0.09 | 5.02 | 1 | 0.025 | |
| HC | −0.05 | 0.03 | 3.20 | 1 | 0.033 | |
| TIQR Side A | 8.89 | 5.34 | 2.77 | 1 | 0.026 | |
| Constant | 8.92 | 5.08 | 3.08 | 1 | 0.049 |
| Phenotypic Traits | t-Value | df | p-Value |
|---|---|---|---|
| Height | −0.299 | 42 | >0.05 |
| Diameter | −3.722 | 42 | <0.05 |
| Shoots ratio | 2.522 | 42 | <0.01 |
| Crown area | 2.665 | 42 | <0.01 |
| Healthy crown | 4.997 | 42 | <0.001 |
| Age | −3.722 | 42 | <0.05 |
| Cavities ratio | −2.938 | 42 | <0.01 |
| Productivity | 6.645 | 42 | <0.001 |
| Productivity range | 2.124 | 42 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zevgolis, Y.G.; Kouris, A.; Christopoulos, A.; Dimitrakopoulos, P.G. Linking Thermal Indices, Productivity, Phenotypic Traits, and Stressors for Assessing the Health of Centennial Traditional Olive Trees. Appl. Sci. 2023, 13, 11443. https://doi.org/10.3390/app132011443
Zevgolis YG, Kouris A, Christopoulos A, Dimitrakopoulos PG. Linking Thermal Indices, Productivity, Phenotypic Traits, and Stressors for Assessing the Health of Centennial Traditional Olive Trees. Applied Sciences. 2023; 13(20):11443. https://doi.org/10.3390/app132011443
Chicago/Turabian StyleZevgolis, Yiannis G., Alexandros Kouris, Apostolos Christopoulos, and Panayiotis G. Dimitrakopoulos. 2023. "Linking Thermal Indices, Productivity, Phenotypic Traits, and Stressors for Assessing the Health of Centennial Traditional Olive Trees" Applied Sciences 13, no. 20: 11443. https://doi.org/10.3390/app132011443





