Evaluation of Fire Blight Resistance of Eleven Apple Rootstocks Grown in Kazakhstani Fields
Abstract
:1. Introduction
2. Materials and Methods
2.1. Disease Monitoring, Sampling, and DNA Isolation
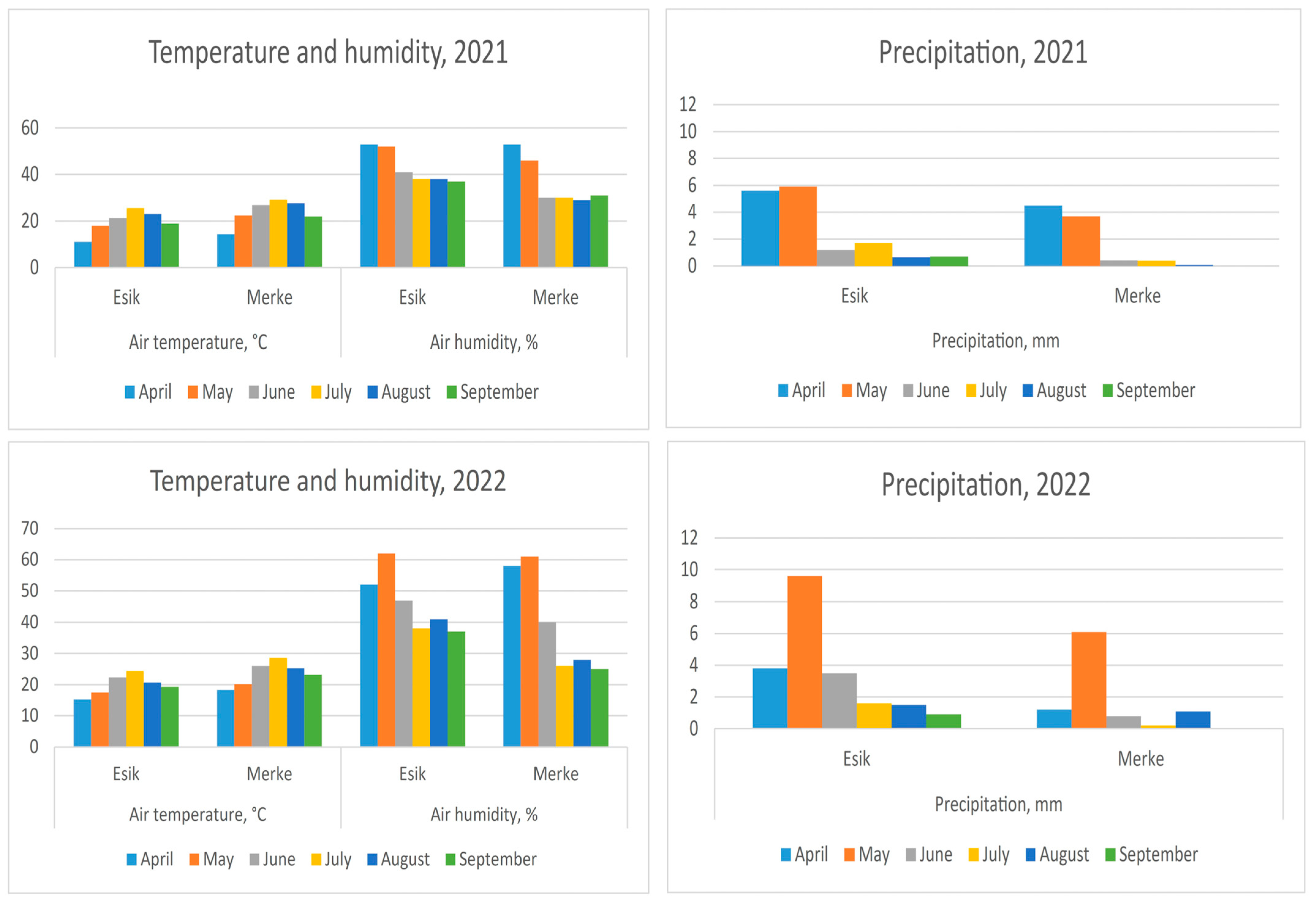
2.2. Weather Data Acquisition
2.3. SCAR Marker Amplification
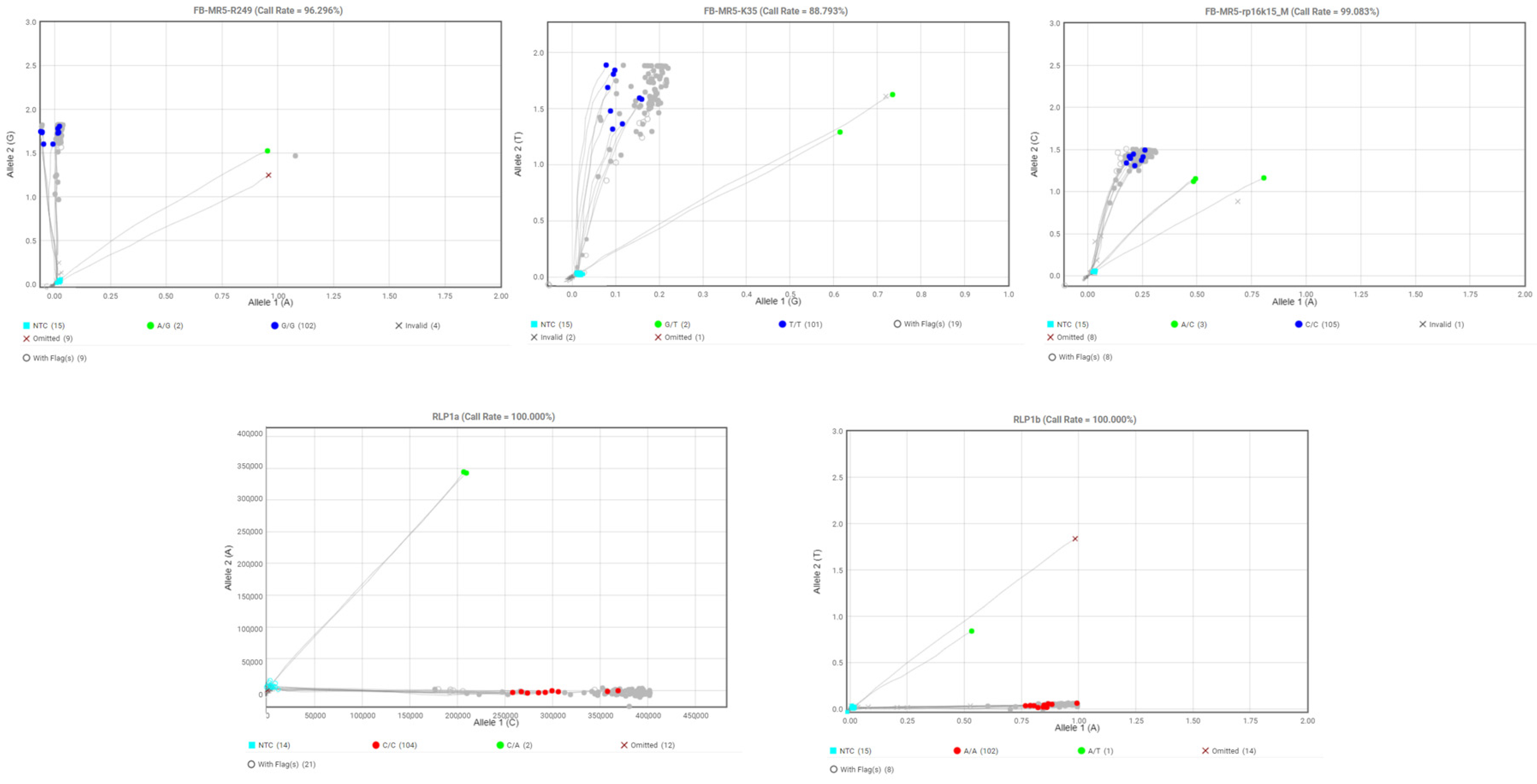
2.4. SNP Genotyping
3. Results
3.1. Observing and Tracking the Occurrence of Fire Blight in Rootstock Fields
3.2. Genetic Determinants Influencing Resistance to Erwinia amylovora in Apple Rootstocks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.A.; Zhao, Y.; Korban, S.S. Molecular Mechanisms of Pathogenesis and Resistance to the Bacterial Pathogen Erwinia amylovora, Causal Agent of Fire Blight Disease in Rosaceae. Plant Mol. Biol. Report. 2012, 30, 247–260. [Google Scholar] [CrossRef]
- van der Zwet, T.; Orolaza-Halbrendt, N.; Zeller, W. Fire Blight: History, Biology, and Management; Scientific Societies: St. Paul, MN, USA, 2016. [Google Scholar] [CrossRef]
- Winslow, C.-E.A.; Broadhurst, J.; Buchanan, R.E.; Krumwiede, C.; Rogers, L.A.; Smith, G.H. The Families and Genera of the Bacteria: Final Report of the Committee of the Society of American Bacteriologists on Characterization and Classification of Bacterial Types. J. Bacteriol. 1920, 5, 191–229. [Google Scholar] [CrossRef] [PubMed]
- Norelli, J.L.; Jones, A.L.; Aldwinckle, H.S. Fire Blight Management in the Twenty-First Century: Using New Technologies That Enhance Host Resistance in Apple. Plant Dis. 2003, 87, 756–765. [Google Scholar] [CrossRef]
- Maltseva, E.R.; Zharmukhamedova, G.A.; Jumanova, Z.K.; Naizabayeva, D.A.; Berdygulova, Z.A.; Dmitriyeva, K.A.; Soltanbekov, S.S.; Argynbayeva, A.M.; Skiba, Y.A.; Malakhova, N.P.; et al. Assessment of Fire Blight Introduction in the Wild Apple Forests of Kazakhstan. Biodiversity 2022, 23, 123–128. [Google Scholar] [CrossRef]
- Calenge, F.; Drouet, D.; Denancé, C.; Van De Weg, W.E.; Brisset, M.N.; Paulin, J.P.; Durel, C.E. Identification of a Major QTL Together with Several Minor Additive or Epistatic QTLs for Resistance to Fire Blight in Apple in Two Related Progenies. Theor. Appl. Genet. 2005, 111, 128–135. [Google Scholar] [CrossRef]
- Durel, C.E.; Denancé, C.; Brisset, M.N. Two Distinct Major QTL for Resistance to Fire Blight Co-Localize on Linkage Group 12 in Apple Genotypes “Evereste” and Malus Floribunda Clone 821. Genome 2009, 52, 139–147. [Google Scholar] [CrossRef]
- Emeriewen, O.; Richter, K.; Kilian, A.; Zini, E.; Hanke, M.V.; Malnoy, M.; Peil, A. Identification of a Major Quantitative Trait Locus for Resistance to Fire Blight in the Wild Apple Species Malus Fusca. Mol. Breed. 2014, 34, 407–419. [Google Scholar] [CrossRef]
- Gardiner, S.E.; Norelli, J.L.; de Silva, N.; Fazio, G.; Peil, A.; Malnoy, M.; Horner, M.; Bowatte, D.; Carlisle, C.; Wiedow, C.; et al. Putative Resistance Gene Markers Associated with Quantitative Trait Loci for Fire Blight Resistance in Malus “Robusta 5” Accessions. BMC Genet. 2012, 13, 25. [Google Scholar] [CrossRef]
- Khan, M.A.; Durel, C.E.; Duffy, B.; Drouet, D.; Kellerhals, M.; Gessler, C.; Patocchi, A. Development of Molecular Markers Linked to the “Fiesta” Linkage Group 7 Major QTL for Fire Blight Resistance and Their Application for Marker-Assisted Selection. Genome 2007, 50, 568–577. [Google Scholar] [CrossRef]
- Le Roux, P.M.F.; Khan, M.A.; Broggini, G.A.L.; Duffy, B.; Gessler, C.; Patocchi, A. Mapping of Quantitative Trait Loci for Fire Blight Resistance in the Apple Cultivars “Florina” and “Nova Easygro”. Genome 2010, 53, 710–722. [Google Scholar] [CrossRef]
- Hanke, M. No Flower No Fruit—Genetic Potentials to Trigger Flowering in Fruit Trees. Genes Genomes Genom. 2007, 1, 1–20. [Google Scholar]
- Harshman, J.M.; Evans, K.M.; Allen, H.; Potts, R.; Flamenco, J.; Aldwinckle, H.S.; Wisniewski, M.E.; Norelli, J.L. Fire Blight Resistance in Wild Accessions of Malus Sieversii. Plant Dis. 2017, 101, 1738–1745. [Google Scholar] [CrossRef]
- Fazio, G.; Robinson, T.L.; Aldwinckle, H.S. The Geneva Apple Rootstock Breeding Program. Plant Breed. Rev. 2015, 39, 379–424. [Google Scholar] [CrossRef]
- Peil, A.; Garcia-Libreros, T.; Richter, K.; Trognitz, F.C.; Trognitz, B.; Hanke, M.V.; Flachowsky, H. Strong Evidence for a Fire Blight Resistance Gene of Malus Robusta Located on Linkage Group 3. Plant Breed. 2007, 126, 470–475. [Google Scholar] [CrossRef]
- Aćimović, S.G.; Santander, R.D.; Meredith, C.L.; Pavlović, Ž.M. Fire Blight Rootstock Infections Causing Apple Tree Death: A Case Study in High-Density Apple Orchards with Erwinia amylovora Strain Characterization. Front. Hortic. 2023, 2, 3. [Google Scholar] [CrossRef]
- Hasan, N.; Choudhary, S.; Naaz, N.; Sharma, N.; Laskar, R.A. Recent Advancements in Molecular Marker-Assisted Selection and Applications in Plant Breeding Programmes. J. Genet. Eng. Biotechnol. 2021, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Duffy, B.; Gessler, C.; Patocchi, A. QTL Mapping of Fire Blight Resistance in Apple. Mol. Breed. 2006, 17, 299–306. [Google Scholar] [CrossRef]
- Chagné, D.; Vanderzande, S.; Kirk, C.; Profitt, N.; Weskett, R.; Gardiner, S.E.; Peace, C.P.; Volz, R.K.; Bassil, N.V. Validation of SNP Markers for Fruit Quality and Disease Resistance Loci in Apple (Malus × Domestica Borkh.) Using the OpenArray® Platform. Hortic. Res. 2019, 6, 30. [Google Scholar] [CrossRef]
- Carpenter, S.C.D.; Mishra, P.; Ghoshal, C.; Dash, P.K.; Wang, L.; Midha, S.; Laha, G.S.; Lore, J.S.; Kositratana, W.; Singh, N.K.; et al. An Xa5 Resistance Gene-Breaking Indian Strain of the Rice Bacterial Blight Pathogen Xanthomonas Oryzae Pv. Oryzae Is Nearly Identical to a Thai Strain. Front. Microbiol. 2020, 11, 579504. [Google Scholar] [CrossRef]
- Wang, Z.; Hobson, N.; Galindo, L.; Zhu, S.; Shi, D.; McDill, J.; Yang, L.; Hawkins, S.; Neutelings, G.; Datla, R.; et al. The Genome of Flax (Linum usitatissimum) Assembled de Novo from Short Shotgun Sequence Reads. Plant J. 2012, 72, 461–473. [Google Scholar] [CrossRef]
- Norelli, J.L.; Holleran, H.T.; Johnson, W.C.; Robinson, T.L.; Aldwinckle, H.S. Resistance of Geneva and Other Apple Rootstocks to Erwinia amylovora. Plant Dis. 2003, 87, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Lyzhin, A.S. Creation of Genetic Passports of Apple Rootstock Forms on the Basis of Microsatellite DNA Polymor Phism. Available online: https://agris.fao.org/agris-search/search.do?recordID=RU2019000910 (accessed on 15 March 2023).
- Webster, A.D.; Hollands, M.S. Apple Rootstock Studies: Comparison of Polish, Russian, USA and UK Selections as Rootstocks for the Apple Cultivar Cox’s Orange Pippin (Malus Domestica Borkh.). J. Hortic. Sci. Biotechnol. 1999, 74, 367–374. [Google Scholar] [CrossRef]
- Piestrzeniewicz, C.; Sadowski, A. Early Orchard Performance of “rubin” Apple on Nineteen Rootstocks. Acta Hortic. 2007, 732, 113–117. [Google Scholar] [CrossRef]
- Samus’, V.A.; Zhabrovskij, I.E. Productivity of New Clonal Rootstocks of Apple in Stock Nursery. Available online: https://agris.fao.org/agris-search/search.do?recordID=BY9600002 (accessed on 15 March 2023).
- Drabudko, N.N.; Ganusenko, M.Y.; Grusheva, T.P.; Levshunov, V.A.; Samus, V.A.; Shkrobova, M.A. Clonal Rootstocks as the Basis to Increase Productivity of Fruit Crop Plantings. Fruit. Grow. 2022, 30, 247–257. (In Russian) [Google Scholar]
- Shaulenova, A.G.; Khamzina, A.K.; Karimov, K.B.; Umurzakova, R.M. Clone Apple Rootstock in the West of Kazakhstan. Sci. Prod. Bus. 2018, 2, 260–265. (In Russian) [Google Scholar]
- Robinson, T.; Aldwinckle, H.; Fazio, G.; Holleran, T. The Geneva Series of Apple Rootstocks from Cornell: Performance, Disease Resistance, and Commercialization. Acta Hortic. 2003, 622, 513–520. [Google Scholar] [CrossRef]
- Russo, N.L.; Robinson, T.L.; Fazio, G.; Aldwinckle, H.S. Fire Blight Resistance of Budagovsky 9 Apple Rootstock. Plant Dis. 2008, 92, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Loreti, S.; Valeria, S.; Kairova, G.; Daulet, N.; Solomadin, M.; Sandybayev, N.; Orkara, S.; Beloussov, V.; Kerimbek, N.; Gritsenko, D.; et al. Identification of Apple Varieties Resistant to Fire Blight (Erwinia amylovora) Using Molecular Markers. Horticulturae 2023, 9, 1000. [Google Scholar] [CrossRef]
- Porebski, S.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA Extraction Protocol for Plants Containing High Polysaccharide and Polyphenol Components. Plant Mol. Biol. Rep. 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Gottsberger, R.A. Development and Evaluation of a Real-Time PCR Assay Targeting Chromosomal DNA of Erwinia amylovora. Lett. Appl. Microbiol. 2010, 51, 285–292. [Google Scholar] [CrossRef]
- WMO. Guide to the WMO Integrated Global Observing System (WMO-No. 1165); World Meteorological Organization: Geneva, Switzerland, 2019; 111p. [Google Scholar]
- Jänsch, M.; Broggini, G.A.L.; Weger, J.; Bus, V.G.M.; Gardiner, S.E.; Bassett, H.; Patocchi, A. Identification of SNPs Linked to Eight Apple Disease Resistance Loci. Mol. Breed. 2015, 35, 45. [Google Scholar] [CrossRef]
- Broggini, G.A.L.; Wöhner, T.; Fahrentrapp, J.; Kost, T.D.; Flachowsky, H.; Peil, A.; Hanke, M.V.; Richter, K.; Patocchi, A.; Gessler, C. Engineering Fire Blight Resistance into the Apple Cultivar “Gala” Using the FB_MR5 CC-NBS-LRR Resistance Gene of Malus × Robusta 5. Plant Biotechnol. J. 2014, 12, 728–733. [Google Scholar] [CrossRef]
- Santander, R.D.; Biosca, E.G. Erwinia amylovora Psychrotrophic Adaptations: Evidence of Pathogenic Potential and Survival at Temperate and Low Environmental Temperatures. PeerJ 2017, 2017, e3931. [Google Scholar] [CrossRef]
- Pusey, P.L.; Curry, E.A. Temperature and Pomaceous Flower Age Related to Colonization by Erwinia amylovora and Antagonists. Phytopathology 2004, 94, 901–911. [Google Scholar] [CrossRef]
- Turechek, W.W.; Biggs, A.R. Maryblyt v. 7.1 for Windows: An Improved Fire Blight Forecasting Program for Apples and Pears. Plant Health Prog. 2015, 16, 16–22. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Xu, X.; Qiu, C.; Wu, T.; Wei, Q.; Ma, F.; Han, Z. Progress of Apple Rootstock Breeding and Its Use. Hortic. Plant J. 2019, 5, 183–191. [Google Scholar] [CrossRef]
- Fallahi, E.; Michael Colt, W.; Fallahi, B.; Chun, I.-J. The Importance of Apple Rootstocks on Tree Growth, Yield, Fruit Quality, Leaf Nutrition, and Photosynthesis with an Emphasis on “Fuji”. HortTechnology 2002, 12, 38–44. [Google Scholar] [CrossRef]
- Warschefsky, E.J.; Klein, L.L.; Frank, M.H.; Chitwood, D.H.; Londo, J.P.; von Wettberg, E.J.B.; Miller, A.J. Rootstocks: Diversity, Domestication, and Impacts on Shoot Phenotypes. Trends Plant Sci. 2016, 21, 418–437. [Google Scholar] [CrossRef] [PubMed]
- Djaimurzina, A.; Umiralieva, Z.; Zharmukhamedova, G.; Born, Y.; Bühlmann, A.; Rezzonico, F. Detection of the Causative Agent of Fire Blight—Erwinia amylovora (Burrill) Winslow et al.—In the Southeast of Kazakhstan. Acta Hortic. 2014, 1056, 129–132. [Google Scholar] [CrossRef]
- Czynczyk, A.; Bielicki, P. Eleven Year Evaluation of American (Geneva®) and Polish Rootstocks with ‘Golden Delicious Reinders’ Apple in Poland. J. Fruit Ornam. Plant Res. 2012, 20, 11–21. [Google Scholar] [CrossRef]
- Zhao, Y.-Q.; Tian, Y.-L.; Wang, L.-M.; Geng, G.-M.; Zhao, W.-J.; Hu, B.-S.; Zhao, Y.-F. Fire Blight Disease, a Fast-Approaching Threat to Apple and Pear Production in China. J. Integr. Agric. 2019, 18, 815–820. [Google Scholar] [CrossRef]
- Rakhimova, E.; Byzova, Z.; Valiyeva, B.; Dernovskaya, L. Diversity of microfungi in fruit forests of ili-alatau national park (Kazakhstan). Phytopath. Polonica 2005, 35, 203–213. [Google Scholar]
- Lemaire, C.; De Gracia, M.; Leroy, T.; Michalecka, M.; Lindhard-Pedersen, H.; Guerin, F.; Gladieux, P.; Le Cam, B. Emergence of New Virulent Populations of Apple Scab from Nonagricultural Disease Reservoirs. New Phytol. 2016, 209, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Soltanbekov, S.; Dolgikh, S.; Zhumagulova, M.; Madenova, A.; Isina, Z.; Kabylbekova, B. Physiological and Phyto-Pathological Assessment Scion-Rootstock Combinations for Apple Cv. Aport and M. sieversii. Res. Crops 2022, 23, 795–800. [Google Scholar] [CrossRef]
- Singh, J.; Sun, M.; Cannon, S.B.; Wu, J.; Khan, A. An Accumulation of Genetic Variation and Selection across the Disease-Related Genes during Apple Domestication. Tree Genet. Genomes 2021, 17, 29. [Google Scholar] [CrossRef]
- Gritsenko, D.A.; Aubakirova, K.P.; Voitsekhovskiy, I.; Soldatova, I.; Galiakparov, N.N. Simultaneous Detection of Five Apple Viruses by RT-PCR. Int. J. Biol. Chem. 2020, 13, 129–134. [Google Scholar] [CrossRef]
- Romadanova, N.V.; Tolegen, A.B.; Koken, T.E.; Nurmanov, M.M.; Kushnarenko, S.V. Chemotherapy of Apple Shoots in Vitro as Method of Viruses Eradication. Int. J. Biol. Chem. 2021, 14, 48–55. [Google Scholar] [CrossRef]
- Romadanova, N.V.; Mishustina, S.A.; Gritsenko, D.A.; Omasheva, M.Y.; Galiakparov, N.N.; Reed, B.M.; Kushnarenko, S.V. Cryotherapy as a Method for Reducing the Virus Infection of Apples (Malus Sp.). Cryobiology 2016, 71, 559. [Google Scholar] [CrossRef]
- Emeriewen, O.F.; Peil, A.; Richter, K.; Zini, E.; Hanke, M.V.; Malnoy, M. Fire Blight Resistance of Malus ×arnoldiana Is Controlled by a Quantitative Trait Locus Located at the Distal End of Linkage Group 12. Eur. J. Plant Pathol. 2017, 148, 1011–1018. [Google Scholar] [CrossRef]
- Omasheva, M.Y.; Pozharskiy, A.S.; Maulenbay, A.D.; Ryabushkina, N.A.; Galiakparov, N.N. SSR Genotyping of KazakhstaniApple Varieties: Identification of Alleles Associated with Resistance to Highly Destructive Pathogens. Eurasian J. Appl. Biotechnol. 2016, 14, 1–16. [Google Scholar]
- Kolchenko, M.; Nurtaza, A.; Pozharskiy, A.; Dyussembekova, D.; Kapytina, A.; Nizamdinova, G.; Khusnitdinova, M.; Taskuzhina, A.; Kakimzhanova, A.; Gritsenko, D. Wild Malus Niedzwetzkyana Dieck Ex Koehne as a Genetic Resource for Fire Blight Resistance. Horticulturae 2023, 9, 1066. [Google Scholar] [CrossRef]
- Russo, N.L.; Robinson, T.L.; Fazio, G.; Aldwinckle, H.S. Field Evaluation of 64 Apple Rootstocks for Orchard Performance and Fire Blight Resistance. HortScience 2007, 42, 1517–1525. [Google Scholar] [CrossRef]
- Robinson, T.L.; Hoying, S.A.; Fazio, G. Performance of Geneva® Rootstocks in On-Farm Trials in New York State. Acta Hortic. 2011, 903, 249–255. [Google Scholar] [CrossRef]
- Robinson, T.; Anderson, L.; Autio, W.; Barritt, B.; Cline, J.; Cowgill, W.; Crassweller, R.; Embree, C.; Ferree, D.; Garcia, E.; et al. A Multi-Location Comparison of “Geneva® 16”, “geneva® 41” and “m.9” Apple Rootstocks in North America. Acta Hortic. 2007, 732, 59–65. [Google Scholar] [CrossRef]
- Cummins, J.N.; Aldwinckle, H.S. Breeding Apple Rootstocks. Plant Breed. Rev. 2011, 1, 294–394. [Google Scholar] [CrossRef]
- Shamshin, I.N.; Maslova, M.V.; Drenova, N.V.; Dubrovsky, M.L.; Parusova, O.V. Assessment of Fire Blight Resistance in Apple Clonal Rootstocks Using Molecular Markers. Proc. Appl. Bot. Genet. Breed. 2020, 181, 185–191. [Google Scholar] [CrossRef]
- Peil, A.; Emeriewen, O.F.; Khan, A.; Kostick, S.; Malnoy, M. Status of Fire Blight Resistance Breeding in Malus. J. Plant Pathol. 2021, 103, 3–12. [Google Scholar] [CrossRef]
- Egea-Gilabert, C.; Pagnotta, M.A.; Tripodi, P. Genotype × Environment Interactions in Crop Breeding. J. Plant Pathol. 2021, 103, 1644. [Google Scholar] [CrossRef]
- El-Soda, M.; Malosetti, M.; Zwaan, B.J.; Koornneef, M.; Aarts, M.G.M. Genotype × Environment Interaction QTL Mapping in Plants: Lessons from Arabidopsis. Trends Plant Sci. 2014, 19, 390–398. [Google Scholar] [CrossRef] [PubMed]
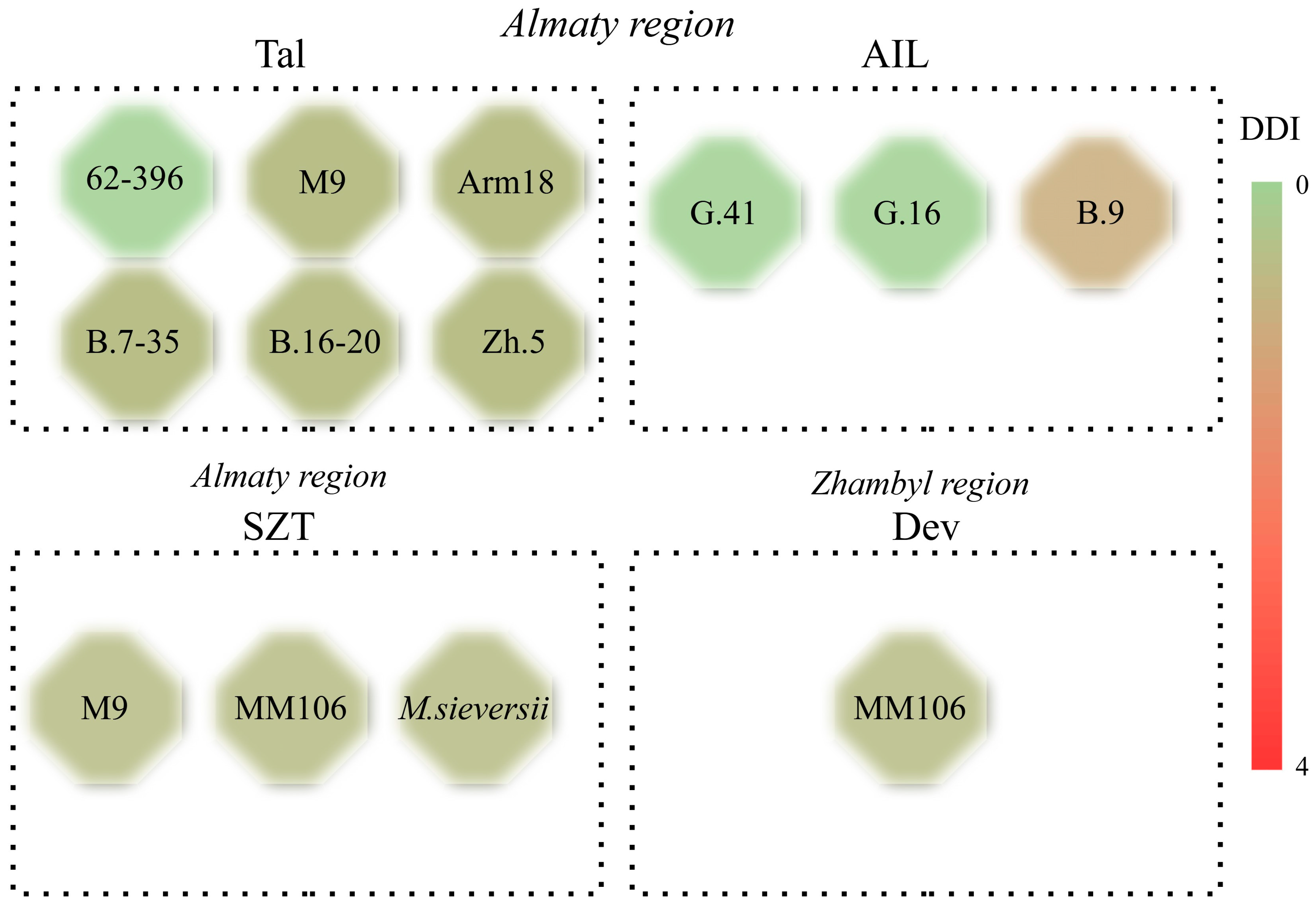

| Designation | Parentage and Origin | Short Description | Field * | References |
|---|---|---|---|---|
| 62-396 | 13–14 × “Paradise Budagovskij”, Michurinsk State Agrarian University, Russia. Approved for use in the southern region of Kazakhstan since 1997. | Dwarfing. The rootstock is char acterized by high resistance to frost, cold, and drought. Cultivars grafted on this rootstock are early-bearing and productive. In the nursery, it provides a high yield of seedlings. | 1 | [23] |
| M9 | Source is not confirmed, East Malling Research Station, England. Approved for use in the southern region of Kazakhstan since 1961. | Dwarfing. It is characterized by a shallow root system. Cultivars grafted on this rootstock require physical support and do not regularly bear fruit. The rootstock is not sufficiently cold-resistant for planting in northern regions of Kazakhstan. | 1; 3 | [24] |
| MM 106 | Northern Spy × M.1, East Malling Research Station, England. Approved for use in 1961 in the southern region of Kazakhstan. | Semi-dwarfing. The rootstock is not sufficiently drought- and frost-resistant. Cultivars grafted on this rootstock begin bearing fruit in 4–5 years. | 3; 4 | [24] |
| Arm 18 | Free pollination of M9. Scientific Center of Viticulture Fruit-Growing and Wine-Making, Armenia. Approved for use since 1997 in the southern region of Kazakhstan. | Dwarfing. The rootstock is highly frost-resistant, productive in the nursery, and shows excellent compatibility in rootstock–scion interactions. | 1 | [25] |
| B.7-35 | M4 × M-9, Buynaksk Experimental Production Station, Russia. Approved for use in 1991 in the southern region of Kazakhstan. | Dwarfing. The rootstock is characterized by high winter hardiness, productivity in the nursery, robust growth and development, and rootstock–scion compatibility. | 1 | [26] |
| B.16-20 | M;4 × M-9, Buynaksk Experimental Production Station, Russia. Approved for use in 1991 in the southern region of Kazakhstan. | Semi-dwarfing. The rootstock is characterized by high winter hardiness and productivity. | 1 | [27] |
| Zhetysu 5 (Local breeding) | The crossing of a semi-dwarf rootstock 57-146 with Malus niedzwetzkyana by open pollination, Kazakh Research Institute of Fruit Growing and Viticulture, Kazakhstan. Approved for use since 2009 in the southern region of Kazakhstan. | Semi-dwarfing. The rootstock is characterized by rapid development, drought resistance, and sufficient production of mother plants. Fruit-bearing occurs 5 years after planting of cultivars grafted onto this rootstock. | 1 | [28] |
| Malus sieversii | Free pollination of wild apple. Approved for use in 1961 in the southern and eastern regions of Kazakhstan. | Vigorous. Seedling rootstock is included on the Red List of rare and endangered plant species. The rootstock is drought- and frost-resistant. | 3 | Not published |
| Geneva 41 (G 41) | Malling 27 × M. robusta ‘Robusta 5’, Cornell University, USA. | Dwarfing. The rootstock is highly resistant to fire blight and cold. | 2 | [14,22,29] |
| Geneva 16 (G 16) | Ottawa 3 × Malus floribunda, Cornell University, USA. | Dwarfing. Resistant to fire blight and scab but susceptible to powdery mildew and latent viruses. Productive and drought-resistant. | 2 | [14,22,29] |
| B.9 | M8 × Red standard, Michurinsk State Agrarian University, Russia. | Dwarfing. The rootstock is highly frost-resistant. Cultivars grafted onto this rootstock begin bearing fruit in 3–4 years. | 2 | [30] |
| Gene, Locus | Marker | Primer Sequence (5′–3′) | PCR Cycling Program |
|---|---|---|---|
| F7 QTL | AE10-375 | CTGAAGCGCACGTTCTCC-F CTGAAGCGCATCATTTCTGATAG-R | 1× 95 °C—3 min, 35× (95 °C—40 s; 60 °C—40 s; 72 °C—60 s), 1× 72 °C—10 min. |
| F7 QTL | GE-8019 | TTGAGACCGATTTTCGTGTG-F TCTCTCCCAGAGCTTCATTGT-R | 1× 95 °C—3 min, 35× (95 °C—40 s; 60 °C—40 s; 72 °C—60 s), 1× 72 °C—10 min. |
| SNP Marker | LG * | Gene/Locus Name | SNP ID | Taqman Assay ID | SNP Type | Reference |
|---|---|---|---|---|---|---|
| FB-MR5-K35 | 3 | MR5 | FB-MR5-NZsnEH034548_K35 | AH0JFXM | G/T | [35] |
| FB-MR5-R249 | 3 | MR5 | FB-MR5-NZsnEH034548_R249 | AH21B92 | A/G | [35] |
| FB-MR5-rp16k15_M106 | 3 | MR5 | FB-MR5-rp16k15_M106 | AH4AAGA | A/C | [35] |
| RLP1a | 3 | RLP1 | RLP1a | AH5I8MI | C/A | [9] |
| RLP1b | 3 | RLP1 | RLP1b | AH6R6SQ | A/T | [9] |
| Region | Rootstock | 2021 | 2022 |
|---|---|---|---|
| Almaty | 62-396 | 0 | 0 |
| M9 | 1 (3.2%) | 1 (7.1%) | |
| Apm 18 | 1 (5.4%) | 1 (8.5%) | |
| G.41 | 0 | 0 | |
| G.16 | 0 | 0 | |
| B.9 | 1 (7.1%) | 2 (16.9%) | |
| Zh.5 | 1 (6.3%) | 1 (9.7%) | |
| B.7-35 | 1 (4.1%) | 1 (7.4%) | |
| B.16-20 | 1 (2.7%) | 1 (5.2%) | |
| M. siversii | 1 (3.1%) | 1 (6.8%) | |
| Almaty, Turkistan | MM106 | 1 (2.2%) | 1 (5.0%) |
| Rootstock Name | SCAR Markers | SNP Markers | |||||
|---|---|---|---|---|---|---|---|
| AE10-375 | GE-8019 | AH0JFXM | AH21B92 | AH4AAGA | AH5I8MI | AH6R6SQ | |
| 62-396 | 375 bp | - | G/T | A/G | A/C | C/A | A/A |
| M9 | - | - | T/T | G/G | C/C | C/C | A/A |
| Apm 18 | - | - | T/T | G/G | C/C | C/C | A/A |
| G.41 | - | - | G/T | A/G | A/C | C/A | A/T |
| G.16 | 375 bp | - | T/T | G/G | A/C | C/C | A/A |
| B.9 | - | - | T/T | G/G | C/C | C/C | A/A |
| Zh.5 | - | - | T/T | G/G | C/C | C/C | A/A |
| B.7-35 | - | - | T/T | G/G | C/C | C/C | A/A |
| B.16-20 | - | - | T/T | G/G | C/C | C/C | A/A |
| M. siversii | - | - | T/T | G/G | C/C | C/C | A/A |
| MM106 | 375 bp | - | T/T | G/G | C/C | C/C | A/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kairova, G.; Pozharskiy, A.; Daulet, N.; Solomadin, M.; Sandybayev, N.; Khusnitdinova, M.; Nizamdinova, G.; Sapakhova, Z.; Gritsenko, D. Evaluation of Fire Blight Resistance of Eleven Apple Rootstocks Grown in Kazakhstani Fields. Appl. Sci. 2023, 13, 11530. https://doi.org/10.3390/app132011530
Kairova G, Pozharskiy A, Daulet N, Solomadin M, Sandybayev N, Khusnitdinova M, Nizamdinova G, Sapakhova Z, Gritsenko D. Evaluation of Fire Blight Resistance of Eleven Apple Rootstocks Grown in Kazakhstani Fields. Applied Sciences. 2023; 13(20):11530. https://doi.org/10.3390/app132011530
Chicago/Turabian StyleKairova, Gulshariya, Alexandr Pozharskiy, Nurzhan Daulet, Maxim Solomadin, Nurlan Sandybayev, Marina Khusnitdinova, Gulnaz Nizamdinova, Zagipa Sapakhova, and Dilyara Gritsenko. 2023. "Evaluation of Fire Blight Resistance of Eleven Apple Rootstocks Grown in Kazakhstani Fields" Applied Sciences 13, no. 20: 11530. https://doi.org/10.3390/app132011530






