Investigation on the Microstructural Diversity of a Three-Dimensional Porous Hydroxyapatite/Wollastonite Skeleton via Biomineralization in Simulated Body Fluids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
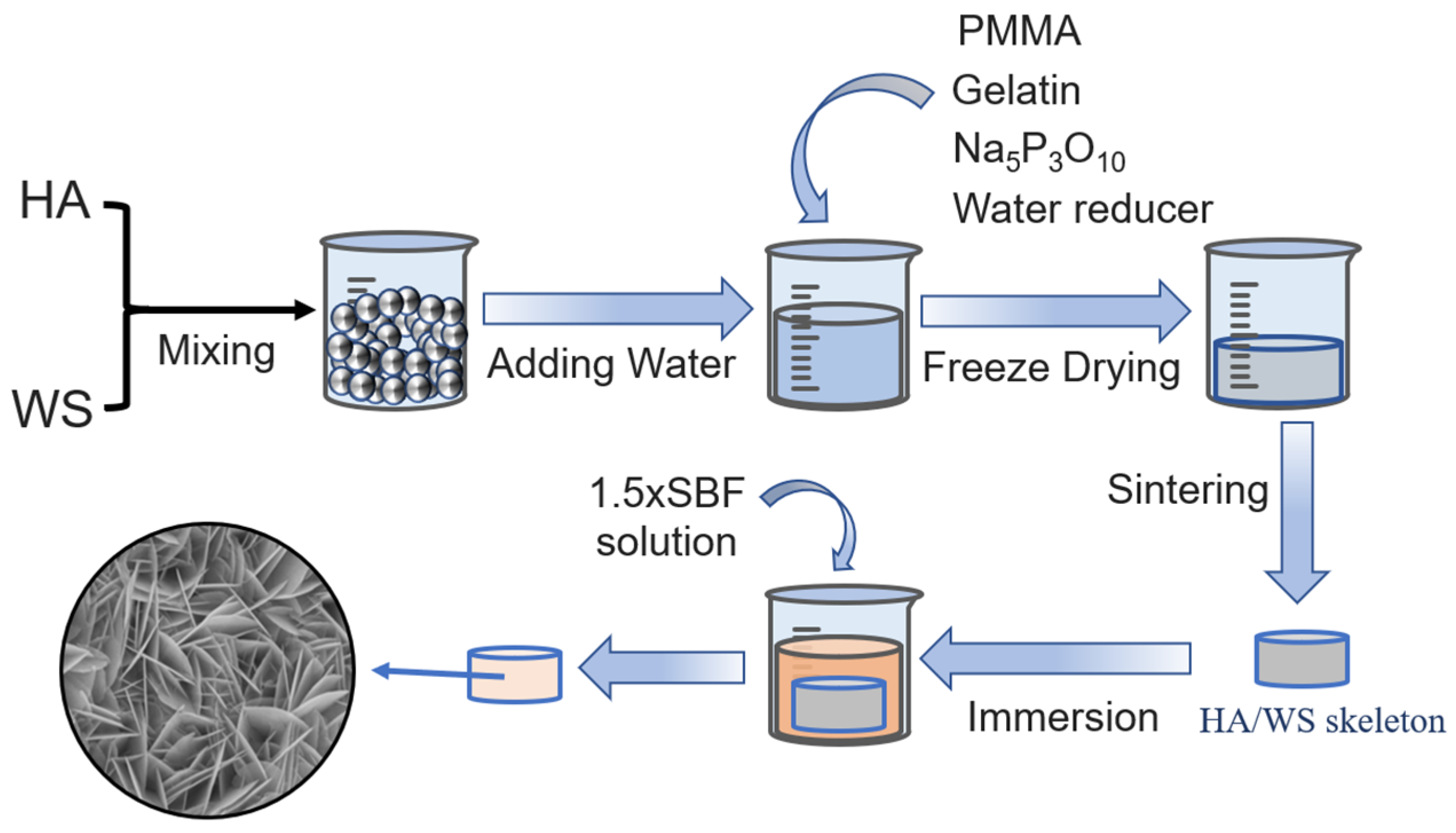
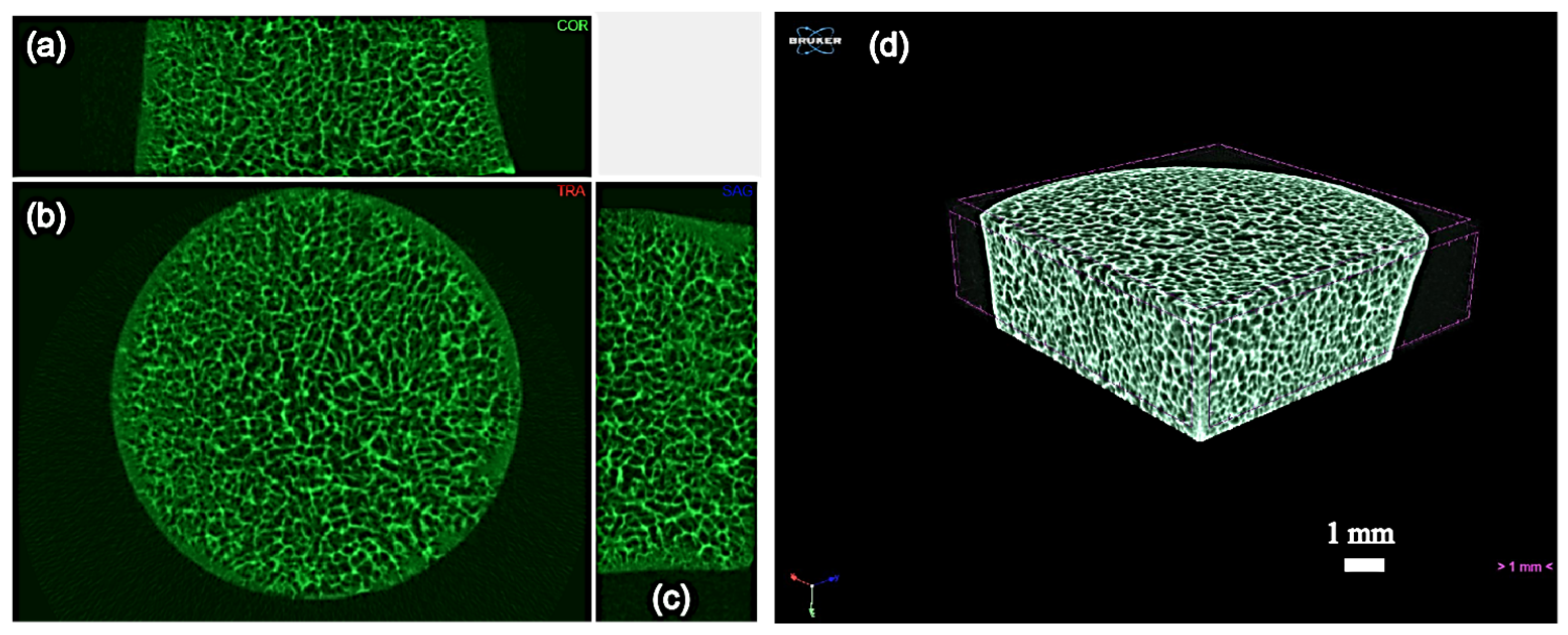
2.2. Preparation of Three-Dimensional Porous HA/WS Skeleton and 1.5 × SBF
2.3. Immersion of Three-Dimensional Porous HA/WS Skeleton in SBFs
2.4. Analysis Method
3. Results and Discussion
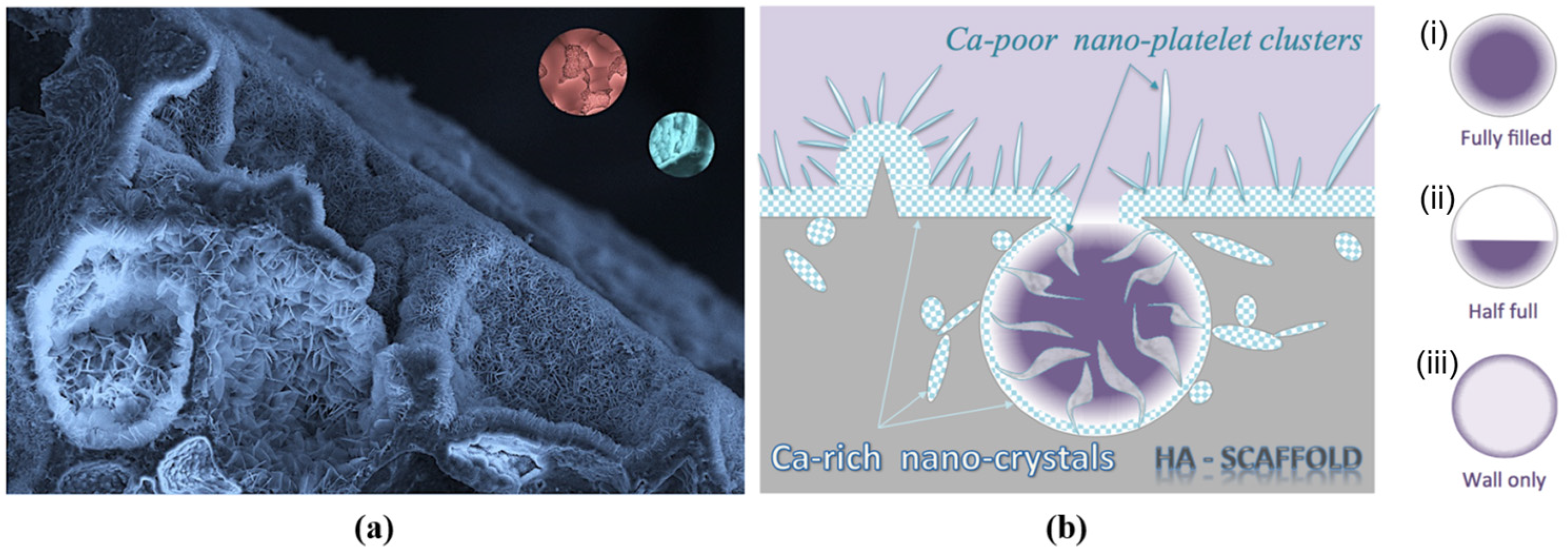
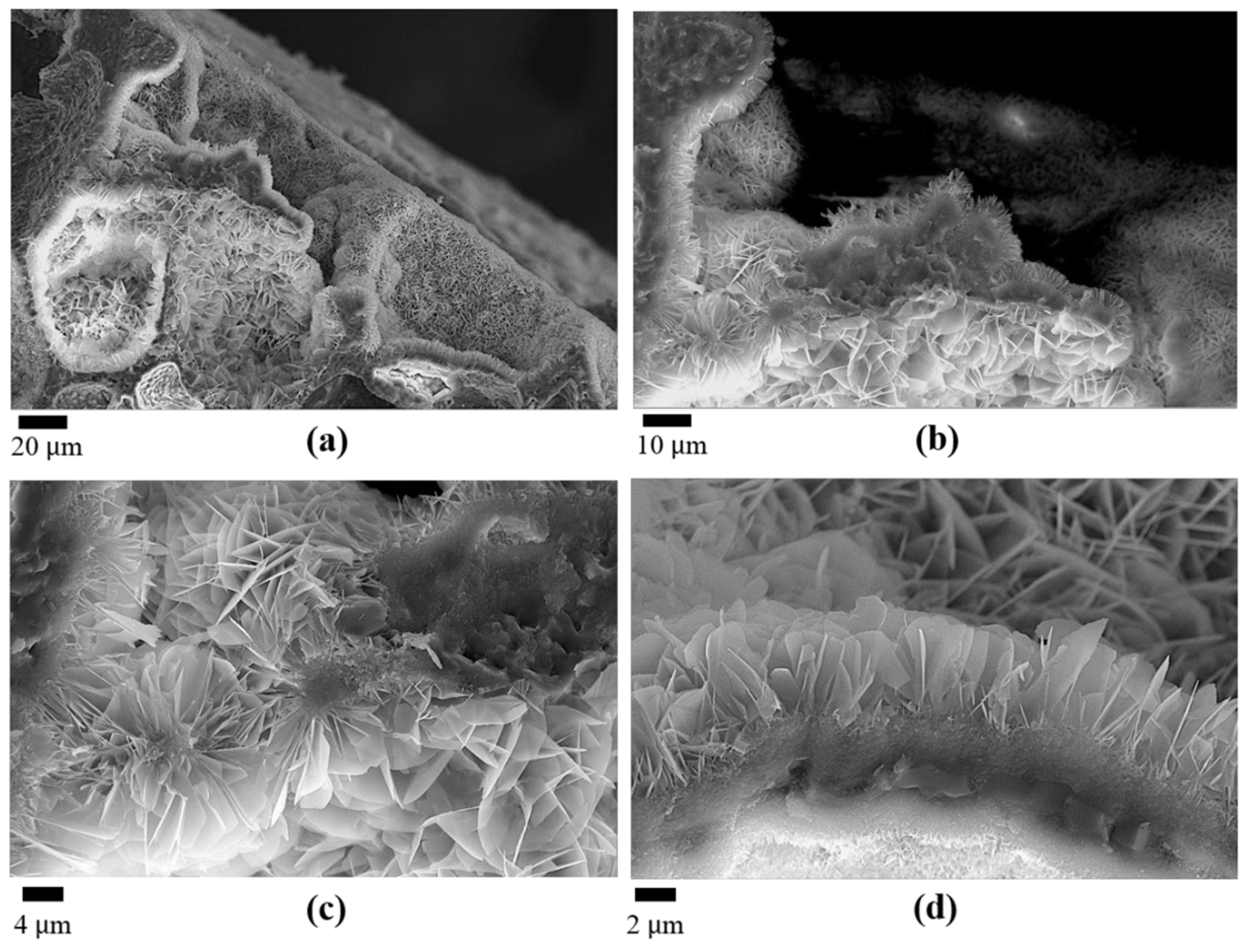
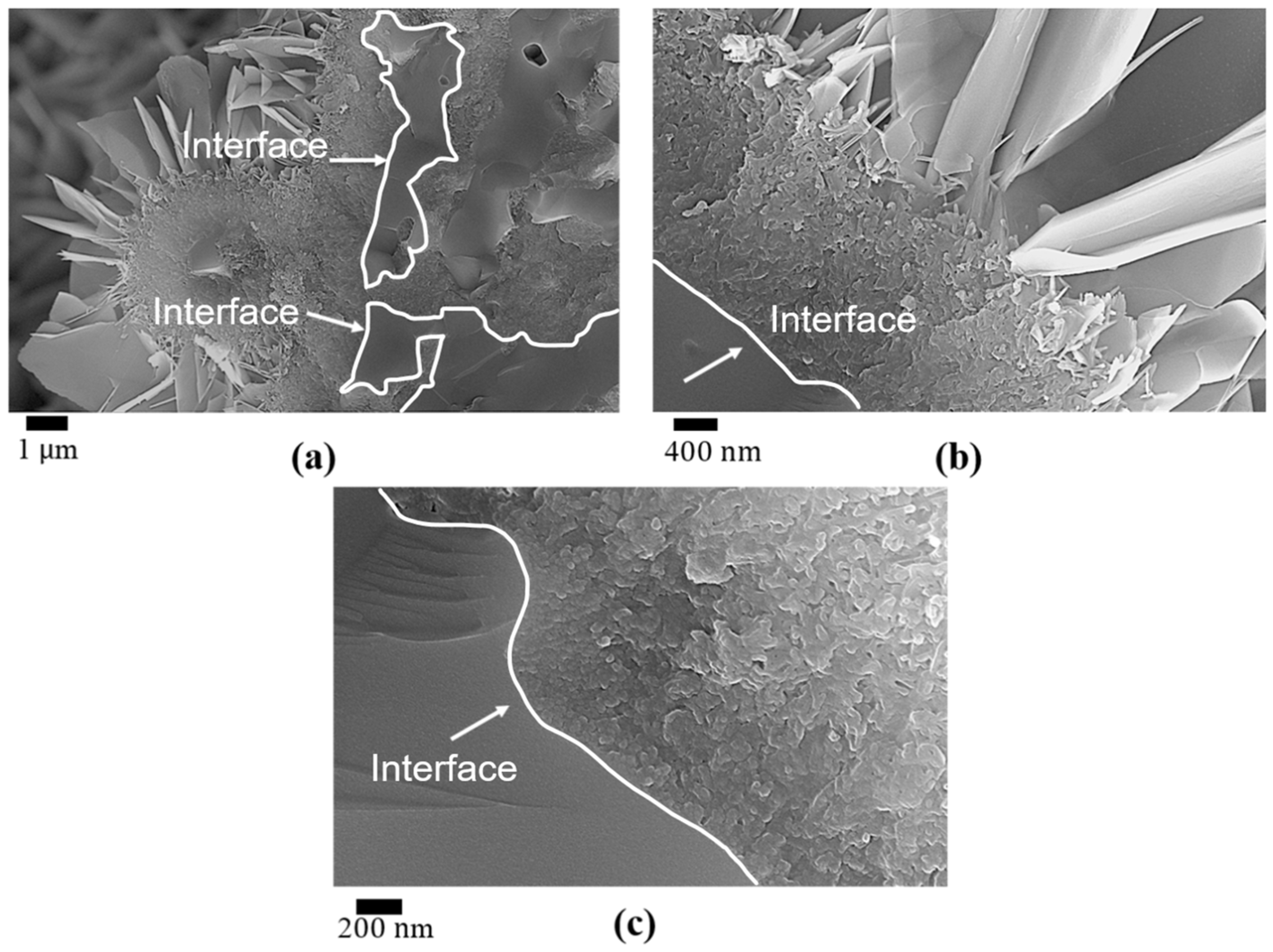
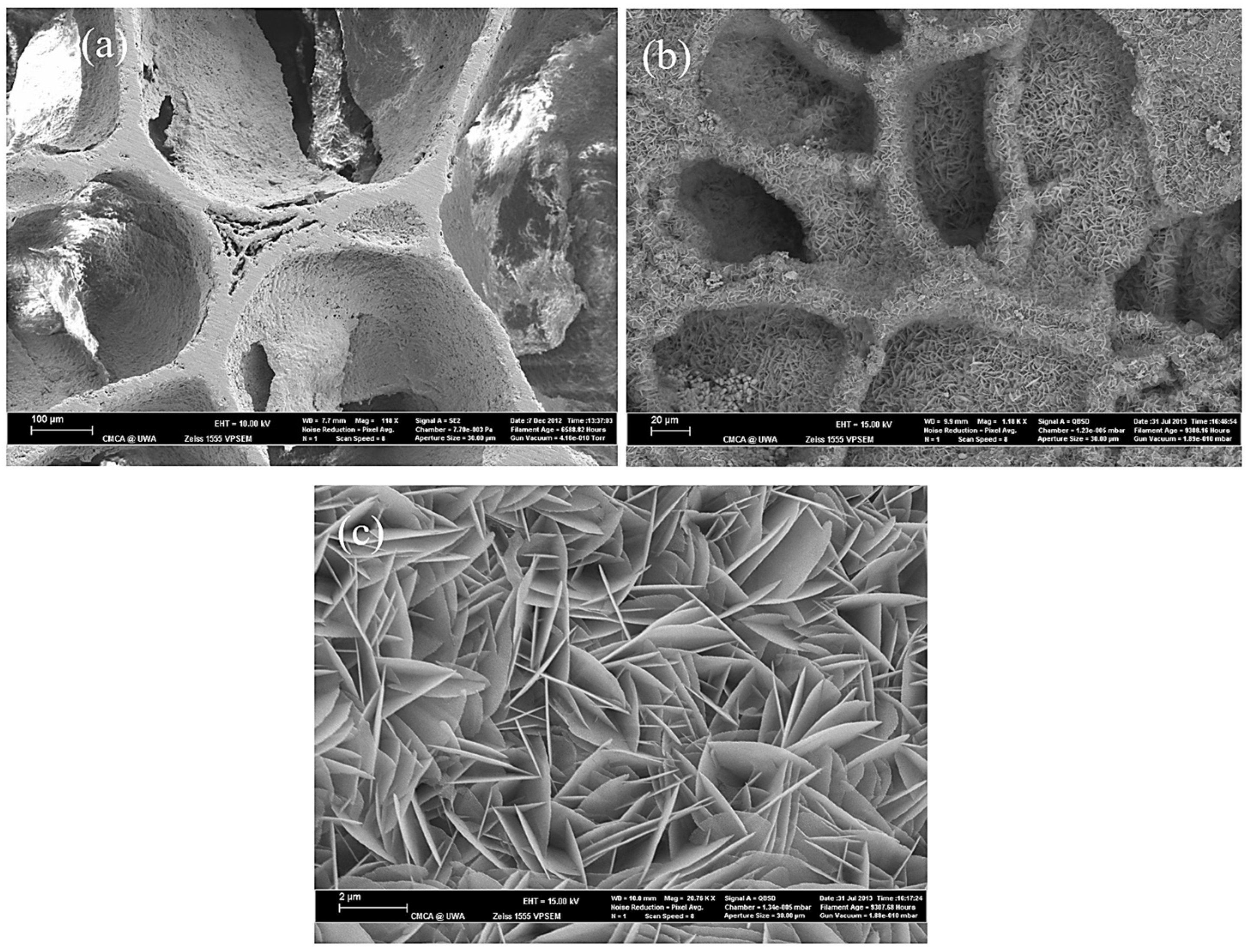
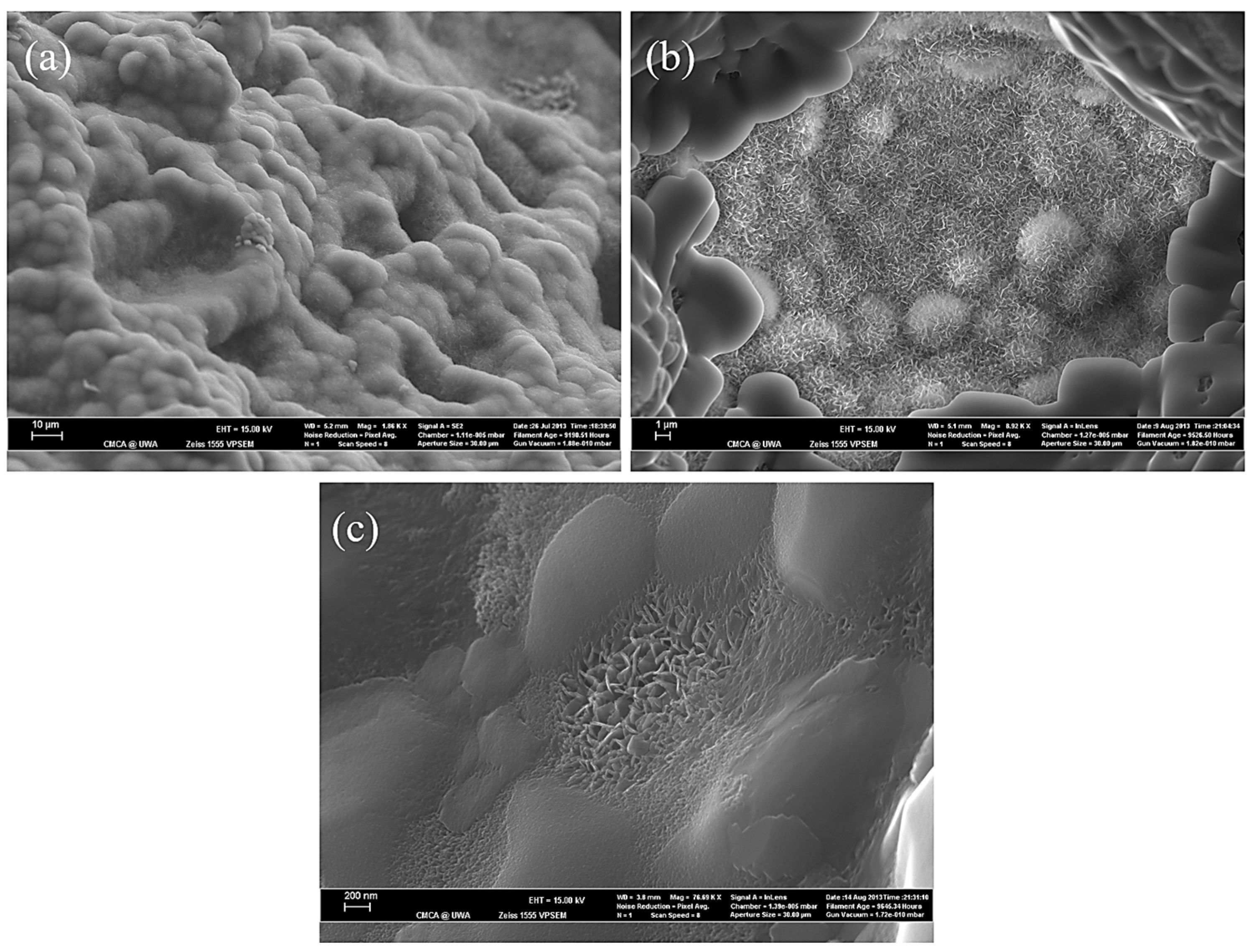
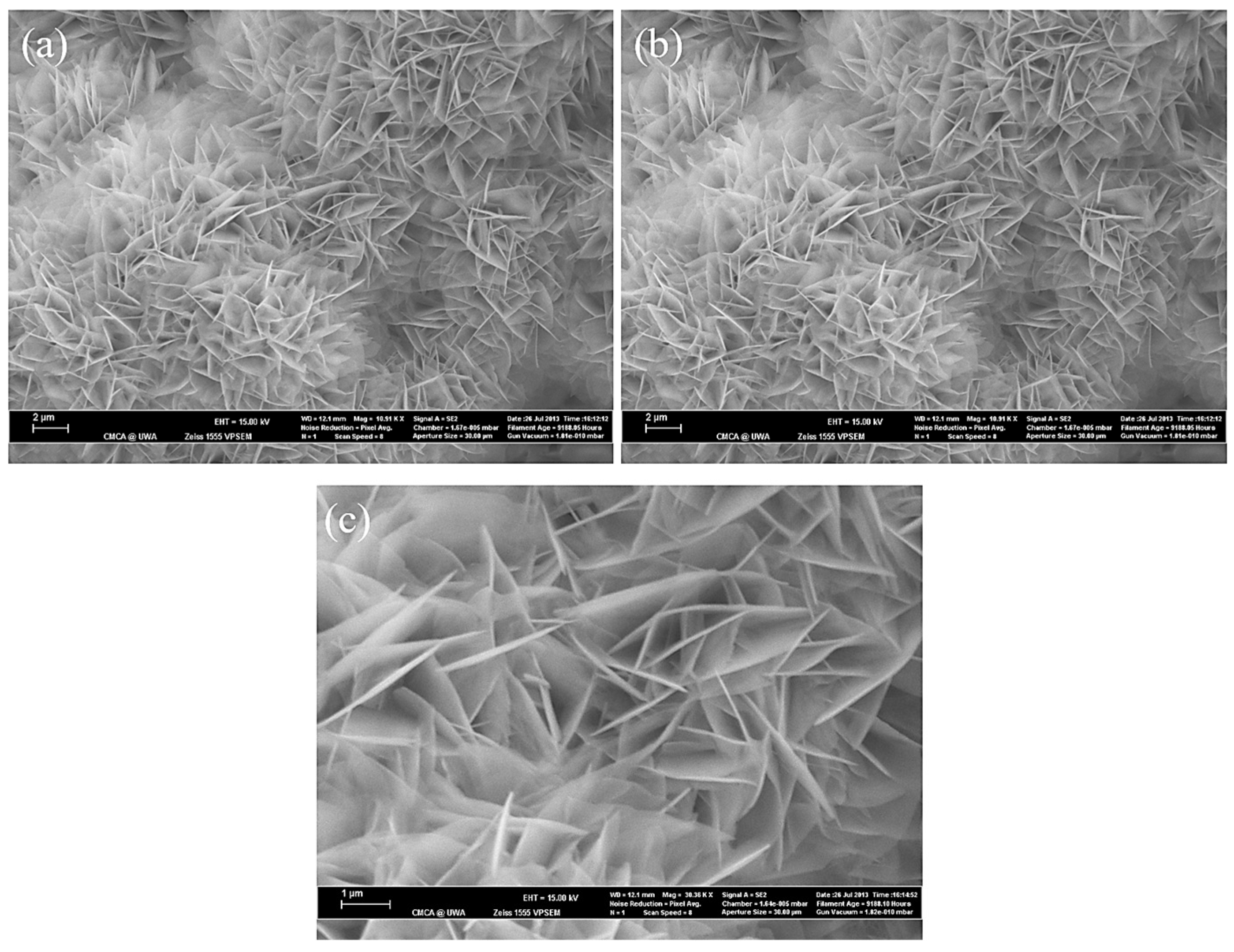
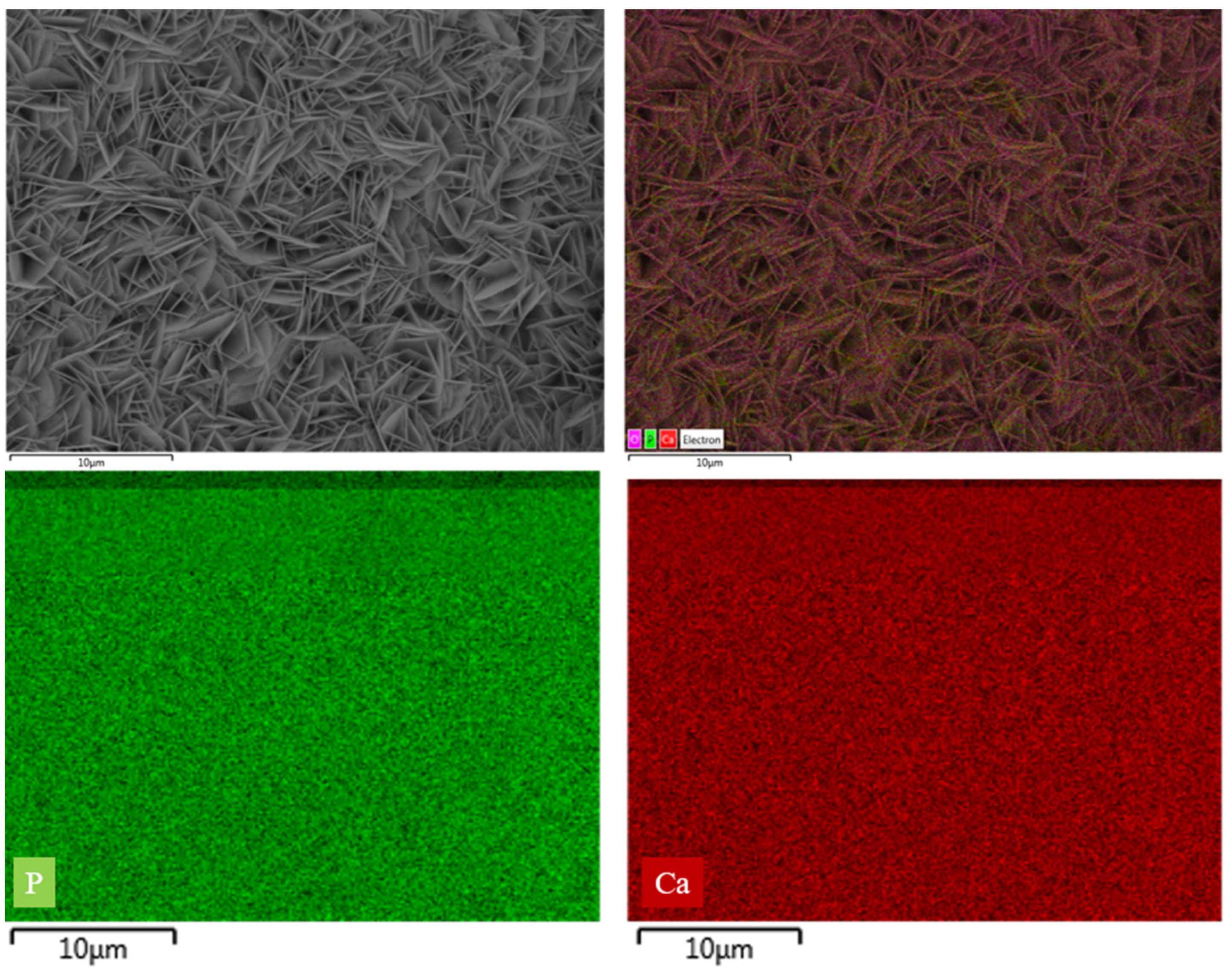
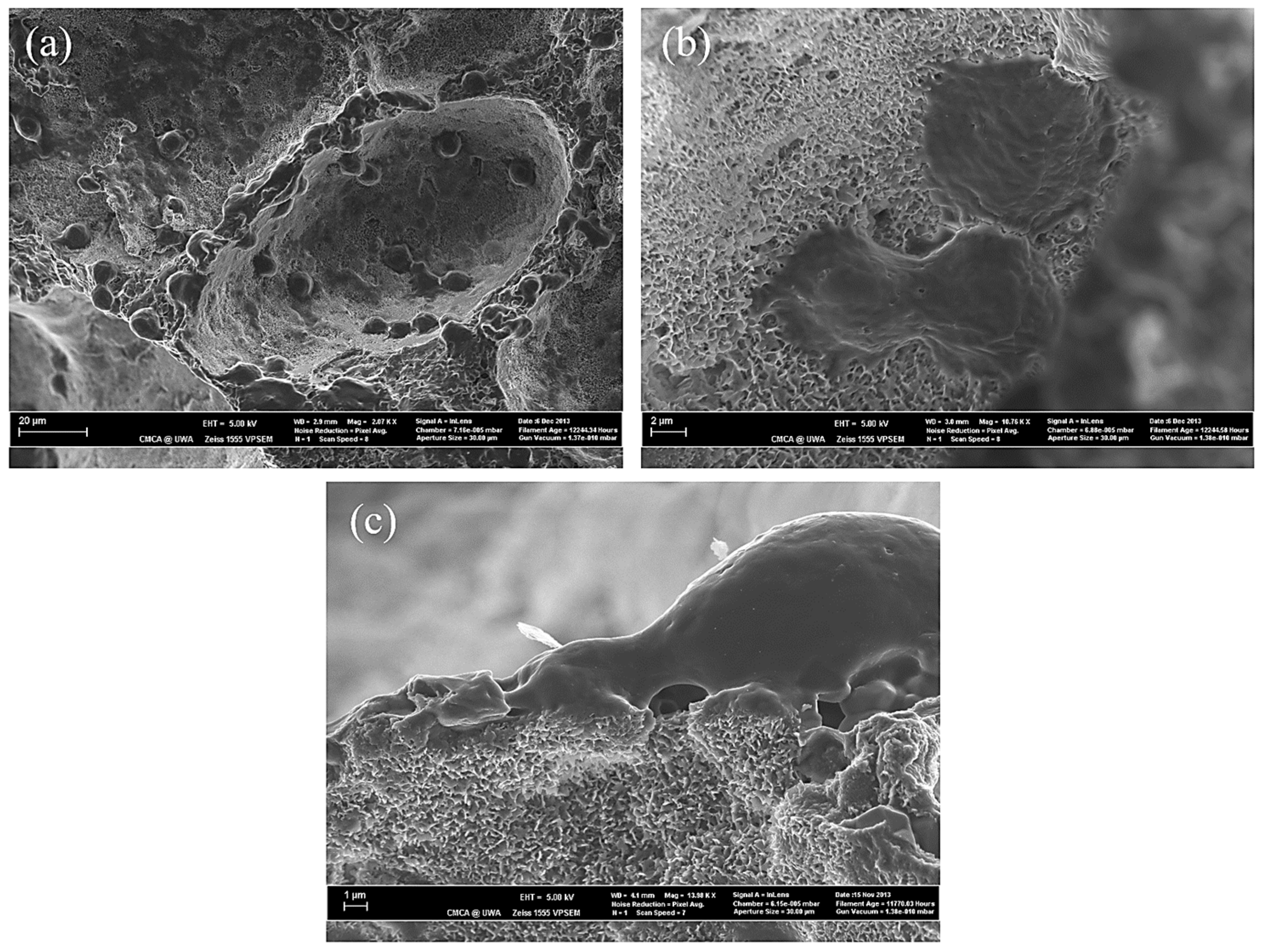
3.1. The Effect of SBF Immersion on the Microstructure of an HA/WS Skeleton
3.2. The Effect of SBF Immersion Time on the Microstructure of the HA/WS Skeleton
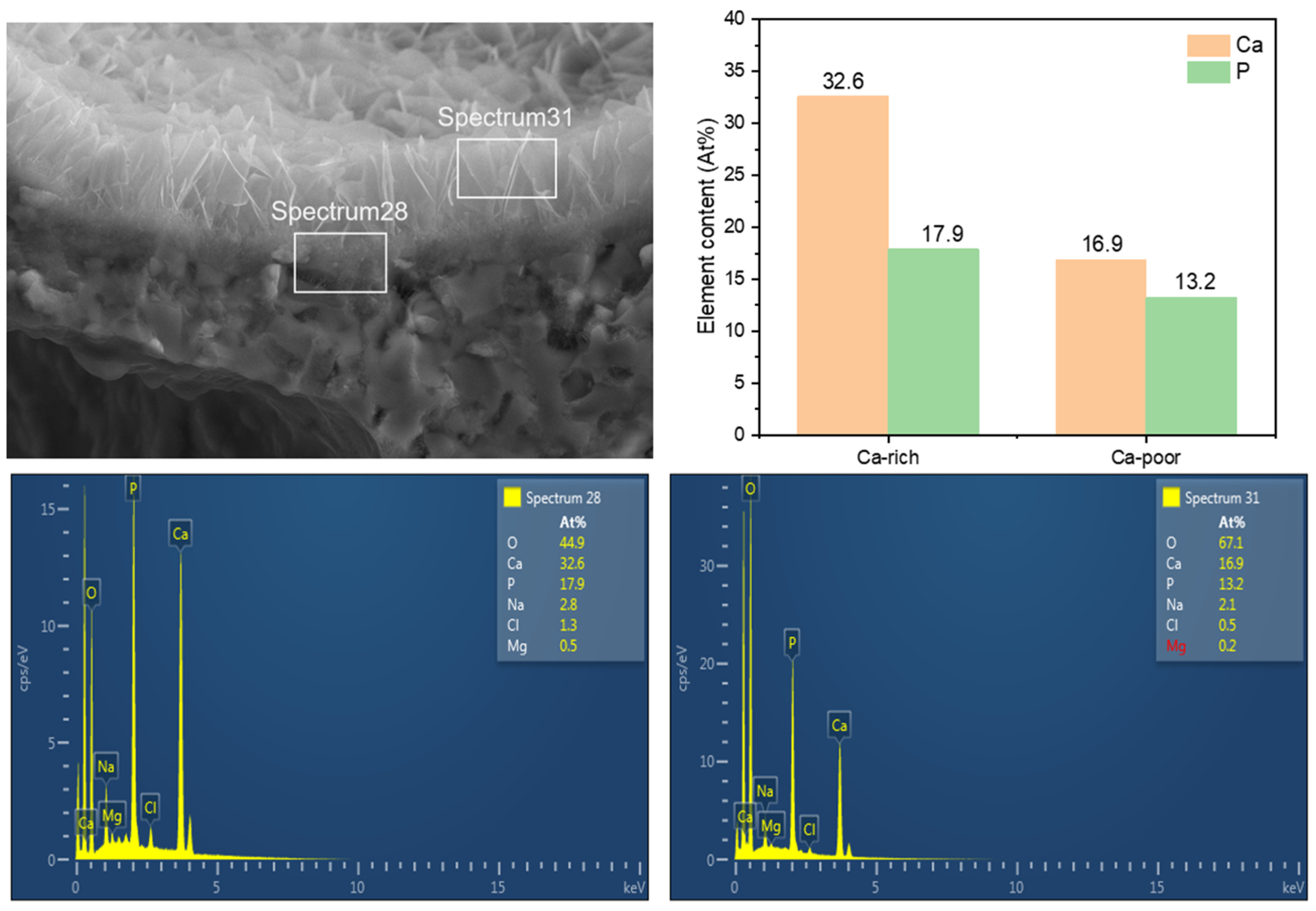
3.3. The Influence of SBF Immersion Time on the Surface Energy Spectrum of an HA/WS Skeleton
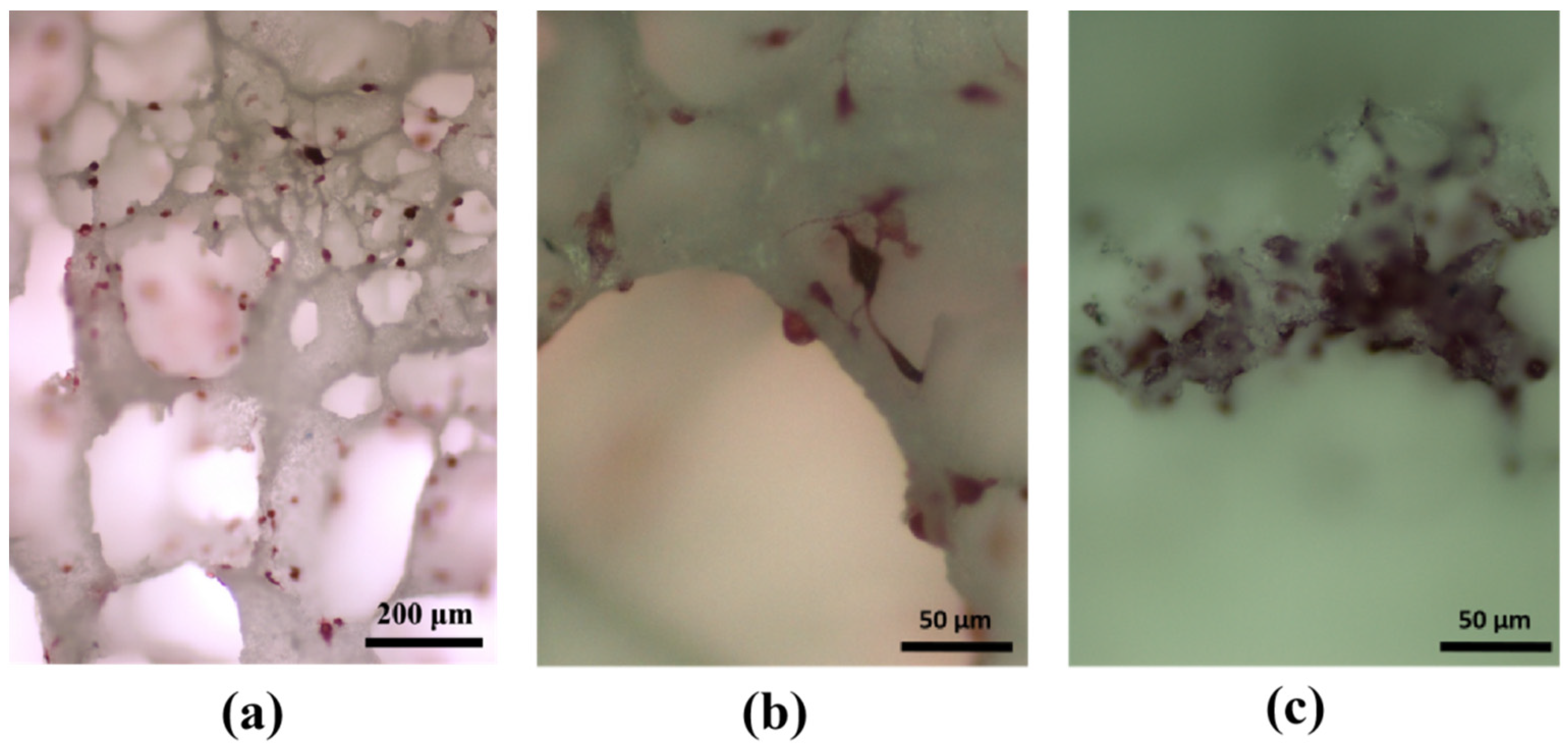
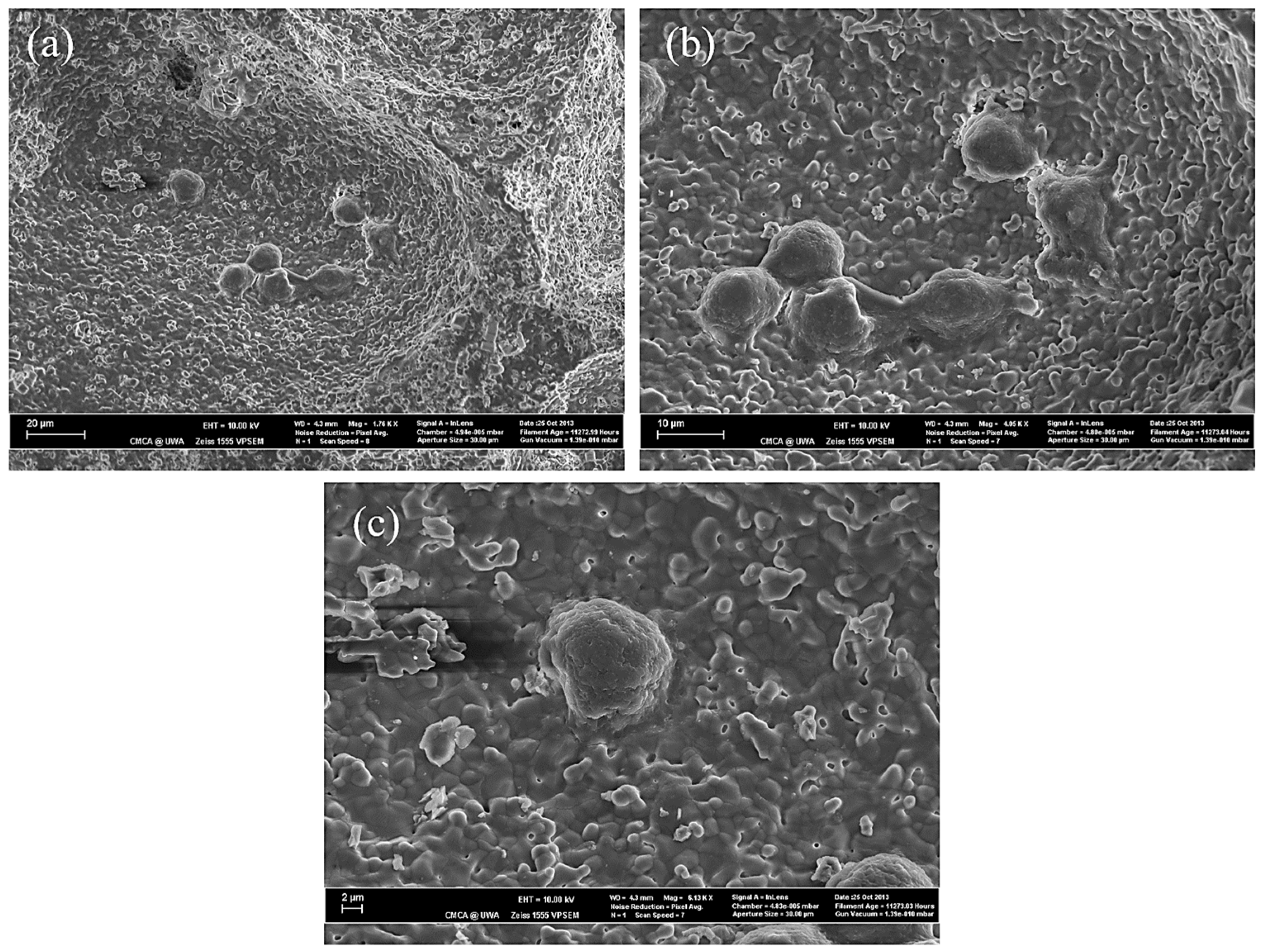
3.4. Experimental Study on Cell Adhesion within HA/WS Skeleton In Vitro
4. Conclusions
- (1)
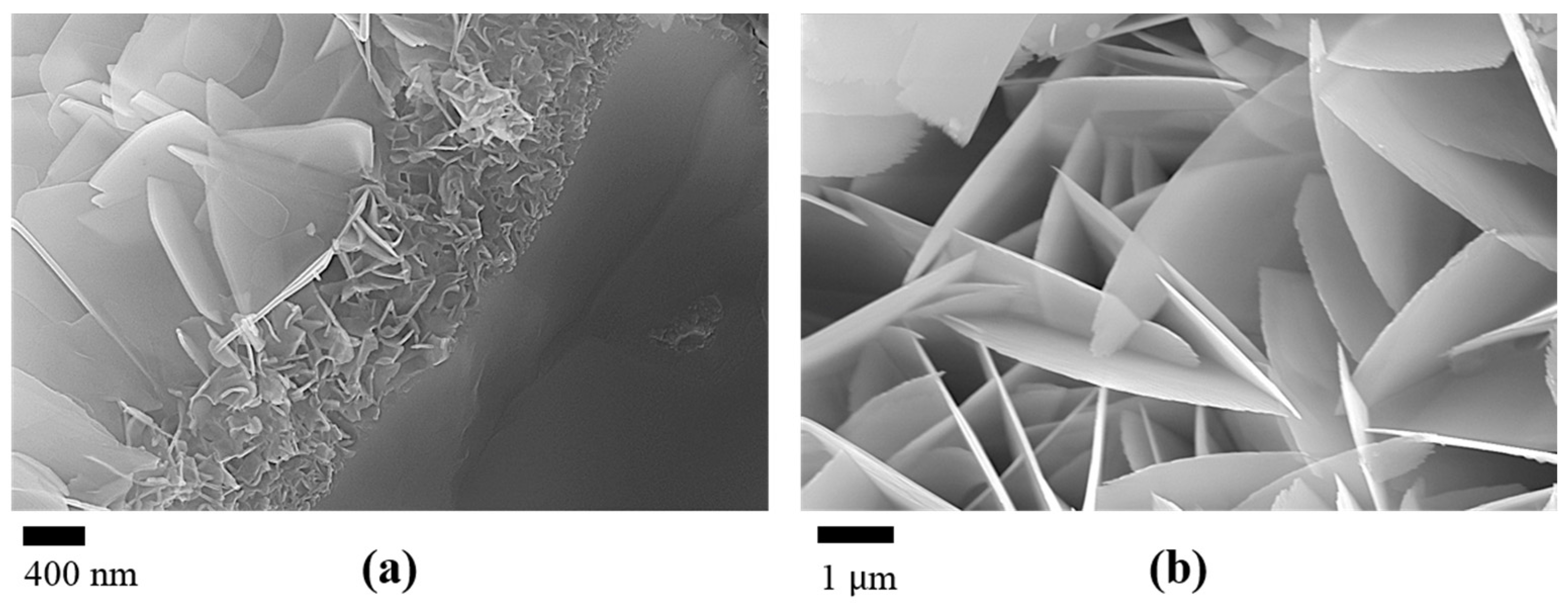
- The biomineralization process was successfully achieved after a 21 day immersion in 1.5 × SBF, resulting in the formation of densely laminar apatite nanocrystals. These flake-shaped nanocrystals were deposited within the three-dimensional porous HA/WS skeleton pores, each with an outer diameter of approximately 2 μm, an inner layer thickness of about 1 μm, and a single thickness ranging in tens of nanometers. This morphology is attributed to the concentration and distribution of Ca ions present in simulated body fluids.
- (2)
- Apatite nanocrystals exhibit exceptional interfacial properties with a three-dimensional porous HA/WS skeleton substrate. The sedimentary environment within the three-dimensional porous HA/WS skeleton is complex and diverse, resulting in diverse apatite nanocrystals being formed through biomineralization in SBF solution. These nanocrystals can enhance pore wall thickness, reduce original pore size, and promote densification. This highlights the practical significance of channel structure design in bio-porous materials. Furthermore, variations in microscopic morphology and particle size in apatite nanocrystals also significantly impact subsequent cell experiments.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, W.; Gao, C.; Feng, P.; Liu, Q.; Liu, C.; Wang, Z.; Deng, Y.; Shuai, C. Dual-functional scaffolds of poly (L-lactic acid)/nanohydroxyapatite encapsulated with metformin: Simultaneous enhancement of bone repair and bone tumor inhibition. Mater. Sci. Eng. C 2021, 120, 111592. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Y.; Zhao, Z.; Wang, X.; Mikos, A.G.; Qiu, Z.; Song, T.; Sun, X.; Zhao, L.; Zhang, C. Mineralized collagen-based composite bone materials for cranial bone regeneration in developing sheep. ACS Biomater. Sci. Eng. 2017, 3, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, R.; Di Filippo, E.S.; Tumedei, M.; Marrone, M.; Fontana, A.; Ettorre, V.; Giordani, S.; Baldrighi, M.; Iezzi, G.; Piattelli, A. Human dental pulp stem cell osteogenic differentiation seeded on equine bone block with graphene and melatonin. Appl. Sci. 2021, 11, 3218. [Google Scholar] [CrossRef]
- Boschetto, F.; Ngoc Doan, H.; Phong Vo, P.; Zanocco, M.; Zhu, W.; Sakai, W.; Adachi, T.; Ohgitani, E.; Tsutsumi, N.; Mazda, O. Antibacterial and osteoconductive effects of chitosan/polyethylene oxide (PEO)/bioactive glass nanofibers for orthopedic applications. Appl. Sci. 2020, 10, 2360. [Google Scholar] [CrossRef]
- Bueno, C.R.E.; Sarmiento, J.L.; Vasques, A.M.V.; da Silva, A.C.R.; Cintra, L.T.A.; Santos, J.M.M.; Dezan-Júnior, E. Biocompatibility, Biomineralization and Induction of Collagen Maturation with the Use of Calcium Hydroxide and Iodoform Intracanal Dressing. J. Funct. Biomater. 2023, 14, 507. [Google Scholar] [CrossRef]
- Chen, J.; Xing, Y.; Bai, X.; Xue, M.; Shi, Q.; Li, B. Strong Bioactive Glass-Based Hybrid Implants with Good Biomineralization Activity Used to Reduce Formation Duration and Improve Biomechanics of Bone Regeneration. Polymers 2023, 15, 3497. [Google Scholar] [CrossRef]
- Scaglione, S.; Giannoni, P.; Bianchini, P.; Sandri, M.; Marotta, R.; Firpo, G.; Valbusa, U.; Tampieri, A.; Diaspro, A.; Bianco, P. Order versus disorder: In vivo bone formation within osteoconductive scaffolds. Sci. Rep. 2012, 2, 274. [Google Scholar] [CrossRef]
- Xia, Z.; Yu, X.; Jiang, X.; Brody, H.D.; Rowe, D.W.; Wei, M. Fabrication and characterization of biomimetic collagen–apatite scaffolds with tunable structures for bone tissue engineering. Acta Biomater. 2013, 9, 7308–7319. [Google Scholar] [CrossRef]
- Wang, Y.; Azaïs, T.; Robin, M.; Vallée, A.; Catania, C.; Legriel, P.; Pehau-Arnaudet, G.; Babonneau, F.; Giraud-Guille, M.-M.; Nassif, N. The predominant role of collagen in the nucleation, growth, structure and orientation of bone apatite. Nat. Mater. 2012, 11, 724–733. [Google Scholar] [CrossRef]
- Zhong, C.; Chu, C.C. Biomimetic mineralization of acid polysaccharide-based hydrogels: Towards porous 3-dimensional bone-like biocomposites. J. Mater. Chem. 2012, 22, 6080–6087. [Google Scholar] [CrossRef]
- Bal, Z.; Korkusuz, F.; Ishiguro, H.; Okada, R.; Kushioka, J.; Chijimatsu, R.; Kodama, J.; Tateiwa, D.; Ukon, Y.; Nakagawa, S. A novel nano-hydroxyapatite/synthetic polymer/bone morphogenetic protein-2 composite for efficient bone regeneration. Spine J. 2021, 21, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Correa-Piña, B.A.; Gomez-Vazquez, O.M.; Londoño-Restrepo, S.M.; Zubieta-Otero, L.F.; Millan-Malo, B.M.; Rodriguez-García, M.E. Synthesis and characterization of nano-hydroxyapatite added with magnesium obtained by wet chemical precipitation. Prog. Nat. Sci. Mater. Int. 2021, 31, 575–582. [Google Scholar] [CrossRef]
- Londono-Restrepo, S.; Jeronimo-Cruz, R.; Millán-Malo, B.; Rivera-Munoz, E.; Rodriguez-García, M. Effect of the Nano crystal size on the X-ray diffraction patterns of biogenic hydroxyapatite from human, bovine, and porcine bones. Sci. Rep. 2019, 9, 5915. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.; Bhaskar, R.; Gupta, M.K.; Sharma, S.; Dasgupta, S.; Kumar, A.; Kumar, P. Mechanical, electrical, and biological properties of mechanochemically processed hydroxyapatite ceramics. Nanomaterials 2021, 11, 2216. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-M.; Himeno, T.; Kokubo, T.; Nakamura, T. Process and kinetics of bonelike apatite formation on sintered hydroxyapatite in a simulated body fluid. Biomaterials 2005, 26, 4366–4373. [Google Scholar] [CrossRef]
- Abe, Y.; Kokubo, T.; Yamamuro, T. Apatite coating on ceramics, metals and polymers utilizing a biological process. J. Mater. Sci. Mater. Med. 1990, 1, 233–238. [Google Scholar] [CrossRef]
- Weng, J.; Liu, Q.; Wolke, J.; Zhang, X.; De Groot, K. Formation and characteristics of the apatite layer on plasma-sprayed hydroxyapatite coatings in simulated body fluid. Biomaterials 1997, 18, 1027–1035. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Feng, X.-S.; Liu, H.-G.; Qian, D.-J.; Zhang, L.; Yu, X.-L.; Cui, F.-Z. Hydroxyapatite/collagen composite materials formation in simulated body fluid environment. Mater. Lett. 2004, 58, 719–722. [Google Scholar] [CrossRef]
- Lindberg, F.; Heinrichs, J.; Ericson, F.; Thomsen, P.; Engqvist, H. Hydroxylapatite growth on single-crystal rutile substrates. Biomaterials 2008, 29, 3317–3323. [Google Scholar] [CrossRef]
- Georgeanu, V.A.; Gingu, O.; Antoniac, I.V.; Manolea, H.O. Current Options and Future Perspectives on Bone Graft and Biomaterials Substitutes for Bone Repair, from Clinical Needs to Advanced Biomaterials Research. Appl. Sci. 2023, 13, 8471. [Google Scholar] [CrossRef]
- Petite, H.; Viateau, V.; Bensaid, W.; Meunier, A.; de Pollak, C.; Bourguignon, M.; Oudina, K.; Sedel, L.; Guillemin, G. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Cesarano, J., III; Dellinger, J.G.; Saavedra, M.P.; Gill, D.D.; Jamison, R.D.; Grosser, B.A.; Sinn-Hanlon, J.M.; Goldwasser, M.S. Customization of load-bearing hydroxyapatite lattice scaffolds. Int. J. Appl. Ceram. Technol. 2005, 2, 212–220. [Google Scholar] [CrossRef]
- Hulbert, S.; Young, F.; Mathews, R.; Klawitter, J.; Talbert, C.; Stelling, F. Potential of ceramic materials as permanently implantable skeletal prostheses. J. Biomed. Mater. Res. 1970, 4, 433–456. [Google Scholar] [CrossRef]
- Guillemin, G.; Meunier, A.; Dallant, P.; Christel, P.; Pouliquen, J.C.; Sedel, L. Comparison of coral resorption and bone apposition with two natural corals of different porosities. J. Biomed. Mater. Res. 1989, 23, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Dinescu, S.; Ionita, M.; Ignat, S.-R.; Costache, M.; Hermenean, A. Graphene oxide enhances chitosan-based 3D scaffold properties for bone tissue engineering. Int. J. Mol. Sci. 2019, 20, 5077. [Google Scholar] [CrossRef]
- Chatzipetros, E.; Christopoulos, P.; Donta, C.; Tosios, K.I.; Tsiambas, E.; Tsiourvas, D.; Kalogirou, E.-M.; Tsiklakis, K. Application of nano-hydroxyapatite/chitosan scaffolds on rat calvarial critical-sized defects: A pilot study. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e625. [Google Scholar] [CrossRef]
- Chatzipetros, E.; Yfanti, Z.; Christopoulos, P.; Donta, C.; Damaskos, S.; Tsiambas, E.; Tsiourvas, D.; Kalogirou, E.-M.; Tosios, K.I.; Tsiklakis, K. Imaging of nano-hydroxyapatite/chitosan scaffolds using a cone beam computed tomography device on rat calvarial defects with histological verification. Clin. Oral Investig. 2020, 24, 437–446. [Google Scholar] [CrossRef]
- Dey, A.; Bomans, P.H.; Müller, F.A.; Will, J.; Frederik, P.M.; de With, G.; Sommerdijk, N.A. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nat. Mater. 2010, 9, 1010–1014. [Google Scholar] [CrossRef]
- Cölfen, H. A crystal-clear view. Nat. Mater. 2010, 9, 960–961. [Google Scholar] [CrossRef]
- Liang, H.; Mirinejad, M.S.; Asefnejad, A.; Baharifar, H.; Li, X.; Saber-Samandari, S.; Toghraie, D.; Khandan, A. Fabrication of tragacanthin gum-carboxymethyl chitosan bio-nanocomposite wound dressing with silver-titanium nanoparticles using freeze-drying method. Mater. Chem. Phys. 2022, 279, 125770. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Al-Thebaiti, M.A.; Hashmi, M.U.; Aftab, S.; Abd Razak, S.I.; Abu Hassan, S.; Abdul Kadir, M.R.; Amin, R. Synthesis of silver-coated bioactive nanocomposite scaffolds based on grafted beta-glucan/hydroxyapatite via freeze-drying method: Anti-microbial and biocompatibility evaluation for bone tissue engineering. Materials 2020, 13, 971. [Google Scholar] [CrossRef] [PubMed]
- Kardan-Halvaei, M.; Morovvati, M.; Angili, S.N.; Saber-Samandari, S.; Razmjooee, K.; Toghraie, D.; Khandan, A. Fabrication of 3D-printed hydroxyapatite using freeze-drying method for bone regeneration: RVE and finite element simulation analysis. J. Mater. Res. Technol. 2023, 24, 8682–8692. [Google Scholar] [CrossRef]
- Kim, H.-M.; Kim, Y.; Park, S.-J.; Rey, C.; Lee, H.; Glimcher, M.J.; Ko, J.S. Thin film of low-crystalline calcium phosphate apatite formed at low temperature. Biomaterials 2000, 21, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Toledano, D.S.; Salazar-Osorio, A.I.; Medina-Buelvas, D.M.; Romero-Martínez, J.; Estrada-Muñiz, E.; Vega, L. Methylated and ethylated dialkylphosphate metabolites of organophosphate pesticides: DNA damage in bone marrow cells of Balb/c mice. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2023, 889, 503641. [Google Scholar] [CrossRef]
- Wang, Z.; Mi, F.; Li, J.; Chen, D.; Lin, M.; Wang, X.; Wu, S.; Wu, C.; Liu, C. Bone Marrow Stromal Cells Sorted by Semiconducting Polymer Nanodots for Bone Repair. ACS Biomater. Sci. Eng. 2023, 9, 5772–5781. [Google Scholar] [CrossRef]
- Shao, C.; Liu, Y.; Li, J.; Liu, Z.; Zhao, Y.; Jing, Y.; Lv, Z.; Fu, T.; Wang, Z.; Li, G. Up-regulated IL-17 and Tnf signaling in bone marrow cells of young male osteogenesis imperfecta mice. PeerJ 2022, 10, e13963. [Google Scholar] [CrossRef]
- Ducheyne, P.; Mauck, R.L.; Smith, D.H. Biomaterials in the repair of sports injuries. Nat. Mater. 2012, 11, 652–654. [Google Scholar] [CrossRef]
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent advances in hydroxyapatite-based biocomposites for bone tissue regeneration in orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef]
- Uppal, G.; Thakur, A.; Chauhan, A.; Bala, S. Magnesium based implants for functional bone tissue regeneration–A review. J. Magnes. Alloys 2022, 10, 356–386. [Google Scholar] [CrossRef]
- Pupilli, F.; Ruffini, A.; Dapporto, M.; Tavoni, M.; Tampieri, A.; Sprio, S. Design strategies and biomimetic approaches for calcium phosphate scaffolds in bone tissue regeneration. Biomimetics 2022, 7, 112. [Google Scholar] [CrossRef]
- Khvorostina, M.A.; Mironov, A.V.; Nedorubova, I.A.; Bukharova, T.B.; Vasilyev, A.V.; Goldshtein, D.V.; Komlev, V.S.; Popov, V.K. 3D Printed Gene-Activated Sodium Alginate Hydrogel Scaffolds. Gels 2022, 8, 421. [Google Scholar] [CrossRef] [PubMed]
- Nedorubova, I.A.; Bukharova, T.B.; Mokrousova, V.O.; Khvorostina, M.A.; Vasilyev, A.V.; Nedorubov, A.A.; Grigoriev, T.E.; Zagoskin, Y.D.; Chvalun, S.N.; Kutsev, S.I. Comparative Efficiency of Gene-Activated Matrices Based on Chitosan Hydrogel and PRP Impregnated with BMP2 Polyplexes for Bone Regeneration. Int. J. Mol. Sci. 2022, 23, 14720. [Google Scholar] [CrossRef] [PubMed]
- Khvorostina, M.; Mironov, A.; Nedorubova, I.; Bukharova, T.; Vasilyev, A.; Goldshtein, D.; Komlev, V.; Popov, V. Osteogenesis Enhancement with 3D Printed Gene-Activated Sodium Alginate Scaffolds. Gels 2023, 9, 315. [Google Scholar] [CrossRef] [PubMed]
- Power, R.N.; Cavanagh, B.L.; Dixon, J.E.; Curtin, C.M.; O’Brien, F.J. Development of a Gene-Activated Scaffold Incorporating Multifunctional Cell-Penetrating Peptides for pSDF-1α Delivery for Enhanced Angiogenesis in Tissue Engineering Applications. Int. J. Mol. Sci. 2022, 23, 1460. [Google Scholar] [CrossRef] [PubMed]
- Al-Munajjed, A.A.; Plunkett, N.A.; Gleeson, J.P.; Weber, T.; Jungreuthmayer, C.; Levingstone, T.; Hammer, J.; O’Brien, F.J. Development of a biomimetic collagen-hydroxyapatite scaffold for bone tissue engineering using a SBF immersion technique. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Harada, T.; Myerson, A.S.; Alan Hatton, T.; Trout, B.L. The role of nanopore shape in surface-induced crystallization. Nat. Mater. 2011, 10, 867–871. [Google Scholar] [CrossRef]
- De Yoreo, J. More than one pathway. Nat. Mater. 2013, 12, 284–285. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal engineering: From molecule to crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef]
- Bianchi, E.; Vigani, B.; Viseras, C.; Ferrari, F.; Rossi, S.; Sandri, G. Inorganic nanomaterials in tissue engineering. Pharmaceutics 2022, 14, 1127. [Google Scholar] [CrossRef]
- Xue, N.; Ding, X.; Huang, R.; Jiang, R.; Huang, H.; Pan, X.; Min, W.; Chen, J.; Duan, J.-A.; Liu, P. Bone tissue engineering in the treatment of bone defects. Pharmaceuticals 2022, 15, 879. [Google Scholar] [CrossRef]
- Ressler, A. Chitosan-Based Biomaterials for Bone Tissue Engineering Applications: A Short Review. Polymers 2022, 14, 3430. [Google Scholar] [CrossRef] [PubMed]
- Fatimi, A.; Okoro, O.V.; Podstawczyk, D.; Siminska-Stanny, J.; Shavandi, A. Natural hydrogel-based bio-inks for 3D bioprinting in tissue engineering: A review. Gels 2022, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, Z.; Johnston, J.D.; Cooper, D.M.; Chen, X. 3D bioprinted scaffolds for bone tissue engineering: State-of-the-art and emerging technologies. Front. Bioeng. Biotechnol. 2022, 10, 824156. [Google Scholar] [CrossRef] [PubMed]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on nanoparticles and nanostructured materials: Bioimaging, biosensing, drug delivery, tissue engineering, antimicrobial, and agro-food applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, B.; Li, X.; Yang, B.; Yang, S.; Chen, X.; Chen, J.; Fang, M.; Huang, Z.; Min, X.; Hu, X. Investigation on the Microstructural Diversity of a Three-Dimensional Porous Hydroxyapatite/Wollastonite Skeleton via Biomineralization in Simulated Body Fluids. Appl. Sci. 2023, 13, 11593. https://doi.org/10.3390/app132011593
Jiang B, Li X, Yang B, Yang S, Chen X, Chen J, Fang M, Huang Z, Min X, Hu X. Investigation on the Microstructural Diversity of a Three-Dimensional Porous Hydroxyapatite/Wollastonite Skeleton via Biomineralization in Simulated Body Fluids. Applied Sciences. 2023; 13(20):11593. https://doi.org/10.3390/app132011593
Chicago/Turabian StyleJiang, Bin, Xin Li, Bozhi Yang, Shujie Yang, Xinyi Chen, Junhong Chen, Minghao Fang, Zhaohui Huang, Xin Min, and Xiaozhi Hu. 2023. "Investigation on the Microstructural Diversity of a Three-Dimensional Porous Hydroxyapatite/Wollastonite Skeleton via Biomineralization in Simulated Body Fluids" Applied Sciences 13, no. 20: 11593. https://doi.org/10.3390/app132011593



