Protein/Protein and Quantum Dot/Protein Organization in Sequential Monolayer Materials Studied Using Resonance Energy Transfer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Monte Carlo Computation
2.1.1. Geometry Design
2.1.2. Energy Transfer Modeling
2.2. Preparation and Characterization of Sequential Monolayers
2.3. Proteins, Quantum Dots, and Other Chemicals
2.4. Statistical Analysis
3. Results and Discussion
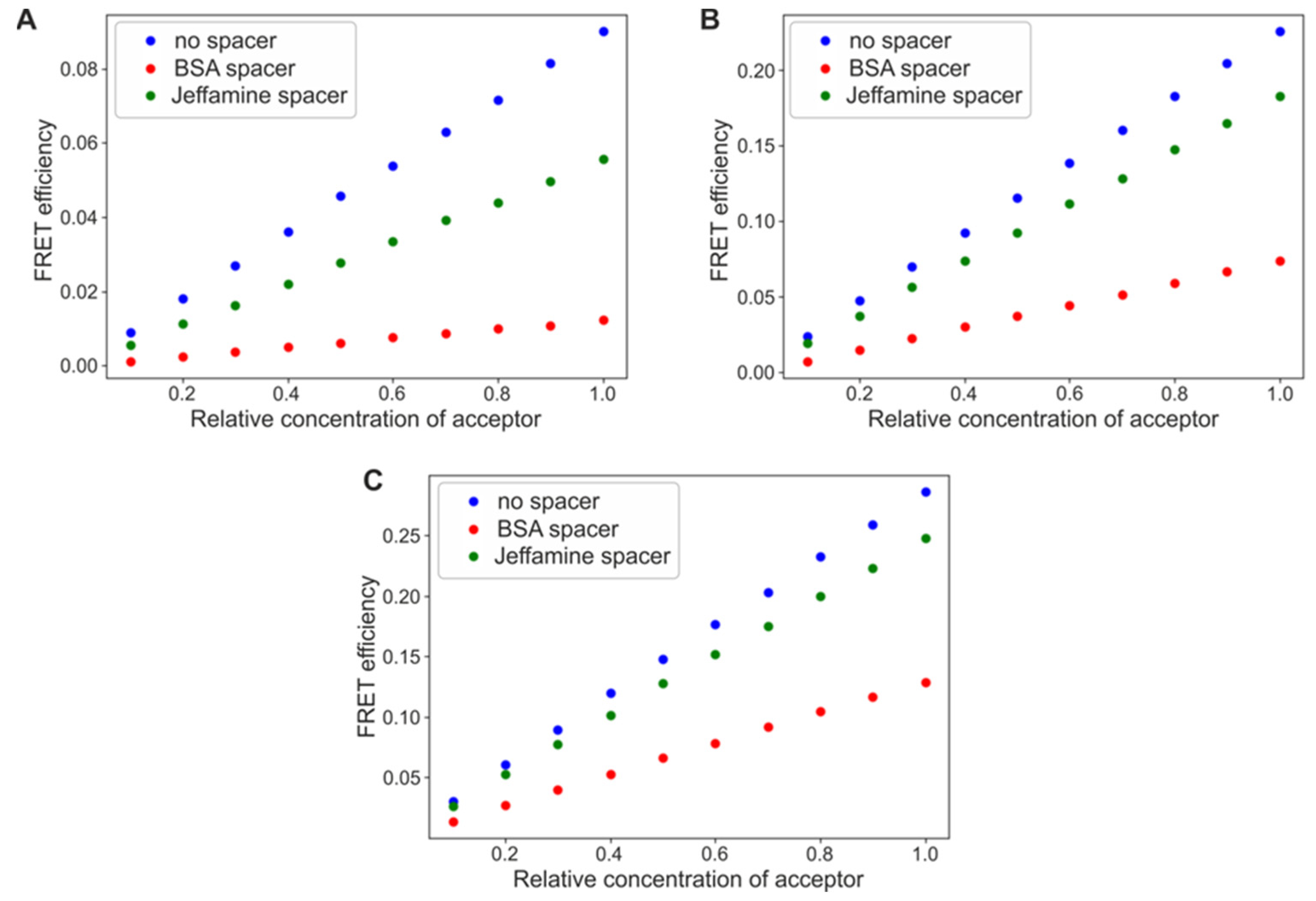
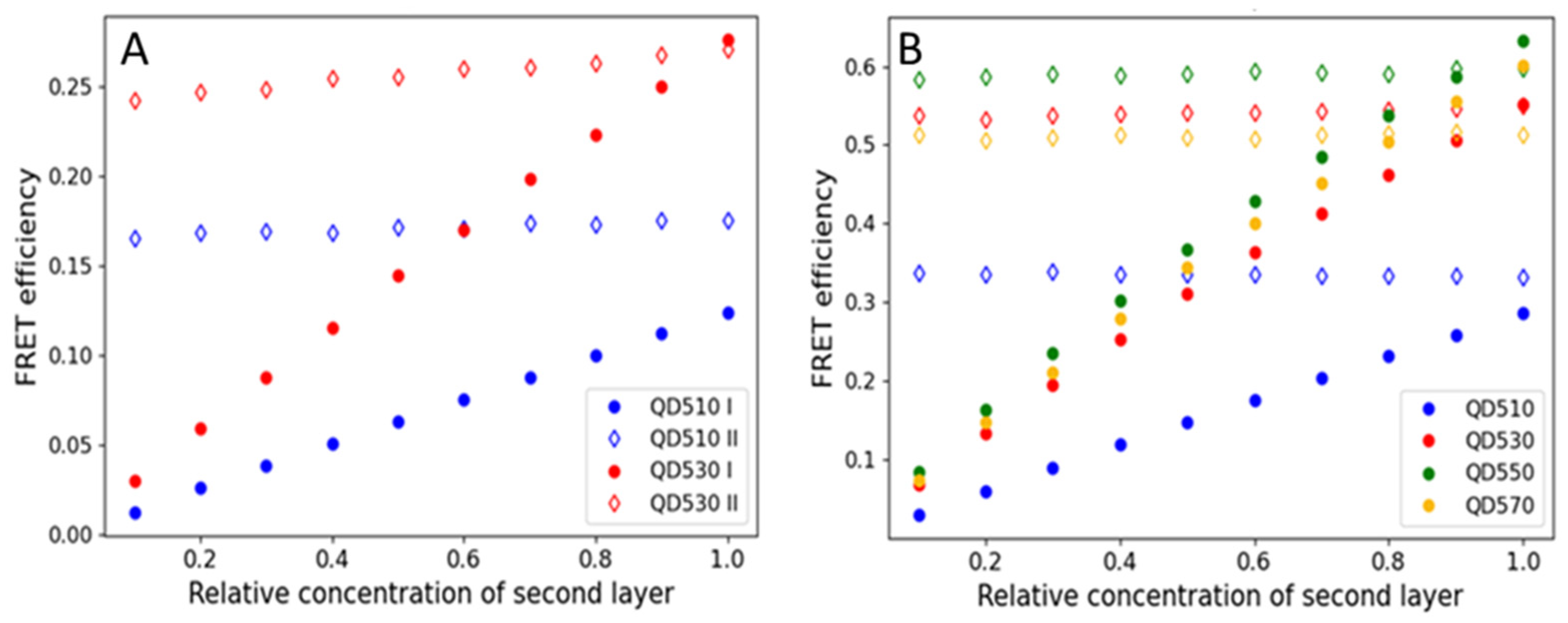
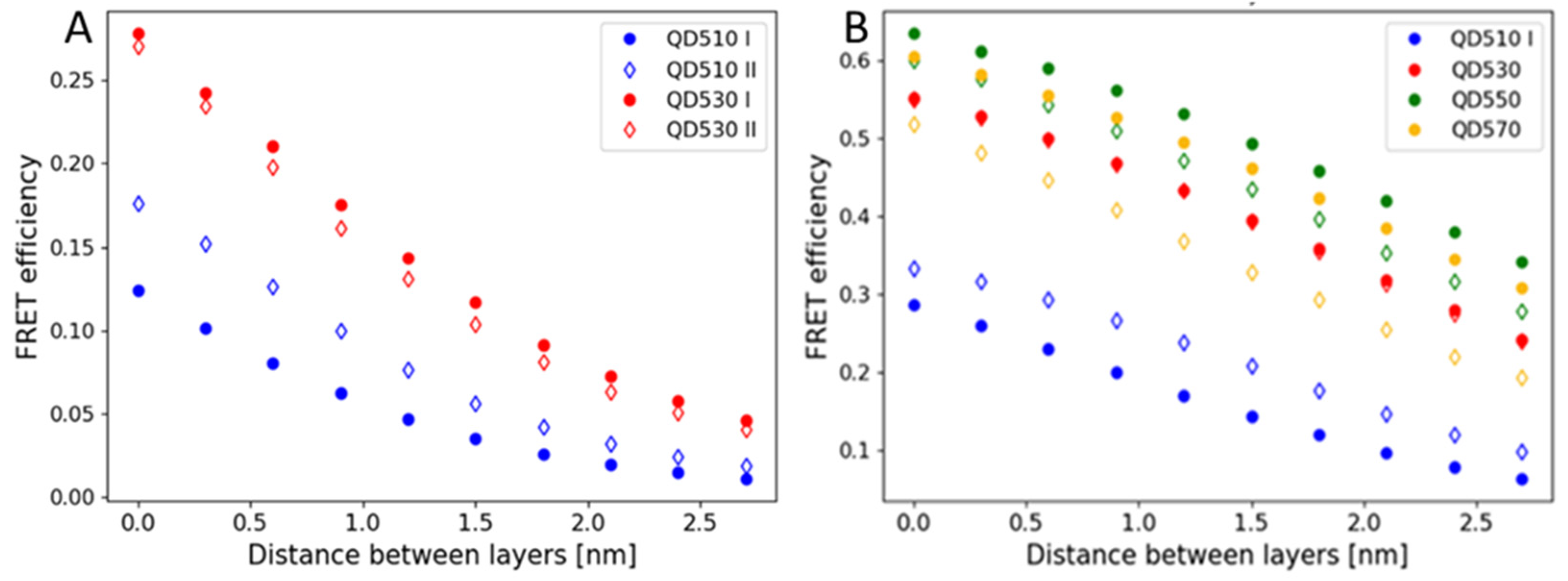
3.1. The Order of Monolayers Defines the Effectiveness of Energy Transfer by Altering the Donor/Acceptor Ratio
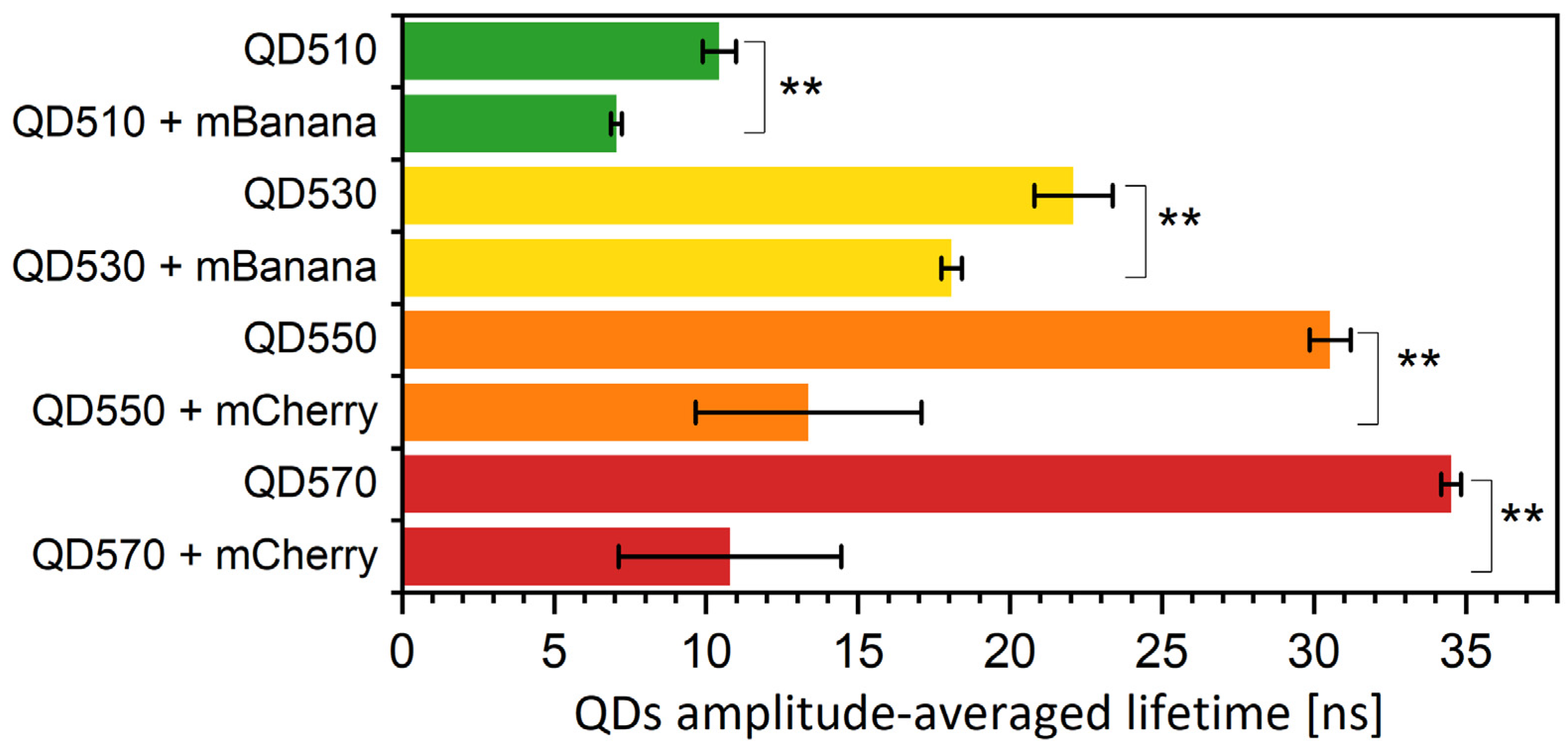
3.2. The QD/Protein Monolayers Are Better with the QD Layer Deposited First
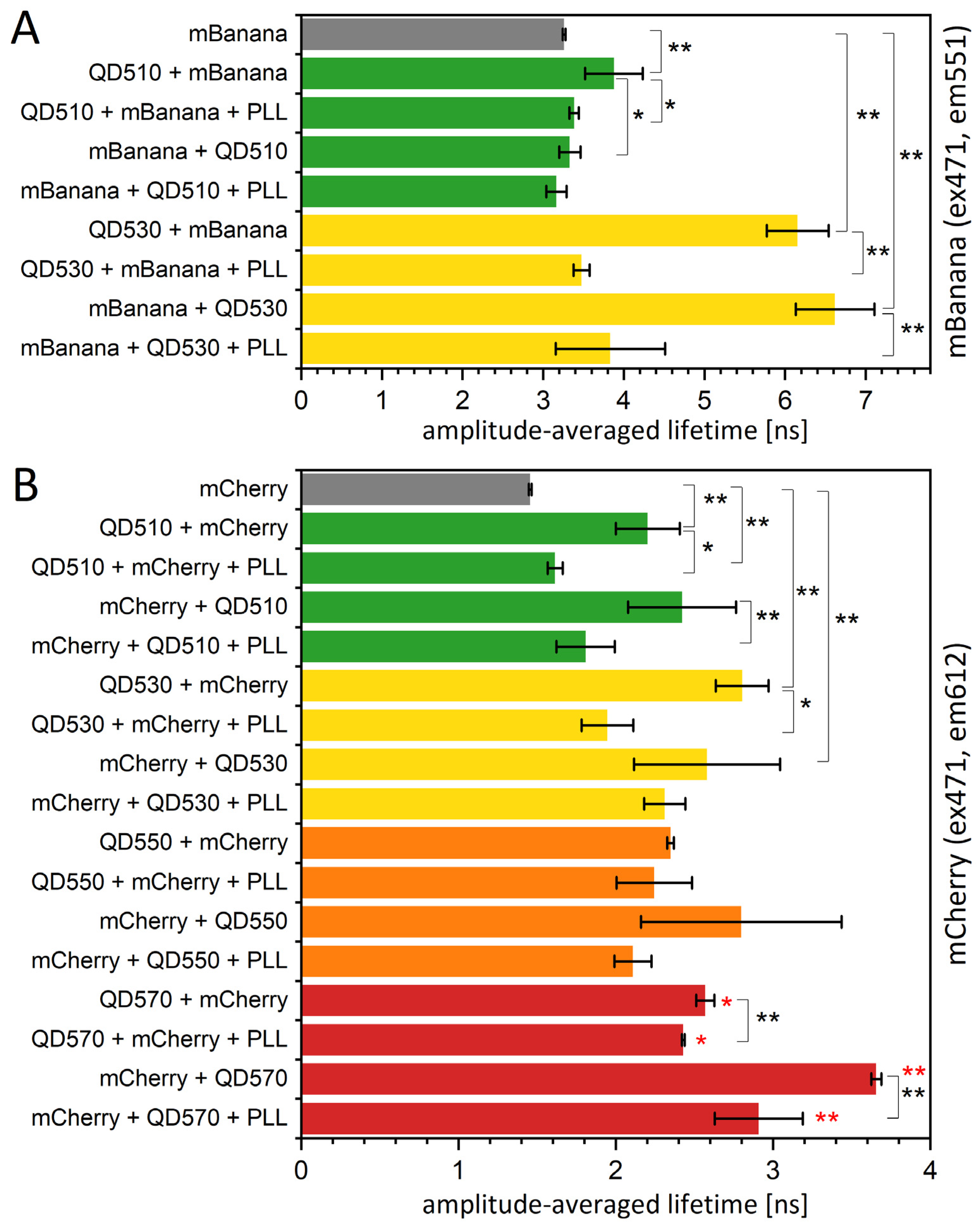
3.3. In a Quest for the FRET Yield Modification in QD-Protein Monolayers—The Effect of Specific Bleaching and Externally Added Poly-L-Lysines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, S.T.; Liu, Y.; Wang, Y.W.; Cao, A. Biosafety and bioapplication of nanomaterials by designing protein–nanoparticle interactions. Small 2013, 9, 1635–1653. [Google Scholar] [CrossRef] [PubMed]
- Edrisi, F.; Baheiraei, N.; Razavi, M.; Roshanbinfar, K.; Imani, R.; Jalilinejad, N. Potential of graphene-based nanomaterials for cardiac tissue engineering. J. Mater. Chem. B 2023, 11, 7280–7299. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.A.; Jung, S.; Ji, T. Protein Biosensors Based on Polymer Nanowires, Carbon Nanotubes and Zinc Oxide Nanorods. Sensors 2011, 11, 5087–5111. [Google Scholar] [CrossRef] [PubMed]
- Mulyana, Y.; Uenuma, M.; Okamoto, N.; Ishikawa, Y.; Yamashita, I.; Uraoka, Y. Creating reversible p–n junction on graphene through ferritin adsorption. ACS Appl. Mater. Interfaces 2016, 8, 8192–8200. [Google Scholar] [CrossRef] [PubMed]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on graphene-, graphene oxide-, reduced graphene oxide-based flexible composites: From fabrication to applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Chen, S.H.; Bell, D.R.; Luan, B. Understanding interactions between biomolecules and two-dimensional nanomaterials using in silico microscopes. Adv. Drug Deliv. Rev. 2022, 186, 114336. [Google Scholar] [CrossRef]
- Remington, S.J. Green fluorescent protein: A perspective. Protein Sci. 2011, 20, 1509–1519. [Google Scholar] [CrossRef]
- Shaner, N.C.; Campbell, R.E.; Steinbach, P.A.; Giepmans, B.N.; Palmer, A.E.; Tsien, R.Y. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004, 22, 1567. [Google Scholar] [CrossRef]
- Woo, E.; Ponvel, K.M.; Ahn, I.-S.; Lee, C.-H. Synthesis of magnetic/silica nanoparticles with a core of magnetic clusters and their application for the immobilization of His-tagged enzymes. J. Mater. Chem. 2010, 20, 1511–1515. [Google Scholar] [CrossRef]
- Gaberc-Porekar, V.; Menart, V. Potential for using histidine tags in purification of proteins at large scale. Chem. Eng. Technol. Ind. Chem.-Plant Equip.-Process Eng.-Biotechnol. 2005, 28, 1306–1314. [Google Scholar] [CrossRef]
- Miyazaki, M.; Kaneno, J.; Yamaori, S.; Honda, T.; Briones, M.P.; Uehara, M.; Arima, K.; Kanno, K.; Yamashita, K.; Yamaguchi, Y. Efficient immobilization of enzymes on microchannel surface through His-tag and application for microreactor. Protein Pept. Lett. 2005, 12, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.; Brus, L. Chemical synthesis and luminescence applications of colloidal semiconductor quantum dots. J. Am. Chem. Soc. 2017, 139, 10939–10943. [Google Scholar] [CrossRef] [PubMed]
- Zito, J.; Infante, I. The future of ligand engineering in colloidal semiconductor nanocrystals. Acc. Chem. Res. 2021, 54, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.C.; Algar, W.R.; Medintz, I.L.; Hildebrandt, N. Quantum dots for Förster resonance energy transfer (FRET). TrAC Trends Anal. Chem. 2020, 125, 115819. [Google Scholar] [CrossRef]
- Filali, S.; Pirot, F.; Miossec, P. Biological applications and toxicity minimization of semiconductor quantum dots. Trends Biotechnol. 2020, 38, 163–177. [Google Scholar] [CrossRef]
- Gidwani, B.; Sahu, V.; Shukla, S.S.; Pandey, R.; Joshi, V.; Jain, V.K.; Vyas, A. Quantum dots: Prospectives, toxicity, advances and applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102308. [Google Scholar] [CrossRef]
- Richert, L.; Arntz, Y.; Schaaf, P.; Voegel, J.-C.; Picart, C. pH dependent growth of poly (L-lysine)/poly (L-glutamic) acid multilayer films and their cell adhesion properties. Surf. Sci. 2004, 570, 13–29. [Google Scholar] [CrossRef]
- Zheng, S.; Guan, Y.; Yu, H.; Huang, G.; Zheng, C. Poly-l-lysine-coated PLGA/poly (amino acid)-modified hydroxyapatite porous scaffolds as efficient tissue engineering scaffolds for cell adhesion, proliferation, and differentiation. New J. Chem. 2019, 43, 9989–10002. [Google Scholar] [CrossRef]
- Khan, Z.G.; Patil, P.O. Design and synthesis of poly-L-lysine-functionalized graphene quantum dots sensor for specific detection of cysteine and homocysteine. Mater. Chem. Phys. 2022, 276, 125383. [Google Scholar] [CrossRef]
- Simões, M.G.; Urstöger, G.; Schennach, R.; Hirn, U. Quantification and Imaging of Nanoscale Contact with Förster Resonance Energy Transfer. ACS Appl. Mater. Interfaces 2021, 13, 19521–19529. [Google Scholar] [CrossRef] [PubMed]
- Algar, W.R.; Krause, K.D. Developing FRET networks for sensing. Annu. Rev. Anal. Chem. 2022, 15, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Corry, B.; Huang, L.; Hildebrandt, N. FRET-modulated multihybrid nanoparticles for brightness-equalized single-wavelength barcoding. J. Am. Chem. Soc. 2019, 141, 11123–11141. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer Science & Business Media: New York, NY, USA, 2007. [Google Scholar]
- Sorout, N.; Chandra, A. Interactions of the Aβ (1–42) Peptide with Boron Nitride Nanoparticles of Varying Curvature in an Aqueous Medium: Different Pathways to Inhibit β-Sheet Formation. J. Phys. Chem. B 2021, 125, 11159–11178. [Google Scholar] [CrossRef]
- Zhang, J.; Kuang, Z.; Li, H.; Li, S.; Xia, F. Electrode surface roughness greatly enhances the sensitivity of electrochemical non-enzymatic glucose sensors. J. Electroanal. Chem. 2022, 919, 116541. [Google Scholar] [CrossRef]
- Yang, F.; Moss, L.G.; Phillips, G.N., Jr. The molecular structure of green fluorescent protein. Nat. Biotechnol. 1996, 14, 1246–1251. [Google Scholar] [CrossRef]
- Shu, X.; Shaner, N.C.; Yarbrough, C.A.; Tsien, R.Y.; Remington, S.J. Novel chromophores and buried charges control color in mFruits. Biochemistry 2006, 45, 9639–9647. [Google Scholar] [CrossRef]
- Grzyb, J.; Walczewska-Szewc, K.; Slawski, J.; Trojnar, M. Quantum dot clusters as self-assembled antennae with phycocyanine and phycobilisomes as energy acceptors. Phys. Chem. Chem. Phys. 2021, 23, 24505–24517. [Google Scholar] [CrossRef]
- Sztatelman, O.; Kopec, K.; Pedziwiatr, M.; Trojnar, M.; Worch, R.; Wielgus-Kutrowska, B.; Jemiola-Rzeminska, M.; Bzowska, A.; Grzyb, J. Heterodimerizing helices as tools for nanoscale control of the organization of protein-protein and protein-quantum dots. Biochimie 2019, 167, 93–105. [Google Scholar] [CrossRef]
- R Core Team, R. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Kressin, A.M.; Doan, V.V.; Klein, J.D.; Sailor, M.J. Synthesis of stoichiometric cadmium selenide films via sequential monolayer electrodeposition. Chem. Mater. 1991, 3, 1015–1020. [Google Scholar] [CrossRef]
- Chiodini, S.; D’avino, G.; Muccioli, L.; Bartolini, L.; Gentili, D.; Toffanin, S.; Albonetti, C. Self-organization of complete organic monolayers via sequential post-deposition annealing. Prog. Org. Coat. 2020, 138, 105408. [Google Scholar] [CrossRef]
- Basabe-Desmonts, L.; Beld, J.; Zimmerman, R.S.; Hernando, J.; Mela, P.; García Parajó, M.F.; van Hulst, N.F.; van den Berg, A.; Reinhoudt, D.N.; Crego-Calama, M. A simple approach to sensor discovery and fabrication on self-assembled monolayers on glass. J. Am. Chem. Soc. 2004, 126, 7293–7299. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Maldarelli, C.; Steiner, C.; Couzis, A. Formation of nanometer domains of one chemical functionality in a continuous matrix of a second chemical functionality by sequential adsorption of silane self-assembled monolayers. Langmuir 2001, 17, 7789–7797. [Google Scholar] [CrossRef]
- Yang, G.; Hallinan, D.T. Gold nanoparticle monolayers from sequential interfacial ligand exchange and migration in a three-phase system. Sci. Rep. 2016, 6, 35339. [Google Scholar] [CrossRef]
- Suzuki, M.; Husimi, Y.; Komatsu, H.; Suzuki, K.; Douglas, K.T. Quantum dot FRET biosensors that respond to pH, to proteolytic or nucleolytic cleavage, to DNA synthesis, or to a multiplexing combination. J. Am. Chem. Soc. 2008, 130, 5720–5725. [Google Scholar] [CrossRef]
- Mattoussi, H.; Mauro, J.M.; Goldman, E.R.; Anderson, G.P.; Sundar, V.C.; Mikulec, F.V.; Bawendi, M.G. Self-assembly of CdSe-ZnS quantum dot bioconjugates using an engineered recombinant protein. J. Am. Chem. Soc. 2000, 122, 12142–12150. [Google Scholar] [CrossRef]
- Mattoussi, H.; Medintz, I.L.; Clapp, A.R.; Goldman, E.R.; Jaiswal, J.K.; Simon, S.M.; Mauro, J.M. Luminescent quantum dot-bioconjugates in immunoassays, FRET, biosensing, and imaging applications. JALA J. Assoc. Lab. Autom. 2004, 9, 28–32. [Google Scholar] [CrossRef]
- Marx, V. Probes: FRET sensor design and optimization. Nat. Methods 2017, 14, 949–953. [Google Scholar] [CrossRef]
- Wu, L.; Huang, C.; Emery, B.P.; Sedgwick, A.C.; Bull, S.D.; He, X.-P.; Tian, H.; Yoon, J.; Sessler, J.L.; James, T.D. Förster resonance energy transfer (FRET)-based small-molecule sensors and imaging agents. Chem. Soc. Rev. 2020, 49, 5110–5139. [Google Scholar] [CrossRef]
- Babic, M.; Horák, D.; Trchová, M.; Jendelová, P.; Glogarová, K.; Lesný, P.; Herynek, V.; Hájek, M.; Syková, E. Poly (L-lysine)-modified iron oxide nanoparticles for stem cell labeling. Bioconjug. Chem. 2008, 19, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Yang, H.; Han, D.; Zhang, Q.; Ivaska, A.; Niu, L. Water-soluble graphene covalently functionalized by biocompatible poly-L-lysine. Langmuir 2009, 25, 12030–12033. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.A.; Custódio, D.A.; Dorneles, E.M.; Coura, F.M.; Carvalho, I.C.; Lage, A.P.; Mansur, H.S. Nanoplexes of ZnS quantum dot-poly-l-lysine/iron oxide nanoparticle-carboxymethylcellulose for photocatalytic degradation of dyes and antibacterial activity in wastewater treatment. Int. J. Biol. Macromol. 2023, 231, 123363. [Google Scholar] [CrossRef] [PubMed]
- Mirtič, A.; Grdadolnik, J. The structure of poly-L-lysine in different solvents. Biophys. Chem. 2013, 175, 47–53. [Google Scholar] [CrossRef]
- Boulesbaa, A.; Huang, Z.; Wu, D.; Lian, T. Competition between energy and electron transfer from CdSe QDs to adsorbed rhodamine B. J. Phys. Chem. C 2010, 962–969. [Google Scholar] [CrossRef]
- Sławski, J.; Szewczyk, S.; Burdziński, G.; Gibasiewicz, K.; Grzyb, J. Time-resolved absorption measurements quantify the competition of energy and electron transfer between quantum dots and cytochrome c. Spectrochim. Acta Part A 2023, 295, 122627. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sławski, J.; Walczewska-Szewc, K.; Grzyb, J. Protein/Protein and Quantum Dot/Protein Organization in Sequential Monolayer Materials Studied Using Resonance Energy Transfer. Appl. Sci. 2023, 13, 11917. https://doi.org/10.3390/app132111917
Sławski J, Walczewska-Szewc K, Grzyb J. Protein/Protein and Quantum Dot/Protein Organization in Sequential Monolayer Materials Studied Using Resonance Energy Transfer. Applied Sciences. 2023; 13(21):11917. https://doi.org/10.3390/app132111917
Chicago/Turabian StyleSławski, Jakub, Katarzyna Walczewska-Szewc, and Joanna Grzyb. 2023. "Protein/Protein and Quantum Dot/Protein Organization in Sequential Monolayer Materials Studied Using Resonance Energy Transfer" Applied Sciences 13, no. 21: 11917. https://doi.org/10.3390/app132111917
APA StyleSławski, J., Walczewska-Szewc, K., & Grzyb, J. (2023). Protein/Protein and Quantum Dot/Protein Organization in Sequential Monolayer Materials Studied Using Resonance Energy Transfer. Applied Sciences, 13(21), 11917. https://doi.org/10.3390/app132111917





