Optimization of the Antifungal Property in a Composite of Polyurethane and Silver Nanoparticles against the Trichophyton rubrum Fungus
Abstract
:1. Introduction
1.1. Properties of AgNPs against Microorganisms and Fungi
1.2. The Shape of Nanoparticles and Their Applications on Polyurethanes
1.3. References for Laboratory Testing
2. Materials and Methods
2.1. Materials
- 5 g of the 15 nm AgNPs/ethanol dispersion for a concentration of 12.66 mg of AgNPs per 1 kg of polyol. (Small AgNPs size, low concentration)
- 5 g of the 45 nm AgNPs/ethanol dispersion for a concentration of 12.66 mg of AgNPs per 1 kg of polyol. (Large AgNPs size, low concentration)
- 15 g of the 15 nm AgNPs/ethanol dispersion for a concentration of 38 mg of AgNPs per 1 kg of polyol. (Small AgNPs size, high concentration)
- 15 g of the 45 nm AgNPs/ethanol dispersion for a concentration of 38 mg of AgNPs per 1 kg of polyol. (Large AgNPs size, high concentration)
2.2. Definition of Experimental Design
2.3. Sample Preparation
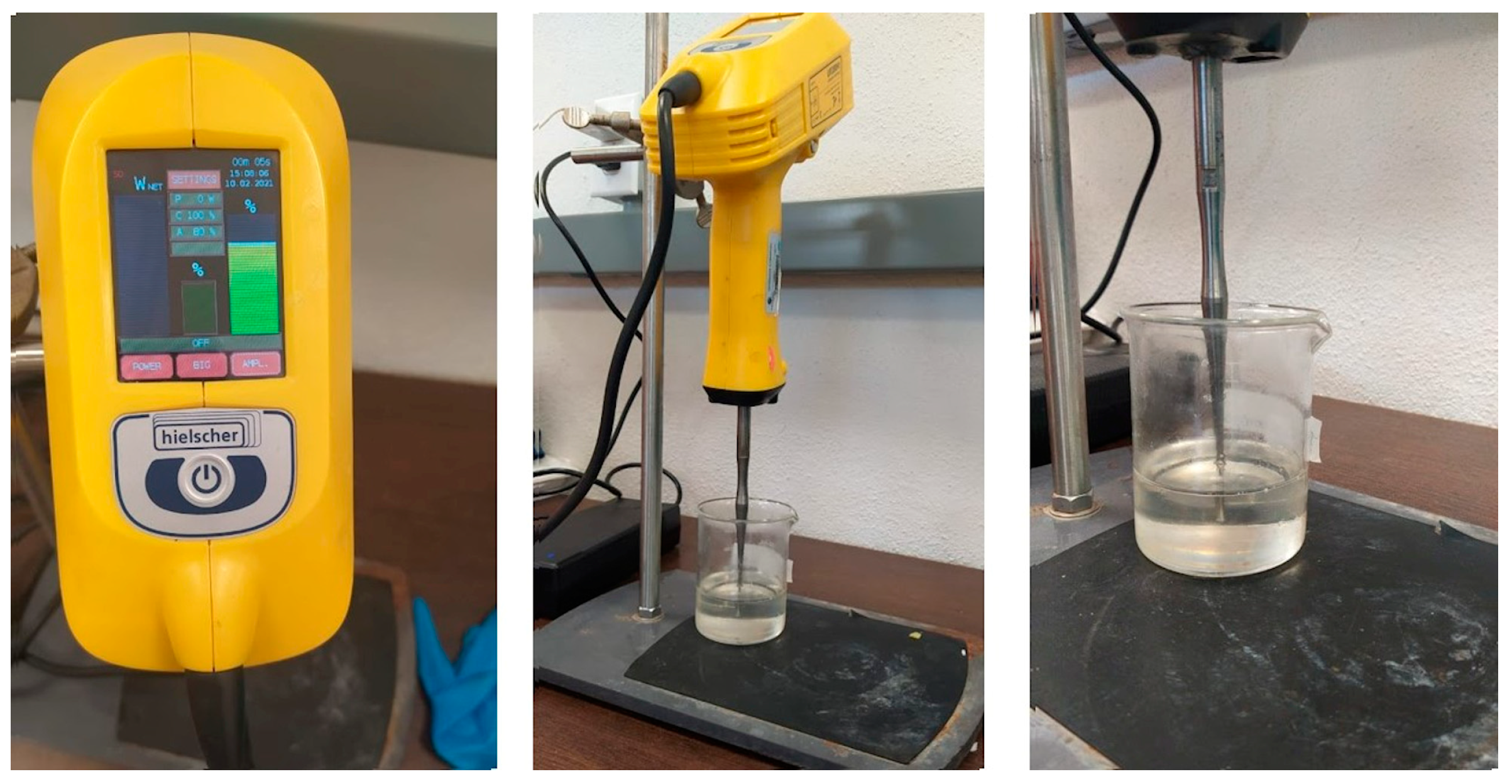
2.4. Ultrasonic Treatment Application
2.5. Obtention of PUR/AgNPs Composite Materials
2.6. Characterization of the PUR/AgNPs Composite Materials
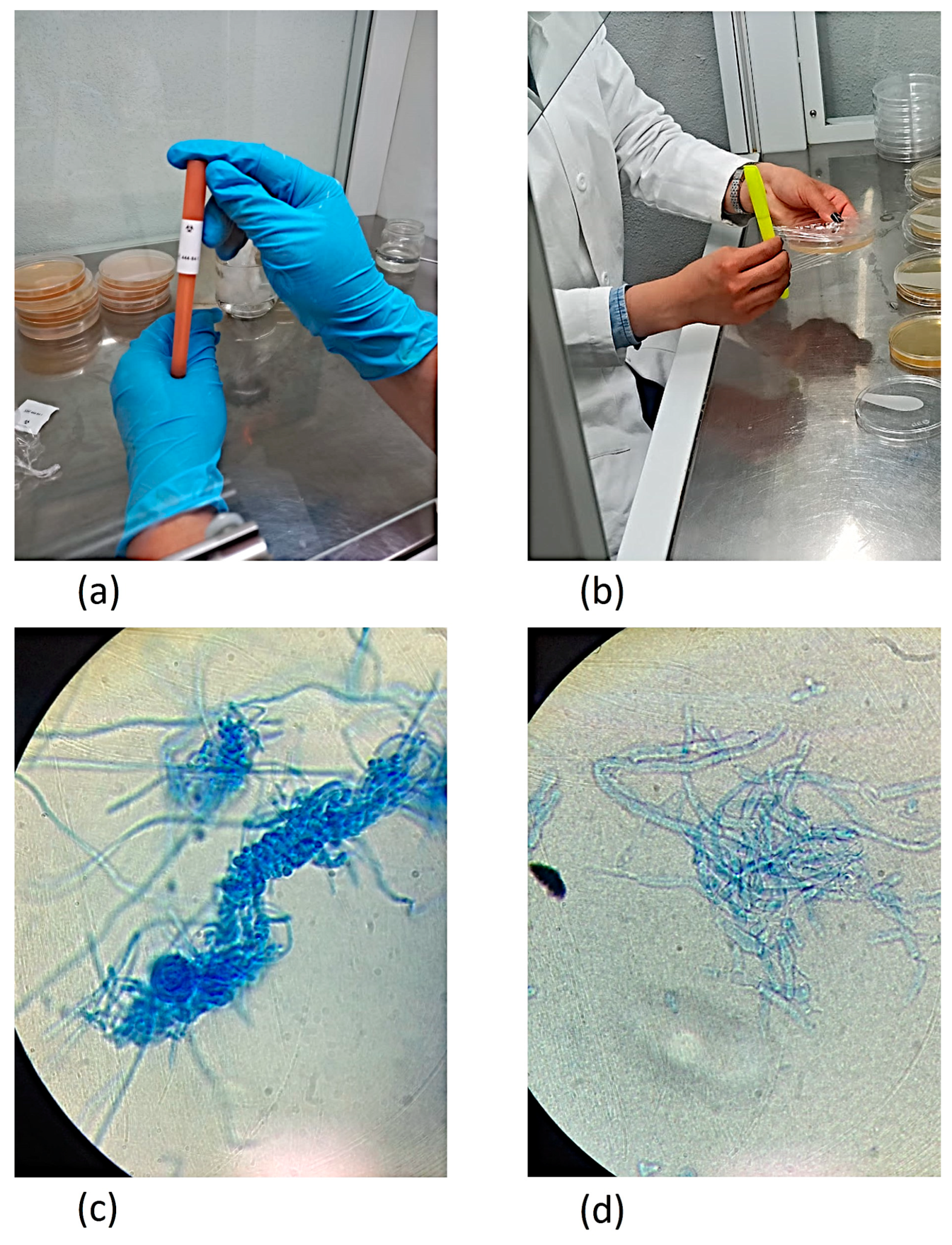
2.7. Culture Medium Preparation
2.8. Activation and Inoculation of the Fungi on the Culture Medium
2.9. Fungal Incubation
2.10. Fungal Growth Evaluation
2.11. Ordinal Logistic Regression Model
2.12. Optimization by Genetic Algorithms
3. Results
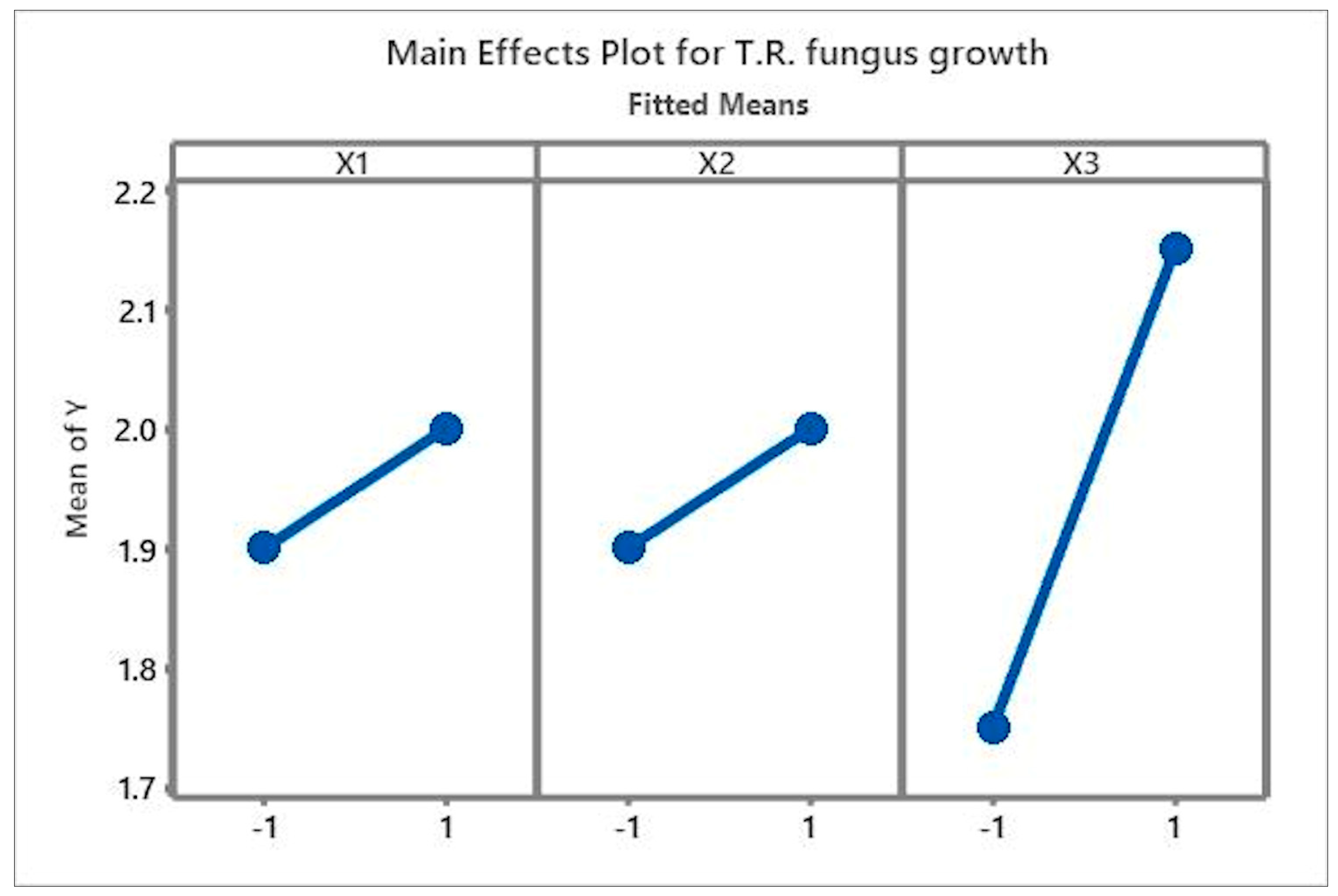
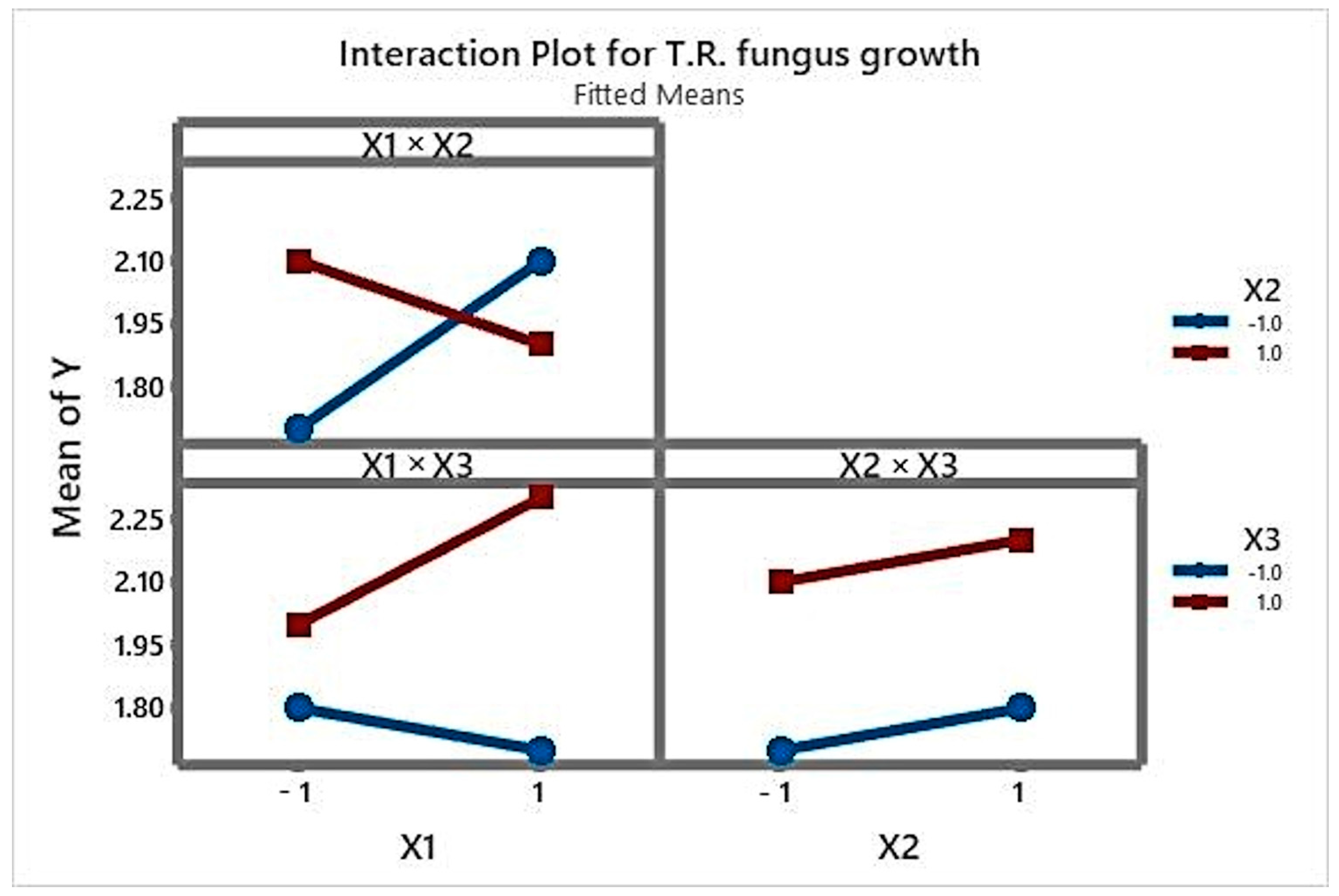
3.1. Analysis of the Main Effects and Interactions
3.2. Adjusting the Ordinal Logistic Regression Model
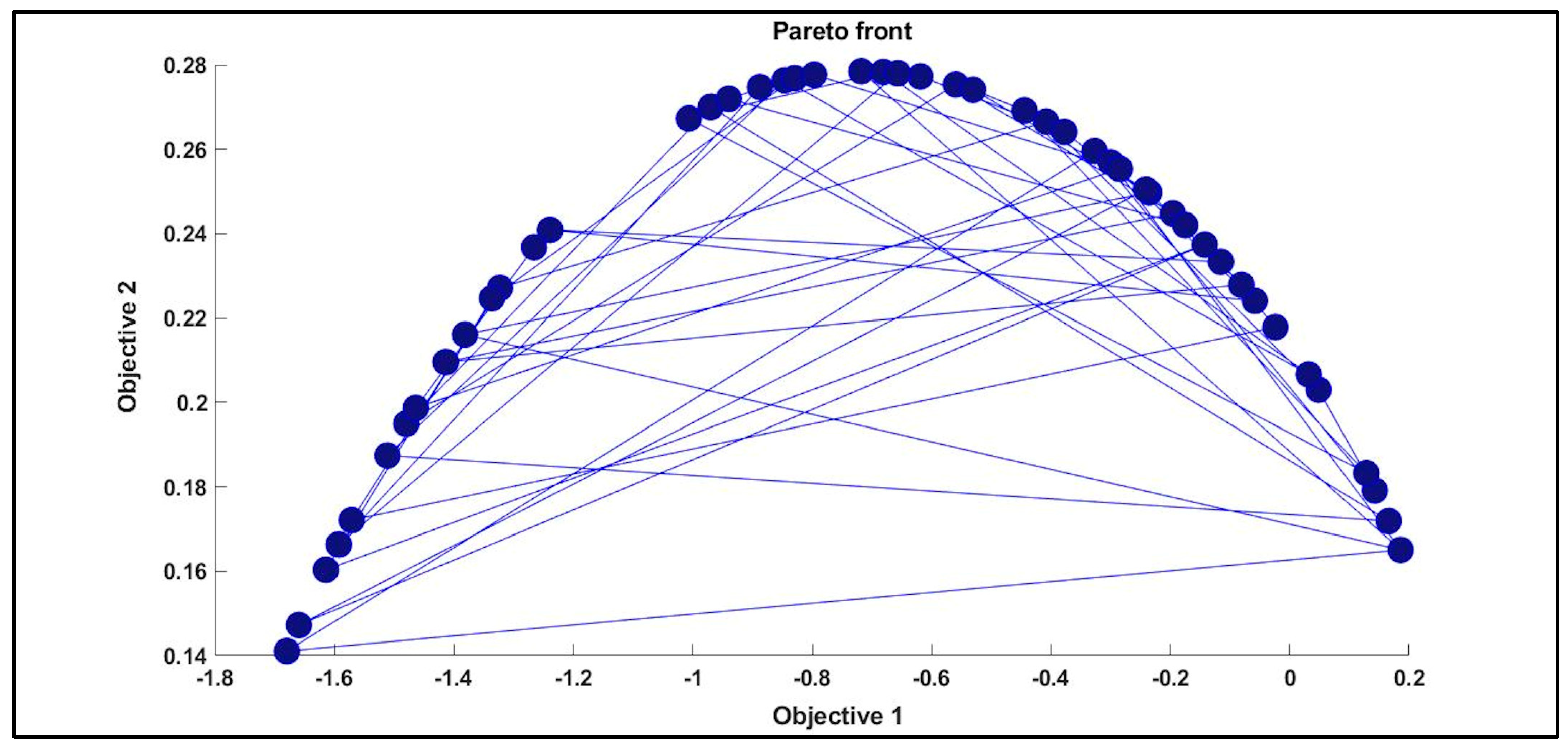
3.3. MOGAs Optimization
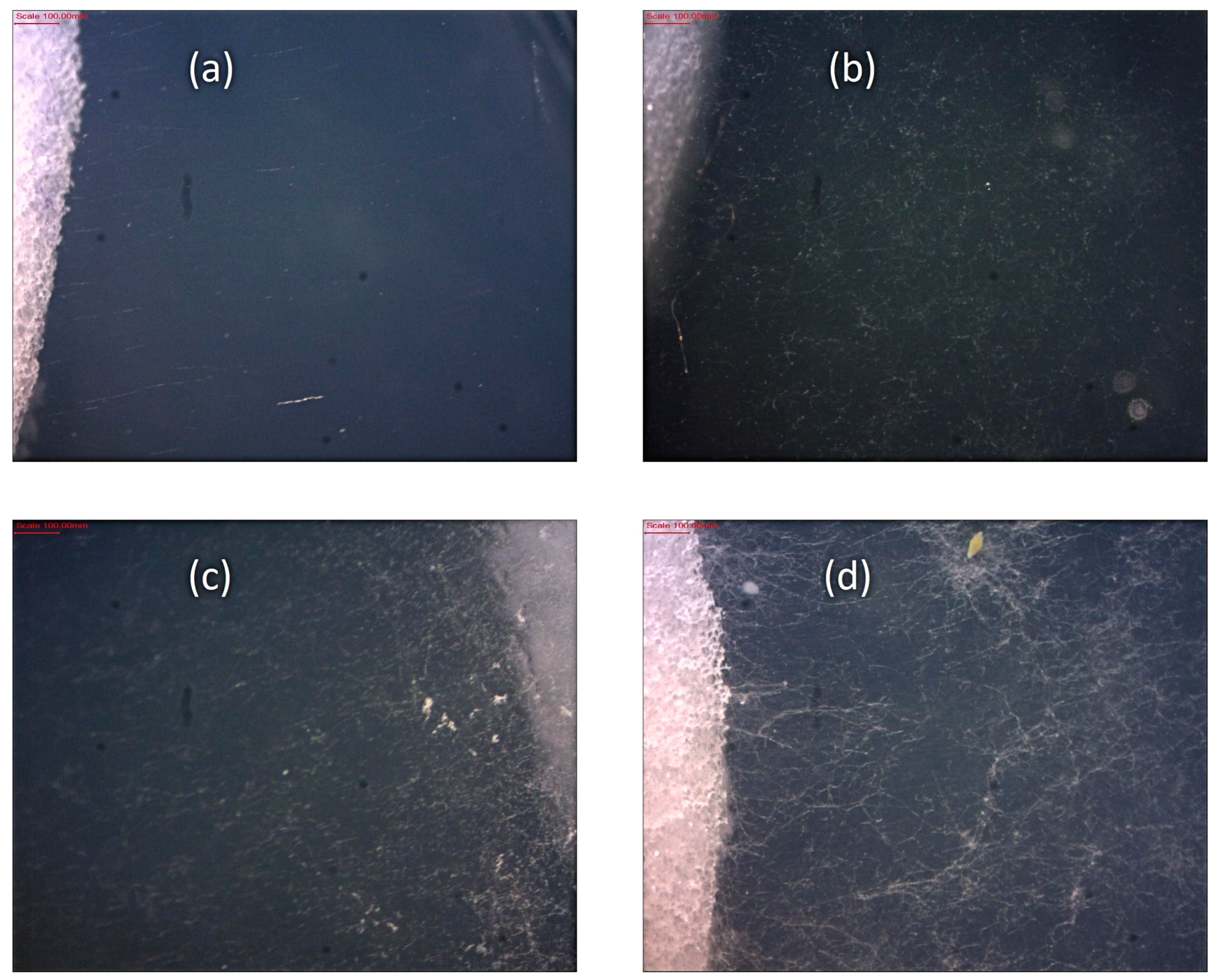
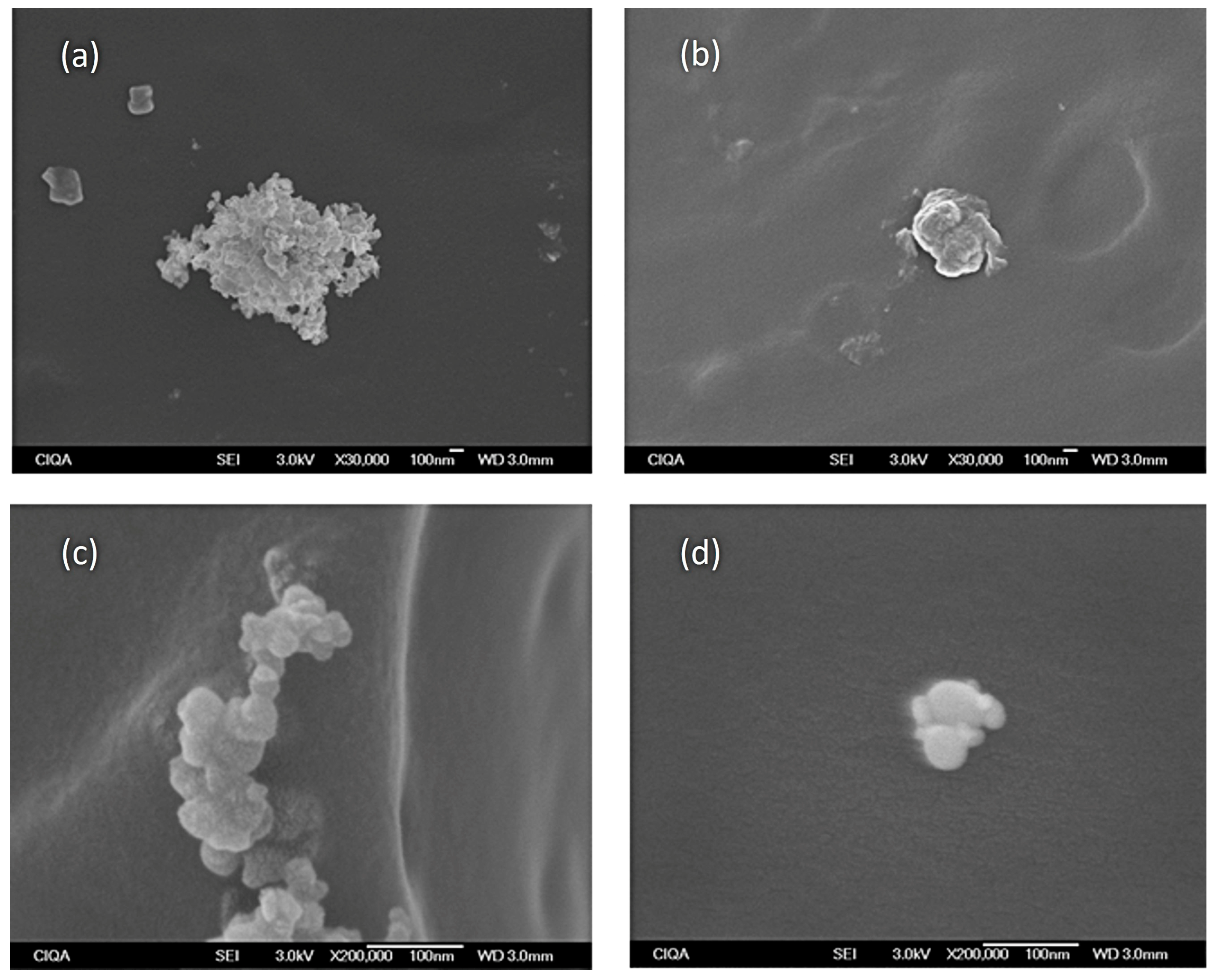
3.4. SEM Analysis of the Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mobeen, H.; Safdar, M.; Fatima, A.; Afzal, S.; Zaman, H.; Mehdi, Z. Emerging Applications of Nanotechnology in Context to Immunology: A Comprehensive Review. Front. Bioeng. Biotechnol. 2022, 10, 1024871. [Google Scholar] [CrossRef]
- Kim, H.-A.; Lee, B.-T.; Na, S.-Y.; Kim, K.-W.; Ranville, J.F.; Kim, S.-O.; Jo, E.; Eom, I.-C. Characterization of Silver Nanoparticle Aggregates Using Single Particle-Inductively Coupled Plasma-Mass Spectrometry (SpICP-MS). Chemosphere 2017, 171, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnology 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Hormozi-Nezhad, M.R. Gold and Silver Nanoparticles: Synthesis and Applications; Elsevier: Amsterdam, The Netherlands, 2023; ISBN 9780323994545. [Google Scholar]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, A.; Chand Mali, S.; Sharma, S.; Trivedi, R. A Review on Biological Synthesis of Silver Nanoparticles and Their Potential Applications. Results Chem. 2023, 6, 101108. [Google Scholar] [CrossRef]
- Chanyachailert, P.; Leeyaphan, C.; Bunyaratavej, S. Cutaneous Fungal Infections Caused by Dermatophytes and Non-Dermatophytes: An Updated Comprehensive Review of Epidemiology, Clinical Presentations, and Diagnostic Testing. J. Fungi 2023, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Ahirwar, D.; Telang, A.; Purohit, R.; Namdev, A. A Short Review on Polyurethane Polymer Composite. Mater. Today Proc. 2022, 62, 3804–3810. [Google Scholar] [CrossRef]
- Witkiewicz, W.; Zieliński, A. Properties of the Polyurethane (PU) Light Foams. Adv. Mater. Sci. 2006, 6, 35–51. [Google Scholar]
- Eugenia, M.M.; Adolfo, T.L.; Rodolfo, E. Characterization of Polyurethane Nanocomposites for Flame Retardant Applications. J. Chem. Chem. Eng. 2018, 12, 60–73. [Google Scholar] [CrossRef]
- Cui, M.; Chai, Z.; Lu, Y.; Zhu, J.; Chen, J. Developments of Polyurethane in Biomedical Applications: A Review. Resour. Chem. Mater. 2023, 2, 262–276. [Google Scholar] [CrossRef]
- Wendels, S.; Avérous, L. Biobased Polyurethanes for Biomedical Applications. Bioact. Mater. 2021, 6, 1083–1106. [Google Scholar] [CrossRef] [PubMed]
- Grzęda, D.; Węgrzyk, G.; Nowak, A.; Idaszek, J.; Szczepkowski, L.; Ryszkowska, J. Cytotoxic Properties of Polyurethane Foams for Biomedical Applications as a Function of Isocyanate Index. Polymers 2023, 15, 2754. [Google Scholar] [CrossRef] [PubMed]
- Okrasa, M.; Leszczyńska, M.; Sałasińska, K.; Szczepkowski, L.; Kozikowski, P.; Majchrzycka, K.; Ryszkowska, J. Viscoelastic Polyurethane Foams for Use in Seals of Respiratory Protective Devices. Materials 2021, 14, 1600. [Google Scholar] [CrossRef] [PubMed]
- Sana, S.S.; Haldhar, R.; Parameswaranpillai, J.; Chavali, M.; Kim, S.-C. Silver Nanoparticles-Based Composite for Dye Removal: A Comprehensive Review. Clean. Mater. 2022, 6, 100161. [Google Scholar] [CrossRef]
- Morena, A.G.; Stefanov, I.; Ivanova, K.; Pérez-Rafael, S.; Sánchez-Soto, M.; Tzanov, T. Antibacterial Polyurethane Foams with Incorporated Lignin-Capped Silver Nanoparticles for Chronic Wound Treatment. Ind. Eng. Chem. Res. 2020, 59, 4504–4514. [Google Scholar] [CrossRef]
- Xu, C.; Akakuru, O.U.; Ma, X.; Zheng, J.; Zheng, J.; Wu, A. Nanoparticle-Based Wound Dressing: Recent Progress in the Detection and Therapy of Bacterial Infections. Bioconjug. Chem. 2020, 31, 1708–1723. [Google Scholar] [CrossRef]
- ASTM D ASTM-G21-15-4; Standard Practice for Determining Resistance of Synthetic Polymeric Materials to Fungi. ASTM International: Conshohocken, PA, USA, 2015. Available online: https://www.astm.org/g0021-15r21e01.html (accessed on 5 August 2023).
- Linima, V.K.; Ragunathan, R.; Johney, J. Biogenic Synthesis of RICINUS COMMUNIS Mediated Iron and Silver Nanoparticles and Its Antibacterial and Antifungal Activity. Heliyon 2023, 9, e15743. [Google Scholar] [CrossRef]
- Gil-Korilis, A.; Cojocaru, M.; Berzosa, M.; Gamazo, C.; Andrade, N.J.; Ciuffi, K.J. Comparison of Antibacterial Activity and Cytotoxicity of Silver Nanoparticles and Silver-Loaded Montmorillonite and Saponite. Appl. Clay Sci. 2023, 240, 106968. [Google Scholar] [CrossRef]
- Zhao, G.; Stevens, J.S.E. Multiple Parameters for the Comprehensive Evaluation of the Susceptibility of Escherichia Coli to the Silver Ion. Biometals 1998, 11, 27–32. [Google Scholar] [CrossRef]
- Slawson, R.M.; Trevors, J.T.; Lee, H. Silver Accumulation and Resistance in Pseudomonas Stutzeri. Arch. Microbiol. 1992, 158, 398–404. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Ashtaputre, N.M.; Varadarajan, P.V.; Nachane, R.P.; Paralikar, K.M.; Balasubramanya, R.H. Biological Synthesis of Silver Nanoparticles Using the Fungus Aspergillus Flavus. Mater. Lett. 2007, 61, 1413–1418. [Google Scholar] [CrossRef]
- Skanda, S.; Bharadwaj, P.S.J.; Datta Darshan, V.M.; Sivaramakrishnan, V.; Vijayakumar, B.S. Proficient Mycogenic Synthesis of Silver Nanoparticles by Soil Derived Fungus Aspergillus Melleus SSS-10 with Cytotoxic and Antibacterial Potency. J. Microbiol. Methods 2022, 199, 106517. [Google Scholar] [CrossRef]
- Gajbhiye, M.; Kesharwani, J.; Ingle, A.; Gade, A.; Rai, M. Fungus-Mediated Synthesis of Silver Nanoparticles and Their Activity against Pathogenic Fungi in Combination with Fluconazole. Nanomedicine 2009, 5, 382–386. [Google Scholar] [CrossRef]
- Mansoor, S.; Zahoor, I.; Baba, T.R.; Padder, S.A.; Bhat, Z.A.; Koul, A.M.; Jiang, L. Fabrication of Silver Nanoparticles against Fungal Pathogens. Front. Nanotechnol. 2021, 3, 679358. [Google Scholar] [CrossRef]
- Robles-Martínez, M.; González, J.F.C.; Pérez-Vázquez, F.J.; Montejano-Carrizales, J.M.; Pérez, E.; Patiño-Herrera, R. Antimycotic Activity Potentiation of Allium Sativum Extract and Silver Nanoparticles against Trichophyton Rubrum. Chem. Biodivers. 2019, 16, e1800525. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, L.Y.; Fadhil Alsaffar, M.; Ahmed Lilo, R.; Khalil Al-Shamari, A. Silver Nanoparticles That Synthesis by Using Trichophyton Rubrum and Evaluate Antifungal Activity. Arch. Razi Inst. 2022, 77, 2145–2149. [Google Scholar]
- da Silva, C.A.; Ribeiro, B.M.; Trotta, C.D.V.; Perina, F.C.; Martins, R.; Abessa, D.M.d.S.; Barbieri, E.; Simões, M.F.; Ottoni, C.A. Effects of Mycogenic Silver Nanoparticles on Organisms of Different Trophic Levels. Chemosphere 2022, 308, 136540. [Google Scholar] [CrossRef]
- Wijesiri, N.; Yu, Z.; Tang, H.; Zhang, P. Antifungal Photodynamic Inactivation against Dermatophyte Trichophyton Rubrum Using Nanoparticle-Based Hybrid Photosensitizers. Photodiagnosis Photodyn. Ther. 2018, 23, 202–208. [Google Scholar] [CrossRef]
- Patiño-Herrera, R.; Catarino-Centeno, R.; Robles-Martínez, M.; Zarate, M.G.M.; Flores-Arriaga, J.C.; Pérez, E. Antimycotic Activity of Zinc Oxide Decorated with Silver Nanoparticles against Trichophyton Mentagrophytes. Powder Technol. 2018, 327, 381–391. [Google Scholar] [CrossRef]
- Helmlinger, J.; Sengstock, C.; Groß-Heitfeld, C.; Mayer, C.; Schildhauer, T.A.; Köller, M.; Epple, M. Silver Nanoparticles with Different Size and Shape: Equal Cytotoxicity, but Different Antibacterial Effects. RSC Adv. 2016, 6, 18490–18501. [Google Scholar] [CrossRef]
- Sadeghi, B.; Garmaroudi, F.S.; Hashemi, M.; Nezhad, H.R.; Nasrollahi, A.; Ardalan, S.; Ardalan, S. Comparison of the Anti-Bacterial Activity on the Nanosilver Shapes: Nanoparticles, Nanorods and Nanoplates. Adv. Powder Technol. 2012, 23, 22–26. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Kim, M.; Park, H.-S.; Shin, U.S.; Gong, M.-S.; Kim, H.-W. Size-Dependent Cellular Toxicity of Silver Nanoparticles. J. Biomed. Mater. Res. A 2012, 100A, 1033–1043. [Google Scholar] [CrossRef]
- Díaz-Puertas, R.; Rodríguez-Cañas, E.; Bello-Perez, M.; Fernández-Oliver, M.; Mallavia, R.; Falco, A. Viricidal Activity of Thermoplastic Polyurethane Materials with Silver Nanoparticles. Nanomaterials 2023, 13, 1467. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, M.; Valério, A.; de Souza, A.A.U.; de Oliveira, D.; Franco, C.V.; Serafim, R.; Souza, S.M.A.G.U. Synthesis and Application of Silver Nanoparticles as Biocidal Agent in Polyurethane Coating. J. Coatings Technol. Res. 2020, 17, 613–620. [Google Scholar] [CrossRef]
- Savelyev, Y.; Gonchar, A.; Movchan, B.; Gornostay, A.; Vozianov, S.; Rudenko, A.; Rozhnova, R.; Travinskaya, T. Antibacterial Polyurethane Materials with Silver and Copper Nanoparticles. Mater. Today Proc. 2017, 4, 87–94. [Google Scholar] [CrossRef]
- El-Rahman, N.R.A.; Bekhit, M.; Fekry, M. Fabrication and Evaluation of Polyurethane Cationic Surfactants, and Their Potential on Silver Nanoparticles Stability, Surface Activity, and Biological Activity. Egypt. J. Pet. 2022, 31, 23–31. [Google Scholar] [CrossRef]
- Wijnhoven, S.W.P.; Peijnenburg, W.J.G.M.; Herberts, C.A.; Hagens, W.I.; Oomen, A.G.; Heugens, E.H.W.; Roszek, B.; Bisschops, J.; Gosens, I.; Van De Meent, D.; et al. Nano-Silver—A Review of Available Data and Knowledge Gaps in Human and Environmental Risk Assessment. Nanotoxicology 2009, 3, 109–138. [Google Scholar] [CrossRef]
- Monje, A.E.; Reséndiz, J.R.H. Synthesis of Urethane Base Composite Materials with Metallic Nanoparticles. MRS Proc. 2013, 1547, 141–147. [Google Scholar] [CrossRef]
- McCullagh, P.; Nelder, J.A. Generalized Linear Models; Chapman and Hall: London, UK, 1983; pp. 1–19. [Google Scholar]
- Dobson Annette, J. Normal Linear Models. In An Intruction to Generalized Linear models; Chatfield, C., Zidek, J., Eds.; Chapman and Hall: Vancouver, BC, Canada, 2001; pp. 179–196. [Google Scholar]
- Bae, E.; Lee, B.-C.; Kim, Y.; Choi, K.; Yi, J. Effect of Agglomeration of Silver Nanoparticle on Nanotoxicity Depression. Korean J. Chem. Eng. 2013, 30, 364–368. [Google Scholar] [CrossRef]



| Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 15 nm | 5 g | 0 s | |||||
| 2 | 45 nm | 5 g | 0 s | |||||
| 3 | 15 nm | 10 g | 0 s | |||||
| 4 | 45 nm | 10 g | 0 s | |||||
| 5 | 15 nm | 5 g | 10 s | |||||
| 6 | 45 nm | 5 g | 10 s | |||||
| 7 | 15 nm | 10 g | 10 s | |||||
| 8 | 45 nm | 10 g | 10 s |
| Observed Growth on Specimens | Rating |
|---|---|
| None | 0 |
| Traces of growth (less than 10%) | 1 |
| Light growth (10 to 30%) | 2 |
| Medium growth (30 to 60%) | 3 |
| Heavy growth (60% to complete coverage) | 4 |
| Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 15 nm | 5 g | 0 s | 2 | 1 | 1 | 1 | 2 |
| 2 | 45 nm | 5 g | 0 s | 2 | 2 | 2 | 3 | 1 |
| 3 | 15 nm | 10 g | 0 s | 4 | 2 | 2 | 2 | 1 |
| 4 | 45 nm | 10 g | 0 s | 1 | 1 | 1 | 2 | 2 |
| 5 | 15 nm | 5 g | 10 s | 2 | 2 | 1 | 3 | 2 |
| 6 | 45 nm | 5 g | 10 s | 4 | 2 | 1 | 1 | 3 |
| 7 | 15 nm | 10 g | 10 s | 1 | 1 | 3 | 2 | 3 |
| 8 | 45 nm | 10 g | 10 s | 3 | 2 | 3 | 2 | 2 |
| Parameter | Value |
|---|---|
| Number of variables | 3 |
| Population size | 50 |
| Limits | [−1, −1, −1]; [1, 1, 1] |
| Selection function | Uniform stochastic |
| Initial value | [−10, 10] |
| Elite counting | 0.05 × initial population |
| Crossover fraction | 0.8 |
| Mutation function | Dependent on restrictions |
| Migration direction | Forward |
| Generations | 100 × Number of variables |
| Stagnant generations | 50 |
| Tolerance of the function | 1 × 10−6 |
| Predictor | Coef | SE Coef | Z | p |
|---|---|---|---|---|
| α1 | −0.844483 | 0.368023 | −2.29 | 0.022 |
| α2 | 1.41937 | 0.403828 | 3.51 | 0.000 |
| α3 | 3.20650 | 0.748000 | 4.29 | 0.000 |
| X1 | −0.115466 | 0.304130 | −0.38 | 0.704 |
| X2 | −0.103887 | 0.304001 | −0.34 | 0.733 |
| X3 | −0.503327 | 0.310272 | −1.62 | 0.105 |
| X1 ×X2 | 0.328445 | 0.305680 | 1.07 | 0.283 |
| X1 × X3 | −0.159936 | 0.304776 | −0.52 | 0.600 |
| X2 × X3 | −0.0594171 | 0.303632 | −0.20 | 0.845 |
| X1 × X2 × X3 | −0.517629 | 0.310828 | −1.67 | 0.096 |
| Pareto Front Solution | ||||||
|---|---|---|---|---|---|---|
| 28 | 0.98357 | 0.98818 | −0.66040 | 0.03183 | 0.20663 | 0.06601 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mares Castro, A.; Estrada Monje, A.; Saldívar Campos, A.I.; Zaragoza Estrada, A. Optimization of the Antifungal Property in a Composite of Polyurethane and Silver Nanoparticles against the Trichophyton rubrum Fungus. Appl. Sci. 2023, 13, 12028. https://doi.org/10.3390/app132112028
Mares Castro A, Estrada Monje A, Saldívar Campos AI, Zaragoza Estrada A. Optimization of the Antifungal Property in a Composite of Polyurethane and Silver Nanoparticles against the Trichophyton rubrum Fungus. Applied Sciences. 2023; 13(21):12028. https://doi.org/10.3390/app132112028
Chicago/Turabian StyleMares Castro, Armando, Anayansi Estrada Monje, Alejandra Imelda Saldívar Campos, and Anayansi Zaragoza Estrada. 2023. "Optimization of the Antifungal Property in a Composite of Polyurethane and Silver Nanoparticles against the Trichophyton rubrum Fungus" Applied Sciences 13, no. 21: 12028. https://doi.org/10.3390/app132112028
APA StyleMares Castro, A., Estrada Monje, A., Saldívar Campos, A. I., & Zaragoza Estrada, A. (2023). Optimization of the Antifungal Property in a Composite of Polyurethane and Silver Nanoparticles against the Trichophyton rubrum Fungus. Applied Sciences, 13(21), 12028. https://doi.org/10.3390/app132112028









