Abstract
The structurally diverse bioactive compounds found in marine organisms represent valuable resources for the food and pharmaceutical industries. The marine ecosystem encompasses over half of the world’s biota, providing an extensive range of bioactive compounds that can be extracted from various marine life forms, including marine microorganisms (such as bacteria, cyanobacteria, and actinobacteria), algae (both macroalgae and microalgae), invertebrates (including sponges, mollusks, echinoderms, and crustaceans), and, most importantly, fish. Many of these organisms thrive in extreme marine environments, leading to the production of complex molecules with unique biological functions. Consequently, marine biomolecules, such as lipids (especially polyunsaturated fatty acids), proteins/peptides, polysaccharides, carotenoids, phenolics, and saponins, exhibit a wide range of biological properties and can serve as valuable components in nutraceuticals and functional foods. Nevertheless, most of these biomolecules are susceptible to oxidation and degradation; encapsulation-based technologies tend to preserve them and increase their bioavailability and functions. These biological compounds demonstrate diverse activities, including antioxidant, anticancer, antithrombotic, anticoagulant, anti-inflammatory, antiproliferative, antidiabetic, antimicrobial, and cardioprotective effects, making them promising candidates for applications in the food industry. Despite their numerous health benefits, marine bioactive compounds have remained underutilized, not only in the food industry but also in the pharmaceutical and nutraceutical sectors. Therefore, this review aims to provide an overview of the various sources of marine bioactive compounds and their potential contributions to the food industry.
1. Introduction
Bioactive compounds originating from the marine environment are considered vital sources of functional ingredients with favorable functions for human health in relation to health promotion and disease risk reduction. Hence, there has been a growing interest in researching and developing marine-derived functional food ingredients, nutraceuticals, pharmaceuticals, and dietary supplements [1]. Recent epidemiological studies have revealed dietary marine food products that contain high bioactive molecules with a direct effect on reducing chronic diseases and cancers and promoting human health. Thus, the current demand for seafood and its derivatives is associated with their potential as functional foods [2,3]. Moreover, this abundance of functional foods and sources of favorable compounds have given immense commercial value to marine bioactive compounds in the food industry. Given the basic definitions and descriptions of bioactives and commercially important functional foods and nutraceuticals, it is important to understand the importance of marine-origin foods to derive bioactives and their use in these sectors. Due to the fact that oceans cover over 70% of the Earth’s surface, their abundant biodiversity makes them a prime resource for natural products [4,5]. Due to the unique characteristics of the oceanic ecosystem, such as differing levels of salinity, temperature, and light exposure, a wide range of marine bioactive components can be obtained from various origins, encompassing marine animals, plants, and other organisms, each with their distinct assortment of biomolecules. These include polyunsaturated fatty acids (PUFAs), polysaccharides, proteins/peptides/protein hydrolysates/enzymes, natural pigments, phenolic compounds, vitamins, and essential minerals [2,3].
Marine by-products, including head, viscera, skin, and bones, often constitute over 50% of the biomass, which contains a wide range of biomolecules and is mostly underutilized [6]. For example, sea cucumber by-products, mainly gut materials, represent up to 50% of sea cucumber body weight, which is a rich source of PUFAs and phenolic compounds (phenolic acids and flavonoids), exhibiting antioxidant activity [7]. Hence, marine bioactive compounds hold a significant potential to be a potential candidate in the value-added nutraceutical, functional food ingredient, and natural health product sector that can be used for health promotion and food preservation [8]. In particular, due to tremendous functional (e.g., stabilizing, emulsifying, gelling, and water-thickening) and biological (e.g., anticancer, antitumor, anti-inflammatory, antihypertensive, antidiabetic, wound healing, and antimicrobial) properties, marine-derived bioactive compounds have gained significant interest as a promising source of functional ingredients. For instance, due to antibacterial and antihypertensive properties as well as foaming and gel-forming capacities, proteins/peptides are considered one of the most promising ingredients in the functional food sector. Nowadays, polysaccharides (alginate, agar, carrageenan, and fucoidan) derived from seaweeds are commonly used in the food industry because of their distinct physiological benefits [8]. Although the abundance of the sources of bioactives in the marine environment and their application in the functional food industry is promising, numerous obstacles must be overcome on the road to the commercialization of these products. The successful transfer of the technology to the market requires standardization of analytical methods for the assessment of sensory properties for consumer acceptance, product quality assurance, and well-designed clinical trials to afford robust evidence for supporting health claims. Apart from these constraints, the lack of conclusive evidence for the feasibility of marine bioactives for human consumption causes vast resources to be abandoned [9,10]. Therefore, to facilitate this discussion, this review focuses mainly on the sources of marine-derived bioactive compounds and their potential utilization, particularly in the food industry. Moreover, this contribution is focused on discussing the major sources of bioactives, their chemical properties and synthesis, as well as the limitations of developing and commercializing them.
2. Marine Sources of Bioactive Molecules
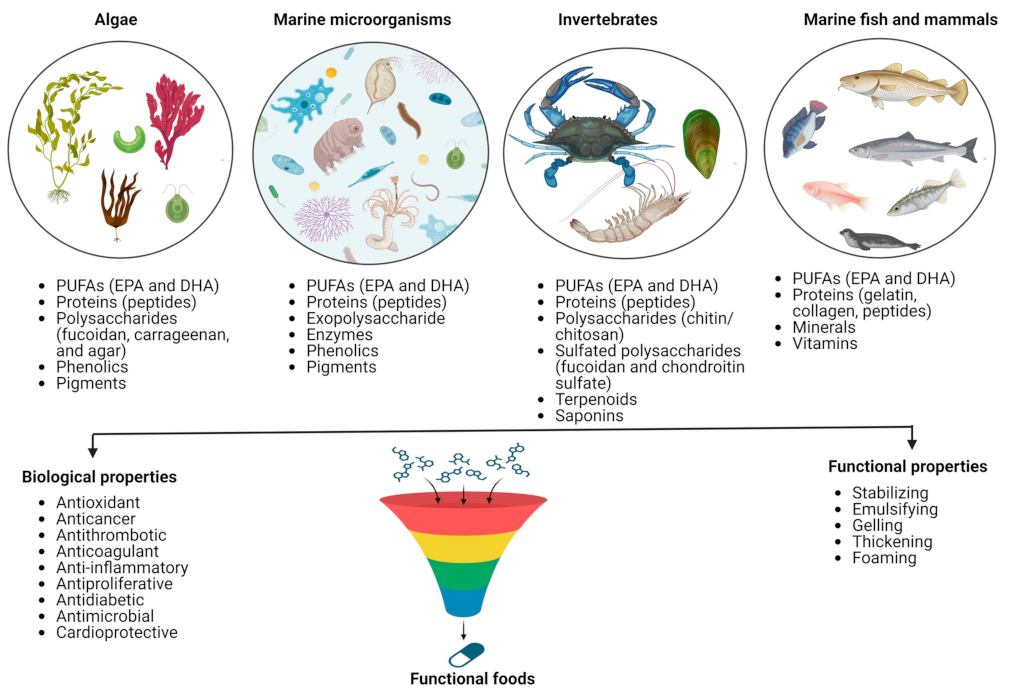
The marine ecosystem is the richest source of life and has phenomenal biodiversity. It represents untapped functional ingredient resources linked with diverse biological activities. Based on their bioactive properties, their discovery and inclusion in food products are relatively new compared to bioactive compounds derived from terrestrial sources. Many unutilized and underutilized species in the marine world possess high nutritional value. A plethora of compounds such as lipids (polyunsaturated fatty acids, PUFAs), proteins (peptides), polysaccharides, phenolics, pigments, and other secondary metabolites from different marine sources, including prokaryotes micro- and macroalgae, seaweeds, invertebrates (e.g., sponges, mollusks, echinoderms, and crustaceans), and vertebrates like marine fish and marine mammals and also all their by-products, can be of much support to the functional food industry (Figure 1) [1,7,8].
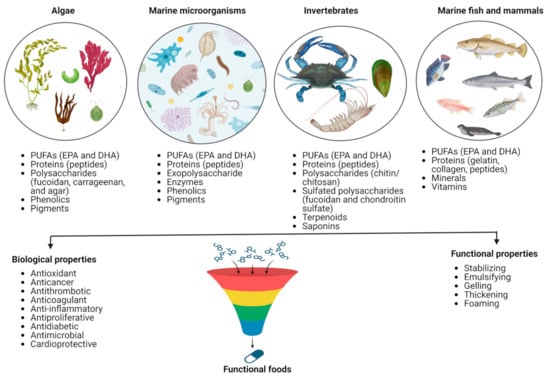
Figure 1.
Marine-derived bioactive compounds and their major functions.
2.1. Marine Microorganisms
Ocean microbial life is considered one of the most dynamic parts of the marine environment. Thus, marine ecology is well known for its highly diversified nature and has been subjected to numerous investigations. Moreover, it has received growing attention as a potential source of bioactive compounds, mainly due to its unique characteristics associated with the adaptations to extreme conditions of the deep sea [11]. The ability to thrive in extreme marine habitats with a wide range of factors, such as pressure, salinity, and temperature, under the competition for nutrients and symbiosis and with highly oxidative environments has facilitated the development of unique metabolic and physiological capabilities. These capabilities have resulted in the production of a plethora of bioactive compounds, such as enzymes, vitamins, antibiotics, biosurfactants, and bio-emulsifiers, which have been identified as contributors to both the pharmaceutical and food industries [12]. When considering the nutraceutical and functional food sectors, metabolite profiles of microorganisms are directly related. In particular, marine bacteria, fungi, and microalgae play a vital role in producing natural products compared to terrestrial microorganisms. It is stated that marine microorganisms possess some distinguished characteristics related to their structural and functional properties as compared to microorganisms present in other habitats such as soil, air, freshwater, industrial effluents, and wastewater [13]. For instance, marine-derived fungi (Aspergillus sydowii) contain various constituents, including alkaloids, terpenes, xanthones, anthraquinones, pyrones, sterols, diphenyl ethers, cyclopentenones, and polyketides, as well as various enzymes such as α-amylases, lipases, keratinases, xylanases, cellulases, and tannase, which have industrial and biotechnological potential [14]. However, all marine microbial products, including their metabolites, are not suited for food product development due to a lack of knowledge and research related to food regulatory requirements.
2.1.1. Bioactive Polysaccharides from Marine Microbiota
Marine microbially derived polysaccharides are commonly used in the food industry as stabilizers, emulsifiers, gelling, and water-thickening agents. According to their location in the microbial cell, these microbial polysaccharides can be categorized into three major groups: cell wall, intercellular, and exocellular polysaccharides. In particular, exopolysaccharides, or exocellular polysaccharides (EPSs), are promising types of polysaccharides for industrial purposes due to their purity and ease of isolation. Pentoses, hexoses, amino sugars, uronic acid, acetic acid, pyruvic acid, phosphoric acid, and sulfuric acid are common sugar and non-sugar components frequently found in bacterial exopolysaccharides. Most microorganisms discharge these EPSs into their exocellular environment, and their primary role is to protect the cell in its environment; in other words, their physiological role depends on their ecological niche as they assist the microorganisms in growth by adhering to solid surfaces and surviving adverse conditions [15,16]. Moreover, most EPSs are synthesized by microorganisms and may be excreted out of the cell as soluble or insoluble polymers. EPSs can further be classified into two major groups, homopolysaccharides and heteropolysaccharides, while the majority of EPSs are heteropolysaccharides. Generally, heteropolysaccharides are represented by guaran, heparin, mannan, and chondroitin or di-heteropolysaccharides, including fucoidan, hyaluronic acid, and agarose, showing various biological activities, including antitumor activity [17]. Generally, EPSs of marine bacteria are comprised of heteropolysaccharides that contain three or four different monosaccharides. Most of the EPSs are polyanionic, with the presence of either uronic acid, ketal-linked pyruvate, or inorganic residues, while some EPSs function as neutral macromolecules. Additionally, most of the reduced carbon in the ocean is in the form of EPSs, and it could facilitate the growth of microorganisms by maintaining their physiochemical environment. Under extreme marine environmental conditions, marine microorganisms produce structurally and functionally diverse EPSs [15,16].
Marine Bacteria
Among marine microorganisms, many species of gram-negative and gram-positive bacteria, fungi, archaea, and cyanobacteria are considered to be EPS producers in the marine environment. When considering marine bacterial species, Pseudoalteromonas sp., Pseudoalteromonas sp., Alteromonas macleodii subsp., Pseudoalteromonas sp., Pseudoalteromonas sp., and Vibrio sp. are the major heteropolysaccharide producers. Table 1 reports several gram-positive and gram-negative bacteria present in the marine environment and their functions.

Table 1.
Exopolysaccharides produced by different bacterial species.
Bacterial EPSs are widely used in numerous fields, such as medicinal, food, and other industrial applications, including heavy metal removal and oil recovery [22]. Their properties, such as thickening, coagulation, adhesion, stabilization, and gelling, enhance their potential application in various sectors. Due to novel combinations of EPSs and their structurally diversified nature, they play a vital role in the pharmaceutical industry. For example, antitumor, antiviral, and immunostimulant activities in polysaccharides produced by marine Vibrio and Pseudomonas, Bacillus licheniformis, and Geobacillus thermodenitrificans produced EPSs, which are considered to be immunomodulatory agents [19]. In addition to their pharmaceutical applications, EPSs can be used as gelling agents and exhibit preferred thermal stability and clarity. Moreover, their participation in removing heavy metals also plays a vital role in marine microorganisms’ EPSs when it comes to environmental pollution. EPSs produced by the marine bacterium Zooglea sp. have been identified as adsorbents of metal ions such as chromium, lead, and iron in a solution resembling a natural environment and remove these types of pollutant and toxic elements [22,23]. Nevertheless, the emulsification and surface-active properties of EPSs have attracted attention for bioremediation and oil recovery. Apart from the above bacterial species, lactic acid bacteria (LAB) also play a major role in producing EPSs and have a better potential to be applied in foods. Lactobacilli can produce diversified EPSs and are popular for their application in fermented food products as texturing agents. Their role in dairy products is explained in the literature to be due to their contribution to the consistency and rheology of fermented milk products. Besides, lactobacilli are well-known as bacteria that are generally recognized as safe (GRAS), which may help their application in food product development [23,24].
On the other hand, marine actinobacteria are considered one of the emerging secondary metabolite producers among marine microorganisms, which have gained much industrial attention. Representatives of the order Actinomycetes are usually filamentous gram-positive bacteria and are known for producing biologically active secondary metabolites. These actinomycete-derived compounds are considered to be antitumor, antibiotic, anthelmintic, and antifungal agents [25].
Marine Cyanobacteria
There is a competitive and appealing interest in marine cyanobacteria compared to terrestrial cyanobacteria as they produce polysaccharides, and their EPSs possess unique biochemical properties. Polysaccharides produced by cyanobacteria are complex heteropolymers comprised of at least 10 different monosaccharides, including hexoses, pentoses, deoxyhexoses, and acidic hexoses. Moreover, the presence of pentoses is considered a unique property in cyanobacterial EPSs as it is often absent in prokaryotic origin acidic sugars and exhibits an anionic character due to the presence of anionic substances such as pyruvyl, acetyl, phosphate, and sulfate [2,26,27]. Since the 1950s, various EPSs have been discovered from different strains of cyanobacteria. For example, Sing et al. [28] reported that EPSs produced by most cyanobacteria are present as sheaths, capsules, and slimes. These polysaccharides are mostly known as released polysaccharides (RPSs) from cyanobacteria, and most of the RPS-producing monosaccharides are obtained from Chroococcus, Gloeothece, and Synechococcus. Several studies have reported that glucose is the most abundant monosaccharide found in RPS, and arabinose, galactose, or fucose may also frequently be found in cyanobacterial RPS. Furthermore, cyanobacterial RPS has been studied for the presence of sulfated polysaccharides (SPs), and research remains in the developing stages [19,27,28]. However, according to Sardari et al. [18], industrial interest is more in microbial polysaccharides, mainly those composed of a lower number of monomers. The most common industrial use of microbial polysaccharides is as thickening agents for stabilizing the flow properties in aqueous solutions under different conditions, such as changes in temperature, ionic strength, and pH [29]. For instance, Anabaena ATCC 33047, Cyanospira capsulata, Nostoc, and Cyanothece, among other strains, are capable of producing RPSs that have considerable rheological properties. Furthermore, RPSs produced by cyanobacterial strains like Aphanocapsa, Cyanothece, Nostoc, Phormidium, and Synechocystis are considered promising sources as stabilizers, emulsifiers, or bio-flocculants [30].
2.1.2. Proteins and Bioactive Peptides from Microbiota
Microorganisms, including Chlorella, Dunaliella, Micractinium, Spirulina, Oscillatoria, Scenedesmus, Chlamydomonas, and Euglena, are considered high-quality protein sources [31]. Moreover, these microalgal species are considered alternative protein sources for food applications due to their favorable amino acid profiles. In particular, S. platensis is identified as a major source of bioactive proteins as more than 75% of the protein nitrogen could be extracted from hexane-defatted biomass [5]. Furthermore, several studies, based on compositional analysis of microalgal proteins, have revealed that they could be used directly as supplements or for formulating other health products such as nutraceuticals [31]. This is mainly due to their enormous diversity and metabolic versatility. In particular, marine microorganisms have attracted considerable attention as a resource for new enzymes as they are more stable and active compared to enzymes from plant or animal origin [32]. Numerous studies have identified a wide range of enzymes with special functions from marine bacteria, fungi, actinomycetes, and other marine microorganisms. These microbial enzymes are being utilized in numerous industries, such as food, leather, textile, and animal feed, as well as in bioconversion and bioremediation [33]. The complexity of the marine environment and extreme environmental conditions, including low temperature, high salinity, and extreme pressures, may determine the differences between enzymes originating from marine microorganisms and enzymes derived from terrestrial sources [31] (Table 2).
In addition to this, bioactive peptides derived from marine organisms possess numerous therapeutic activities, including antihypertensive, anticancer, anti-inflammatory, antioxidant, antimicrobial, and immunomodulatory activities, mainly due to their amino acid composition. Moreover, they are often used in the pharmaceutical industry because of their significant therapeutic potential [31,34]. For example, protein hydrolysate and peptides obtained from Porphyridium sp. showed the potential to prevent inflammation, hypertension, and pain. In particular, protein hydrolysates were incorporated into food carriers (jelly candies) and found their antihypertensive effects in spontaneously hypertensive rats [35].

Table 2.
Enzymes derived from marine microorganisms and their applications.
Table 2.
Enzymes derived from marine microorganisms and their applications.
| Type of Enzymes | Responsible Microorganism | Applications | References |
|---|---|---|---|
| Protease | Aureobasidium pullulans, Bacillus mojavensis, Psychrobacter, Clostridium, Rhizopus, Penicillium, and Aspergillus | Pharmaceutical (digestive drugs and anti-inflammatory drugs), detergent, and leather industries | [34,36] |
| Lipase | Penicillium Oxalicum, Aspergillus flavus, Moraxella, Candida intermedia, Pichia guilliermondii, Lodderomyces elongisporus, Candida parapsilosis, Candia rugosa, Candida quercitrusa, and Yarrowia lipolytica | Detergents, cosmetic production, paper production, and food flavoring | [33,34,37] |
| Chitinase and chitosanase | Aspergillus, Penicillium, Rhizopus Myxobacter, Sporocytophaga, Bacillus, Enterobacter, Flavobacterium, Arthrobacter, Vibrio parahaemolyticus, S Vibrio fluvialis, Vibrio mimicus, Vibrio alginolyticus, Listonella anguillarum, Klebsiella, Pseudomonas, Serratia, Chromobacterium, Clostridium, and Aeromonas hydrophila | Pharmaceutical, functional food, and cosmetic sectors | [33,38,39] |
| Agarases | Pseudomonas galatica, Cytophaga, Alteromonas, Bacillus, Vibrio, Pseudoalteromonas, Streptomyces, and Pseudomonas | Food industry (beverages, bread, cookies, and some low-calorie food) | [33,34,40] |
| Cellulase | Cytophaga, Cellulomonas, Vibrio, and Clostridium, Nocardia, Streptomyces, Trichoderma, Aspergillus, Fusarium, Chaetomium, Phoma, Sporotrichum, and Penicillium | Cotton and linen product processing, bio-textile auxiliaries, and bio-fertilizer processing | [33,34,38,41] |
| Xylanases | Aspergillus niger and Penicillium chrysogenum | Paper and pulp industries and beverage clarification | [33,42] |
2.1.3. Fatty Acids from Microbiota
Marine microorganisms such as bacteria, protists, yeast, dinoflagellates, and filamentous fungi have been identified as producers of long-chain PUFAs in the marine environment. Moreover, numerous bacterial species (e.g., Vibrio marinus, Moritella marina, and Shewanella marinintestina) of marine origin have been found to have long-chain PUFAs, especially the omega-3 and omega-6 series of fatty acids, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). In particular, EPA production is mainly observed in fungi and microalgae [43]. In addition, microalgae, including Spirulina, provide an interesting source of fatty acids, including lauric, palmitic, oleic, and gamma-linolenic acids with the omega-3 fatty acid DHA, and it has been reported to contain sterols, including clionasterol [44]. The content of PUFAs in marine microalgae is higher than that of freshwater microalgal species, as marine microalgae deliver higher amounts of PUFAs to survive in extreme marine environments. Furthermore, microalgae are also able to alter PUFA production rates with the growth phases. Nannochloropsis spp., Phaeodactylum tricornutum, Porphyridium cruentum, and Chaetoceros calcitrans have been reported as the best EPA-producing microalgae, while Cryptecodinium spp. and Isochrysis galbana are more suitable for extracting DHA [45].
As conventional sources of PUFAs, fish oils encounter several natural constraints and limitations, such as producing undesirable odors and suffering from low stability. Furthermore, the purification of PUFAs (e.g., DHA) from fish sources is relatively expensive due to technical difficulties [46]. In contrast, microbial PUFA production is sustainable and independent of climatic or seasonal changes as marine microorganisms, including microalgae and fungi, are able to grow on a diverse range of substrates with relatively rapid growth rates. For instance, microalgae, such as Amphidinium sp., contain up to 48% of their cell fatty acids as DHA [47]. Various yeast and fungal species have also been identified as potential alternative sources to produce long-chain PUFAs. For example, Cunninghamella echinulate, Mucor circinelloides strainsa, Thamnidium elegans strains, Yarrowia lipolyticab, and Zygorhynchus moelleri have been reported as PUFA-producing fungi [46]. Various microbial oils are already commercially produced and can be manipulated by maintaining culture conditions, such as providing unique substrates for microbial enzymes and genetic engineering to enhance microbial lipid-producing biosynthetic pathways. Large-scale production of PUFA-rich microbial oils has already been popular in the industrial world with genetically modified strains [46,48].
2.2. Marine Algae
Marine algae are a significant source of bioactive compounds in the ecosystem, constituting over 90% of all marine plants. These photosynthetic organisms lack true stems or roots and encompass a wide array of species, ranging from unicellular microscopic microalgae to multicellular macroalgae of varying sizes [49]. The latter are commonly referred to as seaweed and are categorized into three distinct groups based on their pigmentation: red (Rhodophyta), brown (Pheophyta), and green (Chlorophyta). Their nutritional and chemical compositions are subject to various influences, including geographical origin, species, seasonal and environmental factors, physiological variations, and processing methods, among others.
2.2.1. Macroalgae
Seaweeds are well known for their abundance of dietary fiber, polysaccharides, proteins, lipids, minerals, and vitamins, as well as other phytochemicals. Furthermore, they encompass a range of bioactive compounds, including phenolic compounds, known for their antibacterial, antiviral, antifungal, anticancer, and antioxidant functions [50]. Owing to their diverse biological activities, they have gained increasing interest as a promising source of pharmaceutical products and functional food ingredients. Due to their functional and health-beneficial properties, most East Asian consumers incorporate seaweeds into their cuisine as a functional food ingredient. On the other hand, microalgae are microscopic organisms (around 20 µm) and are frequently characterized as phytoplankton, including diatoms (phylum Ochrophyta, class Bacillariophyceae), dinoflagellates (phylum Myzozoa, class Dinophyceae), blue-green algae (phylum Cyanobacteria, class Cyanophyceae), and green and yellow-brown flagellates such as prymnesiophyta, prasinophyta, chlorophyta, and cryptophyta, among others [51,52]. Among them, Nostoc, Arthrospira (Spirulina), edible blue-green algae, and Aphanizomenon are very common species that have been used for centuries [53]. They have a rich profile of PUFAs, polysaccharides, carotenoids, and chlorophyll pigments, which have been used in the food and pharmaceutical sectors. For example, Dunaliella salina is a rich source of vitamin A and PUFAs and is cultured on a commercial scale [54].
Macroalgae Polysaccharides
Seaweed is a rich source of polysaccharides, mainly SPs, which demonstrate strong biological and physicochemical characteristics, suggesting their potential to be used in different sectors, including food production. A wide range of polysaccharides have been identified in seaweed, mainly observed as storage polysaccharides and in cell wall structures. The primary components of cell wall polysaccharides consist of hemicelluloses, cellulose, and neutral polysaccharides, which serve to impart structural strength to the thallus when submerged in water. For instance, polysaccharides in brown algae consist of laminarin (β-1,3 glucan), fucoidan (sulfated fucose), cellulose, sargassan, alginates, and mannitol, while green algae contain sulfated galactan, SPs, and xylans. Moreover, red algal polysaccharides are mostly carrageenans, water-soluble sulfated galactan, agars, xylans, floridean starch (amylopectin-like glucan), and porphyran [19,50,55]. In particular, polysaccharides with sulfated hemi-ester groups linked to sugar moieties are observed as galactan (agar and carrageenan) in red algae (Rhodophyceae), fucoidan (fucoidans, ascophyllan, and sargassum) in brown algae (Phaeophyceae), and arabinogalactans with a lesser content of sugars in green algae (Chlorophyceae) [7,19,56]. Generally, SPs can be categorized into three distinct groups such as water-soluble ulvan found in green algae (which consists of sulfated aldobiouronic acid and sulfated rhamnose), linear carrageenan and galactan found in red algae, and water-soluble fucoidan found in brown algae (xylose, uronic acid, fucose, and galactose) [19,56].
The percentage of polysaccharides in seaweed species can go up to 75% on a dry weight basis, while green seaweeds, mainly Ulva, contain a high level of polysaccharides [53]. For example, U. lactuca contains over 80% polysaccharides, mainly ulvan, a functional, structural, and storage polysaccharide [57]. Most of these polysaccharides are mainly dietary fibers, which promote gut health by maintaining the intestinal environment, and the total fiber content of seaweed can vary between 29 and 62% [55]. These dietary fibers can be categorized into two groups: water-soluble, namely alginic acid, agars, porphyrin, furonan, and laminaran, and insoluble dietary fibers, such as mannans, cellulose, and xylan. Soluble fibers can transfer through the gastrointestinal (GI) tract without digestion, and they can enhance the feeling of satiety due to their bulking capacity [58]. For instance, brown seaweed alginates could involve fecal bulking, water-binding, and reducing transit time in the gut, inhibiting colon cancer. Apart from controlling and maintaining intestinal flora, algal fibers or SPs can act as prebiotics, enhancing the growth promotion of gut microbiota in the GI tract. In particular, SPs obtained from marine algae demonstrate a wide range of bioactivities, including anticoagulant and antioxidant activities. For instance, fucoidan in brown algae (Phaeophyceae), ulvan in green algae (Chlorophyceae), and carrageenan in red algae (Rhodophyceae) are the common SPs that showed antioxidant activity [55,59]. The effectiveness of these polysaccharides in terms of their antioxidant activities primarily depends on factors such as the degree of sulfation, molecular weight, glycosidic bonds, and type of main sugars, among others. Specifically, the antioxidant activity of SPs could be due to the donating ability of hydrogen atoms/electrons to free radicals or chelating transition metal ions (e.g., Cu2+ and Fe2+) since they are extremely nucleophilic [60]. Apart from their antioxidant potential, these polysaccharides, due to their unique structural composition, possess antiproliferative, anticoagulant, antithrombotic, antitumoral, anti-inflammatory, anticomplementary, and antiviral activities (Table 3). In addition to fucoidans, the other storage and structural polysaccharides, such as carrageenan, agar, and alginate, are widely utilized commercial polysaccharides found in macroalgae. They exhibit stabilizing and textural characteristics that are broadly used in the food industry for purposes such as gel formation, thickening, product stabilizing, and the development of water-soluble films [49].

Table 3.
Sulfated polysaccharides derived from marine algae and their health benefits.
Macroalgae Proteins, Peptides, and Amino Acids
Macroalgae are a significant source of marine-derived proteins, amino acids, and peptides with various biological activities. These proteins are found within cell walls, often in conjunction with enzymes, polysaccharides, and pigments [49]. The composition of protein in macroalgae varies based on factors like species, geographic location, season, and cultivation conditions. Generally, red and green seaweeds contain a higher protein content (9–47%) than brown seaweeds (4–24%). Despite fluctuations in essential amino acid concentrations, research indicates that macroalgae encompass all essential amino acids. The amino acid levels in seaweed are aligned with the dietary protein requirements outlined by the FAO/WHO, suggesting their potential as an alternative protein source [70]. Moreover, seaweed is rich in acidic amino acids like glutamic and aspartic acids but may lack cysteine, tryptophan, lysine, and methionine, which are graded limiting amino acids [49]. For instance, Palmaria palmate (dulse) is abundant in essential amino acids such as valine, leucine, and methionine, resembling the amino acid composition of ovalbumin. Additionally, the protein content in most seaweeds is substantial, which is akin to that found in terrestrial plants like soybeans [71].
The attention directed towards the compositional, structural, and sequential attributes of peptide sources from marine algae has significantly increased, owing to their extensive application in the food and pharmaceutical sectors. Developing these bioactive peptides from most seaweeds poses challenges because of the significant amount of polysaccharides in their cell walls. Nevertheless, in comparison to methods like solvent extraction and microbial fermentation, enzymatic hydrolysis is widely used in the food and pharmaceutical industries. For instance, enhancing peptide production from seaweed has often involved the application of digestive enzymes or proteolytic microorganisms for enzymatic hydrolysis [60]. Typically consisting of 3–20 amino acid residues, bioactive peptides’ biological activities, such as their antihypertensive, antithrombotic, immunomodulation, anticancer, and antioxidant properties, are determined by their amino acid composition and sequence [7]. Hydrophobic amino acids like valine, leucine, and proline are commonly found in the peptide sequences of marine-derived peptides known for their antioxidant activities. Marine microalgae have yielded antioxidant peptides like glutathione and carnosine, which are typically associated with animal muscles [72]. Additionally, protein hydrolysates sourced from macroalgae have been identified as a promising source of antioxidants. The principal constituents of free amino acids in seaweed encompass aminobutyric acid, taurine, alanine, citrulline, ornithine, and hydroxyproline, with their proportions depending upon seaweed species. Taurine (2-aminoethanesulfonic acid) stands out among these amino acids, playing a critical role in various biological functions such as blood pressure regulation, conjugating bile acids, and acting as an anti-inflammatory, antioxidant, and hypocholesterolemic agent [73]. Additionally, various types of seaweeds contain uncommon amino acids, including mycosporine-like amino acids (MAAs), which are renowned for their antioxidant properties [49]. Nevertheless, the most extensively studied functional algal proteins fall into two main categories: phycobiliproteins (phycocyanins, phycoerythrin, and allophycocyanins) and lectins. Phycobiliproteins form complexes of proteins and pigments (phycobilins) and are considered highly soluble fluorescent proteins. These are broadly applied as fluorescent markers in the biotechnology field and serve as natural colorants for the cosmetic and food industries. Furthermore, certain phycobiliproteins exhibit anti-inflammatory, antioxidant, antiviral, hypocholesterolemic, neuroprotective, and lipase inhibition activities [74]. On the other hand, macroalgal lectins, which are typically bound with glycoproteins and carbohydrates, have also been obtained from both green and red algae (e.g., Ulva sp.). These bioactive lectins play diverse roles in biological processes, including antibacterial and antiviral activities, intercellular communication, antitumor and anti-inflammatory effects, and binding and recognition of carbohydrates [75,76]. Nonetheless, seaweed proteins face limitations in digestibility due to their elevated phenolic content, especially in brown algae. Conversely, red and green algae generally offer better protein availability due to their low phenolic content [77]. Aside from phenolic compounds, the digestibility of proteins can also be influenced by abundant dietary fibers, polysaccharides, and lectins, as well as the extent of protein glycosylation. Consequently, augmenting the digestibility of these proteins can be accomplished by eliminating polysaccharides through enzymatic methods. However, more research is required to uncover the in vivo digestibility of algal proteins while devising effective strategies for enhancing the bioaccessibility of these proteins [49,55].
Macroalgae Lipids and Fatty Acids
Phospholipids and glycolipids are the predominant lipid types in seaweed, making up roughly 1–5% of their cell walls. While seaweed has a lower lipid level than terrestrial plants like soy and sunflower, it contains a high amount of PUFAs. The lipid composition of seaweed differs based on factors such as species, season, location, salinity, temperature, and light intensity [49]. In particular, lower temperatures lead to increased PUFA accumulation in algae [78]. Various species of macroalgae have substantial quantities of EPA and DHA. Specifically, EPA stands out as the primary fatty acid in most seaweeds, making up over 50% of the total fatty acids. Notably, brown and red algae are rich in EPA, linoleic acid, and α-linolenic acid, whereas green seaweeds have higher levels of oleic, hexadecatetraenoic, and palmitic acids. For example, Ulva sp. (e.g., Ulva pertusa) are high in hexadecatetraenoic acid, while Laminaria sp. (e.g., Laminaria ochroleuca and Undaria pinnatifida) are abundant in octadecatetraenoic acid [79]. Nevertheless, their role as a food energy source seems limited due to their relatively low lipid level (~4.5%). Nonetheless, different red seaweed species have phospholipid levels ranging from 10 to 21% of their total lipid level, together with nearly equivalent amounts of glycolipids such as monogalactosyldiacylglycerol (MGDG), sulfoquinovosyl diacylglycerol (SQDG), and digalactosyldiacylglycerol (DGDG). Green algae, on the other hand, predominantly contain MGDG and DGDG as their major glycolipids [51]. Thus, the growing interest among research groups and consumers in lipid products rich in PUFAs has spurred the exploration of alternative methods for extracting lipids from algal sources. One such method, using chloroform/methanol to extract PUFAs from seaweed, has demonstrated higher PUFA yields, though their safety is always a concern [80].
2.2.2. Microalgae
Microscopic marine organisms known as microalgae (~20 µm) are located in both littoral and benthic environments and exist in the ocean as phytoplankton, which are considered the earliest and most organized members of the plant kingdom. These phytoplankton populations often encompass various types, including diatoms, green algae, blue-green algae, dinoflagellates, and yellow-brown flagellates. In particular, dinoflagellates, green and yellow-brown flagellates, and Bacillariophyceae (diatoms) also share many of the same photosynthetic pigments that are similar to those found in terrestrial plants. Consequently, they are rich sources of bioactive compounds and biochemicals such as pigments like carotenoids and chlorophylls, PUFAs, polysaccharides, antioxidants, sterols, and vitamins. For instance, diatoms produce essential PUFAs such as DHA, EPA, and other omega-3 fatty acids, as well as substances like fucoxanthin and chlorophyll [81,82]. Nonetheless, environmental conditions such as abiotic stresses and salinity could affect the pigment and antioxidant components of microalgal species (e.g., Tetraselmis tetrathele) [83]. However, a significant number of these bioactives found in microalgae are not suitable for human consumption due to the potential production of potent neurotoxins or hepatotoxins [51]. Consequently, ensuring their safety is crucial before considering their use for human consumption.
2.2.3. Other Biomolecules from Marine Algae
Some of the other valuable components originating from algae include pigments, phenolic compounds, and vitamins. Many of the pigments found in algae are photosynthetic compounds that are crucial for autotrophic organisms to capture solar energy during photosynthesis [82]. The principal types of photosynthetic pigments in marine life consist of phycobiliproteins, carotenoids, and chlorophylls. Carotenoids act as potent antioxidants, neutralizing reactive oxygen species (ROS). In green algae, lutein, violaxanthin, β-carotene, zeaxanthin, and neoxanthin are abundant, while red algae predominantly contain lutein, α- and β-carotenes, and zeaxanthin. In brown algae, violaxanthin, β-carotene, and fucoxanthin are prevalent [84]. Fucoxanthin, which is among the most abundant carotenoids, displays antioxidant, antidiabetic, antiangiogenic, antiviral, anticancer, and antiphotoaging properties, as well as anti-obesity effects. Other carotenoid derivatives like diatoxanthin, heteroxanthin, peridinin, and siphonaxanthin may also be present in algae [81]. Conversely, β-carotene, with provitamin A potential, has demonstrated cancer prevention abilities and a reduced risk of cardiovascular and ophthalmological ailments. Its antioxidant characteristics counteract the detrimental effects of free radicals, with naturally sourced microalgal β-carotene being more biologically active than its synthetic counterpart [85]. Tocopherol, a potent antioxidant, is found in α-, β-, and δ-tocopherol forms across microalgae species (e.g., Tetraselmis) and can aid in preventing cardiovascular diseases [81]. Apart from this, chlorophylls, lipid-soluble pigments, are often utilized as natural colorants in the food and beverage industries. These pigments can undergo transformations during food processing and ingestion by humans, converting them into pyropheophytin, pheophytin, and pheophorbide. In particular, pigments (e.g., fucoxanthin, zeaxanthin, lutein, and β-carotene), phenolic compounds (e.g., anthocyanin and phlorotannins), proteins (e.g., phycobiliproteins), and glycosides are among the colorants derived from marine algae, which also provide nutritional value to food products in addition to enhancing its acceptability, appeal, and flavor. Additionally, chlorophylls and their derivatives exhibit other bioactivities, including antigenotoxic, anticancer, antioxidant, antimutagenic, and anti-obesogenic properties [52,85,86].
Marine algae are a rich source of phenolic compounds, rendering them highly potent bioactive resources in marine ecosystems. Seaweed species like Undaria sp., Laminaria sp., Porphyra sp. (red algae), and Fucus sp. (brown algae) are notable sources of phenolic compounds. Generally, phenolic acids, such as protocatechuic, caffeic, gallic, chlorogenic, p-hydroxybenzoic, vanillic, and salicylic acids, and flavonoids, such as quercetin, naringenin, apigenin, luteolin, hesperidin, kaempferol, catechin, genistein, and epicatechin, were found in different species of marine algae. Typically, brown algae contain a higher content of tannins, primarily due to elevated phlorotannin levels, than the red and green algae. Phlorotannins contribute to numerous biological activities, encompassing antioxidant, antitumor, anti-inflammatory, antidiabetic, antiallergic, and antihypertensive effects. Moreover, phenolic compounds from marine algae could be used as alternatives to synthetic antioxidants, which are useful for rendering lipid oxidation in oils as well as fish and fish-based products [49,87,88]. For example, Das et al. [89] identified around 100 bioactive compounds, mainly polysaccharides, flavonoids, tannins, glycosides, and steroids, from seagrass (Cymodocea serrulata) and red seaweed (Kappaphycus alvarezii), which exhibited antioxidant and antibacterial properties. Aside from this, marine algae also serve as a valuable vitamin source. To illustrate, red algae contain B vitamins (B1, B2, and B12), while green algae encompass vitamins E and C. Vitamin C is found in all macroalgae, with red algae being notably employed to combat scurvy stemming from vitamin C deficiency. Seaweed can exceed daily vitamin requirements, particularly for vitamins A, B12, B2, and C [90]. Beyond pigments, phenolics, and vitamins, marine algae are also renowned for their richness in essential minerals (e.g., magnesium, sodium, calcium, potassium, and phosphorus) and trace minerals (iodine, zinc, and manganese). Their mineral presence is attributed to their ability to accumulate inorganic matter from seawater [51]. The mineral composition of marine algae is contingent upon the species and can vary due to factors like environment, geography, seasonality, and physiology. In particular, marine algae can contain mineral levels 10 to 100 times higher than those in terrestrial plants. Indeed, the mineral content of specific algal species (e.g., red and brown algae) can extend to approximately 40% when measured on a dry weight basis, whereas spinach, known for its remarkable mineral content, has been documented to possess only 20% dry weight mineral content [88]. As a result, selected marine algae are broadly incorporated into dietary supplements to meet mineral and trace element requirements. Furthermore, owing to the balanced ratio of sodium and potassium, mainly evident in green algae like Ulva rigida, these algae might contribute positively to preventing hypertension [55]. Therefore, marine algae or their products exhibit an array of health benefits, encompassing the prevention of various ailments such as symptoms of osteoporosis, hypocalcemia, goiter, cretinism, and mental disorders [85]. Consequently, it is crucial to make concerted efforts to expand their inclusion in innovative food formulations and to rigorously assess the purported health benefits tied to these novel products.
2.3. Marine Invertebrates
Marine invertebrates, including sponges, mollusks, echinoderms, and crustaceans, have attracted scientific and economic interest worldwide as promising sources of novel bioactive compounds. These multicellular non-chordates belong to a wide range of species, from phylum Porifera to phylum Echinodermata [91]. Their abilities to thrive in extreme halobiotic environments and their metabolic activities have been important drivers in the production of a diverse array of bioactive compounds. These molecules with unique structural features exhibit numerous biological activities with, for example, antioxidant, antibacterial, antiviral, anthelmintic, antifungal, anticancer, antihypertensive, and immune-modulatory properties [92,93]. Moreover, extensive research on marine bioactives highlighted that marine invertebrates (non-chordates) are the most interesting group for the discovery of marine natural products.
2.3.1. Sponges
Sponges, belonging to the phylum Porifera, are the oldest and the most primitive of the metazoans, with a global distribution in all aquatic habitats. They are more abundant (area coverage/biomass/volume) in benthic communities compared to other benthic marine organisms. Sponges are the simplest form of multicellular animals with gelatinous material, mesophyll, and a hollow pitcher-like body that consists of spicules and spongia, as well as often some species associated with dense and complex microbiota [4]. They are ecologically important due to their active involvement in marine nutrient cycling and, in the meantime, play a pioneering role in the discovery of bioactive substances from marine organisms. The very first bioactive compounds from marine sources were the serendipitous isolation of C-nucleosides, spongouridine, and spongothymidine from the Caribbean sponge Cryptothecaa crypt in the early 1950s. Since then, numerous chemically diverse biotechnologically relevant natural products such as peptides, sterols, alkaloids, terpenoids, polyketides, macrolides, and polyphenolic compounds have been isolated from these sponges [91,94] (Table 4).

Table 4.
Bioactive compounds in sponges.
2.3.2. Mollusk
Phylum Mollusca represents one of the largest and most diverse groups of marine animals, and within the phylum, clams, oysters, scallops, and mussels are some of the well-known invertebrates. Several bioactive compounds, including primary metabolites such as amino acids, nucleic acids, simple sugars, and lipids, as well as secondary metabolites, like alkaloids and terpenoids, have been isolated from molluscans [4]. These components have been proven to possess unique biological functions. As a result, they are used for the development of functional foods as well as an alternative medicine for various diseases. Among these organisms, mussel, namely Mytilus and Perna, components have been extensively investigated by many researchers in recent decades. For example, peptides obtained from mussels (Perna canaliculus) exhibited antioxidant and angiotensin-I-converting enzyme (ACE) inhibitory activity [101]. Moreover, Naik and Hayes [102] reported that mussel (Mytilus edulis) by-products, including byssus threads, shell, and discarded meat, contained a wide range of valuable components like polyunsaturated fatty acids, proteins, minerals, pigments, and enzymes, among others.
2.3.3. Echinoderms
Echinoderms are a phylum of invertebrate marine animals that are distributed in five different classes: Asteroidea (starfishe), Holothuroidea (sea cucumber), Echinoidea (sea urchin and sand dollar), Crinoidea (crinoid and sea lilie), and Ophiuroidea (brittle star and basket star). Among these five extant classes of echinoderms, sea urchins (Echinoidea) and sea cucumbers (Holothuroidea) are both fished commercially and have gained much attention due to their abundance in bioactive compounds [10,91]. The major metabolites of echinoderms are mainly saponins, and there has been a recent emphasis on exploring the compositional, structural, and sequential properties of other bioactive compounds such as asterosaponins. Most of these secondary metabolites are well known for their antitumor, cytotoxic, antibacterial, antiviral, antifungal, and anti-inflammatory activities [103]. In particular, sea cucumbers (e.g., Cucumaria frondosa, Apostichopus japonicus, Cucumaria japonica, and Holothuria forskali) are rich in proteins, lipids, and SPs, namely fucosylated chondroitin sulfate and fucoidan, as well as saponins, phenolic compounds (e.g., phenolic acids and flavonoids), and carotenoids, among others [10,60]. The phenolic compounds, including protocatechuic acid, gallic acid, ellagic acid, p-coumaric acid, catechin, and quercetin, of sea cucumbers have been reported to have inhibitory activities against tyrosinase, α-glucosidase, and the formation of advanced glycation end products (AGEs) as well as LDL cholesterol and DNA oxidation, while protein isolates/peptides derived from C. frondosa showed antioxidant and ACE inhibitory effects [104,105]. Therefore, these echinoderms have been, and continue to be, examined as a source of biologically active compounds. Table 5 summarizes some bioactive compounds of echinoderms and their biological activities.

Table 5.
Bioactive compounds from echinoderms.
2.3.4. Crustaceans
The largest group of marine arthropods, the class crustacean, is made up of approximately 30,000 species. They are considered the most economically important class of marine arthropods with regard to their role in the global fisheries market and also make a substantial contribution to the discovery of novel natural ingredients. Marine crustaceans, including shrimp, crabs, lobsters, crayfish, and prawns, have gained great attention due to their abundance of biologically active compounds. The ratios of saturated, monounsaturated, and polyunsaturated fats in shellfish play a role in promoting a healthy diet. Additionally, the presence of high-quality proteins containing all the dietary essential amino acids is vital concerning the maintenance and growth of the human body. Moreover, even their exoskeleton shells are identified as rich sources of functional biopolymers such as chitin/chitosan. By-products of crustaceans are recognized as a rich source of many other compounds, including proteins, lipids, pigments (astaxanthin), flavors, and minerals [114,115].
The exoskeletons of crustaceans comprised about 30–50% minerals (mainly calcium carbonate), 30–40% proteins, and 20–30% chitin, in addition to other compounds such as pigments (e.g., astaxanthin) and lipids [116]. Numerous biologically active compounds associated with shellfish species have been investigated over the decades. For example, peptides derived from crustaceans possess potential therapeutic effects due to their activity as immune modulators and antitumor and antimicrobial agents. In particular, the ACE inhibitory activity of peptides derived from shrimp (Acetes chinensis) and Izumi shrimp (Plesionika izumiae) was found to be most effective as compared to the peptide derived from terrestrial food sources [117,118]. Moreover, protein existing in shrimp waste comprises essential amino acids and non-essential amino acids, approximately 56.8 and 43.2%, respectively, and their protein hydrolysates also have diverse applications, for example, in pharmaceuticals, human nutrition, animal nutrition, cosmetics, and even as a nitrogen source in the growth media for microorganisms [119,120]. For example, shrimp shell discard hydrolysates revealed their potential for application in functional foods, nutraceuticals, and protein supplements, as well as in blood pressure-lowering applications, mainly due to their significant antioxidant activities [114]. Moreover, investigations by Balzano et al. [121] on mantis shrimp (Squilla mantis), caramote prawn (Penaeus kerathurus), and crab (Liocarcinus vernalis) assessed the distribution of fatty acids, mainly n-3 PUFA and furan fatty acids, in the neutral lipid and phospholipids fractions. According to their findings, caramote prawn contained higher furan fatty acids, while the crab edible part represented the most valuable source of dietary omega-3 fatty acids, mainly EPA.
Apart from this, carotenoids are also another essential bioactive constituent that is responsible for the color of marine crustaceans such as shrimp, lobster, and crab. Carotenoids occur either as free pigments or as carotenoproteins in invertebrates. The predominant types of pigments in crustaceans are carotene, hydrocarbon- and oxygen-containing carotenoids, and xanthophyll, which are mainly carotenolipoproteins, carotenoproteins, and chitinocarotenoids [122]. In particular, the crustacean exoskeleton is a rich source of carotenoproteins, which are mainly responsible for the green or orange-red color in shore crabs (Carcinus maenas), the red color in prawns (Pandalus borealis), and the blue color in freshwater prawns (Macrobrachium rosenbergi) [123,124]. Crustaceans can absorb the ingested carotenoids and metabolically alter their keto and hydroxy derivatives [125]. When considering the carotenoprotein complexes in crustacean tissues, prosthetic carotenoid groups in the ovaries/eggs of crustaceans are canthaxanthin, astaxanthin, or astaxanthin ester, while astaxanthin is predominant in their exoskeleton [115]. Several studies have isolated and characterized the different carotenoids and their prosthetic groups. Astaxanthin, astaxanthin esters, and zeaxanthin were identified from shrimp Pandalus borealis and canthaxanthin, astacene, and lutein from processing discards of snow crab Chinoecetes opilio [3,126]. Moreover, a high level of astaxanthin, which exists as a chemical complex with proteins (carotenoproteins) or lipoproteins (carotenolipoproteins), was identified in by-products of shrimp processing discards [114]. This astaxanthin is considered a super antioxidant and possesses various bioactivities, including antioxidative, anticancer, immunomodulating, antidiabetic, and anti-inflammatory effects. Moreover, offals from shrimp processing have been used as a source of carotenoid astaxanthin for aquaculture, particularly for salmon and trout. In addition, carotenoids play a vital role in various physiological processes, and as provitamins, they can also ensure the provision of the daily vitamin A requirements [127].
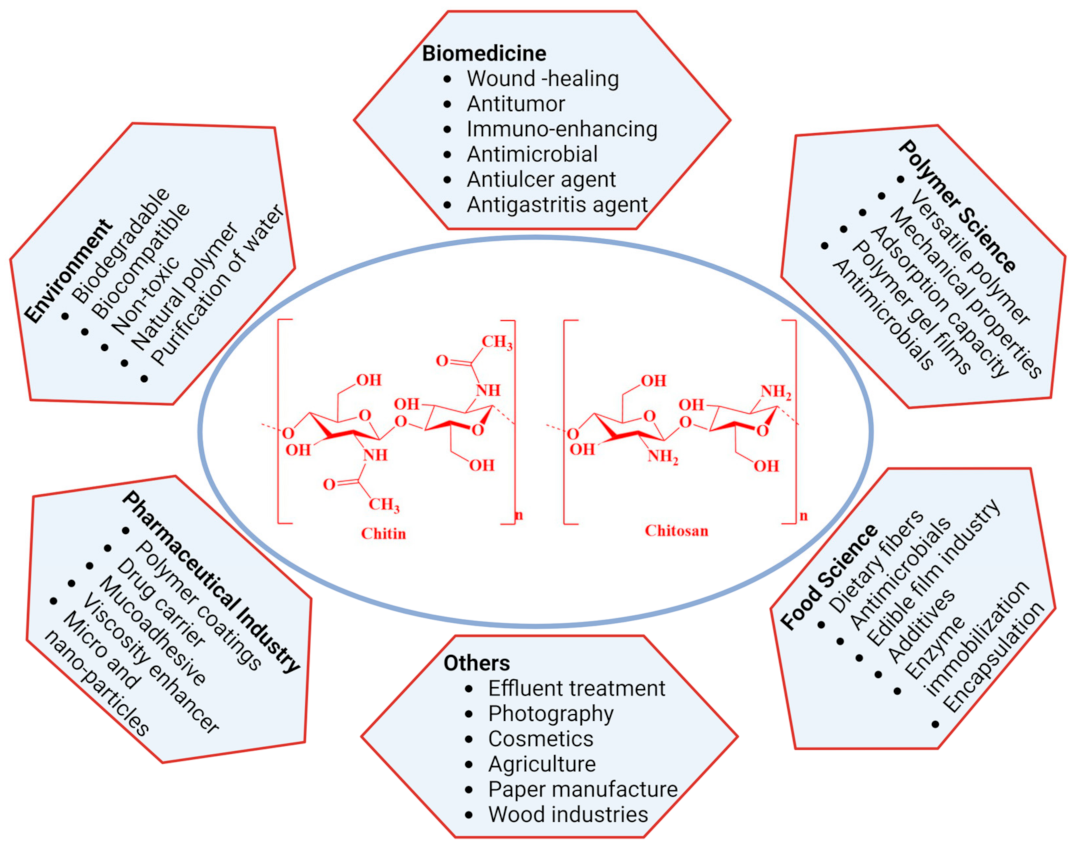
Chitin is another well-known bioactive material derived from the skeletal material of crustacean shells. As a linear amino polysaccharide, chitin is considered the second most abundant natural polymer in the world. From a chemical viewpoint, chitin consists of repeating β-1,4 linked N-acetylated glucosamine units, whereas its N-deacetylated derivative, chitosan, is composed of randomly distributed β-(1→4)-linked-D-glucosamine and N-acetyl-D-glucosamine. Moreover, chitosan is prepared by alkaline or enzymatic deacetylation of chitin and is also widely distributed as a polymeric product in nature. Moreover, one of the limitations of chitin is its hydrophobicity, and as a water-insoluble derivative, chitosan expands its applications in various fields. These two biopolymers possess high biological and mechanical properties due to their unique structures. They have received significant interest as bio-functional, renewable, biocompatible, and biodegradable materials for a wide range of biomedical and biopharmaceutical applications (Figure 2) [116,128]. However, the exoskeletons of crabs and shrimps are widely used as chitin sources for commercial use due to the availability of wastes from the seafood processing industry. Moreover, producing chitin-like important biopolymers using these discards directly helps to manage the waste of the shellfish industry and also mitigate possible environmental hazards.
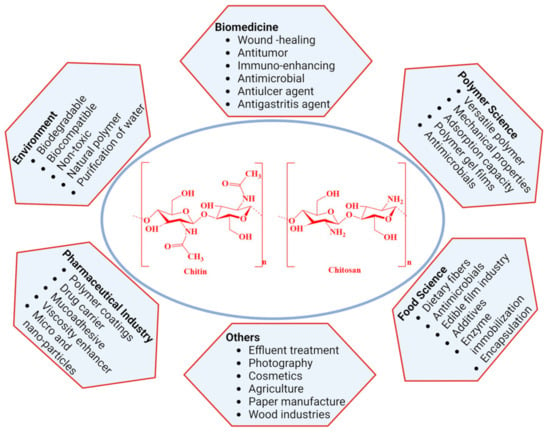
Figure 2.
Major applications of chitin and chitosan.
2.4. Marine Fish
Among the wide range of seafood types, marine fish is the major source of protein in the diet. Marine fish plays a vital role in the world economy, and the per capita finfish demand was around 13.7 kg in 2010, and by 2030, annual fish consumption is predicted to rise by around 202 million tons or between 20 and 22 kg per person [129]. The increasing demand for marine fish for human consumption is linked to the growing population and consumers’ concern about a healthy diet. Generally, fish can serve as an excellent source of functional materials that include PUFAs, minerals, vitamins, enzymes, essential fatty acids, and bioactive peptides. Marine fish, in particular, are considered an excellent source of macromolecules such as high-quality protein, as well as lipids and a wide variety of minerals. Diversified fish products such as fresh, dried, fermented, and salted fish provide a major part of the essential amino acid requirements and can also be the perfect healthy food due to their low content of saturated fat and calories [1,7].
2.4.1. Fish Lipids
Marine lipids are derived from the flesh of fatty fish, the blubber of marine mammals (e.g., seals and whales), and the liver of white lean fish, and they are composed of a mixture of fatty acids with different chain lengths and degrees of unsaturation. Several studies have revealed that the major compound of marine lipids is triacylglycerol (TAG), which is abundant in monounsaturated fatty acids (MUFAs) and PUFAs. These fatty acids occur in different proportions that vary according to their source, the composition of the plankton/feedstuff, and the harvest time [5,7]. Moreover, marine organisms are the best available natural sources of omega-3 PUFA as they are directly or indirectly linked by food webs with marine phytoplankton. These marine phytoplanktons (uni- and multicellular marine plants) are considered primary producers of omega-3 fatty acids in the trophic chain, and they transfer these fatty acids, which can remain in liquid form, even under extreme marine conditions, into fish and marine mammals [85,118]. The long-chain omega-3 PUFAs are mainly comprised of EPA and DHA, and they are considered one of the most abundant essential fatty acids in fish species. In recent decades, marine oils have gained much attention as a rich source of PUFAs due to their beneficial health effects. For example, pelagic species caught in large quantities are considered the main sources of fish oil. Salmon, herring, tuna, mackerel, and small fish such as anchovy and capelin are considered oily fish species that are widely used for fish oil production. Furthermore, several studies have revealed that fish species belonging to Scombridae, Clupeidae, and Salmonidae families contain the highest amounts of EPA and DHA [118]. For example, anchovy contains 21% EPA and 9% DHA, while sardine contains 16% of EPA and DHA. Typically, crude fish oil is considered to be the direct by-product of the fish meal industry. Recently, krill oil has gained great interest due to its unique composition. Krill oil is abundant in phospholipids, and most of the EPAs and DHAs in krill are bound to these phospholipids [130,131].
However, the constitutes and configurations of marine oil from fish species and marine mammals are different. For example, EPA and DHA are present abundantly in most marine oils, while docosapentaenoic acid (DPA) is frequently found mainly in the oil of marine mammals [85,132]. These marine oils are usually extracted from the blubber of marine animals like seals and whales. Moreover, the positional distribution of fatty acids in TAG also varies in fish and marine mammal lipids. Fish oils contain long-chain PUFAs mostly in the sn-2 position of TAG, while marine mammal oils are located primarily at the sn-1 and sn-2 positions [133]. Fish oils are one of the main dietary sources of long-chain PUFAs and contain α-linolenic acid (ALA) and its longer-chain metabolites, namely EPA and DHA. Hence, consumers’ preference for the consumption of fish oil is a growing global trend due to the multiple health benefits associated with the constituents of fish oil. The beneficial effects of omega-3 PUFA, particularly EPA and DHA, are crucial in preventing diseases like cardiovascular, inflammations, hypertension, allergies, and some forms of cancer while enhancing functional development and growth of the central nervous system, which directly influences infant growth and development [134]. Apart from oily fish, fish oil supplements are also considered one of the principal dietary sources of long-chain omega-3 PUFAs. However, the typical ‘Western’ diet is relatively high in omega-6 PUFAs and low in omega-3 PUFAs [7,85,134]. Therefore, alternative methods are needed to overcome this issue and ensure the intake of the required amount of omega-3 PUFAs. In order to achieve this, food-grade fish oils are often introduced to the market as capsules or in liquid forms. Fish liver oil, mainly cod or halibut oil, is generally used as capsules for medical applications [135]. Moreover, salmon oil, omega-3 concentrated oils, and omega-3, -6, and -9 combination oils are already available in the market all over the world. Most of these products are considered health supplements, and the consumption of liver oils provides additional health benefits due to their richness of fat-soluble vitamins (e.g., vitamin A) apart from their omega-3 components. However, the TAG structure of fish oils limits the usage of omega-3 PUFA in these fish oil products and thereby enhances the bioavailability of omega-3 PUFA extraction methods, and their incorporation into food products are important aspects to be discussed [127,134].
2.4.2. Fish Proteins
Fish protein is the dominating source of seafood-derived protein as fish is considered a rich source of easily digestible protein and essential amino acids. Much research has investigated the utilization of fish muscle protein and protein-derived products such as peptides, collagen, and gelatin, which are used as functional ingredients in the food, pharmaceutical, and nutraceutical sectors. Approximately 11–27% of seafood comprises crude proteins and non-protein nitrogenous (NPN) compounds. NPN compounds include free amino acids, peptides, amines, amine oxides, guanidine, quaternary amino acids and ammonium compounds, polyamines, nucleotides, nucleic acids, and urea. In addition, all other muscle and seafood proteins are classified into three groups, namely myofibrillar, sarcoplasmic, and stroma types. Sarcoplasmic protein contains approximately 15–35% of the total muscle tissue protein, while myofibrillar represents 65–75%. Stroma types only represent approximately 3% of the total muscle tissue protein [31,35,136]. Sarcoplasmic proteins such as myoglobin and other albumins can be extracted to water or neutral solutions, whereas myofibrillar proteins like actin, myosin, tropomyosin, and troponins are soluble in high salt solutions. Stroma-type proteins (collagen and elastin from connective tissue) remain in the residue after extraction of myofibrillar and sarcoplasmic proteins [137]. On the other hand, the content of NPN compounds in seafood, including marine fish, is primarily influenced by factors like the specific species, freshness level, and habitat. Fish processing discards also play a key role as a source for obtaining high-quality protein and protein-derived products (e.g., hydrolysates/peptides). Generally, the percentages of proteins in processing by-products are cuts (15–20%), bones (9–15%), heads (9–12%), skin and fins (1–3%), viscera (12–18%), and scales (5%) [138,139]. The global demand for collagen and gelatin continues to grow steadily due to their extensive applications in the food, nutraceutical, pharmaceutical, and cosmeceutical industries. Gelatin, the partially hydrolyzed form of collagen, is a widely employed ingredient in the food sector. The functional properties of the extracted collagen and/or gelatin are closely tied to their quality and their suitability for specific uses. In particular, fish skin collagen and gelatin are gaining more attraction as an alternative to mammalian collagen because of their purity and low risk of possessing unknown pathogens [31,85,118,140].
Development of Fish Protein Hydrolysates/Peptides
Fish protein hydrolysates (FPH) have demonstrated excellent physicochemical and functional properties such as antioxidant, antihypertensive, mainly as inhibitors of ACE, antimicrobial, and immunomodulatory functions, which are often linked to peptide sequences. Hence, these proteins and their hydrolysates have been analyzed for their nutritional and functional properties related to the possibility of recovering biologically active peptides over the years. Bioactive peptides are specific protein fragments acting as a source of nitrogen and amino acids that have a positive impact on body functions or conditions and may ultimately influence health [5,118]. In particular, bioactive peptides with short sequences, typically containing 2–20 amino acids, showed greater biological activities. The nature of bioactive peptides produced from a specific protein is determined by two key factors: the selectivity of the enzyme employed for peptide generation and the primary sequence of the protein substrate [35,141].
Bioactive peptides can be produced using chemical (acid or alkaline) hydrolysis, enzymatic degradation, or microbial fermentation of proteins. When it comes to marine bioactive peptides, they are typically produced through enzymatic hydrolysis using suitable proteolytic enzymes derived from microbes, plants, and animals. Moreover, physicochemical conditions of the reaction media (e.g., pH and temperature of protein solution) are also influenced by the hydrolysis procedure employed. For instance, the recently developed pH-shift process is considered a feasible option for the hydrolysis and recovery of proteins or peptides from fish products. The resultant recovered protein from this pH-shift process can further be hydrolyzed to produce highly bioactive protein hydrolysates. In enzymatic hydrolysis, one of the crucial initial steps includes the selection of enzymes for obtaining the desired bioactive and functional properties with maximum yield of the final product. This can be further explained by considering the exopeptidase and endopeptidase activities of individual enzymes. Enzymes with endopeptidase can cleave the peptide bonds of non-terminal amino acids, while exopeptidases are able to break the terminal ends of proteins or peptides. Exopeptidase enzyme activity subsequently results in a higher degree of hydrolysis and a combination of large and small peptides, whereas endopeptidases produce large peptides [31,111]. The biochemical generation of fish protein hydrolysates can also be carried out by using an autolytic process, which is determined by the digestive enzymes of the fish itself. In particular, fish viscera contain high levels of digestive proteolytic enzymes such as pepsin, trypsin, and chymotrypsin. Furthermore, many bioactive peptides have been produced using commercial proteases such as papain (EC 3.4.22.2), bromelain (EC 3.4.22.32), and ficin (EC 3.4.22.3). Ultrafiltration, nanofiltration, ion exchange membranes, and column chromatography are some of the common methodologies for the separation of the desired bioactive peptides. Moreover, a serial enzymatic digestion system with a combination of a multi-step recycling membrane reactor and an ultrafiltration membrane to separate marine-derived bioactive compounds has been used [111,141,142].
2.4.3. Other Compounds/Elements Derived from Marine Fish
Fish bones constitute a significant part of the fish and are considered a rich source of minerals and collagen proteins. After the removal of muscle proteins on the frame, fish bones comprise 60–70% inorganic compounds and are mostly composed of calcium, phosphorus, and hydroxyapatite, while their organic component represents 30% collagen [5]. Producing calcium-fortified milk powder and fish meal, which contains 10% minerals, are some applications of fish bones, which contain a high content of calcium and phosphorus. Moreover, autografts, allografts, and xenografts are used as bone substitution materials in clinical applications, mainly for bone fractures and damage [5,85]. In addition, the presence of hydroxyapatite has widened the biomedical applications of fish bone due to its role in the structural strength of bone and bone regeneration. Apart from this, fish processing by-products contain a diverse array of enzymes that are found in the stomach and intestine. Enzymes from marine organisms are widely used for the preparation of FPH and also for the processing of herring, pollock, shrimp shells, squid, skate, and tuna [143].
3. Marine Bioactive Compounds and the Food Industry
The marine environment is a valuable source of numerous bioactive compounds with unique biological functions suitable for use as functional food ingredients. Marine products are rich in different kinds of polysaccharides, including SPs and chitin/chitosan, proteins, including both essential and non-essential amino acids, PUFAs, vitamins, minerals, carotenoids, and other bioactive compounds [4,5]. Various sources of marine organisms offer these bioactive compounds in varying quantities, and they can be incorporated into the food production process at different stages, from processing to storage [137]. Moreover, nowadays, consumers are becoming more conscious of the link between a wholesome diet and the prevention of diseases. Hence, marine-derived biologically active components have enormous potential to be the best ingredients for incorporation into foods as they enhance both the nutritional and economic value of the food. In the last decades, a wide range of marine-derived functional foods have been launched into the world market, which could be fortified with many functional ingredients, including antioxidants, vitamins, carotenoids, probiotics, minerals, fiber, PUFAs, polysaccharides, protein hydrolysates, and other dietary supplements. For instance, seaweeds have been incorporated in meat, dairy, confectionery, bakery, and pasta products due to their ability to reduce blood pressure, fight obesity, lower cholesterol content, and tackle free radicals. Most common commercially available seaweed-based supplements include seaweed-derived functional drinks and seaweed biscuits, among others [4,8]. Apart from this, marine-derived polysaccharides (e.g., chitosan, alginate, and carrageenan) and protein (e.g., gelatin) have gained extensive research attention as primary biopolymers for preparing edible films and coatings as they are available worldwide at relatively low costs with high performance [128,144].
3.1. Bioactive Marine Polysaccharides
The biological properties of marine-derived polysaccharides are determined by their chemical composition and structure. They can be derived from a wide range of marine organisms and possess several properties that make them ideal ingredients to incorporate into food. Algae, crustaceans, and most other marine organisms produce fucan/fucoidan, carrageen, hydrocolloids, and glycosaminoglycans like polysaccharides that are widely used in numerous commercial applications, particularly in food, beverages, and supplements [49]. For example, algal polysaccharides such as alginate, agar, and carrageenan are used as gelling, emulsifying, clarifying, thickening, stabilizing, and flocculating agents in various food products, including ice cream, candy, yogurt, meat product, and functional beverages [56]. Most of their functional properties, such as gelling and thickening, are due to the presence of polysaccharide–polysaccharide interactions as their unique structural characteristics. Agar forms stable gels and is widely used as an additive in dairy, bakery, and canned meat/fish products, soups, sauces, and beverages, and also in some culinary applications such as Japanese foods like “Tokoroten” (noodlelike agar gel) and “Mitsumame” (canned fruit salad with agar jelly) [144]. Furthermore, due to the high sugar content of agar, it can be used in confectionery industries, particularly in jellies, jams, fruit candies, puddings, and custards. Like agar, alginate also possesses the ability to form a firm and rigid gel that can be used in jams and puddings, and it is also important as a thickening agent in ice cream, sauces, ketchup, mayonnaise, and purees. Apart from that, the film-forming ability of alginate is used in pastries and fruit fillings to prevent cake moistening [49]. Moreover, some polysaccharides can bind large amounts of water and disperse it in food products. This property is exhibited in carrageenan-like polysaccharides that are used to modify the texture of food products by changing their water-binding and foaming attributes [4]. For example, carrageenan finds application in a variety of water-based gelled desserts and cake frosting. Additionally, it is employed in dairy products both on its own (e.g., processed cheese, flan, sterilized chocolate, and evaporated milk) or in conjunction with other gums such as locust bean gum (e.g., ice cream and cream cheese) [49,145]. Fucoidans are another complex sulfated polysaccharide isolated from different marine species, and because of their structural sulfated groups, they improve biological properties and make them suitable to incorporate into dairy products. Apart from fucoidan, other marine polysaccharides such as chitosans are ideal raw materials for edible and biodegradable films and as antimicrobial agents, additives, and nutraceuticals. In addition, meat products, including sausages, dairy products, and beverages, can be supplemented with polysaccharides like carrageenan, chitosan, and chito-oligosaccharides [7,8,51,128].
3.2. Bioactive Marine Peptides
Proteins and peptides derived from marine organisms display several unique functional properties, such as film-forming, gel-forming, and foaming capacities. For example, marine collagen and gelatin are widely used in the food industry as food additives mainly due to their gel-forming ability, film-forming, texture improvement, and water-holding capacity [144]. Furthermore, there has been much attention and considerable interest in the food industry about bioactive peptides due to their nutritional and therapeutic values. These biologically active peptides play crucial roles in metabolic regulation and modulation. Especially, proteins from fish, mollusks, and crustaceans are among the excellent sources of all essential amino acids. Fish protein powders and fish protein supplements, including fish protein hydrolysates (FPHs), are widely used in fish protein-incorporated food products, and they are available in the market. Fish protein powders are often produced by drying mechanically deboned fish flesh, which is washed with water or diluted in salt solutions (surimi). The amount of collagenous matter and myofibrillar proteins is relatively greater in surimi compared to fish flesh [31,141]. Therefore, dietary proteins can be replaced by fish protein concentrates without any effect on metabolic balance. When considering the functionality of these FPHs, several studies have revealed their high solubility [111]. Due to their high solubility, FPHs could successfully be incorporated into drink formulations. Several research groups have focused their attention on enhancing water-holding capacity in raw and cooked foods by introducing FPHs [146]. Apart from interaction with water, FPHs’ interfacial and surface properties are linked to their interactions with fats and oils. These interactions determined the emulsifying and foaming properties of FPHs. However, bitterness associated with FPHs may hinder their use in several food applications. Therefore, further studies are needed to explore the interfacial properties of FPHs, including their emulsifying and foaming properties in functional foods and nutraceuticals. For example, salt, phosphate, and cyclodextrin were found to be important ingredients to mask the bitter taste of FPH [51]. Moreover, FPHs have the potential to be used as flavorings and as “extracts” to be added to food products like soups, bisques, frozen seafood, fillings, and snacks in order to enhance their flavor. When considering algal proteins, the class of phycobiliproteins (phycoerythrin, phycocyanin, and allophycocyanin) and lectins are two major groups of functionally active proteins, which show several beneficial functions, such as antioxidant and antidiabetic properties. Phycobiliproteins, water-soluble protein pigments, are found mainly in cyanobacteria and red algae (Spirulina) and are used as food dyes [52,118].
3.3. Bioactive Marine Polyunsaturated Fatty Acids
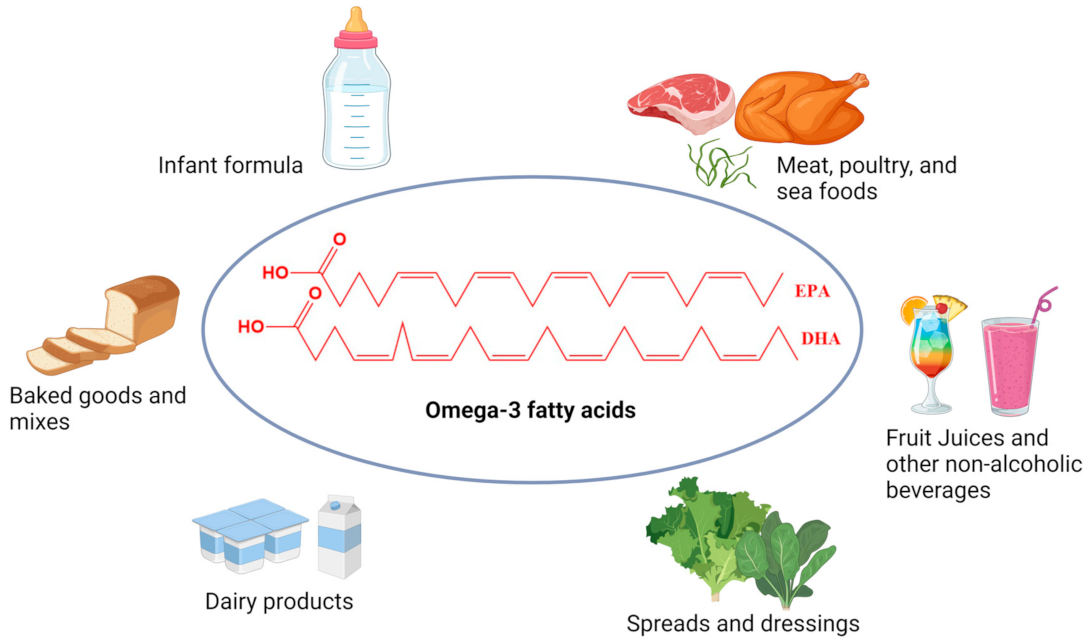
Enhancing food products with omega-3 PUFA fortification has been recognized as an effective approach to increasing their dietary intake (Figure 3).
Figure 3.
Common omega-3 fatty acids enriched foods available in the world market.
Fortification and microencapsulation are the most popular methods in this enrichment process. The inclusion of fish oil in food products such as bakery, dairy, and pasta by using modern technologies is a growing industry, and recent advances in this sector have resulted in a recognizable change in the functional food sector. For example, novel microencapsulated oil, which is known as MEG 3 (produced by Ocean Nutrition Canada; now DSM), has the ability to remain unaffected until it reaches the GI tract. However, preventing lipid oxidation and related fishy off-flavors associated with lipid degradation after incorporation of fish oil and, in the meantime, protecting the sensory attributes of food products are some of the major technical hurdles to overcome during the application [147,148]. The formation of both primary and secondary oxidation products in polyunsaturated fatty acids (PUFAs) over extended periods of storage significantly contributes to the degradation of quality in fish oil and food products that contain fish oil. Nevertheless, the extent to which fish oil can be fortified in products like bakery goods, dairy items, and other frozen foods has been limited primarily because fish oil is highly prone to oxidation over time. Recent studies have indicated that fatty acids present in fish oil might undergo oxidative deterioration [9]. As a consequence of this degradation of the original PUFAs, the possibility of forming reactive lipid radicals and ultimately unhealthy volatile off-flavor molecules is high. Generally, the oxidation of fatty acids (EPA and DHA) results in the production of different volatile aldehydes and ketones. Depending on the substrate and environmental compositions, these volatiles can vary. Moreover, they belong to several groups such as short-chain aldehydes (acetaldehyde, 2-propenal, propanal, and 2-butanal), long-chain aldehydes (hexanal, 2,4-heptadienal, nonal, and nonadienal), diunsaturated aldehydes (nonadienals and 2,4-heptadienal isomers), saturated aldehydes (hexanal, pentanal, and nonanal), ketones (1-penten-3-one), alcohols (1-penten-3-ol), hydrocarbons (pentane, 1-pentene, 2-methyl-1-butene, 1,3-pentadiene, and 1-heptene) and acids (formic acid, propanoic acid, hexanoic acid, and butanoic acid) [147]. However, introducing or incorporating fish oil into foods is a challenging and difficult task as omega-3 PUFAs are extremely liable to oxidation. Moreover, executing preventive measures is not enough for industrial-scale usages of fish oil and fish oil-incorporated products. Hence, the use of antioxidants has gained attention in order to enhance the shelf life of oil-related food products by retarding the oxidation process. Particularly, synthetic antioxidants, such as butylated hydroxyanisole, butylated hydroxytoluene, t-butyl-hydroxyquinone, and propyl gallate, and natural antioxidants, such as tocopherols, ascorbic acid, gallic acid, and lactoferrin, among others, have been studied for preventing oxidation of bulk fish oil, fish oil emulsions, and other marine oils [149,150]. Nevertheless, crude fish oils often contain impurities and oxidation products. Due to their compositional characteristics, these oils are often considered a good source for tanning, margarine oil (hydrogenated), supplement, and aquaculture industries [151]. Therefore, refining and purification procedures should be practiced, especially before their incorporation into food products.
Recent studies on the sensory evaluation of foods enriched with fish oil have also revealed that the addition of fish oil can negatively affect the sensory properties of fortified foods [128]. Nevertheless, there are numerous instances of omega-3 fatty acid-enriched foods that are available in the market worldwide, while maintaining sensory attributes. These include meat and poultry products, eggs and egg products, bread and bakery products, spreadables, milk and dairy products, sauces, pasta, soft drinks, juices, and instant concentrates [127,151]. Therefore, the fortification of food products with long-chain omega-3 PUFA using unhydrogenated marine oils is a popular and growing area in the food sector. However, it is in demand to discover novel methodologies and creativity for devising cost-effective approaches to stabilize these beneficial oils for delivery in food systems for the betterment of consumers and for the industry itself to remain competitive.
4. Challenges and Future Trends
The increasing consumer focus on healthy food ingredients is propelling both research and the development of functional foods, with no exception from those of marine origin. Given this background, the health effect of marine-derived functional foods should be scientifically proven, the institutional barriers to the commercialization of foods with marine-derived ingredients cleared, and the feasibility of marine food for human consumption widely established to ensure the vast resources of marine organisms are optimally utilized. While the wealth of bioactive sources in the marine ecosystem and their potential application in the functional food industry hold great promise, there are several challenges that must be addressed. Achieving a successful transition from technological development to commercialization necessitates the standardization of analytical methods to ensure product quality, evaluation of sensory characteristics to gain consumer acceptance, and well-structured clinical trials to generate substantial evidence for health claims. As an illustration, the utilization of microalgae as a source of food ingredients has seen some progress in the industry but still awaits full exploitation of its biotechnological potential [49].
The incorporation of marine-derived ingredients into the human diet is a multifaceted matter that is influenced by various constraints. These constraints encompass global factors, such as cultural and ethnic considerations like dietary preferences and habits, environmental concerns such as apprehension about ocean pollution and the sustainable use of resources, and also more specific factors like the sensory qualities of food and compliance with legislation [4,49]. There are no clearly defined safety regulations specific to most functional food. In the EU, the functional food sector has only achieved modest growth, mainly due to the lack of European legislation specifically for health benefits, efficacy, and health claims. The legislation in specific countries, which is continuously changing and sometimes requires complex procedures, is a potential obstacle for the new functional foods, mainly marine-derived ones. The financial implication of complying with these regulations is huge. For example, in order to prove the health benefits, scientifically expensive human clinical trials should be conducted. Due to the elevated cost of products due to these costly procedures, the targeted market niche may be somewhat narrow.
Identifying, extracting, incorporating, and commercializing marine-derived functional food ingredients is challenging. The exploration and utilization of marine-derived functional food should be compatible with sustainable harvest practices and food grade compatibility. Moreover, the methods of extraction of concentrated extracts should be efficient and sustainable. It is essential to validate the biological properties of these extracts and purified functional ingredients to facilitate the development of novel functional foods, incorporating them into consumer foods. Furthermore, evaluating the stability and bioavailability of these functional ingredients, as well as substantiating their specific health benefits through human clinical trials, is of paramount importance. For instance, the digestibility of algal proteins remains poorly characterized, and existing studies on their assimilation by humans have not yielded definitive conclusions [4]. Even under the above-discussed challenges, there exists a vast body of literature exposing new opportunities for marine-derived value-added products. For example, encapsulation of bioactives not only preserves nutrients but also improves bioavailability, sensory characteristics, and antioxidant activity; it also reduces toxicity [152]. Amiri et al. [127] reported that encapsulation of fish oils, fish protein hydrolysates, and shrimp lipid extracts using whey protein and chitosan could be a potential approach to retain functional characteristics and improve antioxidant activity.
5. Conclusions
Marine organisms serve as one of the most significant natural sources of bioactive compounds, offering a wide range of such substances. These bioactive compounds derived from marine sources have gained considerable recognition in the food and supplement industries due to their pivotal role in promoting human health and nutrition. The availability and rheological properties associated with important biological functions make marine bioactive compounds promising alternatives to existing natural bioactive molecules. Although the resource is substantial, more research should be conducted to identify efficient and low-cost extraction methods, incorporate foods without changing sensory attributes of the food, and prove they have actual health benefits for people through clinical trials. Therefore, despite the enormous benefits and wide potential of the target compounds, more multidisciplinary research is needed to develop them into functional food ingredients and to explore more of the marine ecosystem, which is still an untapped reservoir. Consequently, it is essential to make concerted efforts to enhance the accessibility and utilization of these bioactive compounds as functional food ingredients in a sustainable manner. This is crucial for the maintenance of health and the prevention of various diseases.
Author Contributions
Conceptualization, T.R.L.S., A.H. and F.S.; investigation, T.R.L.S. and A.H.; resources, F.S.; data curation, T.R.L.S. and A.H.; writing—original draft preparation, T.R.L.S. and A.H.; writing—review and editing, T.R.L.S., A.H. and F.S.; visualization, T.R.L.S. and A.H.; supervision, F.S.; funding acquisition, F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Sciences and Engineering Research Council (NSERC) of Canada, RGPIN-2016-04468.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shahidi, F.; Ambigaipalan, P. Novel functional food ingredients from marine sources. Curr. Opin. Food Sci. 2015, 2, 123–129. [Google Scholar] [CrossRef]
- Huang, I.; Pietrasiak, N.; Gobler, C.J.; Johansen, J.R.; Burkholder, J.M.; D’Antonio, S.; Zimba, P.V. Diversity of bioactive compound content across 71 genera of marine, freshwater, and terrestrial cyanobacteria. Harmful Algae 2021, 109, 102116. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Rezaei, M.; McClements, D.J. Bioactive functional ingredients from aquatic origin: A review of recent progress in marine-derived nutraceuticals. Crit. Rev. Food Sci. Nutr. 2020, 62, 1242–1269. [Google Scholar] [CrossRef] [PubMed]
- Suleria, H.A.R.; Osborne, S.A.; Masci, P.P.; Gobé, G.C. Marine-based nutraceuticals: An innovative trend in the food and supplement industries. Mar. Drugs 2015, 13, 6336–6351. [Google Scholar] [CrossRef]
- Jo, C.; Khan, F.F.; Khan, M.I.; Iqbal, J. Marine bioactive peptides: Types, structures, and physiological functions. Food Rev. Int. 2016, 33, 44–61. [Google Scholar] [CrossRef]
- Ali, A.; Wei, S.; Liu, Z.; Fan, X.; Sun, Q.; Xia, Q.; Liu, S.; Hao, J.; Deng, C. Non-thermal processing technologies for the recovery of bioactive compounds from marine by-products. LWT Food Sci. Technol. 2021, 147, 111549. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Northern sea cucumber (Cucumaria frondosa): A potential candidate for functional food, nutraceutical, and pharmaceutical sector. Mar. Drugs 2020, 18, 274. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sarkar, T.; Pati, S.; Kari, Z.A.; Edinur, H.A.; Chakraborty, R. Novel bioactive compounds from marine sources as a tool for functional food development. Front. Mar. Sci. 2022, 9, 832957. [Google Scholar] [CrossRef]
- Lobine, D.; Rengasamy, K.R.R.; Mahomoodally, M.F. Functional foods and bioactive ingredients harnessed from the ocean: Current status and future perspectives. Crit. Rev. Food Sci. Nutr. 2021, 62, 5794–5823. [Google Scholar] [CrossRef]
- Shahidi, F.; Santhiravel, S. Novel marine bioactives: Application in functional foods, nutraceuticals, and pharmaceuticals. J. Food Bioact. 2022, 19, 4–96. [Google Scholar] [CrossRef]
- Ameen, F.; AlNadhari, S.; Al-Homaidan, A.A. Marine microorganisms as an untapped source of bioactive compounds. Saudi J. Biol. Sci. 2021, 28, 224–231. [Google Scholar] [CrossRef]
- Satheesh, S.; Ba-akdah, M.A.; Al-Sofyani, A.A. Natural antifouling compound production by microbes associated with marine microorganisms—A review. Electron. J. Biotechnol. 2016, 21, 26–35. [Google Scholar] [CrossRef]
- Van Doan, H.; Prakash, P.S.; Hoseinifar, S.H.; Ringø, E.; El-Haroun, E.; Faggio, C.; Olsen, R.E.; Tran, H.Q.; Stejskal, V.; Abdel-Latif, H.M.R.; et al. Marine-derived products as functional feed additives in aquaculture: A review. Aquac. Rep. 2023, 31, 101679. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, S.G.A.; Alsaadi, B.H.; Althubyani, M.M.; Awari, Z.I.; Hussein, H.S.; Aljohani, A.A.; Albasri, J.F.; Faraj, S.A.; Mohamed, G.A. Secondary metabolites, biological activities, and industrial and biotechnological importance of Aspergillus sydowii. Mar. Drugs 2023, 21, 441. [Google Scholar] [CrossRef]
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Exopolysaccharides from marine and marine extremophilic bacteria: Structures, properties, ecological roles and applications. Mar. Drugs 2018, 16, 1–34. [Google Scholar] [CrossRef]
- Qi, M.; Zheng, C.; Wu, W.; Yu, G.; Wang, P. Exopolysaccharides from marine microbes: Source, structure and application. Mar. Drugs 2022, 20, 512. [Google Scholar] [CrossRef]
- Ju, H.; Yu, C.J.; Liu, W.; Li, H.-H.; Fu, Z.; Wu, Y.; Gong, P.-X.; Li, H. Polysaccharides from marine resources exhibit great potential in the treatment of tumor: A review. Carbohydr. Polym. Technol. Appl. 2023, 5, 100308. [Google Scholar] [CrossRef]
- Sardari, R.R.R.; Kulcinskaja, E.; Ron, E.Y.C.; Björnsdóttir, S.H.; Friðjónsson, Ó.H.; Hreggviðsson, G.Ó.; Karlsson, E.N. Evaluation of the production of exopolysaccharides by two strains of the thermophilic bacterium Rhodothermus marinus. Carbohydr. Polym. 2017, 156, 1–8. [Google Scholar] [CrossRef]
- Majee, S.B.; Avlani, D.; Biswas, G.R. Pharmacological, pharmaceutical, cosmetic and diagnostic applications of sulfated polysaccharides from marine algae and bacteria. Afr. J. Pharm. Pharmacol. 2017, 11, 68–77. [Google Scholar]
- Waghmode, S.; Suryavanshi, M.V.; Sharma, D.; Satpute, S.K. Planococcus species—An imminent resource to explore biosurfactant and bioactive metabolites for industrial applications. Front. Bioeng. Biotechnol. 2020, 8, 996. [Google Scholar] [CrossRef]
- Bramhachari, P.V.; Nagaraju, G.P. Extracellular polysaccharide production by bacteria as a mechanism of toxic heavy metal biosorption and biosequestration in the marine environment. In Marine Pollution and Microbial Remediation; Springer: Singapore, 2016; pp. 67–85. [Google Scholar]
- Nadzir, M.M.; Nurhayati, R.W.; Idris, F.N.; Nguyen, M.H. Biomedical applications of bacterial exopolysaccharides: A review. Polymers 2021, 13, 530. [Google Scholar] [CrossRef]
- Dewapriya, P.; Kim, S. Marine microorganisms: An emerging avenue in modern nutraceuticals and functional foods. Food Res. Int. 2014, 56, 115–125. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, Y.; Wang, H.; Zhang, H.; Zhao, J.; Liu, W. Lactic acid bacteria-derived exopolysaccharide: Formation, immunomodulatory ability, health effects, and structure-function relationship. Microbiol. Res. 2023, 274, 127432. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, S.V.; Manemann, E.M.; Rowe, S.E.; Callender, M.C.; Soto, W. Marine actinomycetes, new sources of biotechnological products. Mar. Drugs 2021, 19, 365. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; El-Mallah, M.F.; Yosri, N.; Alajlani, M.; Zhao, C.; Mehmood, M.A.; Du, M.; Ullah, H.; Daglia, M.; Guo, Z.; et al. Review of marine Cyanobacteria and the aspects related to their roles: Chemical, biological properties, nitrogen fixation and climate change. Mar. Drugs 2023, 21, 439. [Google Scholar] [CrossRef] [PubMed]
- Franco-Morgado, M.; Amador-Espejo, G.G.; Pérez-Cortes, M.; Gutiérrez-Uribe, J.A. Microalgae and cyanobacteria polysaccharides: Important link for nutrient recycling and revalorization of agro-industrial wastewater. Appl. Food Res. 2023, 3, 100296. [Google Scholar] [CrossRef]
- Singh, S.R.; Kant, C.; Yadav, R.K.; Reddy, Y.P.; Abraham, G. Cyanobacterial exopolysaccharides: Composition, biosynthesis, and biotechnological applications. In Cyanobacteria: From Basic Science to Applications; Academic Press: Cambridge, MA, USA, 2019; pp. 347–358. [Google Scholar]
- Akan, G.; Oner, E.T. Gel properties of microbial polysaccharides. In Polysaccharides of Microbial Origin; Oliveira, J., Radhouani, H., Reis, R.L., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Laroche, C. Exopolysaccharides from microalgae and cyanobacteria: Diversity of strains, production strategies, and applications. Mar. Drugs 2022, 20, 336. [Google Scholar] [CrossRef]
- Cunha, S.A.; Pintado, M. Bioactive peptides derived from marine sources: Biological and functional properties. Trends Food Sci. Technol. 2022, 119, 348–370. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hur, S.J. Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem. 2017, 228, 506–517. [Google Scholar] [CrossRef]
- Rao, T.E.; Imchen, M.; Kumavath, R. Marine Enzymes. Marine enzymes: Production and applications for human health. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2017; pp. 149–163. [Google Scholar]
- Parte, S.; Sirisha, V.L.; D’Souza, J.S. Biotechnological applications of marine enzymes from algae, bacteria, fungi, and sponges. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2017; pp. 75–106. [Google Scholar]
- Hayes, M.; Aluko, R.E.; Aurino, E.; Mora, L. Generation of bioactive peptides from Porphyridium sp. and assessment of their potential for use in the prevention of hypertension, inflammation and pain. Mar. Drugs 2023, 21, 422. [Google Scholar] [CrossRef]
- Birolli, W.G.; Lima, R.N.; Porto, A.L.M. Applications of marine-derived microorganisms and their enzymes in biocatalysis and biotransformation, the underexplored potentials. Front. Microbiol. 2019, 10, 1453. [Google Scholar] [CrossRef] [PubMed]
- Byreddy, A.R.; Rao, N.M.; Barrow, C.J.; Puri, M. Tween 80 influences the production of intracellular lipase by Schizochytrium S31 in a stirred tank reactor. Process Biochem. 2017, 53, 30–35. [Google Scholar] [CrossRef]
- Bibi, F.; Faheem, M.; Azhar, E.I.; Yasir, M.; Alvi, S.; Kamal, M.A.; Ullah, I.; Naseer, M.I. Bacteria from marine sponges: A source of new drugs. Curr. Drug Metab. 2017, 18, 11–15. [Google Scholar] [CrossRef]
- Sivaperumal, P.; Kamala, K.; Rajaram, R. Bioremediation of industrial waste through enzyme producing marine microorganisms. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2017; pp. 165–179. [Google Scholar]
- Park, S.H.; Lee, C.-R.; Hong, S.-K. Implications of agar and agarase in industrial applications of sustainable marine biomass. Appl. Microbiol. Biotechnol. 2020, 104, 2815–2832. [Google Scholar] [CrossRef] [PubMed]
- John, J.A.; Samuel, M.S.; Govarthan, M.; Ethiraj, S.A. A comprehensive review on strategic study of cellulase producing marine actinobacteria for biofuel applications. Environ. Res. 2022, 214, 114018. [Google Scholar] [CrossRef]
- Qeshmi, F.I.; Homaei, A.; Fernandes, P.; Hemmati, R.; Dijkstra, B.W.; Khajeh, K. Xylanases from marine microorganisms: A brief overview on scope, sources, features and potential applications. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140312. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Yang, W.; Mohamed, H.; Zhang, Y.; Song, Y. Microbes: A hidden treasure of polyunsaturated fatty acids. Front. Nutr. 2022, 9, 827837. [Google Scholar] [CrossRef]
- AlFadhly, N.K.Z.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F.; Narayanankutty, A. Trends and technological advancements in the possible food applications of Spirulina and their health benefits: A review. Molecules 2022, 27, 5584. [Google Scholar] [CrossRef]
- Remize, M.; Brunel, Y.; Silva, J.; Berthon, J.; Filaire, E. Microalgae n-3 PUFAs production and use in food and feed industries. Mar. Drugs 2021, 19, 113. [Google Scholar] [CrossRef]
- Bellou, S.; Triantaphyllidou, I.-E.; Aggeli, D.; Elazzazy, A.M.; Baeshen, M.N.; Aggelis, G. Microbial oils as food additives: Recent approaches for improving microbial oil production and its polyunsaturated fatty acid content. Curr. Opin. Biotechnol. 2016, 37, 24–35. [Google Scholar] [CrossRef]
- Burja, A.M.; Radianingtyas, H. Nutraceuticals and functional foods from marine microbes: An introduction to a diverse group of natural products isolated from marine macroalgae, microalgae, bacteria, fungi, and cyanobacteria. In Marine Nutraceuticals and Functional Foods; Barrow, C., Shahidi, F., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 367–403. [Google Scholar]
- Jovanović, S.; Dietrich, D.; Becker, J.; Kohlstedt, M.; Wittmann, C. Microbial production of polyunsaturated fatty acids—High-value ingredients for aquafeed, superfoods, and pharmaceuticals. Curr. Opin. Biotechnol. 2021, 69, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Rioux, L.; Beaulieu, L.; Turgeon, S.L. Seaweeds: A traditional ingredients for new gastronomic sensation. Food Hydrocoll. 2017, 68, 255–265. [Google Scholar] [CrossRef]
- Pole, R.P.P.; Khan, M.F.; Wesley, S.G. Occurrence of 210Po in marine macroalgae inhabiting a coastal nuclear zone, southeast coast of India. J. Environ. Radioact. 2017, 169, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.C.; Pereira, L.; Rodrigues, D.; Carvalho, A.P.; Panteleitchouk, T.S.L.; Gomes, A.; Duarte, A.C. Marine functional foods. In Handbook of Marine Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 969–994. [Google Scholar]
- Imchen, T.; Singh, K.S. Marine algae colorants: Antioxidant, anti-diabetic properties and applications in food industry. Algal Res. 2023, 69, 102898. [Google Scholar] [CrossRef]
- Ovando, C.A.; De Carvalho, J.C.; De Melo Pereira, G.V.; Jacques, P.; Soccol, V.T.; Soccol, C.R. Functional properties and health benefits of bioactive peptides derived from Spirulina: A review. Food Rev. Int. 2016, 34, 34–51. [Google Scholar] [CrossRef]
- Kratzer, R.; Murkovic, M. Food ingredients and nutraceuticals from microalgae: Main product classes and biotechnological production. Foods 2021, 10, 1626. [Google Scholar] [CrossRef]
- Senadheera, T.R.L.; Dave, D. Marine algae—Ocean based emerging biomass for functional food ingredients. J. Ocean Technol. 2021, 16, 1–10. [Google Scholar]
- Mensah, E.O.; Kanwugu, O.N.; Panda, P.K.; Adadi, P. Marine fucoidans: Structural, extraction, biological activities and their applications in the food industry. Food Hydrocoll. 2023, 142, 108784. [Google Scholar] [CrossRef]
- Harsha, M.E.; Madhusudan, S.; Baskaran, R. The sea lettuce Ulva sensu lato: Future food with health-promoting bioactives. Algal Res. 2023, 71, 103069. [Google Scholar]
- Salleh, S.N.; Fairus, A.A.H.; Zahary, M.N.; Raj, N.B.; Jalil, A.M.M. Unravelling the effects of soluble dietary fibre supplementation on energy intake and perceived satiety in healthy adults: Evidence from systematic review and meta-analysis of randomised-controlled trials. Foods 2019, 8, 1–22. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and potential properties of seaweeds and their recent applications: A review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Dave, D.; Shahidi, F. Antioxidant potential of sea cucumbers and their beneficial effects on human health. Mar. Drugs 2022, 20, 521. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.U.; O’Donnell, C.P.; Dilip, K.; Hossain, M.B.; Burgess, C.M.; Walsh, D.; Tiwari, B.K. Laminarin from Irish brown seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound assisted extraction, characterization and bioactivity. Mar. Drugs 2015, 13, 4270–4280. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.J.; Costa, R.R.; Mano, J.F. Marine origin polysaccharides in drug delivery systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef]
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Shahidi, F.; Rahman, M.J. Bioactives in seaweeds, algae, and fungi and their role in health promotion. J. Food Bioact. 2018, 2, 58–81. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; Burlot, A.-S.; Marty, C.; Critchley, A.T.; Hafting, J.T.; Bedoux, G.; Bourgougnon, N.; Prithiviraj, B. Enzyme assisted extraction of bioactive material from Chondrus crispus and Codium fragile and its effect on Herpes simplex virus (HSV-1). Mar. Drugs 2015, 13, 558–580. [Google Scholar] [CrossRef]
- Panggabean, J.A.; Adiguna, S.P.; Rahmawati, S.I.; Ahmadi, P.; Zainuddin, E.N.; Bayu, A.; Putra, M.Y. Antiviral activities of algal-based sulfated polysaccharides. Molecules 2022, 27, 1178. [Google Scholar] [CrossRef]
- Van Weelden, G.; Bobinski, M.; Okła, K.; Van Weelden, W.J.; Romano, A.; Pijnenborg, J.M.A. Fucoidan structure and activity in relation to anti-cancer mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef]
- Lin, Y.; Qi, X.; Liu, H.-J.; Xue, K.; Xu, S.; Tian, Z. The anti-cancer effects of fucoidan: A review of both in vivo and in vitro investigations. Cancer Cell Int. 2020, 20, 154. [Google Scholar] [CrossRef]
- Riccio, G.; Lauritano, C. Microalgae with immunomodulatory activities. Mar. Drugs 2019, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Thiviya, P.; Gamage, A.; Gama-Arachchige, N.S.; Merah, O.; Madhujith, T. Seaweeds as a source of functional proteins. Phycology 2022, 2, 216–243. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Quitério, E.; Soares, C.; Ferraz, R.; Delerue-Matos, C.; Grosso, C. Marine health-promoting compounds: Recent trends for their characterization and human applications. Foods 2021, 10, 3100. [Google Scholar] [CrossRef] [PubMed]
- Saide, A.; Martínez, K.A.; Ianora, A.; Lauritano, C. Unlocking the health potential of microalgae as sustainable sources of bioactive compounds. Int. J. Mol. Sci. 2021, 22, 4383. [Google Scholar] [CrossRef] [PubMed]
- Dagnino-Leone, J.; Figueroa, C.P.; Castañeda, M.L.; Youlton, A.D.; Vallejos-Almirall, A.; Agurto-Muñoz, A.; Pérez, J.P.; Agurto-Muñoz, C. Phycobiliproteins: Structural aspects, functional characteristics, and biotechnological perspectives. Comput. Struct. Biotechnol. J. 2022, 20, 1506–1527. [Google Scholar] [CrossRef]
- De Arruda, M.C.S.; Da Silva, M.R.O.B.; Cavalcanti, V.L.R.; Brandao, R.M.P.C.; De Araújo Viana Marques, D.; De Lima, L.R.A.; Porto, A.L.F.; Bezerra, R.P. Antitumor lectins from algae: A systematic review. Algal Res. 2023, 70, 102962. [Google Scholar] [CrossRef]
- Maliki, I.M.; Misson, M.; Teoh, P.L.; Rodrigues, K.F.; Yong, W.T.L. Production of lectins from marine algae: Current status, challenges, and opportunities for non-destructive extraction. Mar. Drugs 2022, 20, 102. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal proteins: Extraction, application, and challenges concerning production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Harwood, J.L. Algae: Critical sources of very long-chain polyunsaturated fatty acids. Biomolecules 2019, 9, 708. [Google Scholar] [CrossRef]
- Pradhan, B.; Nayak, R.; Patra, S.; Jit, B.P.; Ragusa, A.; Jena, M. Bioactive metabolites from marine algae as potent pharmacophores against oxidative stress-associated human diseases: A comprehensive review. Molecules 2020, 26, 37. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Guihéneuf, F.; Stengel, D.B. Evaluation of food grade solvents for lipid extraction and impact of storage temperature on fatty acid composition of edible seaweeds Laminaria digitata (Phaeophyceae) and Palmaria palmata (Rhodophyta). Food Chem. 2016, 208, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Li, Y.; Wang, C.; Guo, J.; Zhou, C.; Zhang, R.; Ma, Y.; Ma, X.; Wang, L.; Cheng, Y.; et al. Integrated marine microalgae biorefineries for improved bioactive compounds: A review. Sci. Total Environ. 2022, 817, 152895. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, K.; Duan, X.; Hill, D.R.; Barrow, C.J.; Dunshea, F.R.; Martin, G.M.; Suleria, H.A.R. Bioactive compounds in microalgae and their potential health benefits. Food Biosci. 2022, 49, 101932. [Google Scholar] [CrossRef]
- Nezafatian, E.; Farhadian, O.; Yegdaneh, A.; Safavi, M.; Daneshvar, E.; Bhatnagar, A. Enhanced production of bioactive compounds from marine microalgae Tetraselmis tetrathele under salinity and light stresses: A two-stage cultivation strategy. Bioresour. Technol. 2023, 376, 128899. [Google Scholar] [CrossRef]
- Mekinić, I.G.; Šimat, V.; Rathod, N.B.; Hamed, I.; Čagalj, M. Algal carotenoids: Chemistry, sources, and application. Foods 2023, 12, 2768. [Google Scholar] [CrossRef]
- Hamed, I.; Özoğul, F.; Özoğul, Y.; Regenstein, J.M. Marine bioactive compounds and their health benefits: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Martins, T.; Barros, A.; Rosa, E.; Antunes, L. Enhancing health benefits through chlorophylls and chlorophyll-rich agro-food: A comprehensive review. Molecules 2023, 28, 5344. [Google Scholar] [CrossRef]
- Catarino, M.D.; Amarante, S.J.; Mateus, N.; Silva, A.M.S.; Cardoso, S.M. Brown algae phlorotannins: A marine alternative to break the oxidative stress, inflammation and cancer network. Foods 2021, 10, 1478. [Google Scholar] [CrossRef]
- Circuncisão, A.R.; Catarino, M.D.; Cardoso, S.M.; Silva, A.M.S. Minerals from macroalgae origin: Health benefits and risks for consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef]
- Das, D.; Arulkumar, A.; Paramasivam, S.; López-Santamarina, A.; Del Carmen Mondragón, A.; Miranda, J.M. Phytochemical constituents, antimicrobial properties and bioactivity of marine red seaweed (Kappaphycus alvarezii) and seagrass (Cymodocea serrulata). Foods 2023, 12, 2811. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Philippidis, G.P. The prospects of algae-derived vitamins and their precursors for sustainable cosmeceuticals. Processes 2023, 11, 587. [Google Scholar] [CrossRef]
- Datta, D.; Talapatra, S.N.; Swarnakar, S. Bioactive compounds from marine invertebrates for potential medicines—An overview. Int. Lett. Nat. Sci. 2015, 34, 42–61. [Google Scholar] [CrossRef]
- Hossain, A.; Yeo, J.; Dave, D.; Shahidi, F. Phenolic compounds and antioxidant capacity of sea cucumber (Cucumaria frondosa) processing discards as affected by high-pressure processing (HPP). Antioxidants 2022, 11, 337. [Google Scholar] [CrossRef]
- Romano, G.; Almeida, M.; Coelho, A.V.; Cutignano, A.; Gonçalves, L.G.; Hansen, E.; Khnykin, D.; Mass, T.; Ramšak, A.; Rocha, M.; et al. Bioactive natural products and biomaterials from marine invertebrates: From basic research to innovative applications. Mar. Drugs 2022, 20, 219. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, Y.-Y.; Shao, C.; Wang, C. Metabolites from marine invertebrates and their symbiotic microorganisms: Molecular diversity discovery, mining, and application. Mar. Life Sci. Technol. 2019, 1, 60–94. [Google Scholar] [CrossRef]
- Ko, S.; Jang, J.Y.; Ye, B.-R.; Kim, M.-S.; Choi, I.; Park, W.; Heo, S.; Jung, W. Purification and molecular docking study of angiotensin I-converting enzyme (ACE) inhibitory peptides from hydrolysates of marine sponge Stylotella aurantium. Process Biochem. 2017, 54, 180–187. [Google Scholar] [CrossRef]
- Pailee, P.; Mahidol, C.; Ruchirawat, S.; Prachyawarakorn, V. Sterols from Thai marine sponge Petrosia (Strongylophora) sp. and their cytotoxicity. Mar. Drugs 2017, 15, 54. [Google Scholar] [CrossRef]
- Krishnan, K.; Keerthi, T.R. Comparative evaluation of bioactivities of two marine sponges, Zygomycale parishii and Callyspongia diffusa from Southwest coast of India. Br. J. Pharm. Res. 2016, 11, 1–10. [Google Scholar] [CrossRef]
- Varijakzhan, D.; Loh, J.-Y.; Yap, W.-S.; Yusoff, K.; Seboussi, R.; Lim, S.H.E.; Lai, K.-S.; Chong, C.-M. Bioactive compounds from marine sponges: Fundamentals and applications. Mar. Drugs 2021, 19, 246. [Google Scholar] [CrossRef]
- Xu, J.; Wang, M.; Liu, Z.; Zhang, W.; Ma, J.; Li, G.; Li, P. Terpenoids from the sponge Sarcotragus sp. collected in the South China Sea. J. Nat. Prod. 2023, 86, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Nicácio, K.J.; Ióca, L.P.; Fróes, A.M.; Leomil, L.; Appolinario, L.; Thompson, C.C.; Thompson, F.L.; Ferreira, A.G.; Williams, D.E.; Andersen, R.J.; et al. Cultures of the marine bacterium Pseudovibrio denitrificans Ab134 produce bromotyrosine-derived alkaloids previously only isolated from marine sponges. J. Nat. Prod. 2017, 80, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, R.; Perera, C.O. Partial purification and characterization of bioactive peptides from cooked New Zealand green-lipped mussel (Perna canaliculus) protein hydrolyzates. Foods 2020, 9, 879. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.S.; Hayes, M. Bioprocessing of mussel by-products for value added ingredients. Trends Food Sci. Technol. 2019, 92, 111–121. [Google Scholar] [CrossRef]
- Stonik, V.A.; Kicha, A.A.; Malyarenko, T.V.; Ivanchina, N.V. Asterosaponins: Structures, taxonomic distribution, biogenesis and biological activities. Mar. Drugs 2020, 18, 584. [Google Scholar] [CrossRef]
- Hossain, A.; Senadheera, T.R.L.; Dave, D.; Shahidi, F. Phenolic profiles of Atlantic sea cucumber (Cucumaria frondosa) tentacles and their biological properties. Food Res. Int. 2023, 163, 112262. [Google Scholar] [CrossRef]
- Senadheera, T.R.L.; Hossain, A.; Dave, D.; Shahidi, F. Antioxidant and ACE-inhibitory activity of protein hydrolysates produced from Atlantic sea cucumber. Molecules 2023, 28, 5263. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Sulfated polysaccharides in sea cucumbers and their biological properties: A review. Int. J. Biol. Macromol. 2023, 253, 127329. [Google Scholar] [CrossRef]
- Sridhar, S.C.J.; Deo, S.C.R. Marine and other aquatic dermatoses. Indian J. Dermatol. 2017, 62, 66–78. [Google Scholar] [CrossRef]
- Afzali, M.; Baharara, J.; Shahrokhabadi, K.; Amini, E. Evaluation of the Cytotoxic Effect of the Brittle Star (Ophiocoma Erinaceus) Dichloromethane Extract and Doxorubicin on EL4 Cell Line. Iran J. Pharm. Res. 2017, 16, 216–226. [Google Scholar]
- Zhukova, N.V. Fatty acids of echinoderms: Diversity, current applications and future opportunities. Mar. Drugs 2022, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Senadheera, T.R.L.; Hossain, A.; Dave, D.; Shahidi, F. In silico analysis of bioactive peptides produced from underutilized sea cucumber by-products—A bioinformatics approach. Mar. Drugs 2022, 20, 610. [Google Scholar] [CrossRef] [PubMed]
- Senadheera, T.R.L.; Hossain, A.; Dave, D.; Shahidi, F. Functional and physiochemical properties of protein isolates from different body parts of North Atlantic sea cucumber (Cucumaria frondosa). Food Biosci. 2023, 52, 102511. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Effect of high-pressure processing (HPP) on phenolics of North Atlantic sea cucumber (Cucumaria frondosa). J. Agric. Food Chem. 2022, 70, 3489–3501. [Google Scholar] [CrossRef]
- Marmouzi, I.; Tamsouri, N.; Hamdani, M.E.; Attar, A.E.; Kharbach, M.; Alami, R.; Jemli, M.E.; Cherrah, Y.; Ebada, S.S.; Faouzi, M.E.A. Pharmacological and chemical properties of some marine echinoderms. Rev. Bras. Farmacogn. 2018, 28, 575–581. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Shahidi, F. Bioactive peptides from shrimp shell processing discards: Antioxidant and biological activities. J. Funct. Foods 2017, 34, 7–17. [Google Scholar] [CrossRef]
- Šimat, V.; Rathod, N.B.; Čagalj, M.; Hamed, I.; Mekinić, I.G. Astaxanthin from crustaceans and their byproducts: A bioactive metabolite candidate for therapeutic application. Mar. Drugs 2022, 20, 206. [Google Scholar] [CrossRef]
- Hamed, I.; Özoğul, F.; Regenstein, J.M. Industrial applications of crustacean byproducts (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Amado, I.R.; González, M.P.; Murado, M.A.; Vázquez, J.A. Shrimp wastewater as a source of astaxanthin and bioactive peptides. J. Chem. Technol. Biotechnol. 2016, 91, 793–805. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S. Bioactive peptide of marine origin for the prevention and treatment of non-communicable diseases. Mar. Drugs 2017, 15, 67. [Google Scholar] [CrossRef]
- Da Silva, C.P.; Bezerra, R.S.; Santos, A.C.O.D.; Messias, J.B.; De Castro, C.R.O.B.; Carvalho, L.B. Biological value of shrimp protein hydrolysate by-product produced by autolysis. LWT Food Sci. Technol. 2017, 80, 456–461. [Google Scholar] [CrossRef]
- De Los Ángeles Pereira, N.; Fangio, M.F.; Rodríguez, Y.E.; Bonadero, M.C.; Harán, N.S.; Fernández-Gimenez, A.V. Characterization of liquid protein hydrolysates shrimp industry waste: Analysis of antioxidant and microbiological activity, and shelf life of final product. J. Food Process. Preserv. 2021, 46, e15526. [Google Scholar]
- Balzano, M.; Pacetti, D.; Lucci, P.; Fiorini, D.; Frega, N.G. Bioactive fatty acids in mantis shrimp, crab and caramote prawn: Their content and distribution among the main lipid classes. J. Food Compos. Anal. 2017, 59, 88–94. [Google Scholar] [CrossRef]
- Mezzomo, N.; Ferreira, S.R.S. Carotenoids functionality, sources, and processing by supercritical technology: A review. J. Chem. 2016, 2016, 3164312. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids in marine animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef]
- Pattanaik, S.S.; Sawant, P.B.; Xavier, K.A.M.; Dube, K.; Srivastava, P.P.; Dhanabalan, V.; Chadha, N.K. Characterization of carotenoprotein from different shrimp shell waste for possible use as supplementary nutritive feed ingredient in animal diets. Aquaculture 2020, 515, 734594. [Google Scholar] [CrossRef]
- Fernández-Gimenez, A.V.; Pereira, N.D.L.Á.; Sarasa, M.V. Liposoluble vitamins in crustacean feed: Metabolic and Histological Responses. Indian J. Exp. Biol. 2016, 54, 297–308. [Google Scholar]
- Mao, X.; Guo, N.; Sun, J.; Xue, C. Comprehensive utilization of shrimp waste based on biotechnological methods: A review. J. Clean. Prod. 2017, 143, 814–823. [Google Scholar] [CrossRef]
- Amiri, H.; Shábanpour, B.; Pourashouri, P.; Kashiri, M. Encapsulation of marine bioactive compounds using liposome technique: Evaluation of physicochemical properties and oxidative stability during storage. Food Struct. 2023, 35, 100308. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Preservation of aquatic food using edible films and coatings containing essential oils: A review. Crit. Rev. Food Sci. Nutr. 2020, 62, 66–105. [Google Scholar] [CrossRef]
- FAO. 2022. Available online: https://www.fao.org/newsroom/detail/record-fisheries-aquaculture-production-contributes-food-security-290622/en (accessed on 1 October 2023).
- Colletti, A.; Cravotto, G.; Citi, V.; Martelli, A.; Testai, L.; Cicero, A.F.G. Advances in technologies for highly active omega-3 fatty acids from krill oil: Clinical applications. Mar. Drugs 2021, 19, 306. [Google Scholar] [CrossRef]
- Xie, D.; Jin, J.; Sun, J.; Liang, L.; Wang, X.; Zhang, W.; Wang, X.; Jin, Q. Comparison of solvents for extraction of krill oil from krill meal: Lipid yield, phospholipids content, fatty acids composition and minor components. Food Chem. 2017, 233, 434–441. [Google Scholar] [CrossRef]
- Hill, S.E.; Krishnamurthy, R.G.; Hossain, A.; Shahidi, F. Cooking Oils, Salad Oils, and Dressings. In Bailey’s Industrial Oil and Fat Products, 7th ed.; Shahidi, F., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 1–33. [Google Scholar]
- Zhang, H.; Zhao, H.; Zhang, Y.; Shen, Y.; Su, H.; Jin, J.; Jin, Q.; Wang, X. Characterization of positional distribution of fatty acids and triacylglycerol molecular compositions of marine fish oils rich in omega-3 polyunsaturated fatty acids. Biomed Res. Int. 2018, 2018, 3529682. [Google Scholar] [CrossRef]
- Kaur, G.; Guo, X.; Sinclair, A.J. Short update on docosapentaenoic acid: A bioactive long-chain n-3 fatty acid. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Sala-Vila, A.; Guasch-Ferré, M.; Hu, F.B.; Sánchez-Taínta, A.; Bulló, M.; Serra-Mir, M.; López-Sabater, C.; Sorlí, J.V.; Arós, F.; Fiol, M.; et al. Dietary α-Linolenic Acid, Marine ω-3 Fatty Acids, and Mortality in a Population with High Fish Consumption: Findings from the PREvención con DIeta MEDiterránea (PREDIMED) Study. J. Am. Heart Assoc. 2016, 5, e002543. [Google Scholar] [CrossRef]
- Halim, N.R.A.; Yusof, H.M.; Sarbon, N.M. Functional and bioactive properties of fish protein hydolysates and peptides: A comprehensive review. Trends Food Sci. Technol. 2016, 51, 24–33. [Google Scholar] [CrossRef]
- Villamil, O.; Váquiro, H.A.; Solanilla, J.F. Fish viscera protein hydrolysates: Production, potential applications and functional and bioactive properties. Food Chem. 2017, 224, 160–171. [Google Scholar] [CrossRef]
- Martínez-Álvarez, Ó.; Chamorro, S.; Brenes, A. Protein hydrolysates from animal processing by-products as a source of bioactive molecules with interest in animal feeding: A review. Food Res. Int. 2015, 73, 204–212. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, Z.; Dai, Z.; Wang, G.; Wu, G. Protein hydrolysates in animal nutrition: Industrial production, bioactive peptides, and functional significance. J. Anim. Sci. Biotechnol. 2017, 8, 209–232. [Google Scholar] [CrossRef] [PubMed]
- Yemışken, E.; Jiménez-Rosado, M.; Pérez-Puyana, V.; Sancar, S.; Bektaş, S.; Yıldız, T.; Eryılmaz, L.; Romero, A. Alternative sources of marine bioactive compounds from the Black Sea: Isolation and characterization of fish skin collagen from Neogobius melanostomus (Pallas 1814) (Perciformes: Gobiidae). Reg. Stud. Mar. Sci. 2023, 60, 102887. [Google Scholar]
- Yu, D.; Cui, S.; Chen, L.; Zheng, S.; Zhao, D.; Yin, X.; Yang, F.; Chen, J. Marine-derived bioactive peptides self-assembled multifunctional materials: Antioxidant and wound healing. Antioxidants 2023, 12, 1190. [Google Scholar] [CrossRef] [PubMed]
- Petrova, I.; Tolstorebrov, I.; Eikevik, T.M. Production of fish protein hydrolysates step by step: Technological aspects, equipment used, major energy costs and methods of their minimizing. Int. Aquat. Res. 2018, 10, 223–241. [Google Scholar] [CrossRef]
- Fernandes, P. Enzymes in fish and seafood processing. Front. Bioeng. Biotechnol. 2016, 4, 59. [Google Scholar] [CrossRef] [PubMed]
- Abdelhedi, O.; Salem, A.; Nasri, R.; Nasri, M.; Jridi, M. Food applications of bioactive marine gelatin films. Curr. Opin. Food Sci. 2022, 43, 206–215. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2016, 29, 949–982. [Google Scholar] [CrossRef]
- Dinakarkumar, Y.; Krishnamoorthy, S.; Margavelu, G.; Ramakrishnan, G.; Masi, C. Production and characterization of fish protein hydrolysate: Effective utilization of trawl by-catch. Food Chem. Adv. 2022, 1, 100138. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Role of lipids in food flavor generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M. Enhancing and improving the extraction of omega-3 from fish oil. Sustain. Chem. Pharm. 2017, 5, 54–59. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Bioactives in spices, and spice oleoresins: Phytochemicals and their beneficial effects in food preservation and health promotion. J. Food Bioact. 2018, 3, 8–75. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Y.; Shen, Y.; Zhu, Y.; Wang, H.; Xu, Z. Inhibitory effects of red wine on lipid oxidation in fish oil emulsion and angiogenesis in zebrafish embryo. J. Food Sci. 2017, 82, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzade, T.; Jafari, S.M.; Akhavan, S.; Hadavi, R. Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chem. 2017, 216, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Ramezanzade, L.; McClements, D.J. Recent advances in nanoencapsulation of hydrophobic marine bioactives: Bioavailability, safety, and sensory attributes of nano-fortified functional foods. Trends Food Sci. Technol. 2021, 109, 322–339. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).