Comparative Analysis of Pulsed Electric Fields (PEF) and Traditional Pasteurization Techniques: Comparative Effects on Nutritional Attributes and Bacterial Viability in Milk and Whey Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Milk Preparation
2.2. Whey Preparation
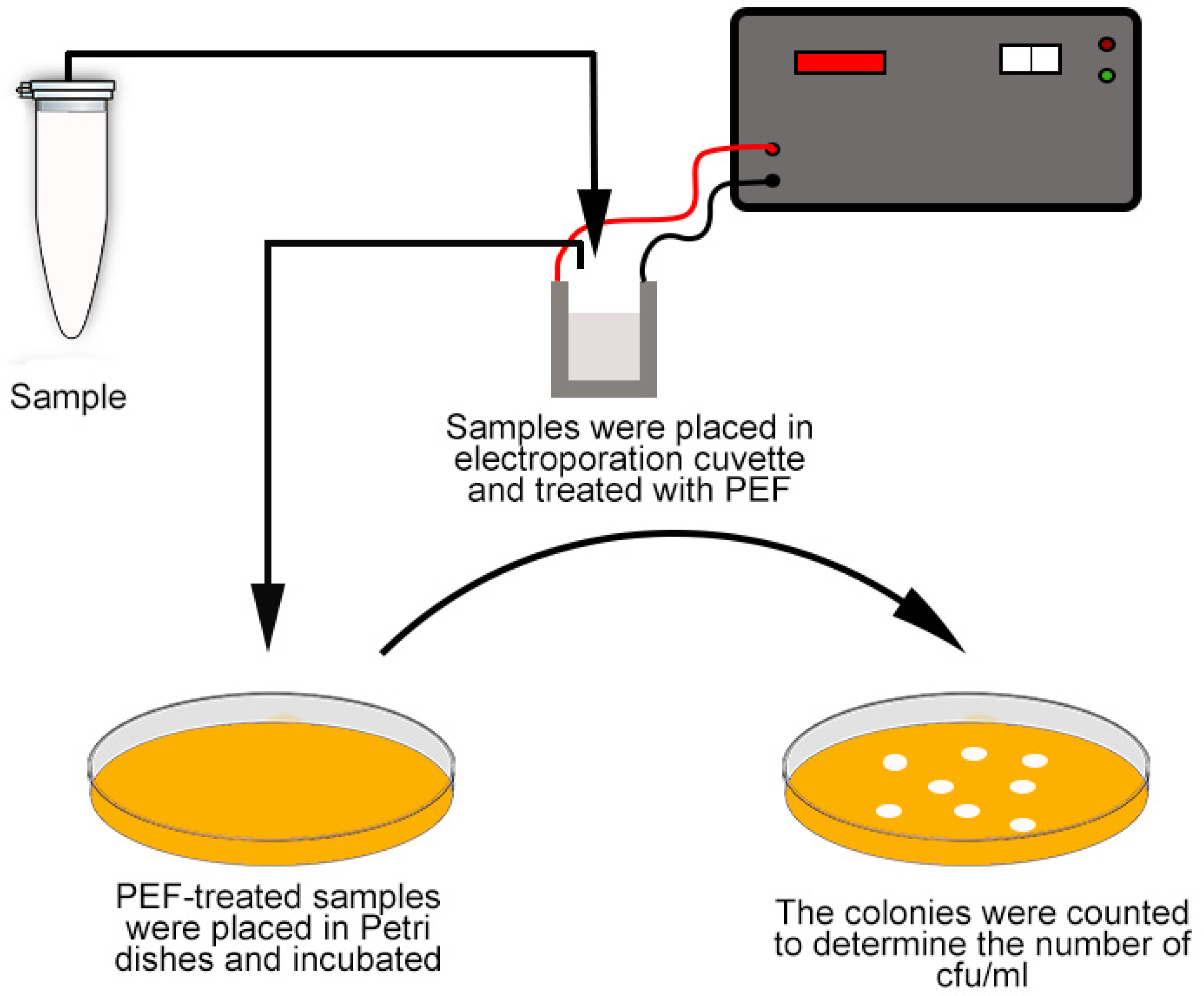
2.3. Determination of Bacterial Load in Samples
2.4. PEF Treatment
2.5. Determination of Vitamins A, D, E
2.6. Determination of Amino Acid Content
2.7. Determination of Fatty Acids Content
2.8. Determination of Biogenic Amine Content
2.9. Determination of Antioxidant Activity
2.10. Determination of Cholesterol Content
2.11. Determination of β-Lactoglobulin Content
2.12. Temperature Measurements
2.13. Statistics
3. Results
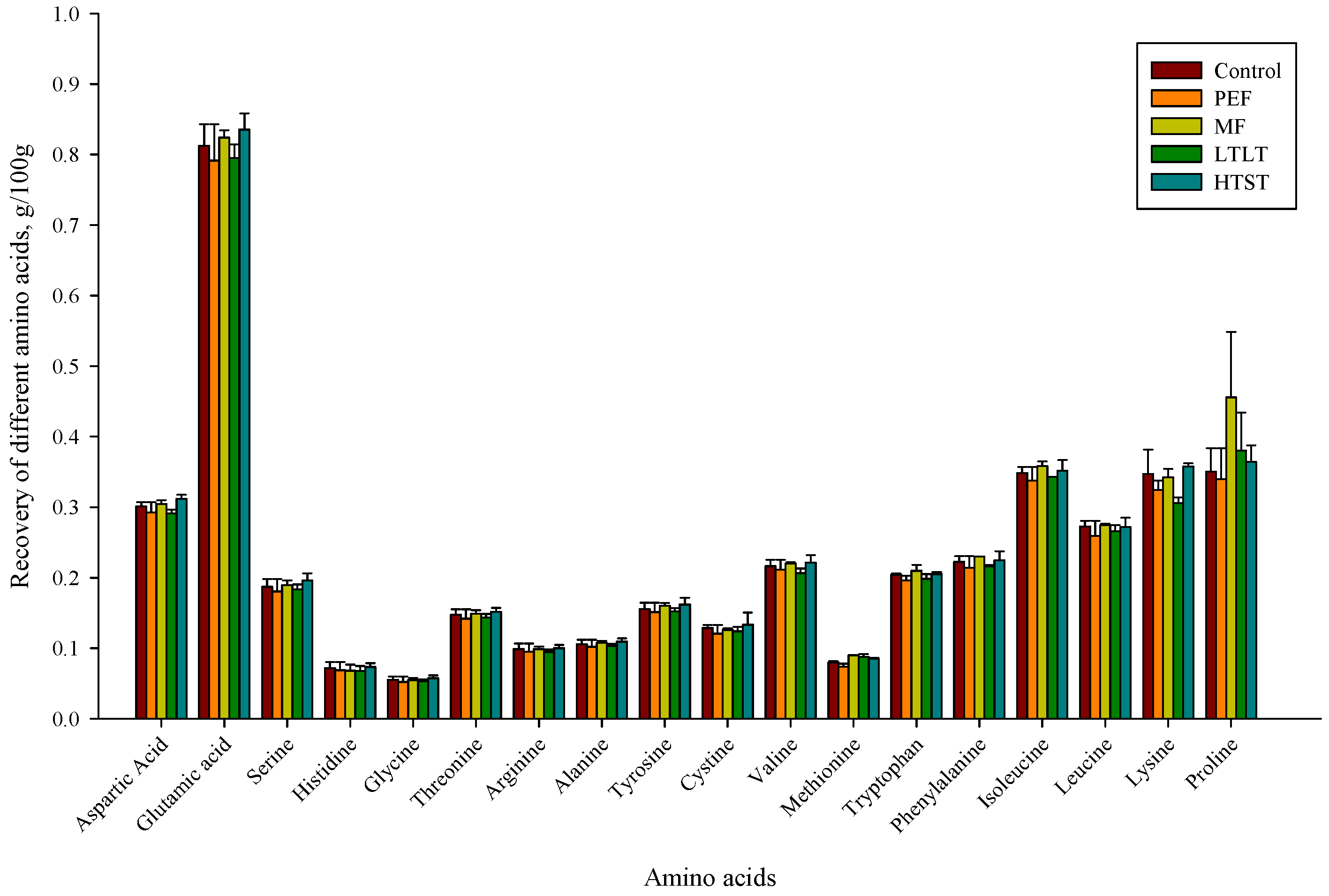
3.1. Amino Acid Composition Analysis after Pasteurization
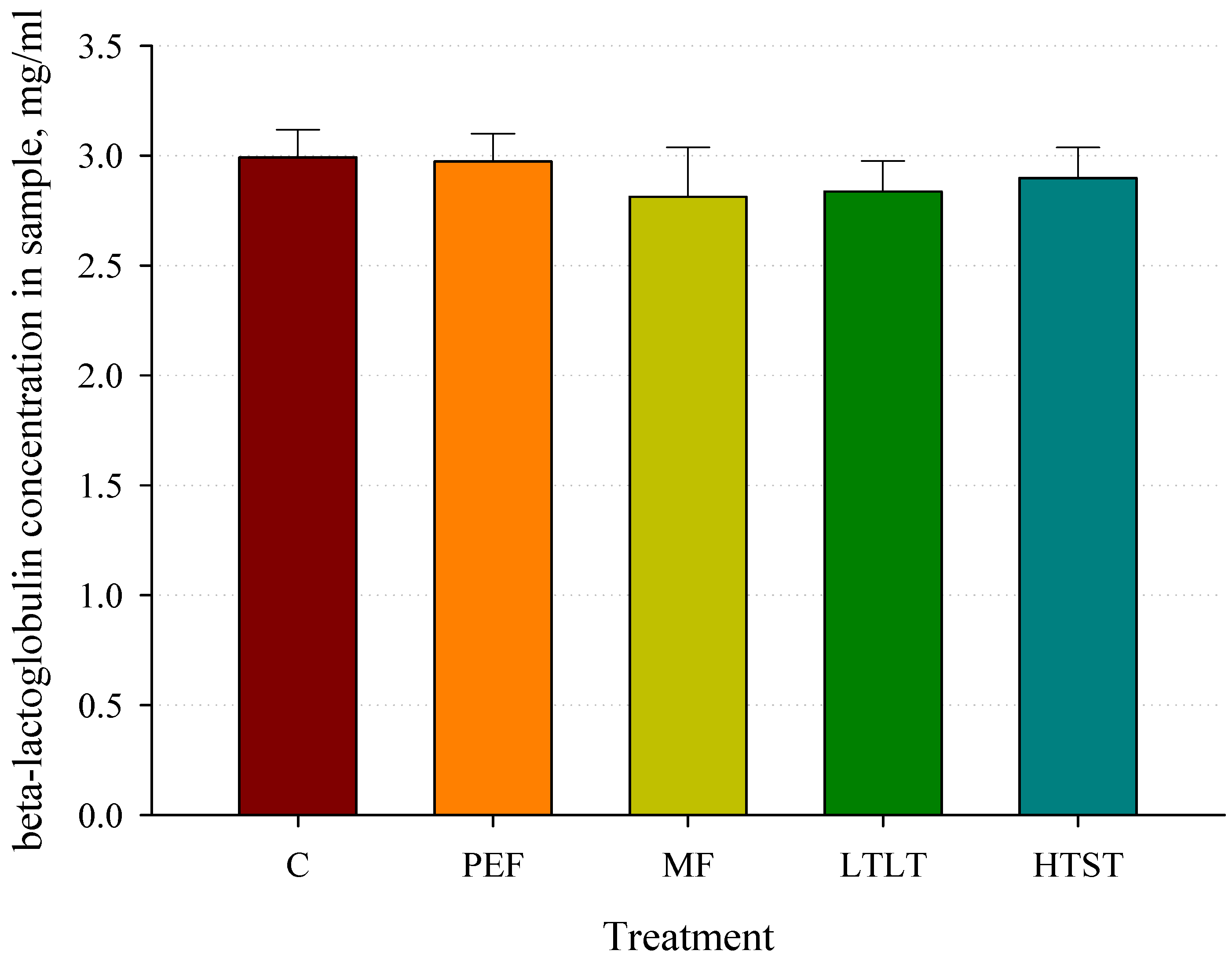
3.2. β-Lactoglobulin Concentration Analysis after Pasteurization
3.3. α-Casein, β-Casein, and κ-Casein Concentrations Analysis after Pasteurization
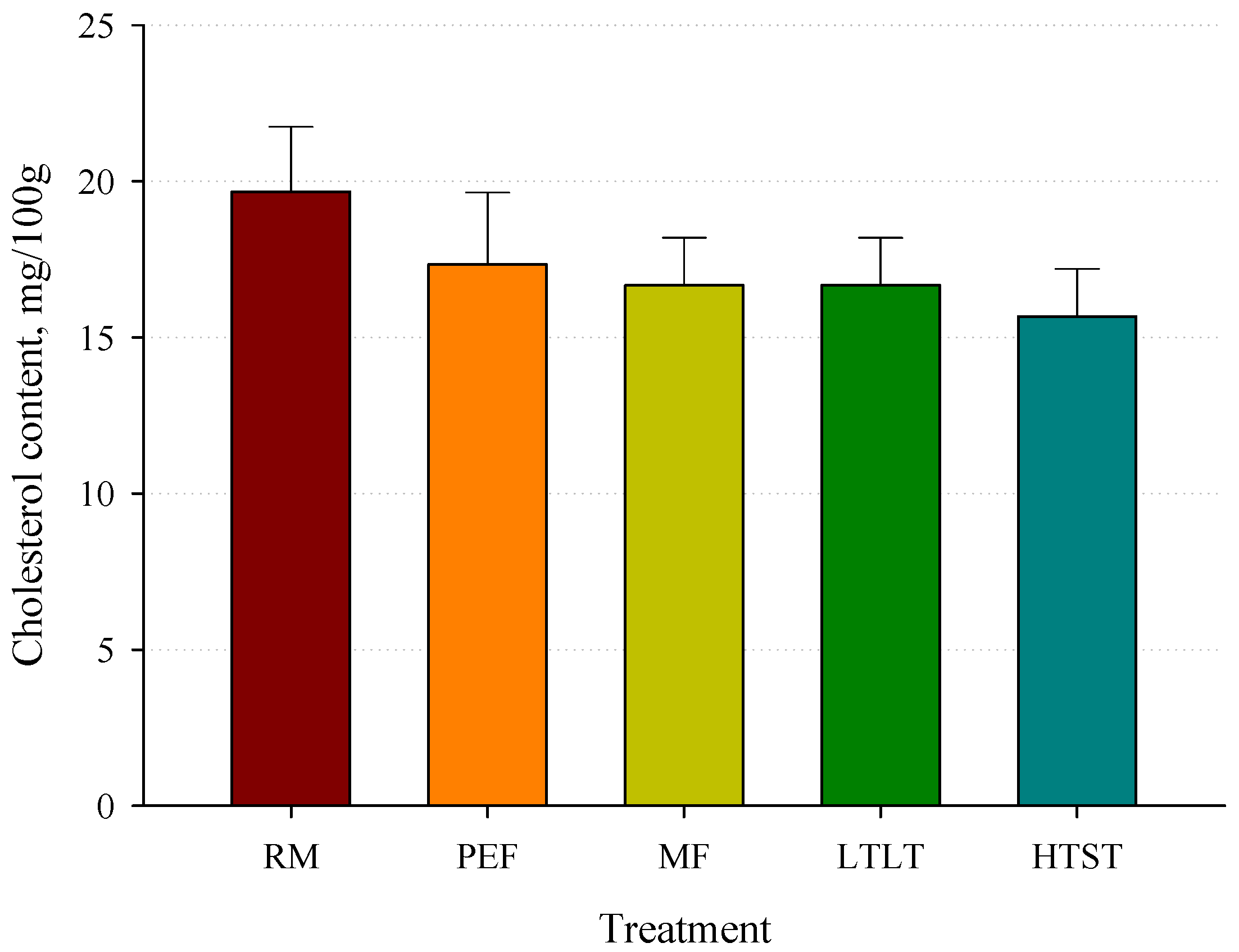
3.4. Cholesterol Concentrations Analysis after Pasteurization
3.5. DPPH Radical Scavenging Activity after Pasteurization
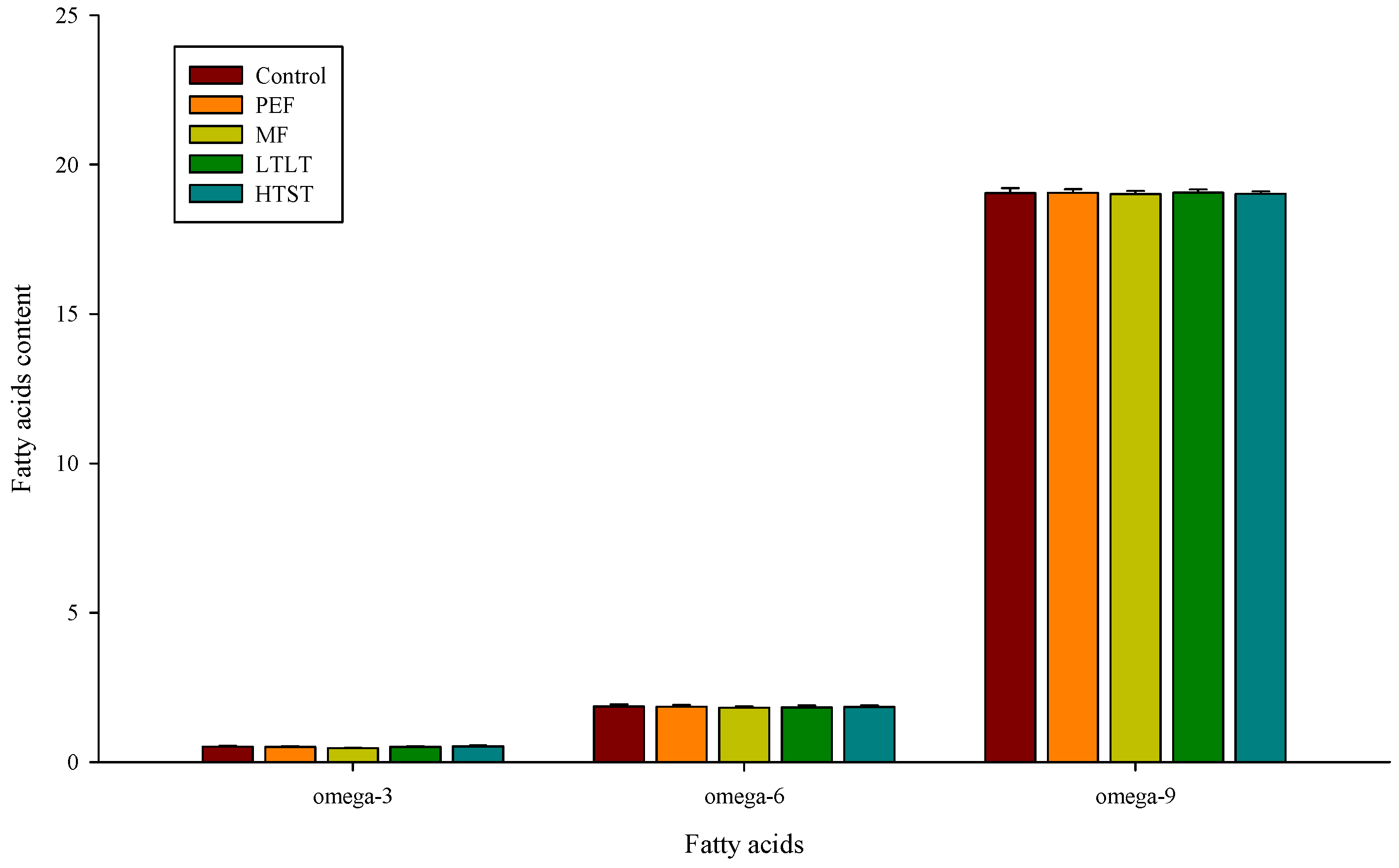
3.6. Fatty Acid Profile of Milk after Pasteurization
3.7. Glycomacropeptide (GMP) Concentrations after Pasteurization
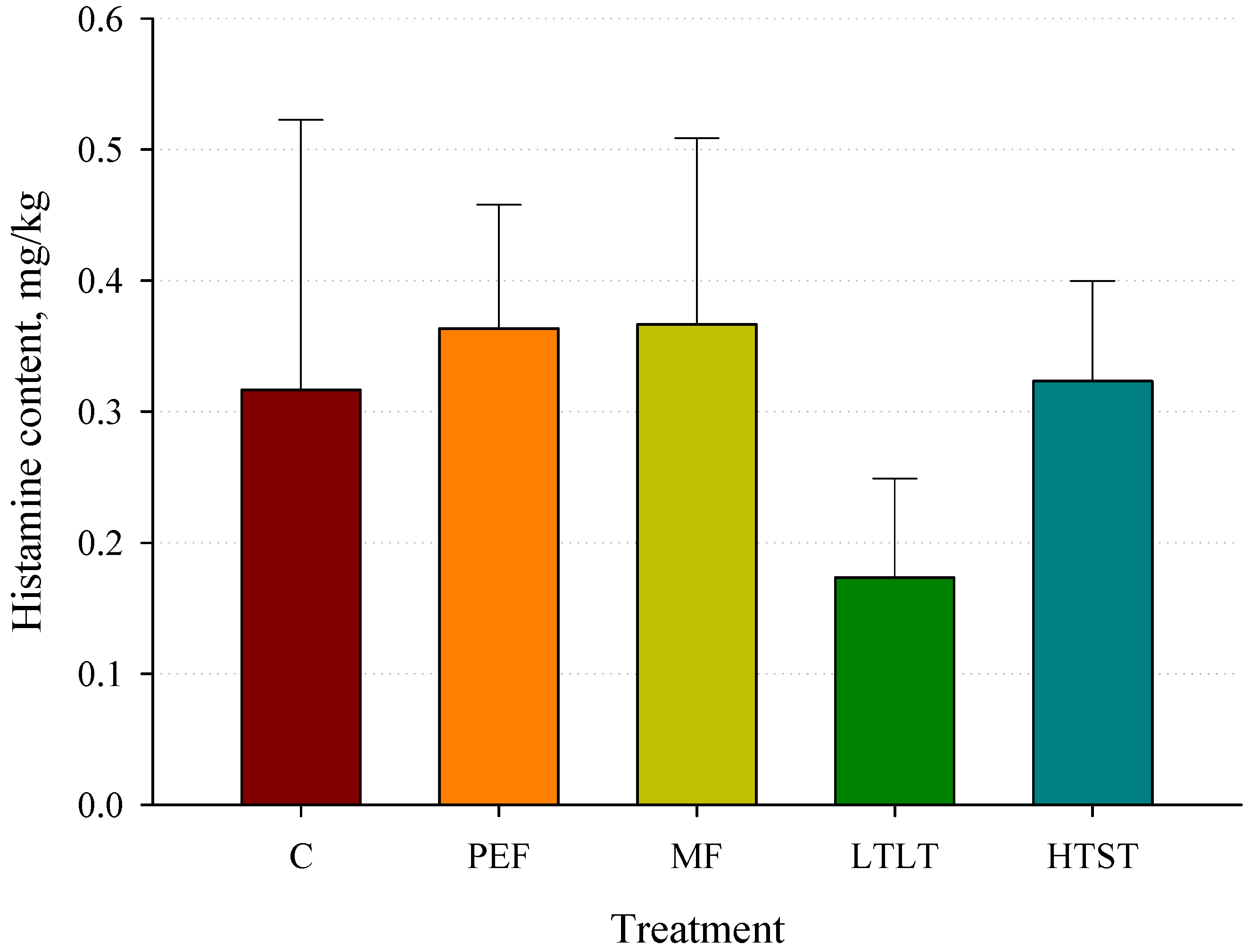
3.8. Histamine Concentrations after Pasteurization
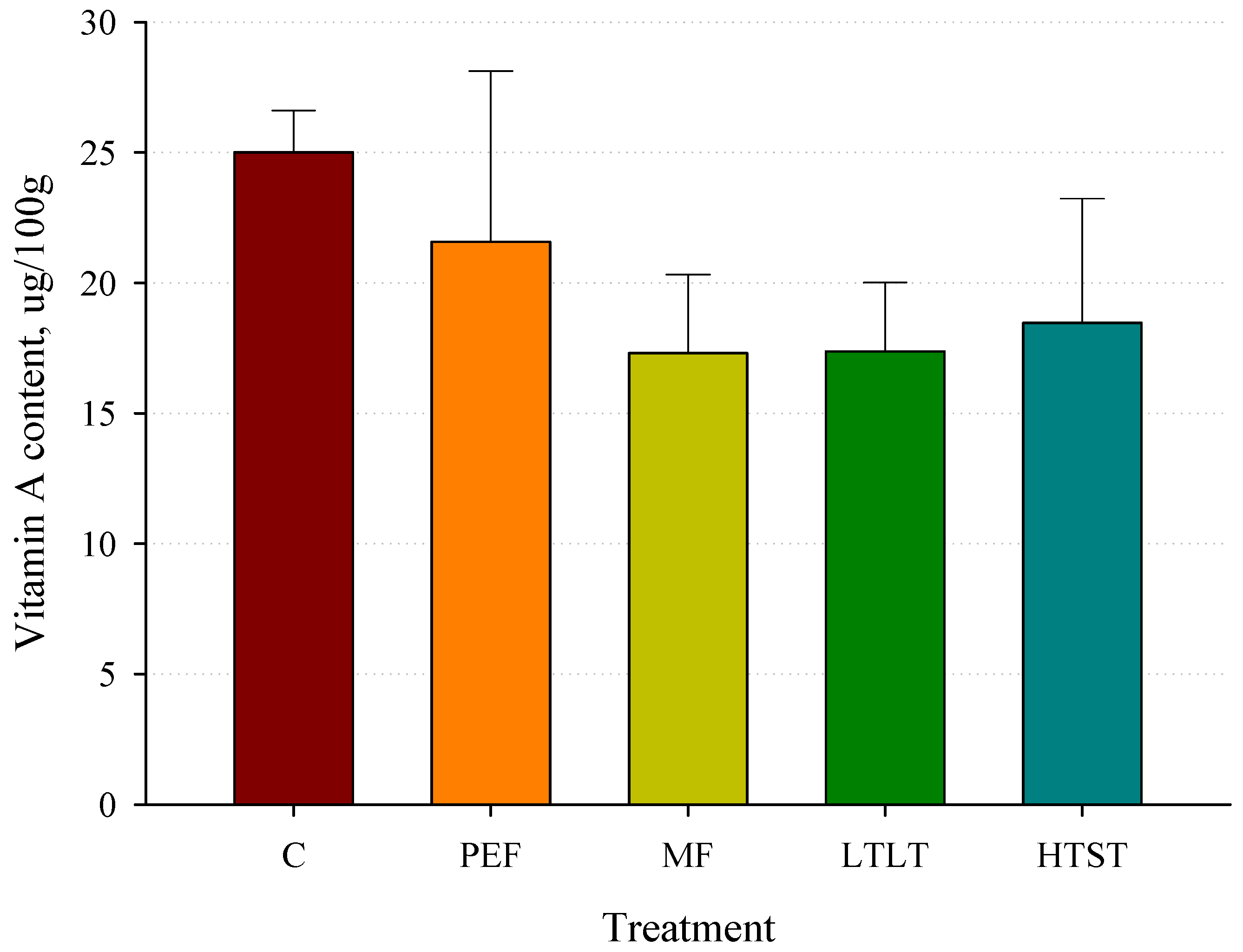
3.9. Vitamin A Concentrations after Pasteurization
3.10. Vitamin D Concentrations after Pasteurization
3.11. Vitamin E Concentrations after Pasteurization
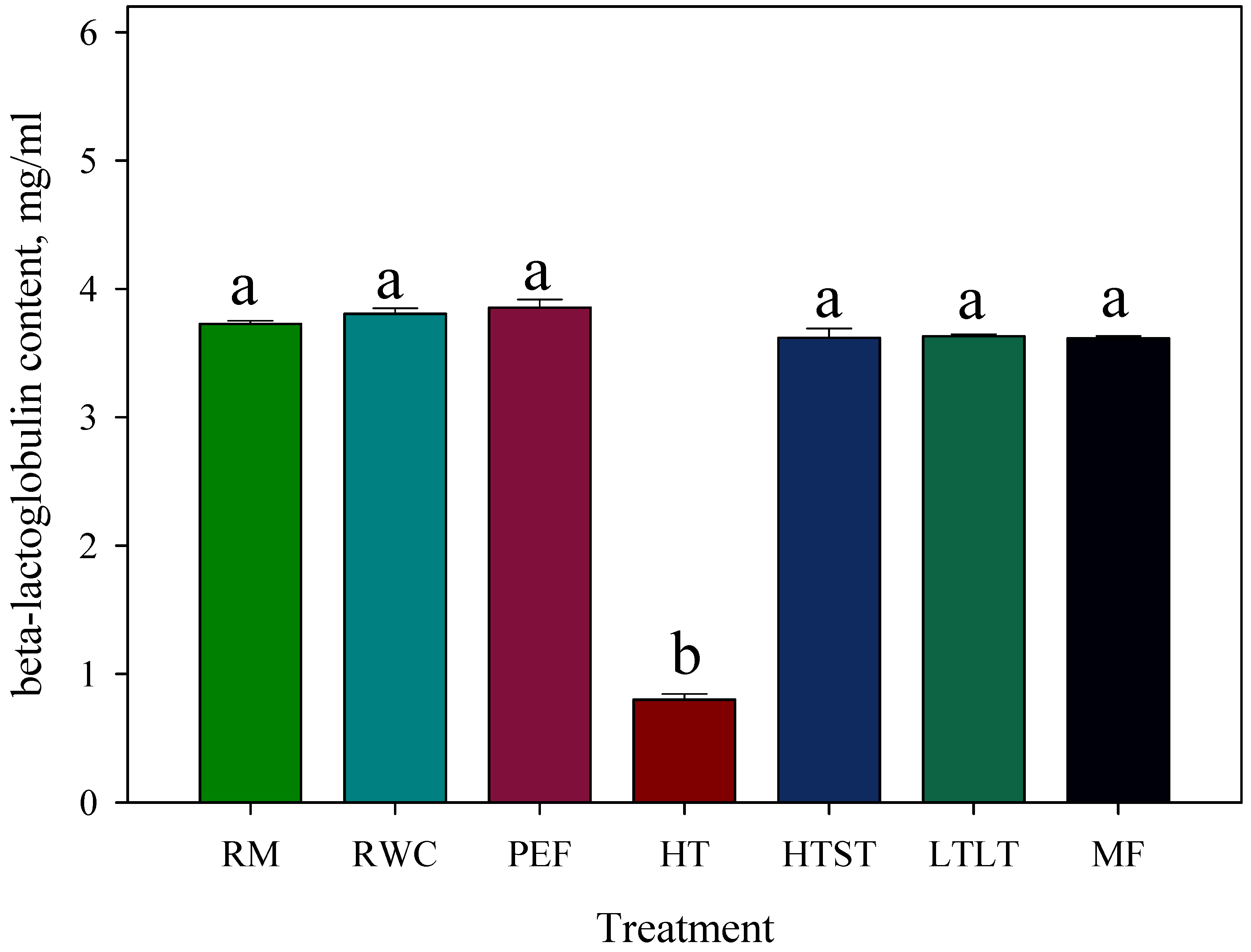
3.12. β-Lactoglobulin Content in Liquid Whey after Curd Removal and Pasteurization
3.13. Bacterial Load in Milk Samples: A Comparison between Raw, Curd-Reduced, and Pasteurized Samples
4. Discussion
4.1. Nutritional Properties of Milk Subjected to Thermal Pasteurization Techniques and PEFs
4.2. Differences in Bacterial Inactivation
4.3. PEF in Production of Liquid Whey Foods
4.4. Potential Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trung, N.D.; Hoang, N.N.; Hieu, D.M. Pulsed Electric Field for Pasteurization of Fresh Sugarcane Juice. ASEAN Eng. J. 2018, 8, 27–35. [Google Scholar] [CrossRef]
- Aguilar-Rosas, S.; Ballinas-Casarrubias, M.; Nevarez-Moorillon, G.; Martin-Belloso, O.; Ortega-Rivas, E. Thermal and pulsed electric fields pasteurization of apple juice: Effects on physicochemical properties and flavour compounds. J. Food Eng. 2007, 83, 41–46. [Google Scholar] [CrossRef]
- Saldaña, G.; Puértolas, E.; Monfort, S.; Raso, J.; Álvarez, I. Defining treatment conditions for pulsed electric field pasteurization of apple juice. Int. J. Food Microbiol. 2011, 151, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Alami Milani, E.; Alkhafaji, S.; Silva, F. Pulsed Electric Field continuous pasteurization of different types of beers. Food Control 2015, 50, 223–229. [Google Scholar] [CrossRef]
- Charles-Rodríguez, A.; Nevárez-Moorillón, G.; Zhang, Q.; Ortega-Rivas, E. Comparison of Thermal Processing and Pulsed Electric Fields Treatment in Pasteurization of Apple Juice. Food Bioprod. Process. 2007, 85, 93–97. [Google Scholar] [CrossRef]
- Lee, S.J.; Choi, H.J.; Min, S.C. Pulsed electric field pasteurization of Mandarin and carrot juices. Korean J. Food Sci. Technol. 2017, 49, 408–414. [Google Scholar] [CrossRef]
- Hariono, B.; Brilliantina, A.; Sari, E.K.N.; Kurnianto, M.; Erawantini, F.; Supriyono; Kautsar, S. Pulsed electric field application on pasteurization of orange milk from low grade orange: Study on nutritional, physical, chemical properties, and total microorganism. IOP Conf. Ser. Earth Environ. Sci. 2022, 980, 012041. [Google Scholar] [CrossRef]
- Rios-Corripio, G.; la Peña, M.M.-D.; Welti-Chanes, J.; Guerrero-Beltrán, J. Pulsed electric field processing of a pomegranate (Punica granatum L.) fermented beverage. Innov. Food Sci. Emerg. Technol. 2022, 79, 103045. [Google Scholar] [CrossRef]
- Eshtiaghi, M.N.; Nakthong, N. Application of Pulsed Electric Field for Inactivation of Yeast S. cerevisiae in Apple Juice. J. Phys. Conf. Ser. 2021, 1893, 012008. [Google Scholar] [CrossRef]
- Preeti, B.; Ravindra, M.R.; Nath, B.S.; Panditrao, D.G. Impact of pulsed electric field treated milk on quality of paneer and khoa. Food Sci. Technol. Int. 2022, 29, 598–609. [Google Scholar] [CrossRef]
- Yiğit, A.; Bielska, P.; Cais-Sokolińska, D.; Samur, G. Whey proteins as a functional food: Health effects, functional properties, and applications in food. J. Am. Nutr. Assoc. 2023, 42, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, M.; Vasiljevic, T. Functional properties of whey proteins affected by heat treatment and hydrodynamic high-pressure shearing. J. Dairy Sci. 2009, 92, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Mezzenga, R.; Fischer, P. The self-assembly, aggregation and phase transitions of food protein systems in one, two and three dimensions. Rep. Prog. Phys. 2013, 76, 046601. [Google Scholar] [CrossRef] [PubMed]
- Wijayanti, H.B.; Bansal, N.; Deeth, H.C. Stability of Whey Proteins during Thermal Processing: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1235–1251. [Google Scholar] [CrossRef]
- Anema, S.G. The whey proteins in milk: Thermal denaturation, physical interactions and effects on the functional properties of milk. In Milk Proteins: From Expression to Food; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Bull, S.P.; Hong, Y.; Khutoryanskiy, V.V.; Parker, J.K.; Faka, M.; Methven, L. Whey protein mouth drying influenced by thermal denaturation. Food Qual. Prefer. 2016, 56, 233–240. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, Y.; Zhang, Y.; Pang, X.; Wang, Y.; Lv, J.; Zhang, S. Effects of different heat treatments on Maillard reaction products and volatile substances of camel milk. Front. Nutr. 2023, 10, 1072261. [Google Scholar] [CrossRef]
- Luis, R.D.; Arias, O.; Puertolas, E.; Benede, S.; Sanchez, L.; Calvo, M.; Perez, M.D. Effect of high—Intensity pulse electric fields on denaturation of bovine whey proteins. Milchwissenschaft 2009, 64, 422–426. [Google Scholar]
- Shamsi, K.; Sherkat, F. Application of pulsed electric field in non-thermal processing of milk. Asian J. Food Agro-Ind. 2011, 2, 216–244. [Google Scholar]
- Griffiths, M.W.; Walkling-Ribeiro, M. Pulsed Electric Field Processing of Liquid Foods and Beverages, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Toepfl, S.; Mathys, A.; Heinz, V.; Knorr, D. Review: Potential of High Hydrostatic Pressure and Pulsed Electric Fields for Energy Efficient and Environmentally Friendly Food Processing. Food Rev. Int. 2006, 22, 405–423. [Google Scholar] [CrossRef]
- Raso, J.; Frey, W.; Ferrari, G.; Pataro, G.; Knorr, D.; Teissie, J.; Miklavčič, D. Recommendations guidelines on the key information to be reported in studies of application of PEF technology in food and biotechnological processes. Innov. Food Sci. Emerg. Technol. 2016, 37, 312–321. [Google Scholar] [CrossRef]
- Marino, R.; Iammarino, M.; Santillo, A.; Muscarella, M.; Caroprese, M.; Albenzio, M. Technical note: Rapid method for determination of amino acids in milk. J. Dairy Sci. 2010, 93, 2367–2370. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Liang, M.; Chen, V.Y.; Chen, H.-L.; Chen, W. A simple and direct isolation of whey components from raw milk by gel filtration chromatography and structural characterization by Fourier transform Raman spectroscopy. Talanta 2006, 69, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Koza, S.; Bouvier, E.S.P. A review size-exclusion chromatography for the analysis of protein biotherapeutics and their aggregates. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 2923–2950. [Google Scholar] [CrossRef] [PubMed]
- Šalaševičius, A.; Uždavinytė, D.; Visockis, M.; Ruzgys, P.; Šatkauskas, S. Effect of pulsed electric field (Pef) on bacterial viability and whey protein in the processing of raw milk. Appl. Sci. 2021, 11, 11281. [Google Scholar] [CrossRef]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Heat-induced changes in milk. In Dairy Chemistry and Biochemistry; Springer: Cham, Switzerland, 2015; pp. 345–375. [Google Scholar]
- Aboshama, K.; Hansen, A. Effect of ultra-high-temperature steam injection processing on sulfur-containing amino acids in milk. J. Dairy Sci. 1977, 60, 1374–1378. [Google Scholar] [CrossRef]
- Broersen, K. Milk Processing Affects Structure, Bioavailability and Immunogenicity of β-lactoglobulin. Foods 2020, 9, 874. [Google Scholar] [CrossRef]
- Krishna, T.C.; Najda, A.; Bains, A.; Tosif, M.M.; Papliński, R.; Kapłan, M.; Chawla, P. Influence of ultra-heat treatment on properties of milk proteins. Polymers 2021, 13, 3164. [Google Scholar] [CrossRef]
- Čurlej, J.; Zajác, P.; Čapla, J.; Golian, J.; Benešová, L.; Partika, A.; Fehér, A.; Jakabová, S. The Effect of Heat Treatment on Cow’s Milk Protein Profiles. Foods 2022, 11, 1023. [Google Scholar] [CrossRef]
- Walstra, P. On the Stability of Casein Micelles. J. Dairy Sci. 1990, 73, 1965–1979. [Google Scholar] [CrossRef]
- Anema, S.G. Heat-induced changes in caseins and casein micelles, including interactions with denatured whey proteins. Int. Dairy J. 2021, 122, 105136. [Google Scholar] [CrossRef]
- El-Fattah, A.A.; Azzam, M.; Elkashef, H.; Elhadydy, A. Antioxidant properties of milk: Effect of milk species, milk fractions and heat treatments. Int. J. Dairy Sci. 2019, 15, 1–9. [Google Scholar] [CrossRef][Green Version]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ayaz, M.; Ajmal, M.; Ellahi, M.Y.; Khalique, A. Antioxidant capacity and fatty acids characterization of heat treated cow and buffalo milk. Lipids Heal. Dis. 2017, 16, 163. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.F.G.; Augusto-Obara, T.R.; Hennebelle, M.; Chantieng, S.; Ozturk, G.; Taha, A.Y.; Vieira, T.M.F.d.S.; Bell, J.M.L.N.d.M. Effects of industrial heat treatments on bovine milk oxylipins and conventional markers of lipid oxidation. Prostaglandins, Leukot. Essent. Fat. Acids 2020, 152, 102040. [Google Scholar] [CrossRef]
- Pestana, J.M.; Gennari, A.; Monteiro, B.W.; Lehn, D.N.; Souza, C.F.V. Effects of pasteurization and ultra-high temperature processes on proximate composition and fatty acid profile in bovine milk. Am. J. Food Technol. 2015, 10, 265–272. [Google Scholar] [CrossRef]
- Paniangvait, P.; King, A.; Jones, A.; German, B. Cholesterol Oxides in Foods of Animal Origin. J. Food Sci. 1995, 60, 1159–1174. [Google Scholar] [CrossRef]
- Sieber, R. Oxidised cholesterol in milk and dairy products. Int. Dairy J. 2005, 15, 191–206. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Isoda, H.; Tanimoto, M.; Dosako, S.I.; Idota, T.; Ahiko, K. Inhibition by lactoferrin and κ-casein glycomacropeptide of binding of cholera toxin to its receptor. Biosci. Biotechnol. Biochem. 1992, 56, 195–198. [Google Scholar] [CrossRef]
- Aimutis, W.R. Bioactive properties of milk proteins with particular focus on anticariogenesis. J. Nutr. 2004, 134, 989S–995S. [Google Scholar] [CrossRef]
- Sawin, E.A.; Murali, S.G.; Ney, D.M. Differential effects of low-phenylalanine protein sources on brain neurotransmitters and behavior in C57Bl/6-Pahenu2 mice. Mol. Genet. Metab. 2014, 111, 452–461. [Google Scholar] [CrossRef]
- Requena, P.; Daddaoua, A.; Martínez-Plata, E.; González, M.; Zarzuelo, A.; Suárez, M.D.; Sánchez de Medina, F.; Martínez-Augustin, O. Bovine glycomacropeptide ameliorates experimental rat ileitis by mechanisms involving downregu-lation of interleukin 17. Br. J. Pharmacol. 2008, 154, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Requena, P.; Daddaoua, A.; Guadix, E.; Zarzuelo, A.; Suárez, M.D.; de Medina, F.S.; Martínez-Augustin, O. Bovine glycomacropeptide induces cytokine production in human monocytes through the stimulation of the MAPK and the NF-κB signal transduction pathways. Br. J. Pharmacol. 2009, 157, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Ortega-González, M.; Capitán-Cañadas, F.; Requena, P.; Ocón, B.; Romero-Calvo, I.; Aranda, C.; Suárez, M.D.; Zarzuelo, A.; De Medina, F.S.; Martínez-Augustin, O. Validation of bovine glycomacropeptide as an intestinal anti-inflammatory nutraceutical in the lymphocyte-transfer model of colitis. Br. J. Nutr. 2014, 111, 1202–1212. [Google Scholar] [CrossRef]
- Ney, D.M.; Blank, R.D.; Hansen, K.E. Advances in the nutritional and pharmacological management of phenylketonuria. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Poppitt, S.D.; Strik, C.M.; McArdle, B.H.; McGill, A.-T.; Hall, R.S. Evidence of enhanced serum amino acid profile but not appetite suppression by dietary glycomacropeptide (GMP): A comparison of dairy whey proteins. J. Am. Coll. Nutr. 2013, 32, 177–186. [Google Scholar] [CrossRef]
- Daddaoua, A.; Puerta, V.; Zarzuelo, A.; Suárez, M.D.; Sánchez de Medina, F.; Martínez-Augustin, O. Bovine glycomacropeptide is anti-inflammatory in rats with hapten-induced colitis. J. Nutr. 2005, 135, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Beucher, S.; Levenez, F.; Yvon, M.; Corring, T. Effects of gastric digestive products from casein on CCK release by intestinal cells in rat. J. Nutr. Biochem. 1994, 5, 578–584. [Google Scholar] [CrossRef]
- de Medina, F.S.; Daddaoua, A.; Requena, P.; Capitán-Cañadas, F.; Zarzuelo, A.; Suárez, M.D.; Martínez-Augustin, O. New insights into the immunological effects of food bioactive peptides in animal models of intestinal inflammation. Proc. Nutr. Soc. 2010, 69, 454–462. [Google Scholar] [CrossRef]
- Brody, E.P. Biological activities of bovine glycomacropeptide. Br. J. Nutr. 2000, 84 (Suppl. S1), 39–46. [Google Scholar] [CrossRef]
- Veldhorst, M.A.; Nieuwenhuizen, A.G.; Hochstenbach-Waelen, A.; Westerterp, K.R.; Engelen, M.P.; Brummer, R.-J.M.; Deutz, N.E.; Westerterp-Plantenga, M.S. Effects of complete whey-protein breakfasts versus whey without GMP-breakfasts on energy intake and satiety. Appetite 2009, 52, 388–395. [Google Scholar] [CrossRef]
- Van Calcar, S.C.; Ney, D.M. Food products made with glycomacropeptide, a low-phenylalanine whey protein, provide a new alternative to amino acid–based medical foods for nutrition management of phenylketonuria. J. Acad. Nutr. Diet. 2012, 112, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Solverson, P.; Murali, S.G.; Litscher, S.J.; Blank, R.D.; Ney, D.M. Low bone strength is a manifestation of phenylketonuria in mice and is attenuated by a glycomacropeptide diet. PLoS ONE 2012, 7, e45165. [Google Scholar] [CrossRef] [PubMed]
- Solverson, P.; Murali, S.G.; Brinkman, A.S.; Nelson, D.W.; Clayton, M.K.; Yen, C.-L.E.; Ney, D.M. Glycomacropeptide, a low-phenylalanine protein isolated from cheese whey, supports growth and attenuates metabolic stress in the murine model of phenylketonuria. Am. J. Physiol. Metab. 2012, 302, E885–E895. [Google Scholar] [CrossRef] [PubMed]
- Wagoner, T.B.; Ward, L.; Foegeding, E.A. Using state diagrams for predicting colloidal stability of whey protein beverages. J. Agric. Food Chem. 2015, 63, 4335–4344. [Google Scholar] [CrossRef] [PubMed]
- Neelima; Sharma, R.; Rajput, Y.S.; Mann, B. Chemical and functional properties of glycomacropeptide (GMP) and its role in the detection of cheese whey adulteration in milk: A review. Dairy Sci. Technol. 2013, 93, 21–43. [Google Scholar] [CrossRef]
- Linares, D.M.; Del Río, B.; Ladero, V.; Martínez, N.; Fernández, M.; Martín, M.C.; Álvarez, M.A. Factors influencing biogenic amines accumulation in dairy products. Front. Microbiol. 2012, 3, 180. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic amine production by lactic acid bacteria: A review. Foods 2019, 8, 17. [Google Scholar] [CrossRef]
- Abbring, S.; Blokhuis, B.R.J.; Miltenburg, J.L.; Olmedo, K.G.J.R.; Garssen, J.; Redegeld, F.A.; van Esch, B.C.A.M. Direct Inhibition of the Allergic Effector Response by Raw Cow’s Milk—An Extensive In Vitro Assessment. Cells 2020, 9, 1258. [Google Scholar] [CrossRef]
- Moniente, M.; García-Gonzalo, D.; Ontañón, I.; Pagán, R.; Botello-Morte, L. Histamine accumulation in dairy products: Microbial causes, techniques for the detection of histamine-producing microbiota, and potential solutions. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1481–1523. [Google Scholar] [CrossRef]
- Chang, G.; Wang, L.; Ma, N.; Zhang, W.; Zhang, H.; Dai, H.; Shen, X. Histamine activates inflammatory response and depresses casein synthesis in mammary gland of dairy cows during SARA. BMC Vet. Res. 2018, 14, 168. [Google Scholar] [CrossRef]
- Claeys, W.L.; Cardoen, S.; Daube, G.; De Block, J.; Dewettinck, K.; Dierick, K.; De Zutter, L.; Huyghebaert, A.; Imberechts, H.; Thiange, P.; et al. Raw or heated cow milk consumption: Review of risks and benefits. Food Control. 2013, 31, 251–262. [Google Scholar] [CrossRef]
- Asadullah; Nisa, K.U.; Tarar, O.M.; Ali, S.A.; Jamil, K.; Begum, A. Study to evaluate the impact of heat treatment on water soluble vitamins in milk. J. Pak. Med. Assoc. 2010, 60, 909–912. [Google Scholar] [PubMed]
- Melini, F.; Melini, V.; Luziatelli, F.; Ruzzi, M. Raw and Heat-Treated Milk: From Public Health Risks to Nutritional Quality. Beverages 2017, 3, 54. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C.; Rielly, C.; Stapley, A. Loss of solubility of α-lactalbumin and β-lactoglobulin during the spray drying of whey proteins. LWT 2008, 41, 270–277. [Google Scholar] [CrossRef]
- Bernard, C.; Regnault, S.; Gendreau, S.; Charbonneau, S.; Relkin, P. Enhancement of emulsifying properties of whey proteins by controlling spray-drying parameters. Food Hydrocoll. 2011, 25, 758–763. [Google Scholar] [CrossRef]
- Chegini, G.; HamidiSepehr, A.; Dizaji, M.F.; Mirnezami, S.V. Study of physical and chemical properties of spray drying whey powder. Int. J. Recycl. Org. Waste Agric. 2014, 3, 62. [Google Scholar] [CrossRef]
- Haque, M.A.; Adhikari, B. Proteins in Spray Drying Process. Handb. Ind. Dry. 2015, 2015, 971–983. [Google Scholar]
- Perdigón, G.; Fuller, R.; Raya, R. Lactic acid bacteria and their effect on the immune system. Curr. Issues Intest. Microbiol. 2001, 2, 27–42. [Google Scholar]
- Saeed, A.H.; Salam, A.I. Current limitations and challenges with lactic acid bacteria: A review. Food Nutr. Sci. 2013, 2013, 73–87. Available online: http://file.scirp.org/Html/10-2700895_40133.htm (accessed on 5 March 2023).
- Gilliland, S.E. Health and nutritional benefits from lactic acid bacteria. FEMS Microbiol. Lett. 1990, 87, 175–188. [Google Scholar] [CrossRef]
- Wei, F.; Zhou, L.; Wang, Q.; Zheng, G.; Su, S. Effect of Compound Lactic Acid Bacteria Capsules on the Small Intestinal Bacterial Overgrowth in Patients with Depression and Diabetes: A Blinded Randomized Controlled Clinical Trial. Dis. Markers 2022, 2022, 1–6. [Google Scholar] [CrossRef]
- Kato, Y.; Shimomura, Y.; Takada, Y.; Furuyashiki, T. Correlation Between Lactic Acid Bacteria Beverage Intake and Stress Resilience. Kobe J. Med. Sci. 2021, 67, E1–E6. [Google Scholar]
- Naidu, A.S.; Bidlack, W.R.; Clemens, R.A. Critical Reviews in Food Science and Nutrition Probiotic Spectra of Lactic Acid Bacteria (LAB). Crit. Rev. Food Sci. Nutr. 2010, 38, 37–41. [Google Scholar]
- Charlier, C.; Cretenet, M.; Even, S.; Le Loir, Y. Interactions between Staphylococcus aureus and lactic acid bacteria: An old story with new perspectives. Int. J. Food Microbiol. 2009, 131, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Medved’ová, A.; Koňuchová, M.; Kvočiková, K.; Hatalová, I.; Valík, L. Effect of Lactic Acid Bacteria Addition on the Microbiological Safety of Pasta-Filata Types of Cheeses. Front. Microbiol. 2020, 11, 612528. [Google Scholar] [CrossRef] [PubMed]
- Wouters, J.T.M.; Ayad, E.H.E.; Hugenholtz, J.; Smit, G. Microbes from raw milk for fermented dairy products. Int. Dairy J. 2001, 12, 91–109. [Google Scholar] [CrossRef]
- Adegbehingbe, K.T.; Bello, M. Antibacterial activities of fermented whey on some selected enteropathogenic bacteria. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 152–161. [Google Scholar]
- Tuttle, A.R.; Trahan, N.D.; Son, M.S. Growth and Maintenance of Escherichia coli Laboratory Strains. Curr. Protoc. 2021, 1, e20. [Google Scholar] [CrossRef] [PubMed]
- Elbing, K.L.; Brent, R. Recipes and Tools for Culture of Escherichia coli. Curr. Protoc. Mol. Biol. 2019, 125, e83. [Google Scholar] [CrossRef]
- Kim, C.; Wilkins, K.; Bowers, M.; Wynn, C.; Ndegwa, E. Influence of PH and Temperature on Growth Characteristics of Leading Foodborne Pathogens in a Laboratory Medium and Select Food Beverages. Austin Food Sci. 2018, 3, 1–8. Available online: https://vtechworks.lib.vt.edu/bitstream/handle/10919/81940/afs-v3-id1031_Bacterial%20growth%20curve.pdf (accessed on 15 March 2023).
- Hervert, C.; Martin, N.; Boor, K.; Wiedmann, M. Survival and detection of coliforms, Enterobacteriaceae, and gram-negative bacteria in Greek yogurt. J. Dairy Sci. 2017, 100, 950–960. [Google Scholar] [CrossRef] [PubMed]
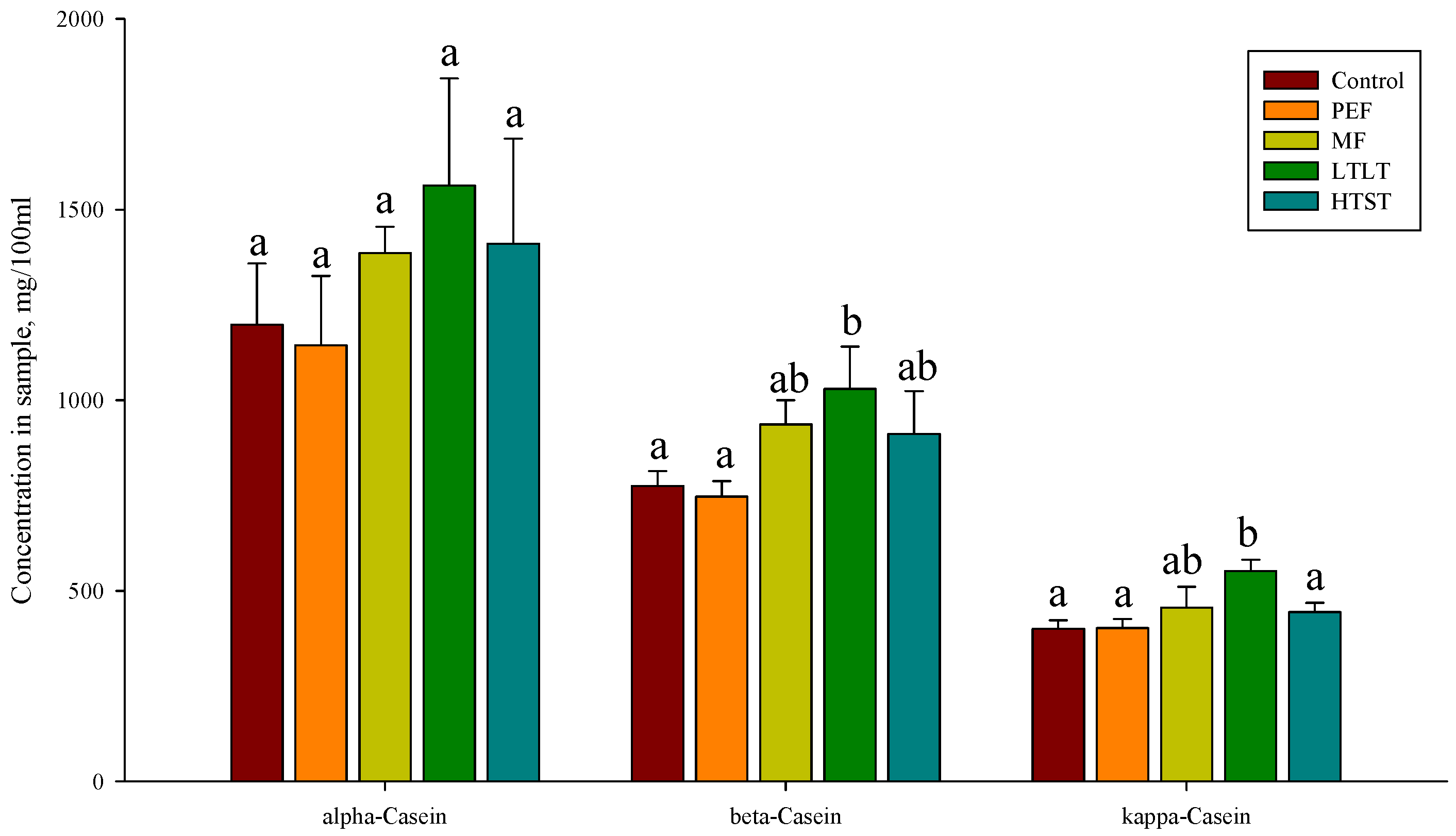
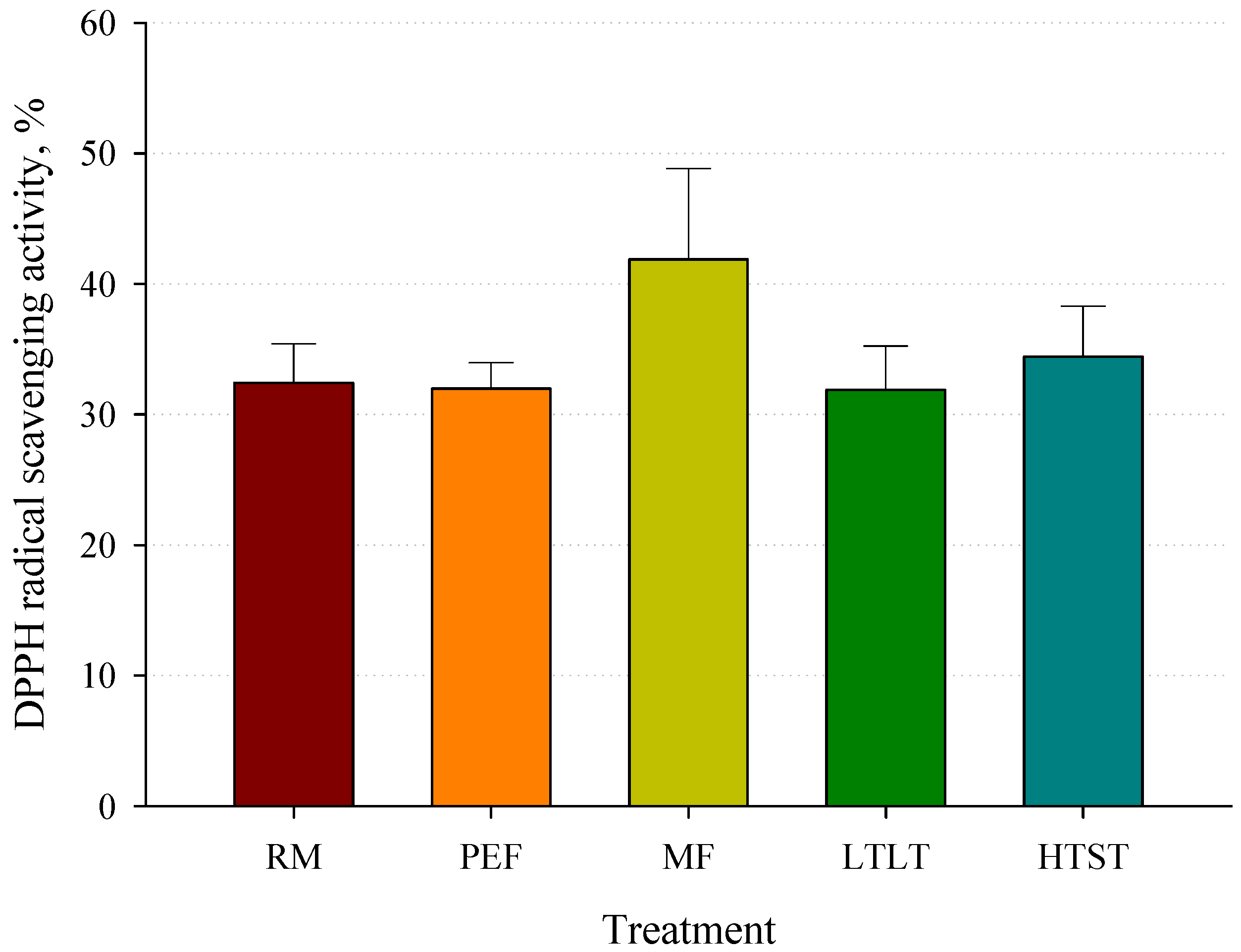

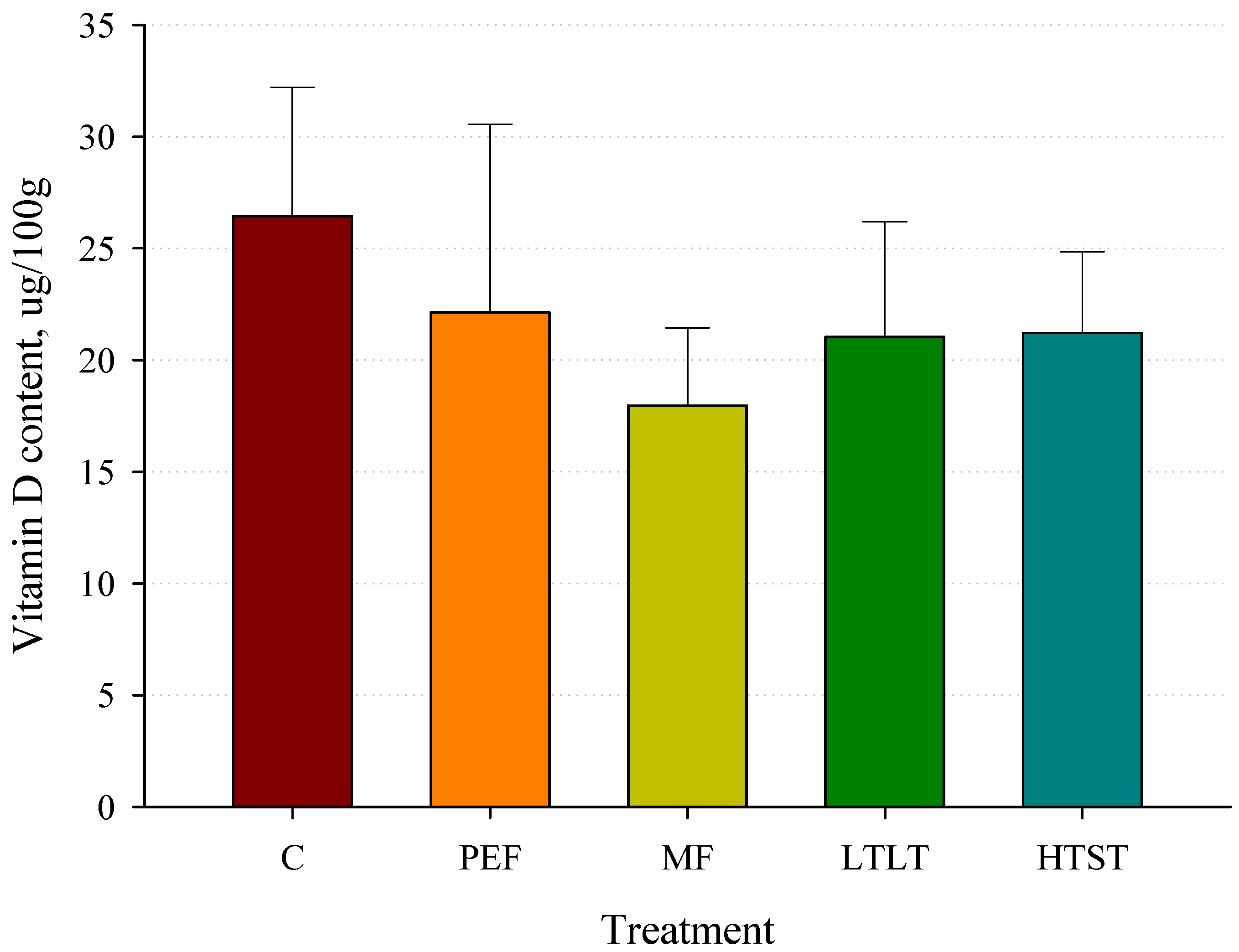
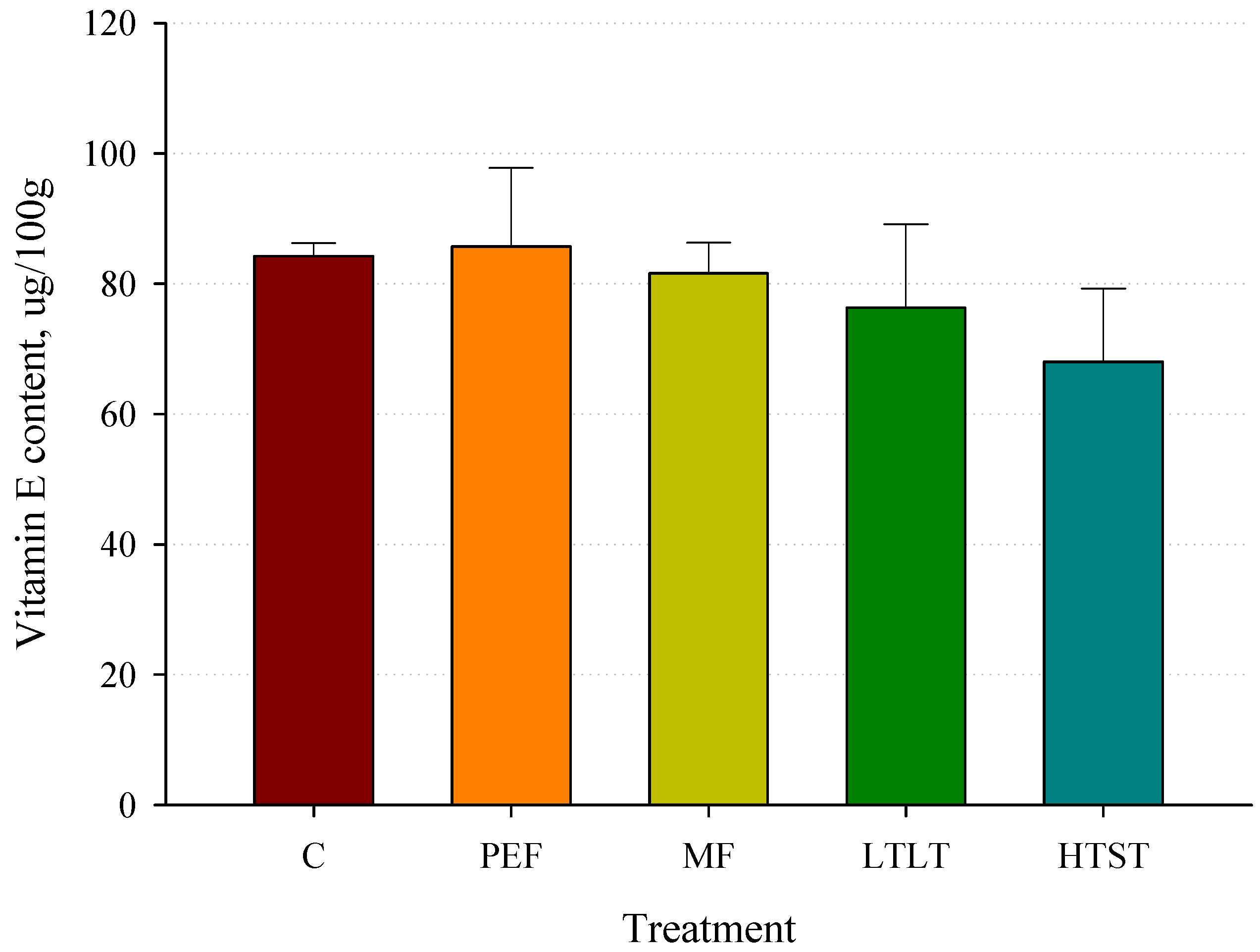

| No. | Milk Samples | Total Number of Bacteria Found in Samples, CFU/mL | Coliform Bacteria, CFU/mL | Mesophilic Lactic Acid Bacteria CFU/mL | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| 1. | RM | 9 × 104 a | 1.5 × 103 | 1.6 × 102 a | 21 | - | - |
| 2. | RWC | 8.3 × 103 b | 3.6 × 102 | 12 b | 2 | 5.5 × 103 a | 1.5 × 10 2 |
| 3. | PEF | 6.2 × 103 c | 1 × 102 | <4 | - | 1.6 × 102 b | 17 |
| 4. | HT | <1 | - | <1 | - | <1 | - |
| 5. | HTST | 2.4 × 102 d | 34 | <1 | - | <1 | - |
| 6. | LTLT | 7 × 102 d | 92 | <1 | - | <1 | - |
| 7. | MF | 36 d | 8 | <1 | - | <1 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šalaševičius, A.; Uždavinytė, D.; Visockis, M.; Ruzgys, P.; Šatkauskas, S. Comparative Analysis of Pulsed Electric Fields (PEF) and Traditional Pasteurization Techniques: Comparative Effects on Nutritional Attributes and Bacterial Viability in Milk and Whey Products. Appl. Sci. 2023, 13, 12127. https://doi.org/10.3390/app132212127
Šalaševičius A, Uždavinytė D, Visockis M, Ruzgys P, Šatkauskas S. Comparative Analysis of Pulsed Electric Fields (PEF) and Traditional Pasteurization Techniques: Comparative Effects on Nutritional Attributes and Bacterial Viability in Milk and Whey Products. Applied Sciences. 2023; 13(22):12127. https://doi.org/10.3390/app132212127
Chicago/Turabian StyleŠalaševičius, Aivaras, Dovilė Uždavinytė, Mindaugas Visockis, Paulius Ruzgys, and Saulius Šatkauskas. 2023. "Comparative Analysis of Pulsed Electric Fields (PEF) and Traditional Pasteurization Techniques: Comparative Effects on Nutritional Attributes and Bacterial Viability in Milk and Whey Products" Applied Sciences 13, no. 22: 12127. https://doi.org/10.3390/app132212127
APA StyleŠalaševičius, A., Uždavinytė, D., Visockis, M., Ruzgys, P., & Šatkauskas, S. (2023). Comparative Analysis of Pulsed Electric Fields (PEF) and Traditional Pasteurization Techniques: Comparative Effects on Nutritional Attributes and Bacterial Viability in Milk and Whey Products. Applied Sciences, 13(22), 12127. https://doi.org/10.3390/app132212127








