Oxidation of Zinc Microparticles by Microwave Plasma to Form Effective Solar-Sensitive Photocatalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Testing
3. Results
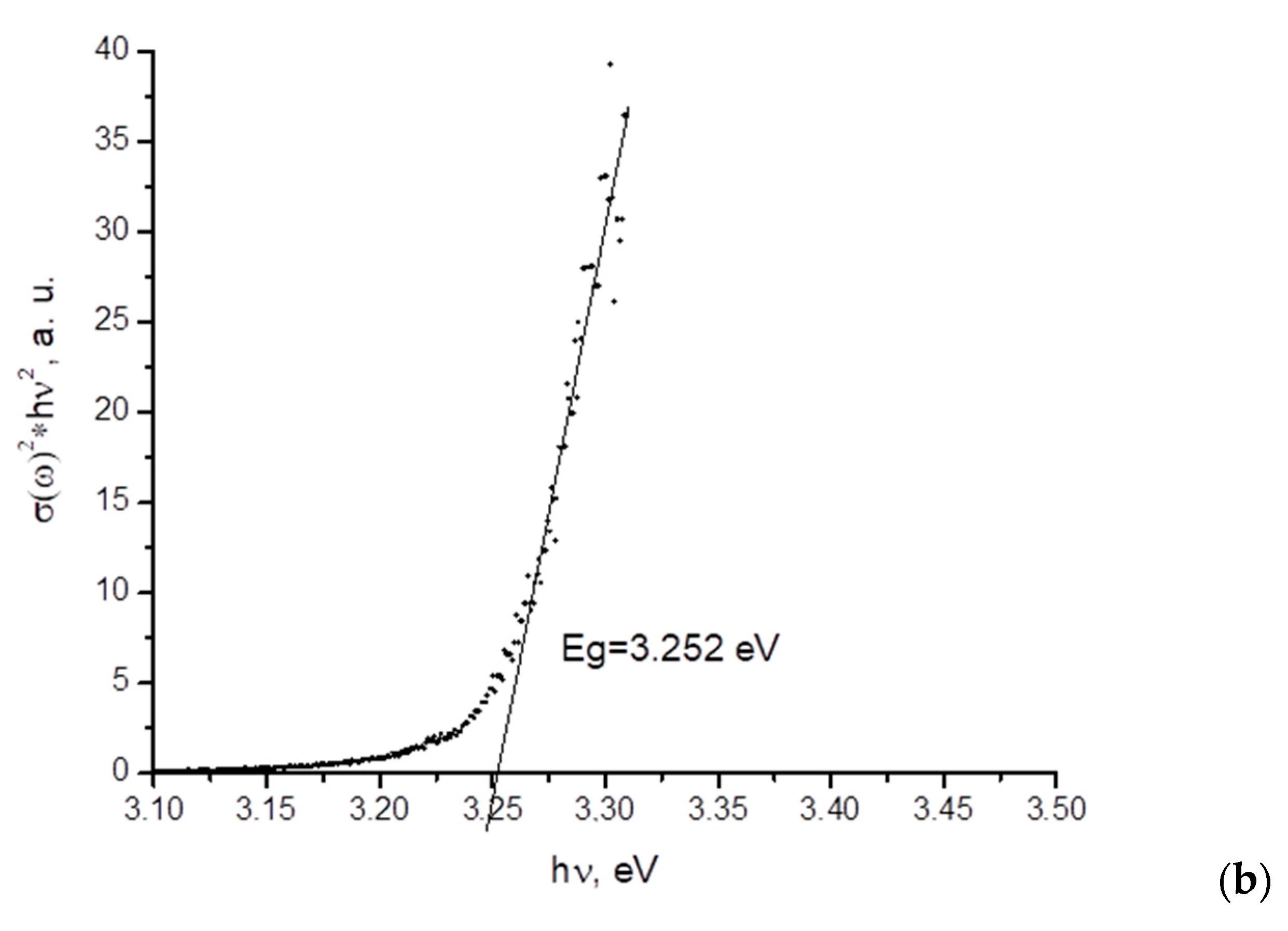
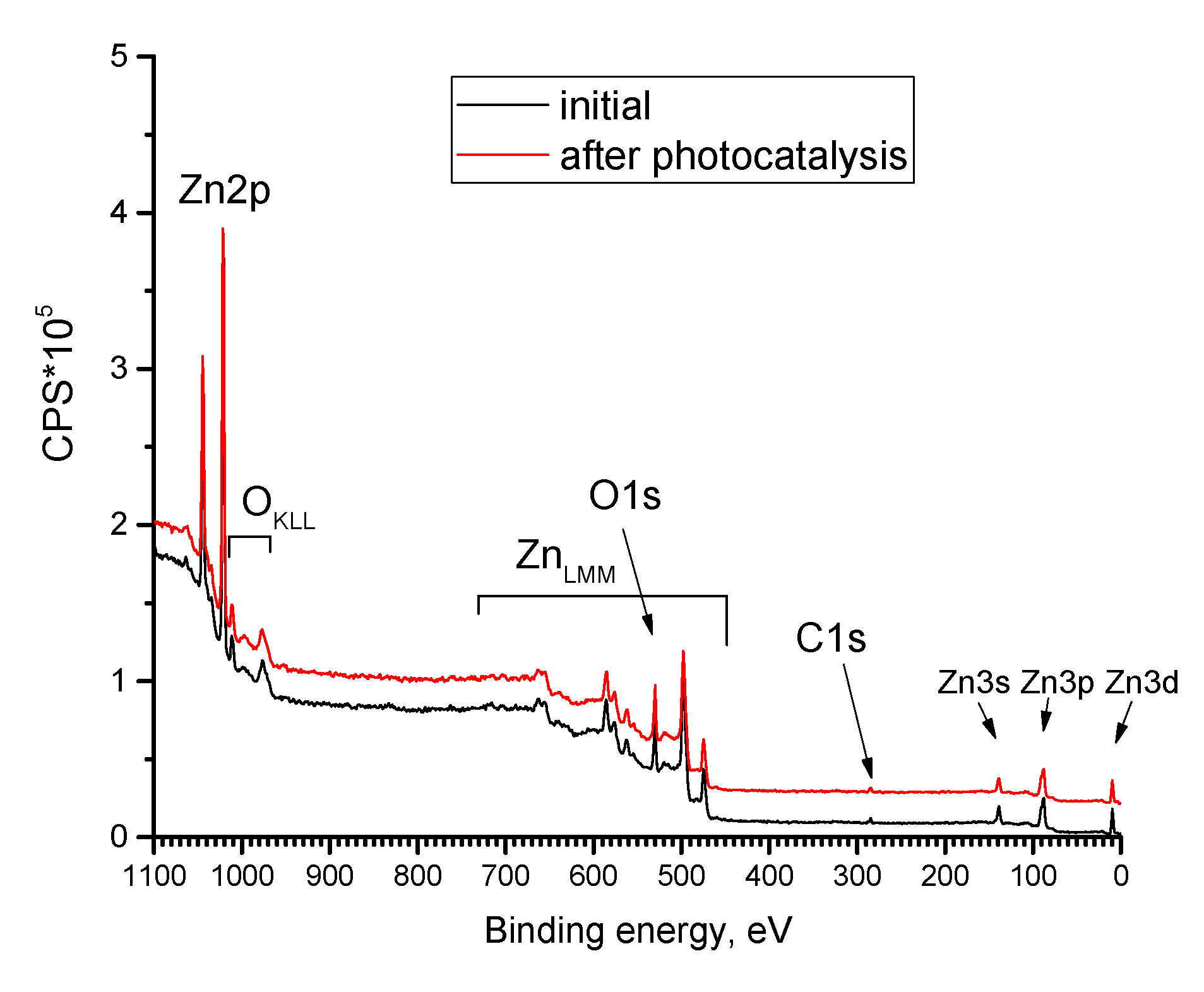
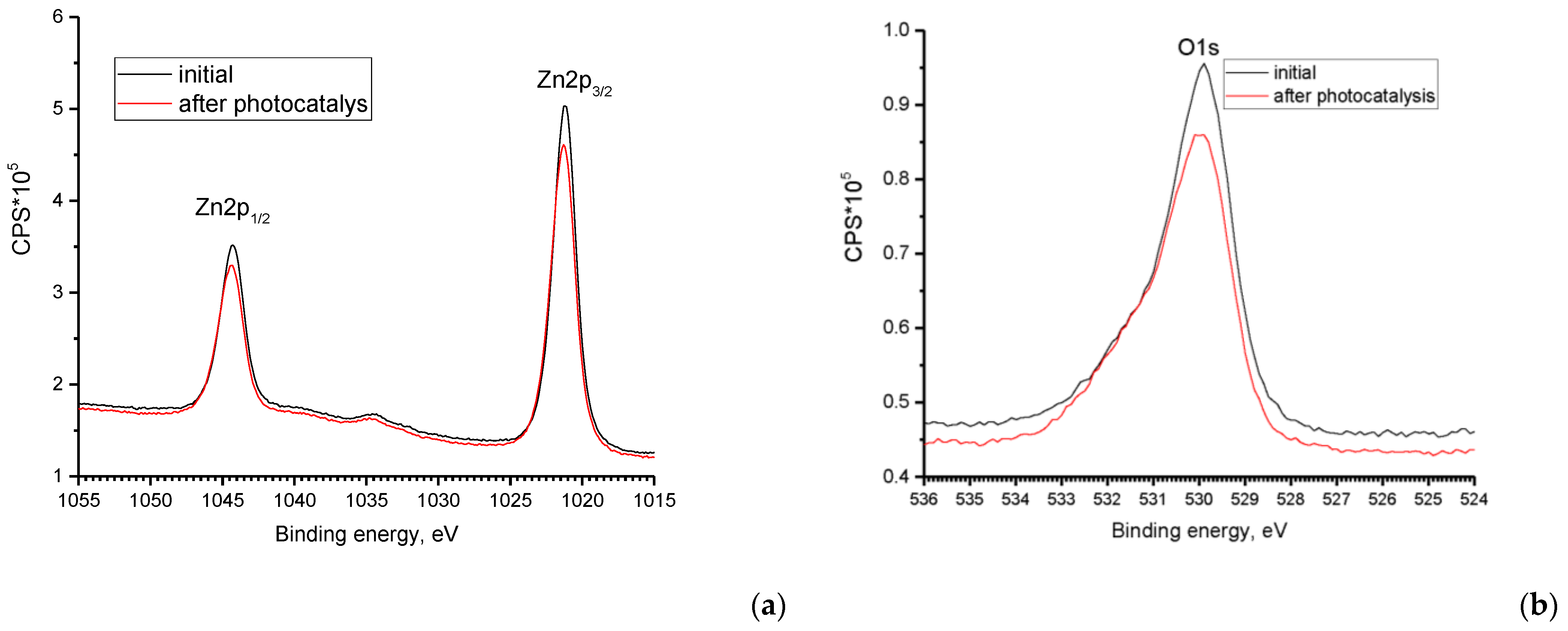
3.1. ZnO Powder Characterization
3.2. Photocatalytic Properties of ZnO Powder
3.3. Reproducibility of Photocatalyst Characteristics in Cyclic Processes
4. Conclusions
- The work proposes an effective plasma method for formation of photosensitive ZnO powders. Zn microparticles were injected into the microwave plasma at a mass rate of 20 g/min;
- Treatment of zinc metal powders using microwave nitrogen plasma has a number of advantages: a high gas temperature that promotes zinc evaporation and charging of microparticles by incoming fluxes of electrons and ions from the surrounding plasma that prevents the formation of agglomerates. Both of these factors contribute to the formation of ZnO structures ranging in size from hundreds of nanometers to several micrometers;
- Study of the band gap parameters of ZnO structures using X-ray photoelectron spectroscopy demonstrates a high density of states near the Fermi level associated with defects contributing to the expansion of the photosensitivity range into the visible range;
- High photoactivity was demonstrated (rate constants 0.036 min−1 and 0.051 min−1) of synthesized ZnO structures during photodegradation of 2,4-dinitrophenol and ciprofloxacin, respectively, when exposed to solar radiation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.A.; Dunlop, P.S.M.; Hamilton, J.W.J.; Fernández-Ibáñez, P.; Polo-López, I.; Sharma, P.K.; Vennard, A.S.M. A Review of Heterogeneous Photocatalysis for Water and Surface Disinfection. Molecules 2015, 20, 5574–5615. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.-M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Matthews, R.; McEvoy, S. Destruction of phenol in water with sun, sand, and photocatalysis. Sol. Energy 1992, 49, 507–513. [Google Scholar] [CrossRef]
- Mugumo, R.; Ichipi, E.; Tichapondwa, S.M.; Chirwa, E.M.N. Visible-Light-Induced Photocatalytic Degradation of Rhodamine B Dye Using a CuS/ZnS p-n Heterojunction Nanocomposite under Visible-Light Irradiation. Catalysts 2023, 13, 1184. [Google Scholar] [CrossRef]
- Chen, H.; Hu, Y.; Ying, Z.; Xia, Y.; Ye, J.; Zhao, J.; Zhang, S. BiOI-SnO2 Heterojunction Design to Boost Visible-Light-Driven Photocatalytic NO Purification. Int. J. Environ. Res. Public Health 2023, 20, 4009. [Google Scholar] [CrossRef]
- Chowdhury, A.; Balu, S.; Lan, K.-W.; Wei-Chih Lee, L.; Yang, T.C.-K. Synergistic Effect of BiVO4/P-g-C3N4 Heterojunction with Enhanced Optoelectronic Properties on Synthetic Colorants under Visible Light. Colorants 2023, 2, 426–442. [Google Scholar] [CrossRef]
- Yan, X.; Ning, G.; Zhao, P. Enhanced Visible Light Photocatalytic Reduction of Cr(VI) over a Novel Square Nanotube Poly (Triazine Imide)/TiO2 Heterojunction. Catalysts 2019, 9, 55. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Sang, T.; Zhong, Y.; Hu, C.-H.; Wang, D.-H.; Ye, J.-C.; Wei, N.-N.; Liu, H. Surfactant-Modified CdS/CdCO3 Composite Photocatalyst Morphology Enhances Visible-Light-Driven Cr(VI) Reduction Performance. Nanomaterials 2022, 12, 3923. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.-W. Visible Light-Responsive CeO2/MoS2 Composite for Photocatalytic Hydrogen Production. Catalysts 2022, 12, 1185. [Google Scholar] [CrossRef]
- Mohamed, K.M.; Benitto, J.J.; Vijaya, J.J.; Bououdina, M. Recent Advances in ZnO-Based Nanostructures for the Photocatalytic Degradation of Hazardous, Non-Biodegradable Medicines. Crystals 2023, 13, 329. [Google Scholar] [CrossRef]
- Ma, S.; Huang, Y.; Hong, R.; Lu, X.; Li, J.; Zheng, Y. Enhancing Photocatalytic Activity of ZnO Nanoparticles in a Circulating Fluidized Bed with Plasma Jets. Catalysts 2021, 11, 77. [Google Scholar] [CrossRef]
- Alkauskas, A.; Pasquarello, A. Band-edge problem in the theoretical determination of defect energy levels: The O vacancy in ZnO as a benchmark case. Phys. Rev. B 2011, 84, 125206. [Google Scholar] [CrossRef]
- Hanif, A.; Kim, Y.S.; Ameen, S.; Kim, H.G.; Kwac, L.K. Boosting the Visible Light Photocatalytic Activity of ZnO through the Incorporation of N-Doped for Wastewater Treatment. Coatings 2022, 12, 579. [Google Scholar] [CrossRef]
- Heo, S.-G.; Jo, S.-I.; Jeong, G.-H. Revealing the enhanced photocatalytic properties of ZnO tetrapods produced by atmospheric-pressure microwave plasma jet system. Curr. Appl. Phys. 2023, 46, 46–54. [Google Scholar] [CrossRef]
- Fortov, V.; Ivlev, A.; Khrapak, S.; Khrapak, A.; Morfill, G. Complex (dusty) plasmas: Current status, open issues, perspectives. Phys. Rep. 2005, 421, 1–103. [Google Scholar] [CrossRef]
- Ishihara, O. Complex plasma: Dusts in plasma. J. Phys. D Appl. Phys. 2007, 40, R121. [Google Scholar] [CrossRef]
- Koohgard, M.; Sarvestani, A.M.; Hosseini-Sarvari, M. Photocatalytic synthesis of unsymmetrical thiourea derivatives via visible-light irradiation using nitrogen-doped ZnO nanorods. New J. Chem. 2020, 44, 14505–14512. [Google Scholar] [CrossRef]
- Moisan, M.; Sauve, G.; Zakrzewski, Z.; Hubert, J. An atmospheric pressure waveguide-fed microwave plasma torch: The TIA design. Plasma Sources Sci. Technol. 1994, 3, 584–592. [Google Scholar] [CrossRef]
- Baltin, L.; Batenin, V.M.; Lebedeva, V.; Tsemko, N.; Devyatkin, I. Stationary microwave discharge in nitrogen at atmospheric pressure. High Temp. 1971, 9, 1019. [Google Scholar]
- Lavand, A.B.; Malghe, Y.S. Synthesis, characterization and visible light photocatalytic activity of nitrogen-doped zinc oxide nanospheres. J. Asian Ceram. Soc. 2015, 3, 305–310. [Google Scholar] [CrossRef]
- García, J.F.; Sánchez, S.; Metz, R. Complete Oxidation of Zinc Powder. Valid. Kinet. Models 2008, 69, 317–325. [Google Scholar] [CrossRef]
- An, Z.; Huang, Y.; Zhang, R. High-temperature multispectral stealth metastructure from the microwave-infrared compatible design. Compos. Part B Eng. 2023, 259, 110737. [Google Scholar] [CrossRef]
- Zheng, J.; Wei, X.; Li, Y.; Dong, W.; Li, X.; E, S.; Wu, Z.; Wen, J. Stretchable polyurethane composite foam triboelectric nanogenerator with tunable microwave absorption properties at elevated temperature. Nano Energy 2021, 89, 106397. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Hassan, N.; Jalil, A.; Fei, I.; Razak, M.; Khusnun, N.; Bahari, M.; Riwayati, Y.; Suprapto, S.; Prasetyoko, D.; Firmansyah, M.; et al. Vanadia as an electron-hole recombination inhibitor on fibrous silica-titania for selective hole oxidation of ciprofloxacin and Congo red photodegradation. Chemosphere 2023, 338, 139502. [Google Scholar] [CrossRef]
- Bano, K.; Kaushal, S.; Lal, B.; Joshi, S.K.; Kumar, R.; Singh, P.P. Fabrication of CuO/ZnO heterojunction photocatalyst for efficient photocatalytic degradation of tetracycline and ciprofloxacin under direct sun light. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100863. [Google Scholar] [CrossRef]
- Rani, M.; Rachna; Yadav, J.; Shanker, U. Efficient degradation of nonylphenol and 2,4-dinitrophenol by sunlight responsive hexacyanocobaltates nanostructures. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100325. [Google Scholar] [CrossRef]
- Van Thuan, D.; Nguyen, T.B.; Pham, T.H.; Kim, J.; Chu, T.T.H.; Nguyen, M.V.; Nguyen, K.D.; Al-Onazi, W.A.; Elshikh, M.S. Photodegradation of ciprofloxacin antibiotic in water by using ZnO-doped g-C3N4 photocatalyst. Chemosphere 2022, 308, 136408. [Google Scholar] [CrossRef]
- Mukherjee, I.; Cilamkoti, V.; Dutta, R.K. Sunlight-Driven Photocatalytic Degradation of Ciprofloxacin by Carbon Dots Embedded in ZnO Nanostructures. ACS Appl. Nano Mater. 2021, 4, 7686–7697. [Google Scholar] [CrossRef]
- Costa, L.N.; Nobre, F.X.; Lobo, A.d.O.; de Matos, J.M.E. Photodegradation of ciprofloxacin using Z-scheme TiO2/SnO2 nanostructures as photocatalyst. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100466. [Google Scholar] [CrossRef]
- Park, S.J.; Das, G.S.; Schütt, F.; Adelung, R.; Mishra, Y.K.; Tripathi, K.; Kim, T.M. Visible-light photocatalysis by carbon-nano-onion-functionalized ZnO tetrapods: Degradation of 2,4-dinitrophenol and a plant-model-based ecological assessment. NPG Asia Mater. 2019, 11, 8. [Google Scholar] [CrossRef]
- Mao, L.; Shen, J.; Ma, X.; Lan, Z.; Zhang, X. Effects of operational parameters on the photodegradation of 2,4-dinitrophenol in TiO2dispersion. Desalination Water Treat. 2014, 56, 744–751. [Google Scholar] [CrossRef]
- Ohno, T.; Sarukawa, K.; Tokieda, K.; Matsumura, M. Morphology of a TiO2 Photocatalyst (Degussa, P-25) Consisting of Anatase and Rutile Crystalline Phases. J. Catal. 2001, 203, 82–86. [Google Scholar] [CrossRef]
- Toe, C.Y.; Scott, J.; Amal, R.; Ng, Y.H. Recent advances in suppressing the photocorrosion of cuprous oxide for photocatalytic and photoelectrochemical energy conversion. J. Photochem. Photobiol. C Photochem. Rev. 2018, 40, 191–211. [Google Scholar] [CrossRef]
- Das, A.; Nikhil, S.K.; Nair, R.G. Influence of surface morphology on photocatalytic performance of zinc oxide: A review. Nano-Struct. Nano-Objects 2019, 19, 100353. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Zhou, Y.; Yang, F.; Kim, E.J.; Hahn, S.H.; Seong, S.G. Rapid room-temperature synthesis of nanosheet-assembled ZnO mesocrystals with excellent photocatalytic activity. CrystEngComm 2012, 15, 754–763. [Google Scholar] [CrossRef]
- Kislov, N.; Lahiri, J.; Verma, H.; Goswami, D.Y.; Stefanakos, E.; Batzill, M. Photocatalytic Degradation of Methyl Orange over Single Crystalline ZnO: Orientation Dependence of Photoactivity and Photostability of ZnO. Langmuir 2009, 25, 3310–3315. [Google Scholar] [CrossRef]
- Vempati, S.; Mitra, J.; Dawson, P. One-step synthesis of ZnO nanosheets: A blue-white fluorophore. Nanoscale Res. Lett. 2012, 7, 470. [Google Scholar] [CrossRef]
- Oba, F.; Togo, A.; Tanaka, I.; Paier, J.; Kresse, G. Defect energetics in ZnO: A hybrid Hartree-Fock density functional study. Phys. Rev. B 2008, 77, 245202. [Google Scholar] [CrossRef]
- Rogozin, I.V.; Georgobiani, A.N.; Kotlyarevsky, M.B.; Marakhovskii, A.V. Compensation mechanism for hole conduction in ZnO: N films. Inorg. Mater. 2009, 45, 391–398. [Google Scholar] [CrossRef]

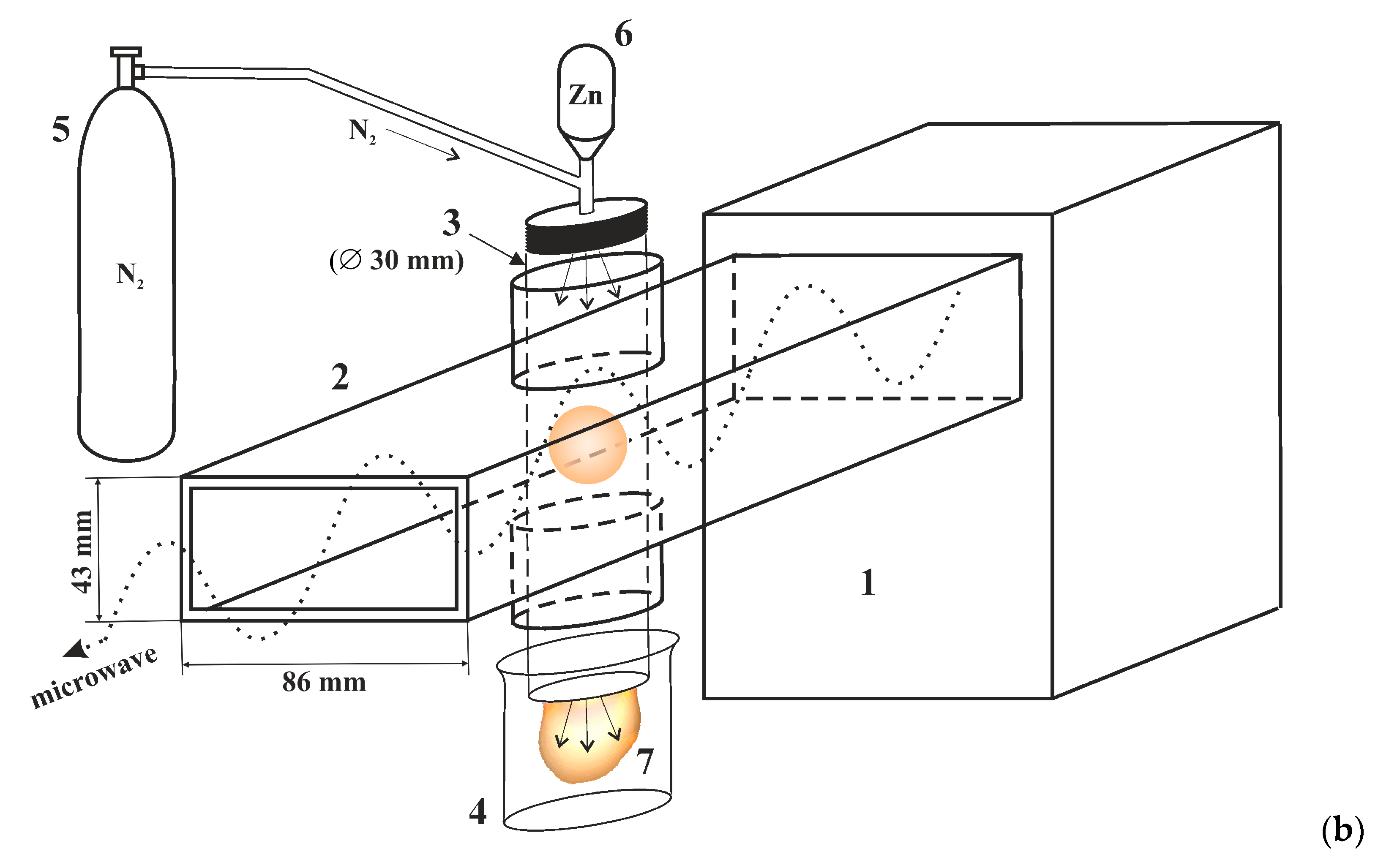
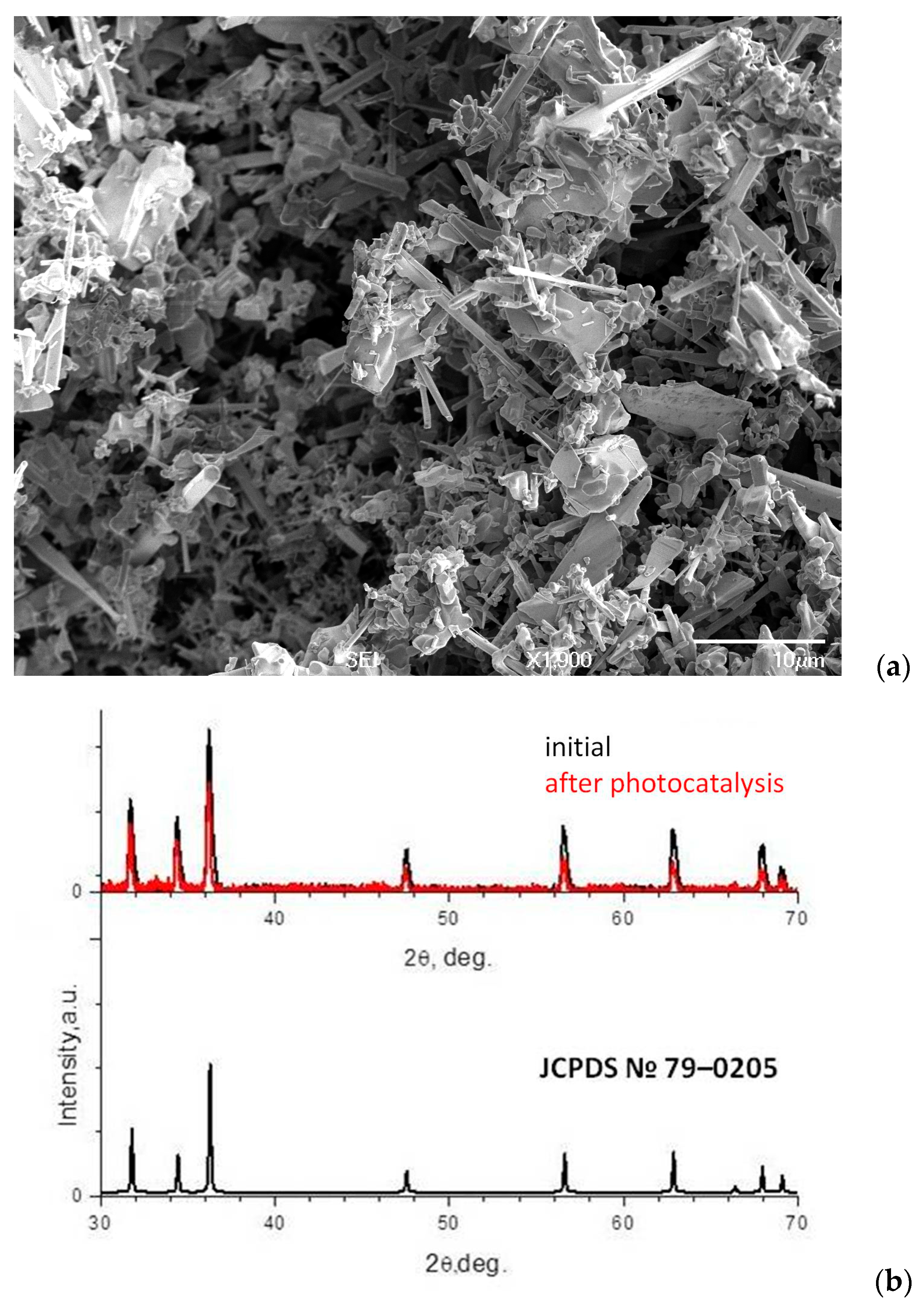
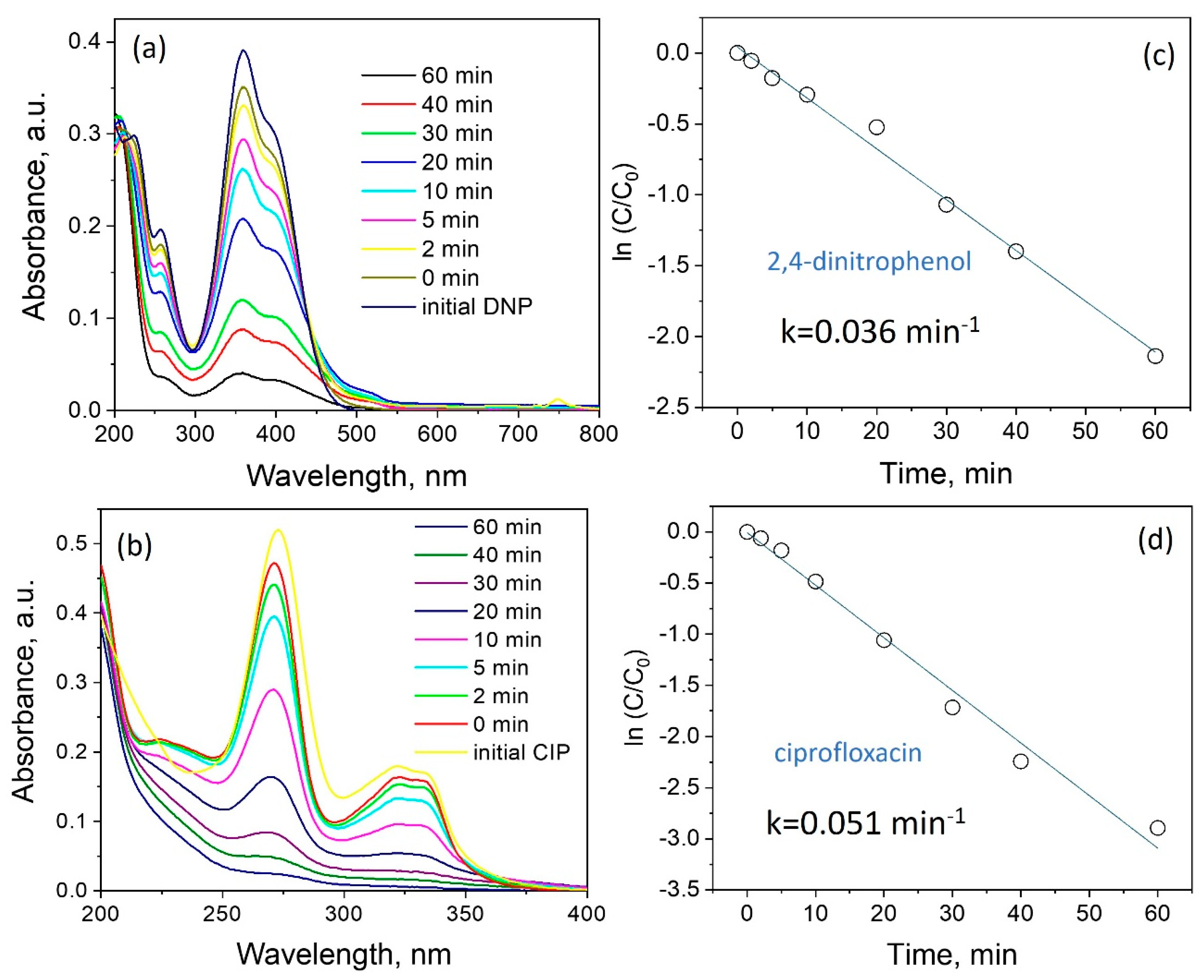
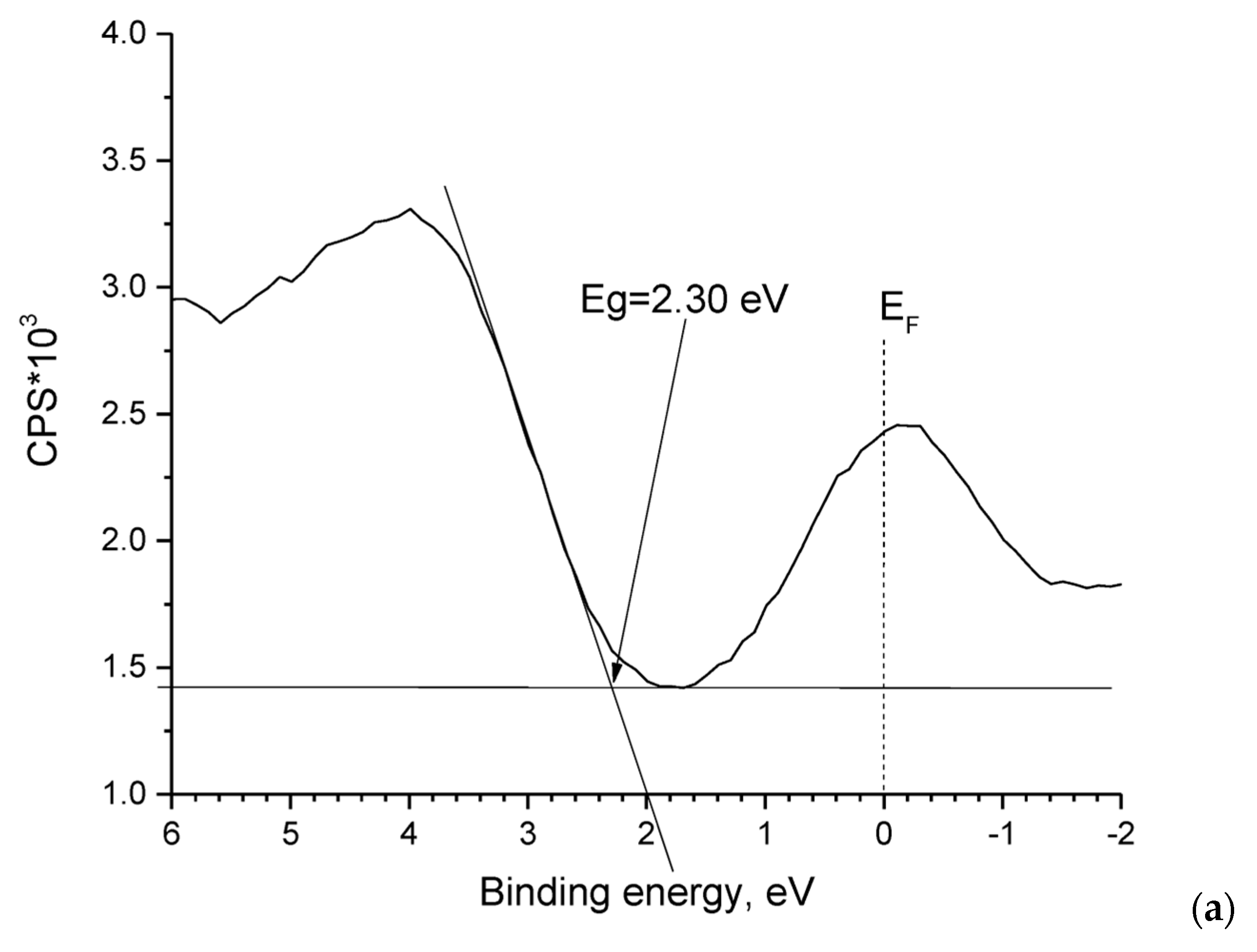
| Element | Zn | O | N |
|---|---|---|---|
| Quantity % | 27.56 | 68.32 | 4.12 |
| Sample | Zn | O | C | N |
|---|---|---|---|---|
| Initial | 49.6 | 40.8 | 9.6 | 0 |
| After photocatalysis | 49.5 | 41.3 | 9.2 | 0 |
| Photocatalyst | Pollutant | Catalyst Dosage | Pollutant Concentration | Light Source (g L−1) | kapp (10−3 min−1) | Reference |
|---|---|---|---|---|---|---|
| ZnO/g-C3N4 | CIP | 0.5 | 5 mg L−1 | 32 W compact fluorescent bulb | 24 | [29] |
| ZnO nanoparticles | CIP | 0.6 | 12 mg L−1 | Natural sunlight, 221 W/m2 | 13 | [30] |
| TiO2/SnO2 nanocomposite | CIP | 0.5 | 5 mg L−1 | Three UVC lamps with 35 W (253 nm) | 28.2 | [31] |
| carbon-nano-onion-functionalized ZnO tetrapods | DNP | 2 | 0.1 mM | 60 W tungsten bulb | 18.34 | [32] |
| Commercial P25 | DNP | 2 | 5 µM | 266 W/m2 Hg lamp | 32 | [33] |
| ZnO powder (microparticles) | CIP | 0.5 | 5 mg L−1 | 500 W Xenon lamp | 51 | This work |
| DNP | 0.5 | 5 mg L−1 | 500 W Xenon lamp | 36 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muslimov, A.; Antipov, S.; Gadzhiev, M.; Ulyankina, A.; Krasnova, V.; Lavrikov, A.; Kanevsky, V. Oxidation of Zinc Microparticles by Microwave Plasma to Form Effective Solar-Sensitive Photocatalysts. Appl. Sci. 2023, 13, 12195. https://doi.org/10.3390/app132212195
Muslimov A, Antipov S, Gadzhiev M, Ulyankina A, Krasnova V, Lavrikov A, Kanevsky V. Oxidation of Zinc Microparticles by Microwave Plasma to Form Effective Solar-Sensitive Photocatalysts. Applied Sciences. 2023; 13(22):12195. https://doi.org/10.3390/app132212195
Chicago/Turabian StyleMuslimov, Arsen, Sergey Antipov, Makhach Gadzhiev, Anna Ulyankina, Valeria Krasnova, Alexander Lavrikov, and Vladimir Kanevsky. 2023. "Oxidation of Zinc Microparticles by Microwave Plasma to Form Effective Solar-Sensitive Photocatalysts" Applied Sciences 13, no. 22: 12195. https://doi.org/10.3390/app132212195
APA StyleMuslimov, A., Antipov, S., Gadzhiev, M., Ulyankina, A., Krasnova, V., Lavrikov, A., & Kanevsky, V. (2023). Oxidation of Zinc Microparticles by Microwave Plasma to Form Effective Solar-Sensitive Photocatalysts. Applied Sciences, 13(22), 12195. https://doi.org/10.3390/app132212195






