Polysaccharides Derived from Drynaria fortunei Attenuated Osteoclast Differentiation Induced by Receptor Activator of Nuclear Factor-κB Ligand by Modulating NFATc1 and c-Fos
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Chemical Profiling of DFPs
2.3. Osteoclast Differentiation Assay
2.4. Quantitative PCR and Western Blotting
2.5. Statistical Analysis
3. Results
3.1. Phytochemical Properties of Polysaccharides
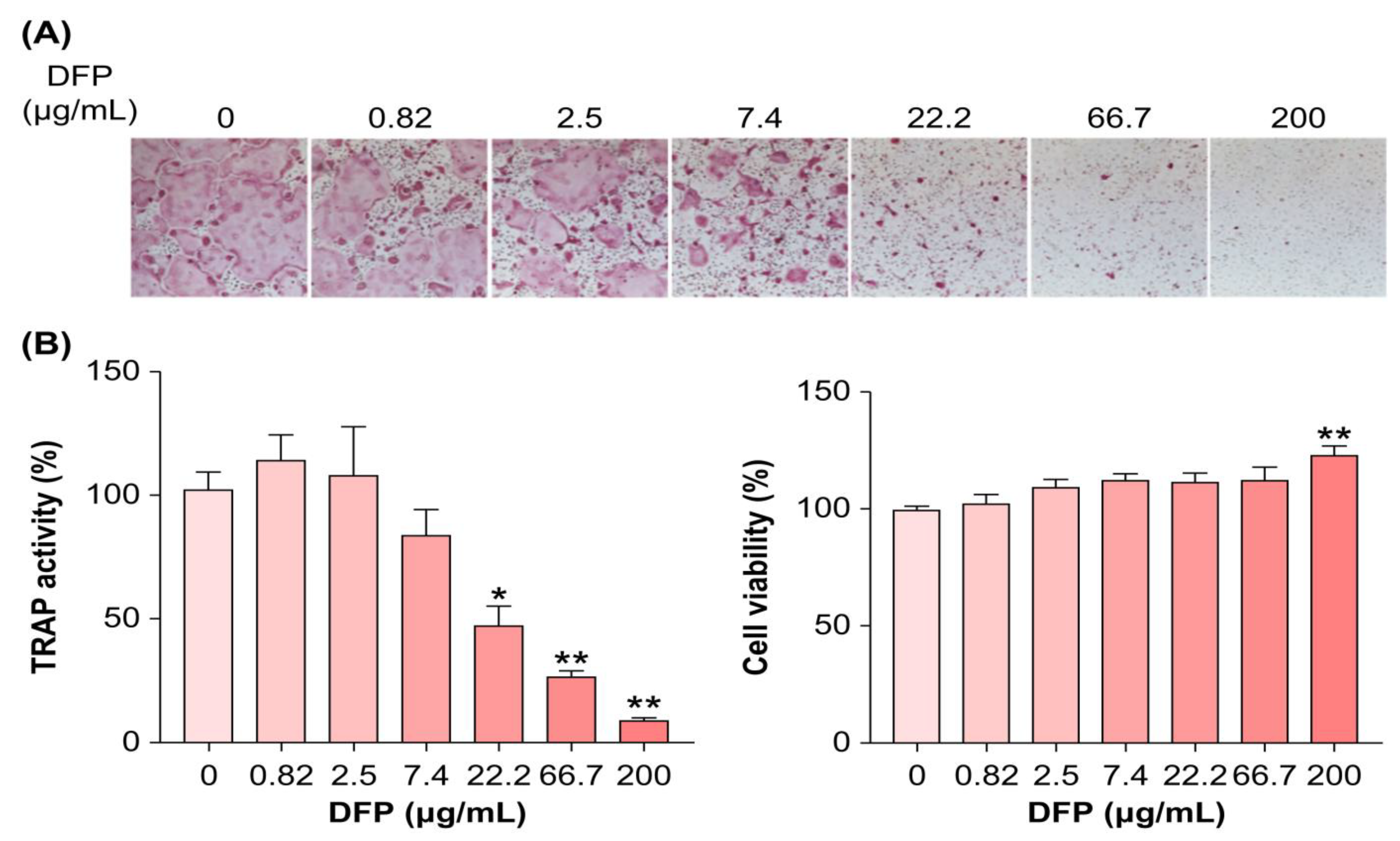
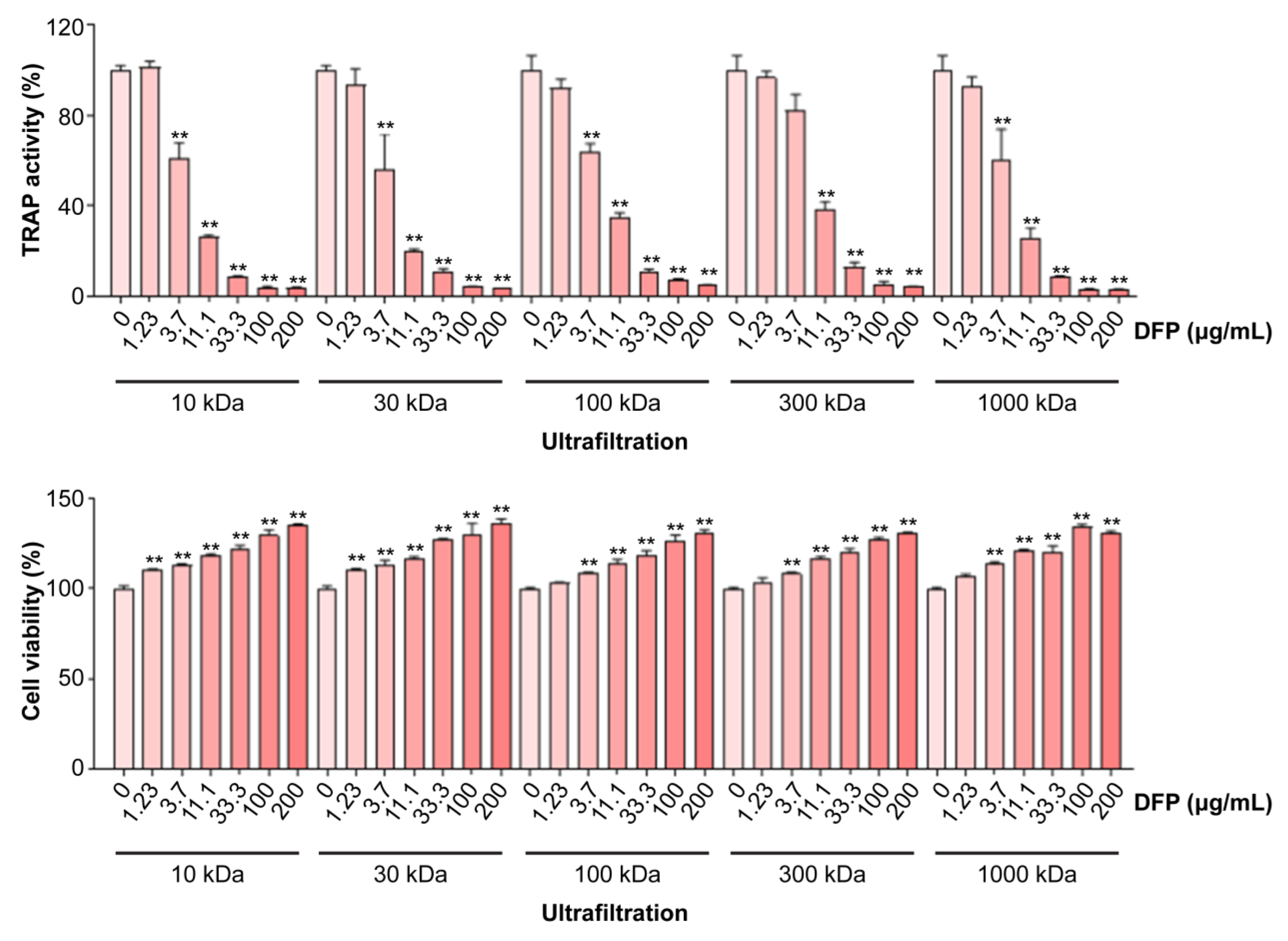
3.2. DFPs Inhibit Osteoclastogenesis
3.3. DFPs Suppress RANKL-Induced Key Osteoclastic Marker Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aubin, J.E.; Triffitt, J.T. Mesenchymal stem cells and osteoblast differentiation. In Principles of Bone Biology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2002; pp. 59–81. [Google Scholar] [CrossRef]
- Maruotti, N.; Corrado, A.; Cantatore, F.P. Osteoporosis and rheumatic diseases. Reumatismo 2014, 66, 125–135. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, T.; Yan, X.; Liu, Z.; Yan, Y.; Yang, K.; Qi, J.; Zhou, H.; Qian, N.; Zhou, Q.; et al. A novel Rhein derivative modulates bone formation and resorption and ameliorates estrogen-dependent bone loss. J. Bone Miner. Res. 2019, 34, 361–374. [Google Scholar] [CrossRef]
- Teitelbaum, S.L.; Ross, F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003, 4, 638–649. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, N. Regulation of NFATc1 in osteoclast differentiation. J. Bone Metab. 2014, 21, 233–241. [Google Scholar] [CrossRef]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Kwak, S.-C.; Moon, S.-Y.; Kwack, H.-B.; Jeon, B.-H.; Min, O.-J.; Choi, M.-K.; Kim, J.-J.; Jang, S.-J. Effect of drynariae rhizoma in RANKL-induced osteoclast differentiation. J. Physiol. Pathol. Korean Med. 2012, 26, 506–510. [Google Scholar]
- Yamashita, T.; Yao, Z.; Li, F.; Zhang, Q.; Badell, I.R.; Schwarz, E.M.; Takeshita, S.; Wagner, E.F.; Noda, M.; Matsuo, K.; et al. NF-κB p50 and p52 regulate receptor activator of NF-κB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J. Biol. Chem. 2007, 282, 18245–18253. [Google Scholar] [CrossRef]
- Ju, Y.S. Ungok’s Illustrated Guide to Medicinal Materials; Woosuk Press: Jeonju, Republic of Korea, 2017; Volume 616, p. 632. [Google Scholar]
- Yoon, S.H.; Kim, H.J. Donguibogam; Donguibogam Publishing Company: Seoul, Republic of Korea, 2006; pp. 297–2189. [Google Scholar]
- Ding, Z. Clinical study on TCM orthopedics in treatment of closed pilon fracture in children. ACTA Chin. Med. 2017, 32, 1799–1801. [Google Scholar]
- Yu, H.-M.; Sun, Y.-J. Clinical study on external fixation by small splint and sequential stages of Chinese medicine treatment on distal epiphyseal fracture of radius of children. J. Liaoning Univ. Tradit. Chin. Med. 2016, 18, 148–150. [Google Scholar]
- Ma, K.-C.; Zhu, T.-Y.; Wang, F.-X. Stimulative effects of gusuibu (Drynaria baronii) injection on chick embryo bone primordium calcification in vitro. Am. J. Chin. Med. 1996, 24, 77–82. [Google Scholar] [CrossRef]
- Jiang, J.-Q.; Cai, W.; Wang, Z.-C.; Ding, Y.; Li, X.-Y. Effect of Drynaria fortunei naringin on the total protein content and ultra-structure of human periodontal ligament cells. Hua Xi Kou Qiang Yi Xue Za Zhi= West China J. Stomatol. 2010, 28, 330–333. [Google Scholar]
- Chang, R. Bioactive polysaccharides from traditional Chinese medicine herbs as anticancer adjuvants. J. Altern. Complement. Med. 2002, 8, 559–565. [Google Scholar] [CrossRef]
- Jiang, M.-H.; Zhu, L.; Jiang, J.-G. Immunoregulatory actions of polysaccharides from Chinese herbal medicine. Expert Opin. Ther. Targets 2010, 14, 1367–1402. [Google Scholar] [CrossRef]
- Lee, T.-T.; Huang, C.-C.; Shieh, X.-H.; Chen, C.-L.; Chen, L.-J.; Yu, B. Flavonoid, phenol and polysaccharide contents of Echinacea purpurea L. and its immunostimulant capacity in vitro. Int. J. Environ. Sci. Dev. 2010, 1, 5. [Google Scholar] [CrossRef]
- Li, Q.; Niu, Y.; Xing, P.; Wang, C. Bioactive polysaccharides from natural resources including Chinese medicinal herbs on tissue repair. Chin. Med. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Wu, J.; Shi, S.; Wang, H.; Wang, S. Mechanisms underlying the effect of polysaccharides in the treatment of type 2 diabetes: A review. Carbohydr. Polym. 2016, 144, 474–494. [Google Scholar] [CrossRef]
- Zheng, Y.; Bai, L.; Zhou, Y.; Tong, R.; Zeng, M.; Li, X.; Shi, J. Polysaccharides from Chinese herbal medicine for anti-diabetes recent advances. Int. J. Biol. Macromol. 2019, 121, 1240–1253. [Google Scholar] [CrossRef]
- Ben Saad, H.; Frikha, D.; Bouallegue, A.; Badraoui, R.; Mellouli, M.; Kallel, H.; Pujo, J.M.; Ben Amara, I. Mitigation of Hepatic Impairment with Polysaccharides from Red Alga Albidum corallinum Supplementation through Promoting the Lipid Profile and Liver Homeostasis in Tebuconazole-Exposed Rats. Pharmaceuticals 2023, 16, 1305. [Google Scholar] [CrossRef]
- Zeng, P.; Li, J.; Chen, Y.; Zhang, L. The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog. Mol. Biol. Transl. Sci. 2019, 163, 423–444. [Google Scholar] [CrossRef]
- Qiao, X.; Lin, X.-H.; Liang, Y.-H.; Dong, J.; Guo, D.-A.; Ye, M. Comprehensive chemical analysis of the rhizomes of Drynaria fortunei by orthogonal pre-separation and liquid chromatography mass spectrometry. Planta Med. 2014, 80, 330–336. [Google Scholar] [CrossRef]
- Sun, X.; Wei, B.; Peng, Z.; Chen, X.; Fu, Q.; Wang, C.; Zhen, J.; Sun, J. A polysaccharide from the dried rhizome of Drynaria fortunei (Kunze) J. Sm. prevents ovariectomized (OVX)-induced osteoporosis in rats. J. Cell Mol. Med. 2020, 24, 3692–3700. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Karkhanis, Y.D.; Zeltner, J.Y.; Jackson, J.J.; Carlo, D.J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal. Biochem. 1978, 85, 595–601. [Google Scholar] [CrossRef]
- Hwang, Y.-H.; Lee, A.; Kim, T.; Jang, S.-A.; Ha, H. Anti-osteoporotic effects of Commiphora Myrrha and its poly-saccharide via osteoclastogenesis inhibition. Plants 2021, 10, 945. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Kim, T.; Kim, R.; Ha, H. Magnolol inhibits osteoclast differentiation via suppression of RANKL expression. Molecules 2018, 23, 1598. [Google Scholar] [CrossRef]
- Jeong, J.-C.; Lee, J.-W.; Yoon, C.-H.; Lee, Y.-C.; Chung, K.-H.; Kim, M.-G.; Kim, C.-H. Stimulative effects of Drynariae Rhizoma extracts on the proliferation and differentiation of osteoblastic MC3T3-E1 cells. J. Ethnopharmacol. 2005, 96, 489–495. [Google Scholar] [CrossRef]
- Fernández, H.; Kumar, A.; Revilla, M.A. Working with Ferns: Issues and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Aoki, K.; Aoki, S.; Saito, H.; Jimi, E.; Shimokawa, H.; Ohya, K. The inhibitory effects of NF-kappa B inhibitor on the osteoclast function. In Proceedings of the Journal of Pharmacological Sciences; Japanese Pharmacological Society: Tokyo, Japan, 2004; p. 188P. [Google Scholar]
- Karin, M.; Cao, Y.; Greten, F.R.; Li, Z.-W. NF-κB in cancer: From innocent bystander to major culprit. Nat. Rev. Cancer 2002, 2, 301–310. [Google Scholar] [CrossRef]
- David, J.-P.; Sabapathy, K.; Hoffmann, O.; Idarraga, M.H.; Wagner, E.F. JNK1 modulates osteoclastogenesis through both c-Jun phosphorylation-dependent and-independent mechanisms. J. Cell Sci. 2002, 115, 4317–4325. [Google Scholar] [CrossRef]
- Silverman, S.; Christiansen, C. Individualizing osteoporosis therapy. Osteoporos. Int. 2012, 23, 797–809. [Google Scholar] [CrossRef]
- Anagnostis, P.; Gkekas, N.K.; Potoupnis, M.; Kenanidis, E.; Tsiridis, E.; Goulis, D.G. New therapeutic targets for osteoporosis. Maturitas 2019, 120, 1–6. [Google Scholar] [CrossRef]
- Barko, P.; McMichael, M.; Swanson, K.S.; Williams, D.A. The gastrointestinal microbiome: A review. J. Vet. Intern. Med. 2018, 32, 9–25. [Google Scholar] [CrossRef]
- He, M.; Shi, B. Gut microbiota as a potential target of metabolic syndrome: The role of probiotics and prebiotics. Cell Biosci. 2017, 7, 54. [Google Scholar] [CrossRef]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T.; et al. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab. 2017, 26, 672–685. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mainali, R.; Nagpal, R.; Sheikh-Zeinoddin, M.; Soleimanian-Zad, S.; Wang, S.; Deep, G.; Mishra, S.K.; Yadav, H. Dietary polysaccharides in the amelioration of gut microbiome dysbiosis and metabolic diseases. Obes. Control Ther. 2017, 4. [Google Scholar] [CrossRef]
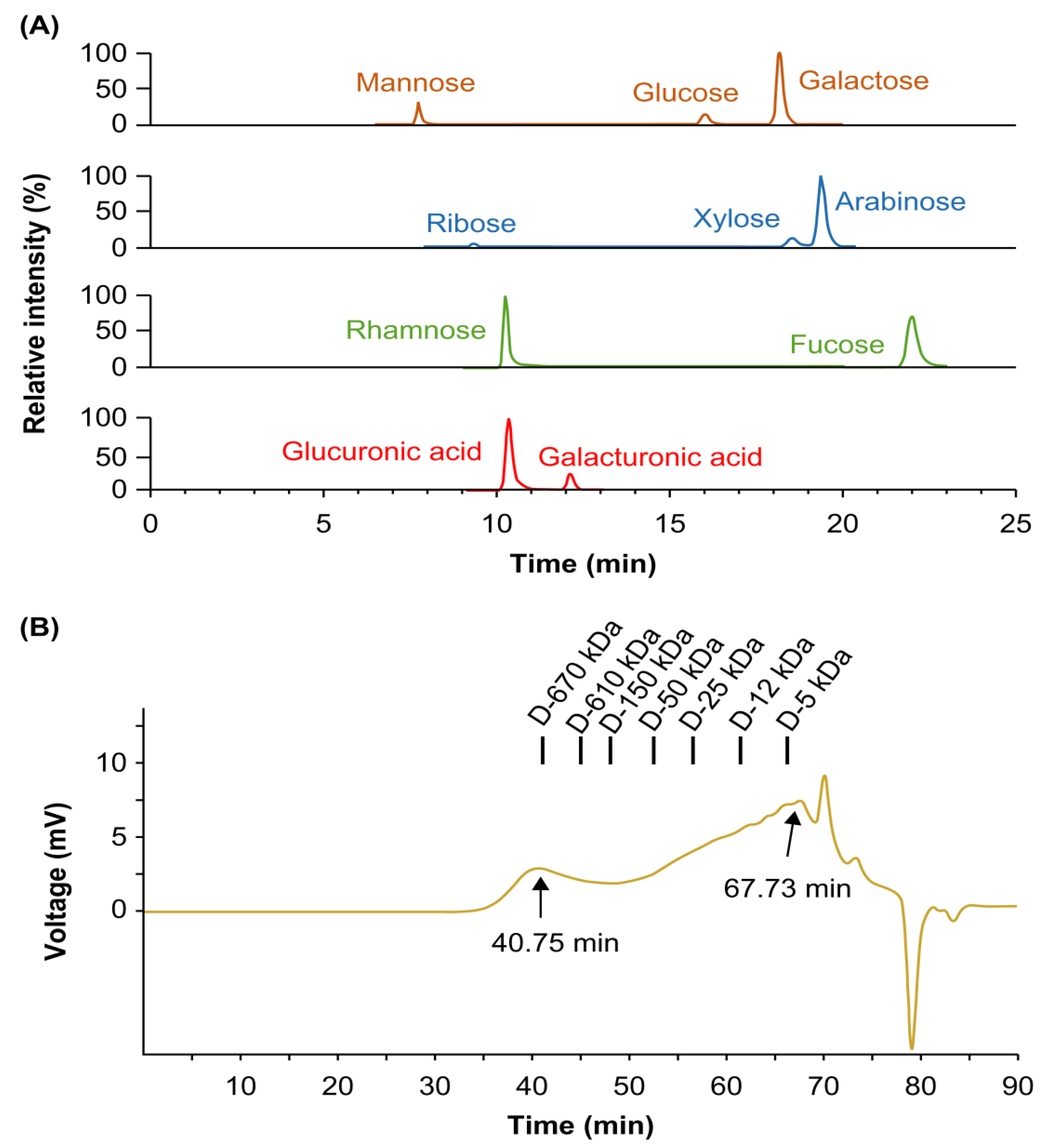

| Parameters | Content a |
|---|---|
| Chemical composition (%) | |
| Neutral sugar | 65.88 ± 2.04 |
| Uronic acid | 23.16 ± 0.63 |
| 2-Keto-3-deoxy-mannooctanoic acid | 0.35 ± 0.01 |
| Protein | 4.47 ± 0.34 |
| Component sugar (Mol% b) | |
| Arabinose | 15.56 ± 0.78 |
| Fucose | 3.73 ± 0.11 |
| Galactose | 23.75 ± 0.51 |
| Glucose | 14.97 ± 0.25 |
| Mannose | 12.93 ± 2.02 |
| Rhamnose | 5.18 ± 0.13 |
| Ribose | 1.83 ± 0.13 |
| Xylose | 3.76 ± 0.12 |
| Galacturonic acid | 3.45 ± 0.47 |
| Glucuronic acid | 14.84 ± 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryuk, J.A.; Lee, A.; Kim, T.; Hwang, Y.-H.; Ha, H. Polysaccharides Derived from Drynaria fortunei Attenuated Osteoclast Differentiation Induced by Receptor Activator of Nuclear Factor-κB Ligand by Modulating NFATc1 and c-Fos. Appl. Sci. 2023, 13, 12201. https://doi.org/10.3390/app132212201
Ryuk JA, Lee A, Kim T, Hwang Y-H, Ha H. Polysaccharides Derived from Drynaria fortunei Attenuated Osteoclast Differentiation Induced by Receptor Activator of Nuclear Factor-κB Ligand by Modulating NFATc1 and c-Fos. Applied Sciences. 2023; 13(22):12201. https://doi.org/10.3390/app132212201
Chicago/Turabian StyleRyuk, Jin Ah, Ami Lee, Taesoo Kim, Youn-Hwan Hwang, and Hyunil Ha. 2023. "Polysaccharides Derived from Drynaria fortunei Attenuated Osteoclast Differentiation Induced by Receptor Activator of Nuclear Factor-κB Ligand by Modulating NFATc1 and c-Fos" Applied Sciences 13, no. 22: 12201. https://doi.org/10.3390/app132212201
APA StyleRyuk, J. A., Lee, A., Kim, T., Hwang, Y.-H., & Ha, H. (2023). Polysaccharides Derived from Drynaria fortunei Attenuated Osteoclast Differentiation Induced by Receptor Activator of Nuclear Factor-κB Ligand by Modulating NFATc1 and c-Fos. Applied Sciences, 13(22), 12201. https://doi.org/10.3390/app132212201






