Ex Vivo Analysis of Ability of Osseodensification to Improve Dental Implant Primary Stability Using Xenograft Bone Walls
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Power Analysis and Sample Size Determination
2.3. Randomization and Allocation of Implants
2.4. Drilling Procedure and Implant Installation
2.5. Microtomographic Analysis and VOI Determination
2.6. Image Analysis
2.7. Implant Stability Measurements
2.8. Statistical Analysis
3. Results
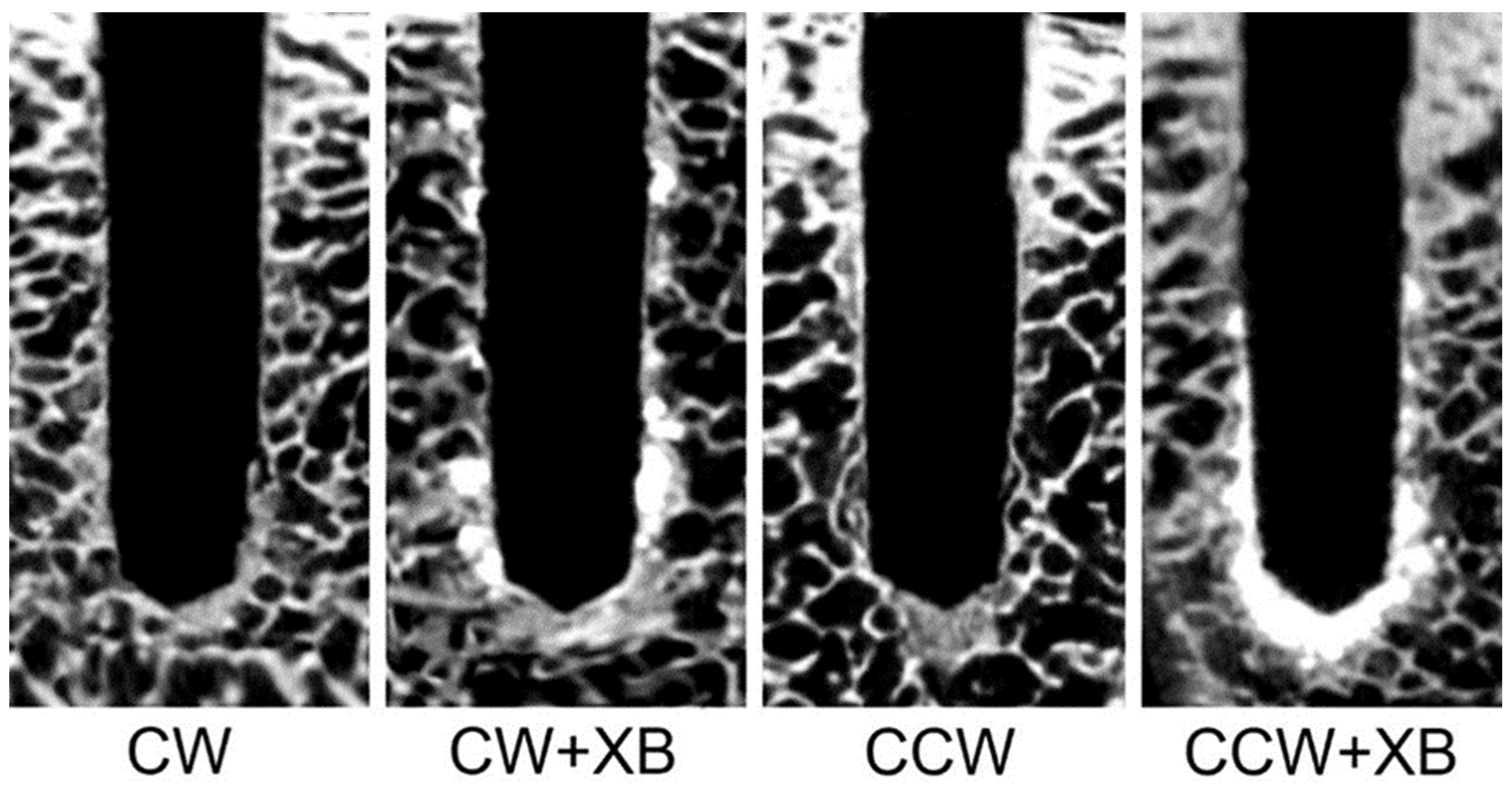
3.1. Analysis of Bone Density and Microstructure Post-Osteotomy
3.2. Evaluation of Implant Stability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trisi, P.; Berardi, D.; Paolantonio, M.; Spoto, G.; D’Addona, A.; Perfetti, G. Primary stability, insertion torque, and bone density of conical implants with internal hexagon: Is there a relationship? J. Craniofac. Surg. 2013, 24, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Punnoose, K.; Kumar, G.A.; Mahesh, B.; Govindarajulu, R.; Amalorpavam, V.; Ebinu, A.; Babu, J.S.; Swarnalatha, C.; Nayyar, A.S. Osseodensification implant site preparation technique and subsequent implant stability: A pilot study. J. Orthod. Sci. 2022, 11, 1–7. [Google Scholar]
- Balshi, T.J.; Wolfinger, G.J.; Balshi, S.F.; Nevins, M.; Kim, D.M. Thirty-Two-Year Success of Dental Implants in Periodontally Compromised Dentition. Int. J. Periodontics Restor. Dent. 2018, 38, 827–831. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant. Dent. Relat. Res. 2019, 21 (Suppl. 1), 4–7. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.; Suzuki, M.; Guimaraes, M.V.; Marin, C.; Granato, R.; Gil, J.N.; Miller, R.J. Early bone healing around different implant bulk designs and surgical techniques: A study in dogs. Clin. Implant. Dent. Relat. Res. 2010, 12, 202–208. [Google Scholar] [CrossRef]
- Barberá-Millán, J.; Larrazábal-Morón, C.; Enciso-Ripoll, J.J.; Pérez-Pevida, E.; Chávarri-Prado, D.; Gómez-Adrián, M.D. Evaluation of the primary stability in dental implants placed in low density bone with a new drilling technique, Osseodensification: An in vitro study. Med. Oral Patol. Oral Cir. Bucal 2021, 26, e361–e367. [Google Scholar] [CrossRef]
- Barone, A.; Alfonsi, F.; Derchi, G.; Tonelli, P.; Toti, P.; Marchionni, S.; Covani, U. The effect of insertion torque on the clinical outcome of single implants: A randomized clinical trial. Clin. Implat. Dent. Relat. Res. 2016, 18, 588–600. [Google Scholar] [CrossRef]
- Bavetta, G.; Bavetta, G.; Randazzo, V.; Cavataio, A.; Paderni, C.; Grassia, V.; Dipalma, G.; Gargiulo Isacco, C.; Scarano, A.; De Vito, D.; et al. A Retrospective Study on Insertion Torque and Implant Stability Quotient (ISQ) as Stability Parameters for Immediate Loading of Implants in Fresh Extraction Sockets. BioMed Res. Int. 2019, 2019, 9720419. [Google Scholar] [CrossRef]
- Beutel, B.G.; Danna, N.R.; Granato, R.; Bonfante, E.A.; Marin, C.; Tovar, N.; Suzuki, M.; Coelho, P.G. Implant design and its effects on osseointegration over time within cortical and trabecular bone. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1091–1097. [Google Scholar] [CrossRef]
- Buser, D.; Schenk, R.; Steinemann, S.; Fiorellini, J.; Fox, C.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef]
- Brizuela-Velasco, A.; Álvarez-Arenal, Á.; Gil-Mur, F.J.; Herrero-Climent, M.; Chávarri-Prado, D.; Chento-Valiente, Y.; Dieguez-Pereira, M. Relationship between Insertion Torque and Resonance Frequency Measurements, Performed by Resonance Frequency Analysis, in Micromobility of Dental Implants: An In Vitro Study. Implant. Dent. 2015, 24, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Granato, R.; Suzuki, M.; Gil, J.N.; Janal, M.N.; Coelho, P.G. Histomorphologic and histomorphometric evaluation of various endosseous implant healing chamber configurations at early implantation times: A study in dogs. Clin. Oral Implant. Res. 2010, 21, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Huwais, S.; Meyer, E.G. A Novel Osseous Densification Approach in Implant Osteotomy Preparation to Increase Biomechanical Primary Stability, Bone Mineral Density, and Bone-to-Implant Contact. Int. J. Oral Maxillofac. Implant. 2017, 32, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Huwais, S.; Mazor, Z.; Ioannou, A.L.; Gluckman, H.; Neiva, R. A Multicenter Retrospective Clinical Study with Up-to-5-Year Follow-up Utilizing a Method that Enhances Bone Density and Allows for Transcrestal Sinus Augmentation through Compaction Grafting. Int. J. Oral Maxillofac. Implant. 2018, 33, 1305–1311. [Google Scholar] [CrossRef]
- Goiato, M.C.; dos Santos, D.M.; Santiago, J.M., Jr.; Moreno, A.; Pellizzer, E.P. Longevity of dental implants in type IV bone: A systematic review. Int. J. Oral Maxillofac. Surg. 2014, 43, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E. Density of bone: Effect on treatment plans, surgical approach, healing, and progressive boen loading. Int. J. Oral Implantol. 1990, 6, 23–31. [Google Scholar]
- Bilhan, H.; Geckili, O.; Mumcu, E.; Bozdag, E.; Sünbüloğlu, E.; Kutay, O. Influence of surgical technique, implant shape and diameter on the primary stability in cancellous bone. J. Oral Rehabil. 2010, 37, 900–907. [Google Scholar] [CrossRef]
- Turkyilmaz, I.; Aksoy, U.; McGlumphy, E.A. Two alternative surgical techniques for enhancing primary implant stability in the posterior maxilla: A clinical study including bone density, insertion torque, and resonance frequency analysis data. Clin. Implat. Dent. Relat. Res. 2008, 10, 231–237. [Google Scholar] [CrossRef]
- Jimbo, R.; Giro, G.; Marin, C.; Granato, R.; Suzuki, M.; Tovar, N.; Lilin, T.; Janal, M.; Coelho, P.G. Simplified drilling technique does not decrease dental implant osseointegration: A preliminary report. J. Periodontol. 2013, 84, 1599–1605. [Google Scholar] [CrossRef]
- Mello-Machado, R.C.; de Almeida Barros Mourão, C.F.; Javid, K.; Ferreira, H.T.; Montemezzi, P.; Calasans-Maia, M.D.; Senna, P.M.S. Clinical Assessment of Dental Implants Placed in Low-Quality Bone Sites Prepared for the Healing Chamber with Osseodensification Concept: A Double-Blind, Randomized Clinical Trial. Appl. Sci. 2021, 11, 640. [Google Scholar] [CrossRef]
- Machado, R.C.M.; da Gama, C.S.; Batista, S.H.; Rizzo, D.; Valiense, H.; Moreira, R.F. Tomographic and clinical findings, pre-, trans-, and post-operative, of osseodensification in immediate loading. Int. J. Growth Factors Stem Cells Dent. 2018, 1, 101–105. [Google Scholar] [CrossRef]
- Lopez, C.D.; Alifarag, A.M.; Torroni, A.; Tovar, N.; Diaz-Siso, J.R.; Witek, L.; Rodriguez, E.D.; Coelho, P.G. Osseodensification for enhancement of spinal surgical hardware fixation. J. Mech. Behav. Biomed. Mater. 2017, 69, 275–281. [Google Scholar] [CrossRef]
- Padhye, N.M.; Padhye, A.M.; Bhatavadekar, N.B. Osseodensification—A systematic review and qualitative analysis of published literature. J. Oral Biol. Craniofac. Res. 2020, 10, 375–380. [Google Scholar] [CrossRef]
- Mello-Machado, R.C.; Sartoretto, S.C.; Granjeiro, J.M.; Calasans-Maia, J.A.; de Uzeda, M.; Mourão, C.; Ghiraldini, B.; Bezerra, F.J.B.; Senna, P.M.; Calasans-Maia, M.D. Osseodensification enables bone healing chambers with improved low-density bone site primary stability: An in vivo study. Sci. Rep. 2021, 11, 15436. [Google Scholar] [CrossRef]
- Pikos, M.A.; Miron, R.J. Osseodensification: An Overview of Scientific Rationale and Biological Background. Compend. Contin. Educ. Dent. 2019, 40, 217–222; quiz 223. [Google Scholar]
- Mikic, M.; Vlahovic, Z.; Stevanović, M.; Arsic, Z.; Mladenovic, R. The Importance of Correlation between CBCT Analysis of Bone Density and Primary Stability when Choosing the Design of Dental Implants-Ex Vivo Study. Tomography 2022, 8, 1293–1306. [Google Scholar] [CrossRef]
- Vidyasagar, L.; Salms, G.; Apse, P.; Teibe, U.J.S. The influence of site preparation (countersinking) on initial dental implant stability, an in vitro study using resonance frequency analysis. Stomatologija 2004, 6, 14–16. [Google Scholar]
- Bhargava, N.; Perrotti, V.; Caponio, V.C.A.; Matsubara, V.H.; Patalwala, D.; Quaranta, A. Comparison of heat production and bone architecture changes in the implant site preparation with compressive osteotomes, osseodensification technique, piezoelectric devices, and standard drills: An ex vivo study on porcine ribs. Odontology 2023, 111, 142–153. [Google Scholar] [CrossRef]
- Lahens, B.; Neiva, R.; Tovar, N.; Alifarag, A.M.; Jimbo, R.; Bonfante, E.A.; Bowers, M.M.; Cuppini, M.; Freitas, H.; Witek, L. Biomechanical and histologic basis of osseodensification drilling for endosteal implant placement in low density bone. An experimental study in sheep. J. Mech. Behav. Biomed. Mater. 2016, 63, 56–65. [Google Scholar] [CrossRef]
- Trisi, P.; Berardini, M.; Falco, A.; Vulpiani, M.P. New osseodensification implant site preparation method to increase bone density in low-density bone: In vivo evaluation in sheep. Implant. Dent. 2016, 25, 24. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Xhajanka, E.; Romeo, D.M.; Romeo, M.; Zappone, C.M.F.; Malcangi, G.; Scarano, A.; Lorusso, F.; et al. The Effectiveness of Osseodensification Drilling Protocol for Implant Site Osteotomy: A Systematic Review of the Literature and Meta-Analysis. Materials 2021, 14, 1147. [Google Scholar] [CrossRef]
- Neiva, R.; Tanello, B.; Duarte, W.; Coelho, P.; Witek, L.; Silva, F.J. Effects of osseodensification on Astra TX and EV implant systems. Clin. Oral Implant. Res. 2018, 29, 444. [Google Scholar] [CrossRef]
- Oliveira, P.; Bergamo, E.T.P.; Neiva, R.; Bonfante, E.A.; Witek, L.; Tovar, N.; Coelho, P.G. Osseodensification outperforms conventional implant subtractive instrumentation: A study in sheep. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 300–307. [Google Scholar] [CrossRef]
- Bätz, J.; Syrigos, S.; Vorbeck, M.; Prüch, E.; Campbell, G.; Morlock, M. The influence of broach design on bone friction and osseodensification in total hip arthroplasty. Clin. Biomech. 2020, 73, 234–240. [Google Scholar] [CrossRef]
- Gaikwad, A.M.; Joshi, A.A.; Nadgere, J.B. Biomechanical and histomorphometric analysis of endosteal implants placed by using the osseodensification technique in animal models: A systematic review and meta-analysis. J. Prosthet. Dent. 2022, 127, 61–70. [Google Scholar] [CrossRef]
- Alifarag, A.M.; Lopez, C.D.; Neiva, R.F.; Tovar, N.; Witek, L.; Coelho, P.G. Atemporal osseointegration: Early biomechanical stability through osseodensification. J. Orthop. Res. 2018, 36, 2516–2523. [Google Scholar] [CrossRef]
- Karl, M.; Grobecker-Karl, T. Effect of bone quality, implant design, and surgical technique on primary implant stability. Quintessence Int. 2018, 22, 189–198. [Google Scholar]
- Lahens, B.; Lopez, C.D.; Neiva, R.F.; Bowers, M.M.; Jimbo, R.; Bonfante, E.A.; Morcos, J.; Witek, L.; Tovar, N.; Coelho, P.G. The effect of osseodensification drilling for endosteal implants with different surface treatments: A study in sheep. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 615–623. [Google Scholar] [CrossRef]
- de Carvalho Formiga, M.; Grzech-Leśniak, K.; Moraschini, V.; Shibli, J.A.; Neiva, R. Effects of Osseodensification on Immediate Implant Placement: Retrospective Analysis of 211 Implants. Materials 2022, 15, 3539. [Google Scholar] [CrossRef]
- de Carvalho Formiga, M.; da Silva, H.D.P.; Ghiraldini, B.; Siroma, R.S.; Ardelean, L.C.; Piattelli, A.; Shibli, J.A. Effects of Osseodensification on Primary Stability of Cylindrical and Conical Implants-An Ex Vivo Study. J. Clin. Med. 2023, 12, 3736. [Google Scholar] [CrossRef]
- Das, N. The New Bone Drilling Concept-Osseodensification (Hydrodynamic Bone Preparation). EC Dent. Sci. 2019, 18, 2345–2355. [Google Scholar]
- Bergamo, E.T.P.; Zahoui, A.; Barrera, R.B.; Huwais, S.; Coelho, P.G.; Karateew, E.D.; Bonfante, E.A. Osseodensification effect on implants primary and secondary stability: Multicenter controlled clinical trial. Clin. Implant. Dent. Relat. Res. 2021, 23, 317–328. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.; Gold, J.; Somohano Marquez, T.; Romanos, G. Under-Drilling versus Hybrid Osseodensification Technique: Differences in Implant Primary Stability and Bone Density of the Implant Bed Walls. Materials 2020, 13, 390. [Google Scholar] [CrossRef]
- Devlin, H.; Horner, K.; Ledgerton, D. A comparison of maxillary and mandibular bone mineral densities. J. Prosthet. Dent. 1998, 79, 323–327. [Google Scholar] [CrossRef]
- do Carmo Filho, L.C.; Faot, F.; de Matos Madruga, M.; Marcello-Machado, R.M.; Bordin, D.; Cury, A.A.D.B. Effect of implant macrogeometry on peri-implant healing outcomes: A randomized clinical trial. Clin. Oral Investig. 2019, 23, 567–575. [Google Scholar] [CrossRef]
- Ren, Y.; Senarathna, J.; Grayson, W.L.; Pathak, A.P. State-of-the-art techniques for imaging the vascular microenvironment in craniofacial bone tissue engineering applications. Am. J. Physiol. Cell Physiol. 2022, 323, C1524–C1538. [Google Scholar] [CrossRef]
- Javed, F.; Ahmed, H.B.; Crespi, R.; Romanos, G.E. Role of primary stability for successful osseointegration of dental implants: Factors of influence and evaluation. Interv. Med. Appl. Sci. 2013, 5, 162–167. [Google Scholar] [CrossRef]




| Group | Drill Direction | Xenograft Bone |
|---|---|---|
| CW | Clockwise | No |
| CW + XB | Clockwise | Yes |
| CCW | Counterclockwise | No |
| CCW + XB | Counterclockwise | Yes |
| Parameters | CW | CW + XB | CCW | CCW + XB |
|---|---|---|---|---|
| BV (%) | 37.0 ± 1.1 A | 55.2 ± 1.9 B | 50.6 ± 2.3 C | 56.8 ± 2.1 B |
| Tb.Th (mm) | 0.41 ± 0.02 A | 0.76 ± 0.02 B | 0.61 ± 0.03 C | 0.78 ± 0.03 B |
| Tb.Sp (mm) | 2.40 ± 0.16 A | 2.11 ± 0.12 B | 2.14 ± 0.12 B | 2.01 ± 0.11 B |
| Po(tot) (%) | 64.0 ± 3.1 A | 48.3 ± 1.9 B | 49.9 ± 2.9 B | 43.5 ± 2.4 C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, H.; Mourão, C.F.; Mello-Machado, R.C.; Javid, K.; dos Santos Pereira, R.; Senna, P.M. Ex Vivo Analysis of Ability of Osseodensification to Improve Dental Implant Primary Stability Using Xenograft Bone Walls. Appl. Sci. 2023, 13, 12337. https://doi.org/10.3390/app132212337
Ferreira H, Mourão CF, Mello-Machado RC, Javid K, dos Santos Pereira R, Senna PM. Ex Vivo Analysis of Ability of Osseodensification to Improve Dental Implant Primary Stability Using Xenograft Bone Walls. Applied Sciences. 2023; 13(22):12337. https://doi.org/10.3390/app132212337
Chicago/Turabian StyleFerreira, Henrique, Carlos Fernando Mourão, Rafael Coutinho Mello-Machado, Kayvon Javid, Rodrigo dos Santos Pereira, and Plinio Mendes Senna. 2023. "Ex Vivo Analysis of Ability of Osseodensification to Improve Dental Implant Primary Stability Using Xenograft Bone Walls" Applied Sciences 13, no. 22: 12337. https://doi.org/10.3390/app132212337
APA StyleFerreira, H., Mourão, C. F., Mello-Machado, R. C., Javid, K., dos Santos Pereira, R., & Senna, P. M. (2023). Ex Vivo Analysis of Ability of Osseodensification to Improve Dental Implant Primary Stability Using Xenograft Bone Walls. Applied Sciences, 13(22), 12337. https://doi.org/10.3390/app132212337






