Patients with Chronic Coronary Syndrome Can Benefit from Consumption of Enriched Chicken Eggs: The Effects on Microvascular Function, Inflammatory Biomarkers, and Oxidative Status—Randomized Clinical Study
Abstract
:1. Introduction
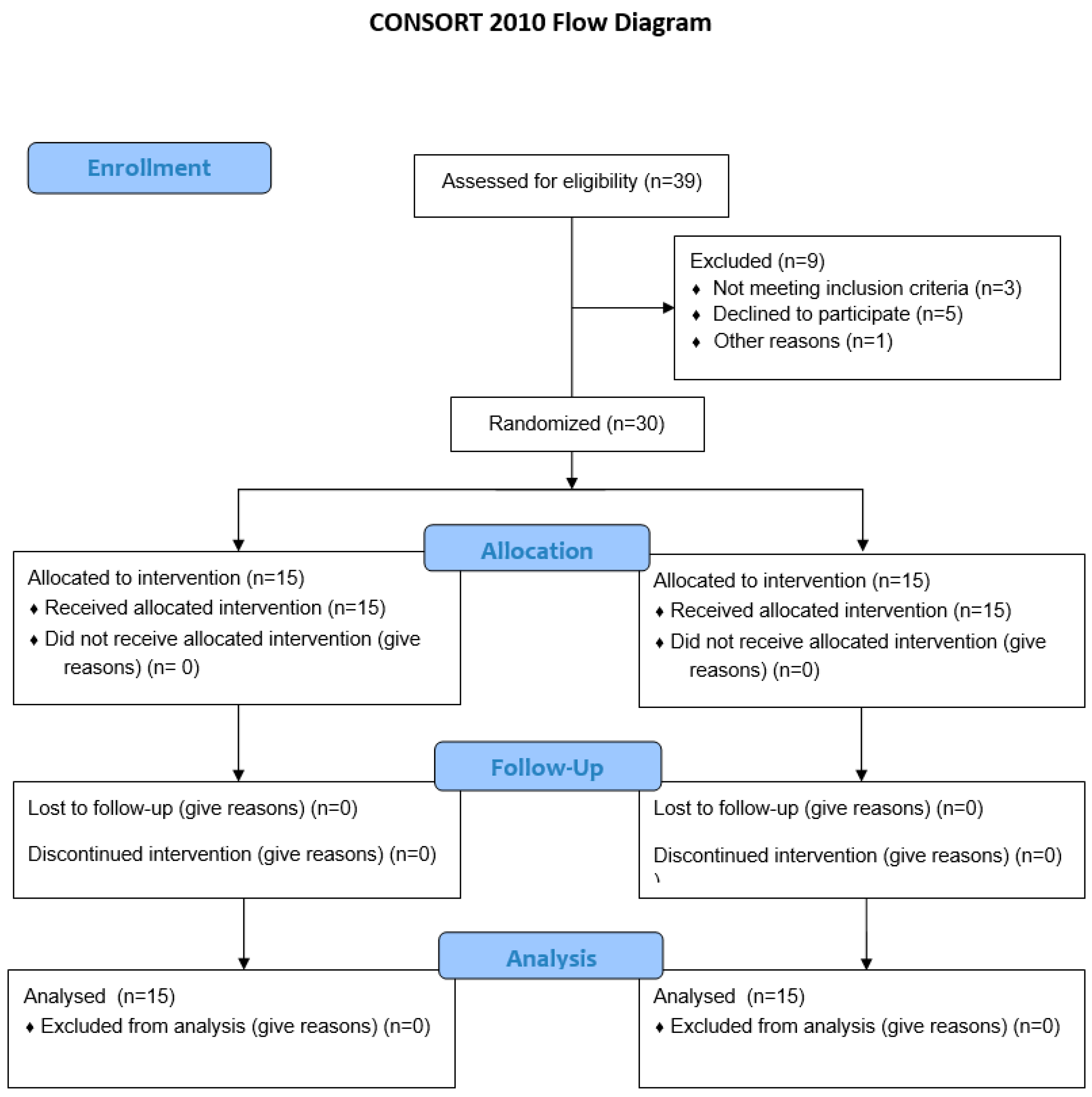
2. Materials and Methods
2.1. Study Population
2.2. The Research Procedure
2.3. Anthropometric Characteristics of the Study Population and Hemodynamic Parameters
2.4. Serum Lipid Levels and Biochemical Marker Analysis
2.5. Serum Free Fatty Acid, Selenium, Vitamin E, and Lutein Concentrations
2.6. Microvascular Endothelium-Dependent and -Independent Vasodilation
2.7. Macrovascular Endothelium-Dependent and -Independent Vasodilation
2.8. Serum Protein Concentrations of eNOS, nNOS, and iNOS
2.9. Biomarkers of Oxidative Stress and Antioxidant Capacity
2.10. Serum Concentration of Anti- and Proinflammatory Cytokines and Chemokines
2.11. Statistical Analysis
3. Results
3.1. Anthropometric Characteristics of the Study Population and Hemodynamic Parameters
3.2. Serum Lipid Levels and Biochemical Marker Analysis
3.3. Serum Free Fatty Acid, Selenium, Vitamin E, and Lutein Concentrations
3.4. Microvascular Endothelium-Dependent and -Independent Vasodilation
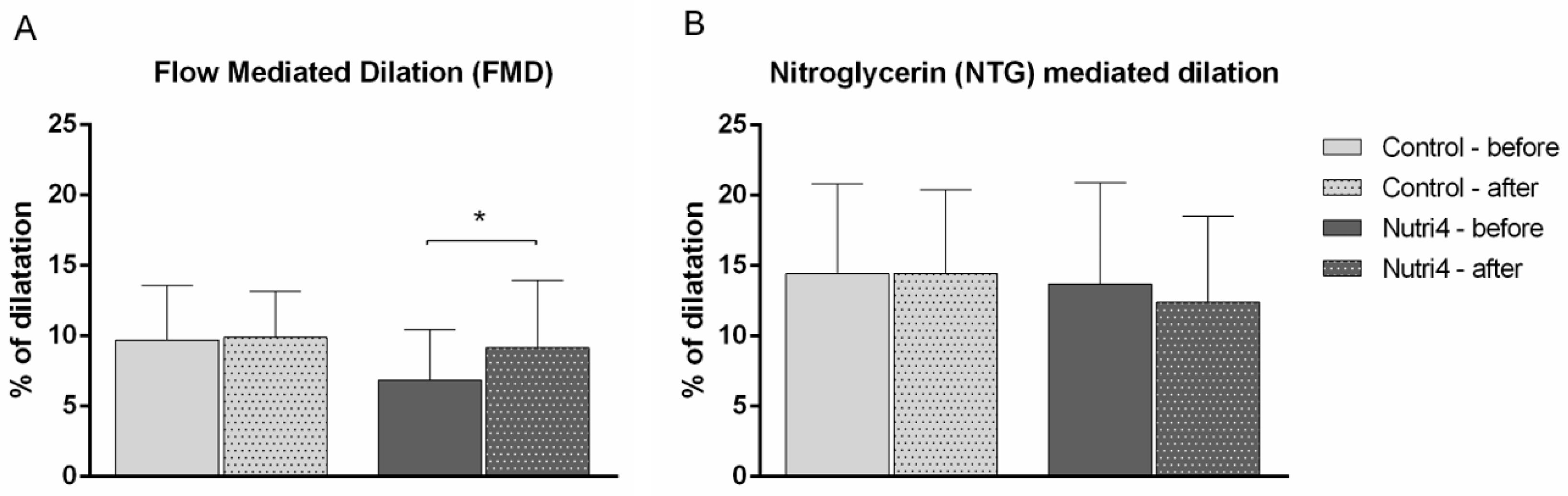
3.5. Macrovascular Endothelium-Dependent and -Independent Vasodilation
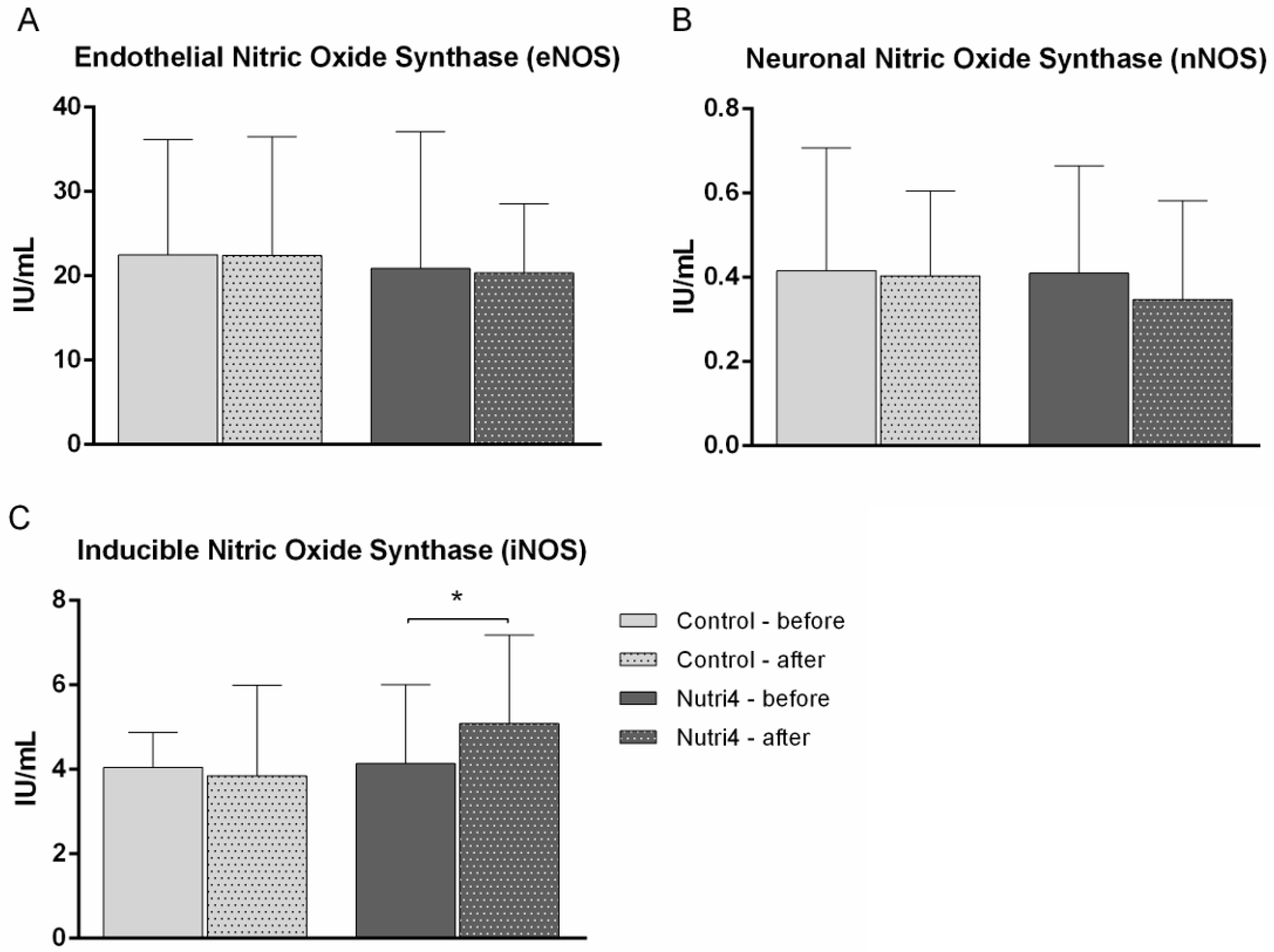
3.6. Serum Protein Concentrations of eNOS, nNOS, and iNOS
3.7. Oxidative Stress Biomarkers and Antioxidant Capacity
3.8. Serum Protein Concentrations of Anti- and Proinflammatory Cytokines and Chemokines
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [PubMed]
- Rana, J.S.; Khan, S.S.; Lloyd-Jones, D.M.; Sidney, S. Changes in Mortality in Top 10 Causes of Death from 2011 to 2018. J. Gen. Intern. Med. 2021, 36, 2517–2518. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; D’Amato, A.; Pucci, M.; Infusino, F.; Adamo, F.; Birtolo, L.I.; Netti, L.; Montefusco, G.; Chimenti, C.; Lavalle, C.; et al. Ischemic Heart Disease Pathophysiology Paradigms Overview: From Plaque Activation to Microvascular Dysfunction. Int. J. Mol. Sci. 2020, 21, 8118. [Google Scholar]
- Li, Y.; Hruby, A.; Bernstein, A.M.; Ley, S.H.; Wang, D.D.; Chiuve, S.E.; Sampson, L.; Rexrode, K.M.; Rimm, E.B.; Willett, W.C.; et al. Saturated Fats Compared with Unsaturated Fats and Sources of Carbohydrates in Relation to Risk of Coronary Heart Disease. J. Am. Coll. Cardiol. 2015, 66, 1538–1548. [Google Scholar] [CrossRef]
- Lăcătușu, C.-M.; Grigorescu, E.-D.; Floria, M.; Onofriescu, A.; Mihai, B.-M. The Mediterranean Diet: From an Environment-Driven Food Culture to an Emerging Medical Prescription. Int. J. Environ. Res. Public Health 2019, 16, 942. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Huang, T.; Yu, Y.; Hu, X.; Yang, B.; Li, D. Fish consumption and CHD mortality: An updated meta-analysis of seventeen cohort studies. Public Health Nutr. 2012, 15, 725–737. [Google Scholar] [PubMed]
- Violi, F.; Nocella, C.; Loffredo, L.; Carnevale, R.; Pignatelli, P. Interventional study with vitamin E in cardiovascular disease and meta-analysis. Free Radic. Biol. Med. 2022, 178, 26–41. [Google Scholar]
- Hajizadeh-Sharafabad, F.; Ghoreishi, Z.; Maleki, V.; Tarighat-Esfanjani, A. Mechanistic insights into the effect of lutein on atherosclerosis, vascular dysfunction, and related risk factors: A systematic review of in vivo, ex vivo and in vitro studies. Pharmacol. Res. 2019, 149, 104477. [Google Scholar]
- Simopoulos, A.P. Omega–3 Fatty Acids, Exercise, Physical Activity and Athletics. In Nutrition and Fitness: Cultural, Genetic and Metabolic Aspects; KARGER: Basel, Switzerland, 2008; pp. 23–50. [Google Scholar]
- Kromhout, D.; de Goede, J. Update on cardiometabolic health effects of ω-3 fatty acids. Curr. Opin. Lipidol. 2014, 25, 85–90. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Kitts, D.; Giovannucci, E.L.; Sahye-Pudaruth, S.; Paquette, M.; Blanco Mejia, S.; Patel, D.; Kavanagh, M.; Tsirakis, T.; Kendall, C.W.; et al. Selenium, antioxidants, cardiovascular disease, and all-cause mortality: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2020, 112, 1642–1652. [Google Scholar] [CrossRef]
- Ćurić, Ž.B.; Masle, A.M.; Kibel, A.; Selthofer-Relatić, K.; Stupin, A.; Mihaljević, Z.; Jukić, I.; Stupin, M.; Matić, A.; Kozina, N.; et al. Effects of n-3 Polyunsaturated Fatty Acid-Enriched Hen Egg Consumption on the Inflammatory Biomarkers and Microvascular Function in Patients with Acute and Chronic Coronary Syndrome—A Randomized Study. Biology 2021, 10, 774. [Google Scholar] [CrossRef] [PubMed]
- Šušnjara, P.; Mihaljević, Z.; Stupin, A.; Kolobarić, N.; Matić, A.; Jukić, I.; Kralik, Z.; Kralik, G.; Miloloža, A.; Pavošević, T.; et al. Consumption of Nutritionally Enriched Hen Eggs Enhances Endothelium-Dependent Vasodilation via Cyclooxygenase Metabolites in Healthy Young People—A Randomized Study. Nutrients 2023, 15, 1599. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J. Role of Endothelial Dysfunction in Atherosclerosis. Circulation 2004, 109, III-27–III-32. [Google Scholar] [CrossRef] [PubMed]
- Rubbo, H.; Trostchansky, A.; Botti, H.; Batthyány, C. Interactions of Nitric Oxide and Peroxynitrite with Low-Density Lipoprotein. Biol. Chem. 2002, 383, 547–552. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Drenjančević, I.; Kralik, G.; Kralik, Z.; Mihalj, M.; Stupin, A.; Novak, S.; Grčević, M. Polyunsaturated Fatty Acids on Cardiovascular Health: Revealing Potentials of Functional Food. In Superfood and Functional Food—The Development of Superfoods and Their Roles as Medicine; InTech: Rijeka, Croatia, 2017. [Google Scholar]
- Wang, L.; Summerhill, K.; Rodriguez-Canas, C.; Mather, I.; Patel, P.; Eiden, M.; Young, S.; Forouhi, N.G.; Koulman, A. Development and validation of a robust automated analysis of plasma phospholipid fatty acids for metabolic phenotyping of large epidemiological studies. Genome Med. 2013, 5, 39. [Google Scholar] [CrossRef]
- Jargar, J.G.; Hattiwale, S.H.; Das, S.; Dhundasi, S.A.; Das, K.K. A modified simple method for determination of serum α-tocopherol (vitamin E). J. Basic Clin. Physiol. Pharmacol. 2012, 23, 45–58. [Google Scholar] [CrossRef]
- Cavka, A.; Cosic, A.; Grizelj, I.; Koller, A.; Jelakovic, B.; Lombard, J.H.; Phillips, S.A.; Drenjancevic, I. Effects of AT1 Receptor Blockade on Plasma Thromboxane A 2 (TXA 2 ) Level and Skin Microcirculation in Young Healthy Women on Low Salt Diet. Kidney Blood Press. Res. 2013, 37, 432–442. [Google Scholar] [CrossRef]
- Lenasi, H.; Štrucl, M. The effect of nitric oxide synthase and cyclooxygenase inhibition on cutaneous microvascular reactivity. Eur. J. Appl. Physiol. 2008, 103, 719–726. [Google Scholar]
- Stupin, M.; Stupin, A.; Rasic, L.; Cosic, A.; Kolar, L.; Seric, V.; Lenasi, H.; Izakovic, K.; Drenjancevic, I. Acute exhaustive rowing exercise reduces skin microvascular dilator function in young adult rowing athletes. Eur. J. Appl. Physiol. 2018, 118, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Oakes, K.D.; Van Der Kraak, G.J. Utility of the TBARS assay in detecting oxidative stress in white sucker (Catostomus commersoni) populations exposed to pulp mill effluent. Aquat. Toxicol. 2003, 63, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Academic Press: San Diego, CA, USA, 1984; pp. 121–126. [Google Scholar]
- Habig, W.H.; Jakoby, W.B. [27] Glutathione S-transferases (rat and human). In Methods in Enzymol; Academic Press: New York, NY, USA, 1981; pp. 218–231. [Google Scholar]
- Flohé, L.; Ötting, F. [10] Superoxide dismutase assays. In Methods in Enzymology; Academic Press: San Diego, CA, USA, 1984; pp. 93–104. [Google Scholar]
- Ference, B.A.; Graham, I.; Tokgozoglu, L.; Catapano, A.L. Impact of Lipids on Cardiovascular Health. J. Am. Coll. Cardiol. 2018, 72, 1141–1156. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, T.A.; Ito, M.K.; Maki, K.C.; Orringer, C.E.; Bays, H.E.; Jones, P.H.; McKenney, J.M.; Grundy, S.M.; Gill, E.A.; Wild, R.A.; et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: Part 1—Executive summary. J. Clin. Lipidol. 2014, 8, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, T.A.; Glickstein, S.B.; Rowe, J.D.; Soni, P.N. Effects of eicosapentaenoic acid and docosahexaenoic acid on low-density lipoprotein cholesterol and other lipids: A review. J. Clin. Lipidol. 2012, 6, 5–18. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk. JAMA 2020, 324, 2268. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar]
- Zehr, K.R.; Walker, M.K. Omega-3 polyunsaturated fatty acids improve endothelial function in humans at risk for atherosclerosis: A review. Prostaglandins Other Lipid Mediat. 2018, 134, 131–140. [Google Scholar] [CrossRef]
- Goodfellow, J.; Bellamy, M.F.; Ramsey, M.W.; Jones, C.J.; Lewis, M.J. Dietary supplementation with marine omega-3 fatty acids improve systemic large artery endothelial function in subjects with hypercholesterolemia. J. Am. Coll. Cardiol. 2000, 35, 265–270. [Google Scholar] [CrossRef]
- Gać, P.; Czerwińska, K.; Macek, P.; Jaremków, A.; Mazur, G.; Pawlas, K.; Poręba, R. The importance of selenium and zinc deficiency in cardiovascular disorders. Environ. Toxicol. Pharmacol. 2021, 82, 103553. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Aaseth, J.; Alexander, J.; Johansson, P. Still reduced cardiovascular mortality 12 years after supplementation with selenium and coenzyme Q10 for four years: A validation of previous 10-year follow-up results of a prospective randomized double-blind placebo-controlled trial in elderly. PLoS ONE 2018, 13, e0193120. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xie, L.; Song, A.; Zhang, C. Selenium Status and Its Antioxidant Role in Metabolic Diseases. Oxid. Med. Cell. Longev. 2022, 2022, 7009863. [Google Scholar] [CrossRef]
- Benstoem, C.; Goetzenich, A.; Kraemer, S.; Borosch, S.; Manzanares, W.; Hardy, G.; Stoppe, C. Selenium and Its Supplementation in Cardiovascular Disease—What do We Know? Nutrients 2015, 7, 3094–3118. [Google Scholar] [CrossRef] [PubMed]
- Neunteufl, T.; Priglinger, U.; Heher, S.; Zehetgruber, M.; Söregi, G.; Lehr, S.; Huber, K.; Maurer, G.; Weidinger, F.; Kostner, K. Effects of vitamin E on chronic and acute endothelial dysfunction in smokers. J. Am. Coll. Cardiol. 2000, 35, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Mah, E.; Pei, R.; Guo, Y.; Masterjohn, C.; Ballard, K.D.; Taylor, B.A.; Taylor, A.W.; Traber, M.G.; Volek, J.S.; Bruno, R.S. Greater γ-tocopherol status during acute smoking abstinence with nicotine replacement therapy improved vascular endothelial function by decreasing 8-iso-15(S)-prostaglandin F 2α. Exp. Biol. Med. 2015, 240, 527–533. [Google Scholar] [CrossRef]
- Zakeri, N.; Kelishadi, M.R.; Asbaghi, O.; Naeini, F.; Afsharfar, M.; Mirzadeh, E.; Naserizadeh, S. kasra Selenium supplementation and oxidative stress: A review. PharmaNutrition 2021, 17, 100263. [Google Scholar] [CrossRef]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K.; Miyazawa, T. Vitamin E: Regulatory Redox Interactions. IUBMB Life 2019, 71, 430–441. [Google Scholar] [CrossRef]
- Barić, L.; Drenjančević, I.; Mihalj, M.; Matić, A.; Stupin, M.; Kolar, L.; Mihaljević, Z.; Mrakovčić-Šutić, I.; Šerić, V.; Stupin, A. Enhanced Antioxidative Defense by Vitamins C and E Consumption Prevents 7-Day High-Salt Diet-Induced Microvascular Endothelial Function Impairment in Young Healthy Individuals. J. Clin. Med. 2020, 9, 843. [Google Scholar] [CrossRef]
- Stirban, A.; Nandrean, S.; Götting, C.; Tamler, R.; Pop, A.; Negrean, M.; Gawlowski, T.; Stratmann, B.; Tschoepe, D. Effects of n–3 fatty acids on macro- and microvascular function in subjects with type 2 diabetes mellitus. Am. J. Clin. Nutr. 2010, 91, 808–813. [Google Scholar] [CrossRef]
- Hammer, A.; Moertl, D.; Schlager, O.; Matschuck, M.; Seidinger, D.; Koppensteiner, R.; Steiner, S. Effects of n-3 PUFA on endothelial function in patients with peripheral arterial disease: A randomised, placebo-controlled, double-blind trial. Br. J. Nutr. 2019, 122, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Ander, B.P.; Dupasquier, C.M.; Prociuk, M.A.; Pierce, G.N. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp. Clin. Cardiol. 2003, 8, 164–172. [Google Scholar] [PubMed]
- Omura, M.; Kobayashi, S.; Mizukami, Y.; Mogami, K.; Todoroki-Ikeda, N.; Miyake, T.; Matsuzaki, M. Eicosapentaenoic acid (EPA) induces Ca2+-independent activation and translocation of endothelial nitric oxide synthase and endothelium-dependent vasorelaxation. FEBS Lett. 2001, 487, 361–366. [Google Scholar] [CrossRef]
- Stebbins, C.L.; Stice, J.P.; Hart, C.M.; Mbai, F.N.; Knowlton, A.A. Effects of Dietary Decosahexaenoic Acid (DHA) on eNOS in Human Coronary Artery Endothelial Cells. J. Cardiovasc. Pharmacol. Ther. 2008, 13, 261–268. [Google Scholar] [CrossRef]
- Wilmes, V.; Scheiper, S.; Roehr, W.; Niess, C.; Kippenberger, S.; Steinhorst, K.; Verhoff, M.A.; Kauferstein, S. Increased inducible nitric oxide synthase (iNOS) expression in human myocardial infarction. Int. J. Legal Med. 2020, 134, 575–581. [Google Scholar] [CrossRef]
- Quaschning, T.; Voss, F.; Herzfeld, S.; Relle, K.; Kalk, P.; Godes, M.; Pfab, T.; Kraemer-Guth, A.; Bonz, A.W.; Theuring, F.; et al. Lack of iNOS Impairs Endothelial Function in Endothelin-1 Transgenic Mice. Kidney Blood Press. Res. 2008, 31, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Kolls, J.K. Interluekin-17A (IL17A). Gene 2017, 614, 8–14. [Google Scholar] [CrossRef]
- Gong, F.; Liu, Z.; Liu, J.; Zhou, P.; Liu, Y.; Lu, X. The paradoxical role of IL-17 in atherosclerosis. Cell. Immunol. 2015, 297, 33–39. [Google Scholar] [CrossRef]
- Allam, G.; Abdel-Moneim, A.; Gaber, A.M. The pleiotropic role of interleukin-17 in atherosclerosis. Biomed. Pharmacother. 2018, 106, 1412–1418. [Google Scholar] [CrossRef]
- Nordlohne, J.; von Vietinghoff, S. Interleukin 17A in atherosclerosis—Regulation and pathophysiologic effector function. Cytokine 2019, 122, 154089. [Google Scholar] [CrossRef] [PubMed]
- Hot, A.; Lavocat, F.; Lenief, V.; Miossec, P. Simvastatin inhibits the pro-inflammatory and pro-thrombotic effects of IL-17 and TNF-α on endothelial cells. Ann. Rheum. Dis. 2013, 72, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Chehimi, M.; Ward, R.; Pestel, J.; Robert, M.; Pesenti, S.; Bendridi, N.; Michalski, M.; Laville, M.; Vidal, H.; Eljaafari, A. Omega-3 Polyunsaturated Fatty Acids Inhibit IL-17A Secretion through Decreased ICAM-1 Expression in T Cells Co-Cultured with Adipose-Derived Stem Cells Harvested from Adipose Tissues of Obese Subjects. Mol. Nutr. Food Res. 2019, 63, 1801148. [Google Scholar] [CrossRef]
- Kim, K.-W.; Kim, B.-M.; Won, J.-Y.; Min, H.K.; Lee, S.J.; Lee, S.-H.; Kim, H.-R. Tocotrienol regulates osteoclastogenesis in rheumatoid arthritis. Korean J. Intern. Med. 2021, 36, S273–S282. [Google Scholar] [CrossRef] [PubMed]
- Hinck, A.P.; Mueller, T.D.; Springer, T.A. Structural Biology and Evolution of the TGF-β Family. Cold Spring Harb. Perspect. Biol. 2016, 8, a022103. [Google Scholar] [CrossRef]
- Xia, M.; Wu, F.; Yang, Y.; Lu, W.; Song, M.; Ma, Z. The possibility of visualizing TGF-β1 expression in ApoE−/− mice atherosclerosis using MR targeted imaging. Acta Radiol. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Nurgazieva, D.; Mickley, A.; Moganti, K.; Ming, W.; Ovsyi, I.; Popova, A.; Sachindra; Awad, K.; Wang, N.; Bieback, K.; et al. TGF-β1, but Not Bone Morphogenetic Proteins, Activates Smad1/5 Pathway in Primary Human Macrophages and Induces Expression of Proatherogenic Genes. J. Immunol. 2015, 194, 709–718. [Google Scholar] [CrossRef]
- Panutsopulos, D.; Papalambros, E.; Sigala, F.; Zafiropoulos, A.; Arvanitis, D.L.; Spandidos, D.A. Protein and mRNA expression levels of VEGF-A and TGF-beta1 in different types of human coronary atherosclerotic lesions. Int. J. Mol. Med. 2005, 15, 603–610. [Google Scholar]
- Chen, P.-Y.; Qin, L.; Li, G.; Wang, Z.; Dahlman, J.E.; Malagon-Lopez, J.; Gujja, S.; Cilfone, N.A.; Kauffman, K.J.; Sun, L.; et al. Endothelial TGF-β signalling drives vascular inflammation and atherosclerosis. Nat. Metab. 2019, 1, 912–926. [Google Scholar] [CrossRef]

| Group | Control | Nutri4 | ||
|---|---|---|---|---|
| Parameter | before | after | before | after |
| N (F/M) | 15 (5/10) | 15 (4/11) | ||
| Age (years) | 59 ± 10 | 59 ± 8 | ||
| BMI (kg/m2) | 30.3 ± 4.7 | 30.2 ± 4.6 | 32.1 ± 6.5 | 32.1 ± 6.6 |
| WHR | 0.93 ± 0.08 | 0.93 ± 0.08 | 0.93 ± 0.07 | 0.94 ± 0.07 |
| HR (beats per minute) | 68 ±11 | 66 ± 10 | 65 ± 8 | 64 ± 9 |
| SBP (mmHg) | 125 ± 21 | 123 ± 16 | 122 ± 17 | 123 ± 15 |
| MBP (mmHg) | 94 ± 14 | 92 ± 11 | 93 ± 13 | 93 ± 11 |
| DBP (mmHg) | 82 ± 17 | 77 ± 10 | 78 ± 12 | 78 ± 10 |
| Glucose (mmol/L) | 7.1 ± 2.4 | 6.5 ± 1.6 | 6.3 ± 1.9 | 6.2 ± 1.9 |
| hsCRP (mg/L) | 1.56 ± 1.30 | 2.14 ± 2.81 | 1.28 ± 0.83 | 1.32 ± 1.26 |
| Urea (mmol/L) | 5.8 ± 1.8 | 6.4 ± 2.7 | 6.4 ± 2.1 | 6.7 ± 2.1 |
| Creatinine (µmol/L) | 74.1 ± 20.1 | 76.3 ± 27.4 | 80.7 ± 18.4 | 80.3 ± 13.1 |
| Iron (μmol/L) | 15.5 ± 4.7 | 14.3 ± 4.0 | 17.0 ± 5.8 | 15.1 ± 5.8 |
| Ferritin (μg/L) | 161.6 ± 133.2 | 149.9 ± 136.8 * | 151.8 ± 117.8 | 138.9 ± 115.7 |
| Transferrin (g/L) | 2.50 ± 0.45 | 2.46 ± 0.38 | 2.53 ± 0.50 | 2.58 ± 0.37 |
| Sodium (mmol/L) | 139.7 ± 2.2 | 139.6 ± 2.1 | 139.5 ± 3.1 | 139.7 ± 2.6 |
| Calcium (mmol/L) | 2.39 ± 0.09 | 2.40 ± 0.10 | 2.37 ± 0.07 | 2.36 ± 0.10 |
| Potassium (mmol/L) | 4.3 ± 0.3 | 4.4 ± 0.3 | 4.4 ± 0.3 | 4.3 ± 0.4 |
| Cholesterol (mmol/L) | 3.53 ± 0.63 | 3.55 ± 0.62 | 4.14 ± 1.50 | 3.81 ± 0.87 |
| HDL cholesterol (mmol/L) | 1.20 ± 0.34 | 1.13 ± 0.34 | 1.20 ± 0.34 | 1.24 ± 0.40 |
| LDL cholesterol (mmol/L) | 2.01 ± 0.50 | 1.98 ± 0.51 | 2.52 ± 1.16 | 2.22 ± 0.54 |
| Triglycerides (mmol/L) | 1.44 ± 0.75 | 1.36 ± 0.66 | 1.58 ± 0.80 | 1.30 ± 0.60 * |
| ALT (U/L) | 39.5 ± 29.0 | 35.1 ± 14.3 | 34.6 ± 14.7 | 33.3 ± 13.8 |
| AST (U/L) | 29.0 ± 5.9 | 27.5 ± 6.3 | 28.8 ± 9.8 | 28.1 ± 7.9 |
| GGT (U/L) | 27.0 ± 20.0 | 25.4 ± 17.8 * | 25.6 ± 9.7 | 23.9 ± 8.7 * |
| Control (N = 15) | Nutri4 (N = 15) | |||||
|---|---|---|---|---|---|---|
| before | after | before | after | |||
| SFAs (μmol/L) | ||||||
| C8:0 Caprylic acid | N/F | N/F | 33.6 | 61.2 | ||
| C10:0 Capric acid | <LOQ | <LOQ | 67.8 ± 34.2 | 70.8 | ||
| C12:0 Lauric acid | 29.9 ± 10.5 | 92.8 | 27.7 ± 1.7 | 27.70 | ||
| C14:0 Myristic acid | 85.6 ± 5.5 | 98.5 ± 49.7 | 86.0 ± 27.6 | 69.1 ± 17.5 *† | ||
| C15:0 Pentadecylic acid | 15.0 ± 3.2 | 15.9 ± 3.2 | 16.4 ± 4.7 | 15.0 ± 2.8 | ||
| C16:0 Palmitic acid | 1936 ± 324 | 1958 ± 372 | 2263 ± 446 | 2112 ± 443 | ||
| C17:0 Margaric acid | 21.0 ± 5.6 | 22.0 ± 3.0 | 22.2 ± 4.3 | 20.9 ± 3.9 | ||
| C18:0 Stearic acid | 886 ± 192 | 815 ± 68 | 887 ± 83 | 826 ± 77 * | ||
| PUFAs (μmol/L) | ||||||
| n-7 | C16:1[cis-9] Palmitoleic acid | 311 ± 113 | 257 ± 135 | 218 ± 121 | 196 ± 105 † | |
| C17:1[cis-10] cis-10-Heptadecenoic acid | 14.0 ± 0.8 | <LOQ | <LOQ | 14.60 | ||
| C18:1[cis-9] Oleic acid | 930 ± 814 | 1421 ± 362 | 1550 ± 423 | 1554 ± 504 | ||
| n-9 | C20:1[cis-11] 11-Eicosenoic acid | 18.6 ± 10.8 | 12.7 ± 4.1 | 14.1 ± 4.6 | 14.4 ± 4.9 | |
| C24:1[cis-15] Nervonic acid | <LOQ | 9.73 ± 0.54 | 7.34 ± 0.96 | 6.86 ± 0.02 | ||
| n-6 | C18:2[cis-9.12] Linoleic acid | 1424 ± 359 | 1540 ± 205 | 1806 ± 407 | 1854 ± 906 | |
| C18:3[cis-6.9.12] gamma-Linolenic acid | 53.8 ± 10.8 | 59.6 ± 15.4 | 60.8 ± 12.8 | 46.2 ± 11.9 * | ||
| C21:2[cis-11.14] Eicosadienoic acid | 16.9 ± 0.5 | 15.8 ± 3.5 | 17.7 ± 3.2 | 16.2 ± 4.3 * | ||
| C20:3[cis-8.11.14] Dihomo-gamma-linolenic acid | 89.6 ± 21.8 | 99.9 ± 22.4 | 187.2 ± 74.8 | 140.8 ± 71.5 * | ||
| C20:4[cis-5.8.11.14] Arachidonic acid | 647 ± 179 | 759 ± 104 | 683 ± 172 | 683 ± 211 | ||
| n-3 | C18:3[cis-9.12.15] alpha-Linolenic acid | 17.4 ± 3.5 | 19.5 ± 4.6 | 21.3 ± 6.9 | 28.8 ± 14.9 | |
| 20:5[cis-5.8.11.14] Eicosa-5.8.11.14.17-pentaenoic acid | 20.7 ± 9.0 | 25.0 ± 13.0 | 29.0 ± 16.2 | 37.3 ± 13.7 *† | ||
| C22:6[cis-4.7.10.13.16.19] cis-4.7.10.13.16.19-Docosahexaenoic acid | 143.4 ± 132.2 | 165.3 ± 81.4 | 244.5 ± 90.5 | 296.0 ± 18.9 | ||
| n6/n3 PUFAs | 26.3 ± 19.9 | 23.3 ± 19.4 | 26.7 ± 24.8 | 17.5 ± 17.1 * | ||
| Group | Control (N = 15) | Nutri4 (N = 15) | ||
|---|---|---|---|---|
| Parameter | before | after | before | after |
| Se (µg/L) | 70.0 ± 16.1 | 75.5 ± 15.9 | 71.1 ± 19.3 | 85.1 ± 27.3 * |
| Vitamin E (ug/mL) | 8.99 ± 4.17 | 8.88 ± 3.97 | 7.17 ± 4.40 | 9.36 ± 4.44 * |
| Lutein (µmol/L) | 0.15 ± 0.06 | 0.13 ± 0.04 | 0.18 ± 0.06 | 0.16 ± 0.09 |
| Group | Control (N = 15) | Nutri4 (N = 15) | ||
|---|---|---|---|---|
| Parameter | before | after | before | after |
| Oxidative stress biomarkers | ||||
| TBARS (mM/L TE) | 0.029 ± 0.007 | 0.032 ± 0.008 | 0.033 ± 0.008 | 0.490 ± 0.007 |
| 8-iso-PGF2α (pg/mL) | 140 ± 58 | 129 ± 48 | 223 ± 266 | 227 ± 282 |
| AOPPs (μmol/L) | 0.056 ± 0.003 | 0.055 ± 0.003 | 0.056 ± 0.004 | 0.055 ± 0.003 |
| OxLDL (pg/mL) | 103 ± 10 | 102 ± 9 | 111 ± 7 | 110 ± 5 |
| Biomarkers of antioxidant defense | ||||
| FRAP (μm/MDA) | 0.250 ± 0.021 | 0.246 ± 0.034 | 0.397 ± 0.166 | 0.355 ± 0.128 |
| GPx (U/mg protein) | 0.021 ± 0.003 | 0.022 ± 0.002 | 0.017 ± 0.003 | 0.017 ± 0.003 |
| CAT (U/mg protein) | 4.716 ± 1.464 | 4.022 ± 1.054 | 5.138 ± 1.462 | 4.507 ± 1.873 |
| SOD (U/mg protein) | 5.073 ± 0.400 | 5.287 ± 0.259 | 5.400 ± 0.343 | 5.387 ± 0.233 |
| Group | Control (N = 15) | Nutri4 (N = 15) | ||
|---|---|---|---|---|
| Parameter | before | after | before | after |
| IL-17A (pg/mL) | 9.0 ± 0.23 | 8.69 ± 0.83 | 9.04 ± 0.25 | 7.73 ± 1.37 * |
| TGF-1β (pg/mL) | 2845 ± 867 | 3181 ± 587 | 2497 ± 1344 | 1556 ± 531 * |
| C3a (pg/mL) | 4232 ± 2639 | 4243 ± 2685 | 4548 ± 2296 | 4635 ± 1946 |
| TNF-α (pg/mL) | 3.42 ± 0.66 | 3.55 ± 0.5 | 3.47 ± 0.52 | 3.3 ± 0.46 |
| INF-γ (pg/mL) | 9.86 ± 1.73 | 9.55 ± 1.34 | 9.83 ± 1.50 | 9.36 ± 1.16 |
| IL-6 (pg/mL) | 18.1 ± 0.6 | 18.0 ± 0.8 | 17.7 ± 0.8 | 17.4 ± 1.3 |
| IL-10 (pg/mL) | 0.86 ± 0.28 | 0.87 ± 0.45 | 0.77 ± 0.09 | 0.77 ± 0.17 |
| IL-23 (pg/mL) | 9.89 ± 0.69 | 9.85 ± 0.58 | 10.24 ± 0.54 | 10.29 ± 0.92 |
| MCP-1 (pg/mL) | 37.4 ± 16.3 | 36.7 ± 22.6 | 24.6 ± 15.4 | 31.2 ± 2.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breškić Ćurić, Ž.; Stupin, A.; Masle, A.M.; Šušnjara, P.; Kozina, N.; Mihaljević, Z.; Jukić, I.; Kibel, A.; Kolobarić, N.; Juranić, B.; et al. Patients with Chronic Coronary Syndrome Can Benefit from Consumption of Enriched Chicken Eggs: The Effects on Microvascular Function, Inflammatory Biomarkers, and Oxidative Status—Randomized Clinical Study. Appl. Sci. 2023, 13, 12442. https://doi.org/10.3390/app132212442
Breškić Ćurić Ž, Stupin A, Masle AM, Šušnjara P, Kozina N, Mihaljević Z, Jukić I, Kibel A, Kolobarić N, Juranić B, et al. Patients with Chronic Coronary Syndrome Can Benefit from Consumption of Enriched Chicken Eggs: The Effects on Microvascular Function, Inflammatory Biomarkers, and Oxidative Status—Randomized Clinical Study. Applied Sciences. 2023; 13(22):12442. https://doi.org/10.3390/app132212442
Chicago/Turabian StyleBreškić Ćurić, Željka, Ana Stupin, Ana Marija Masle, Petar Šušnjara, Nataša Kozina, Zrinka Mihaljević, Ivana Jukić, Aleksandar Kibel, Nikolina Kolobarić, Brankica Juranić, and et al. 2023. "Patients with Chronic Coronary Syndrome Can Benefit from Consumption of Enriched Chicken Eggs: The Effects on Microvascular Function, Inflammatory Biomarkers, and Oxidative Status—Randomized Clinical Study" Applied Sciences 13, no. 22: 12442. https://doi.org/10.3390/app132212442
APA StyleBreškić Ćurić, Ž., Stupin, A., Masle, A. M., Šušnjara, P., Kozina, N., Mihaljević, Z., Jukić, I., Kibel, A., Kolobarić, N., Juranić, B., Nejašmić, D., Šporec, A., Lovrić, M., Selthofer-Relatić, K., & Drenjančević, I. (2023). Patients with Chronic Coronary Syndrome Can Benefit from Consumption of Enriched Chicken Eggs: The Effects on Microvascular Function, Inflammatory Biomarkers, and Oxidative Status—Randomized Clinical Study. Applied Sciences, 13(22), 12442. https://doi.org/10.3390/app132212442








