Zn Doping Improves the Anticancer Efficacy of SnO2 Nanoparticles
Abstract
:1. Introduction
2. Experimental Part
2.1. Chemicals and Reagents
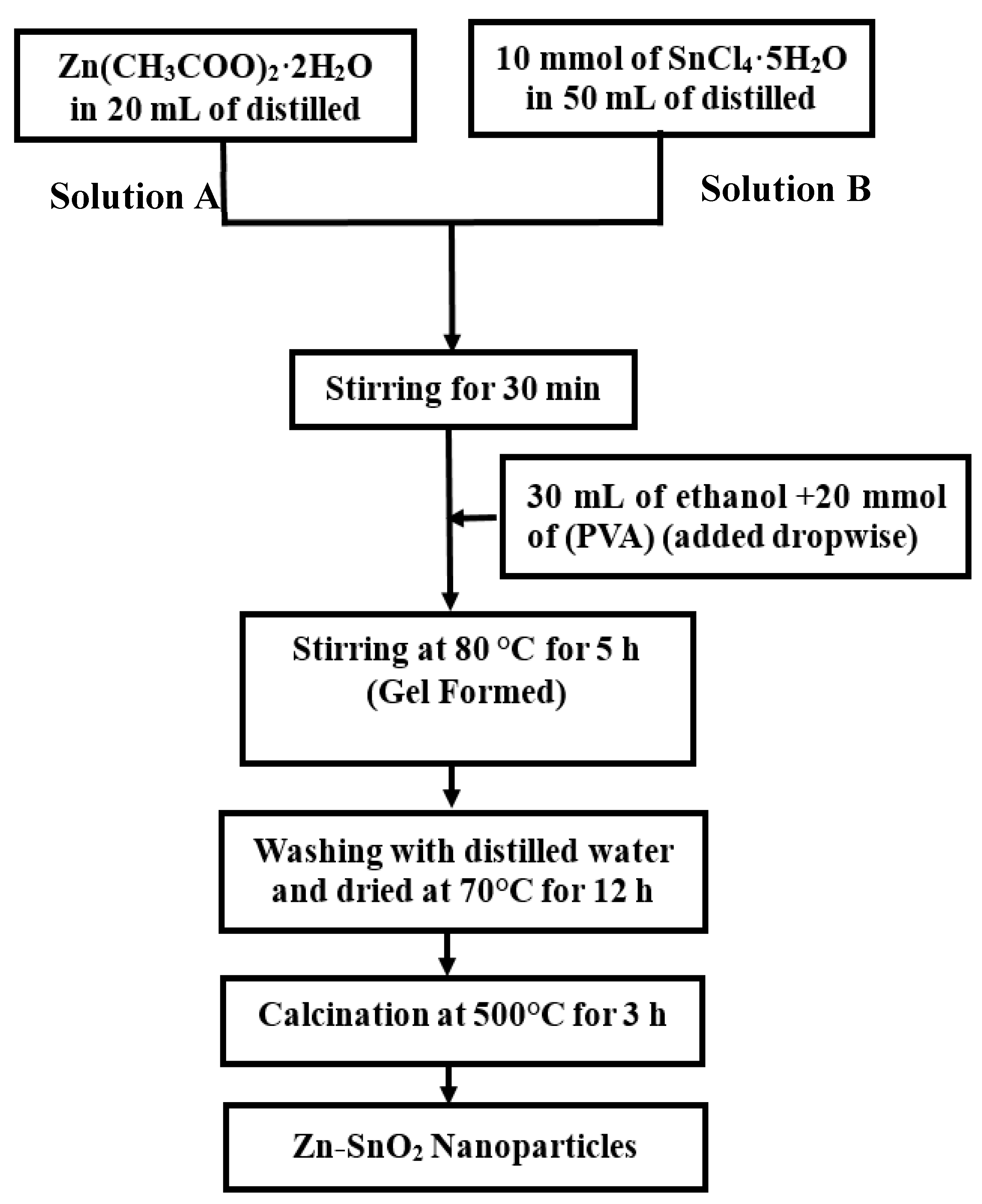
2.2. Synthesis of Zn-SnO2 Nanoparticles
2.3. Characterization of Pure and Zn-SnO2 Nanoparticles
2.4. Cell Culture
2.5. Exposure Protocol
2.6. MTT Assay
2.7. Data Analysis
3. Results and Discussions
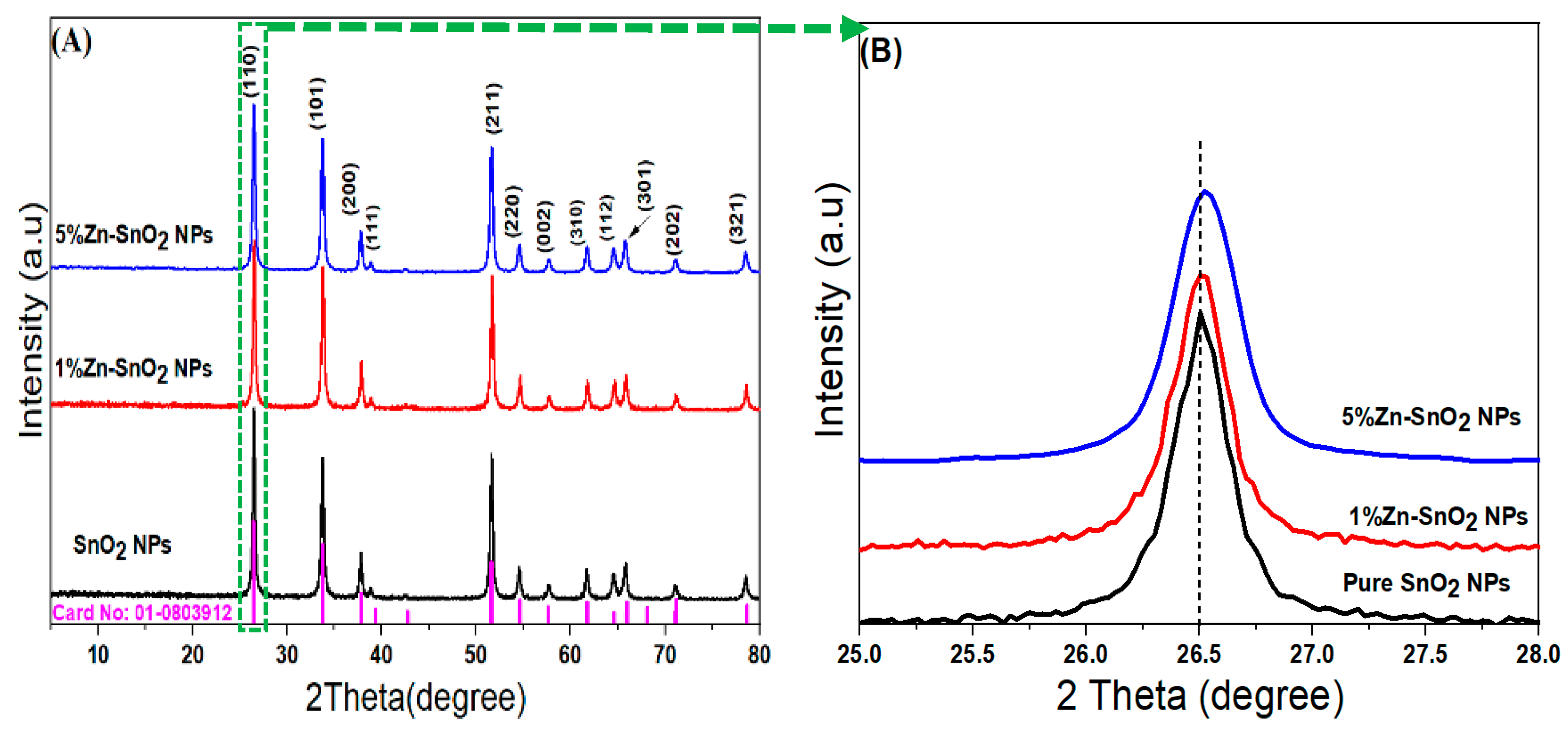
3.1. XRD Analysis
3.2. TEM Analysis
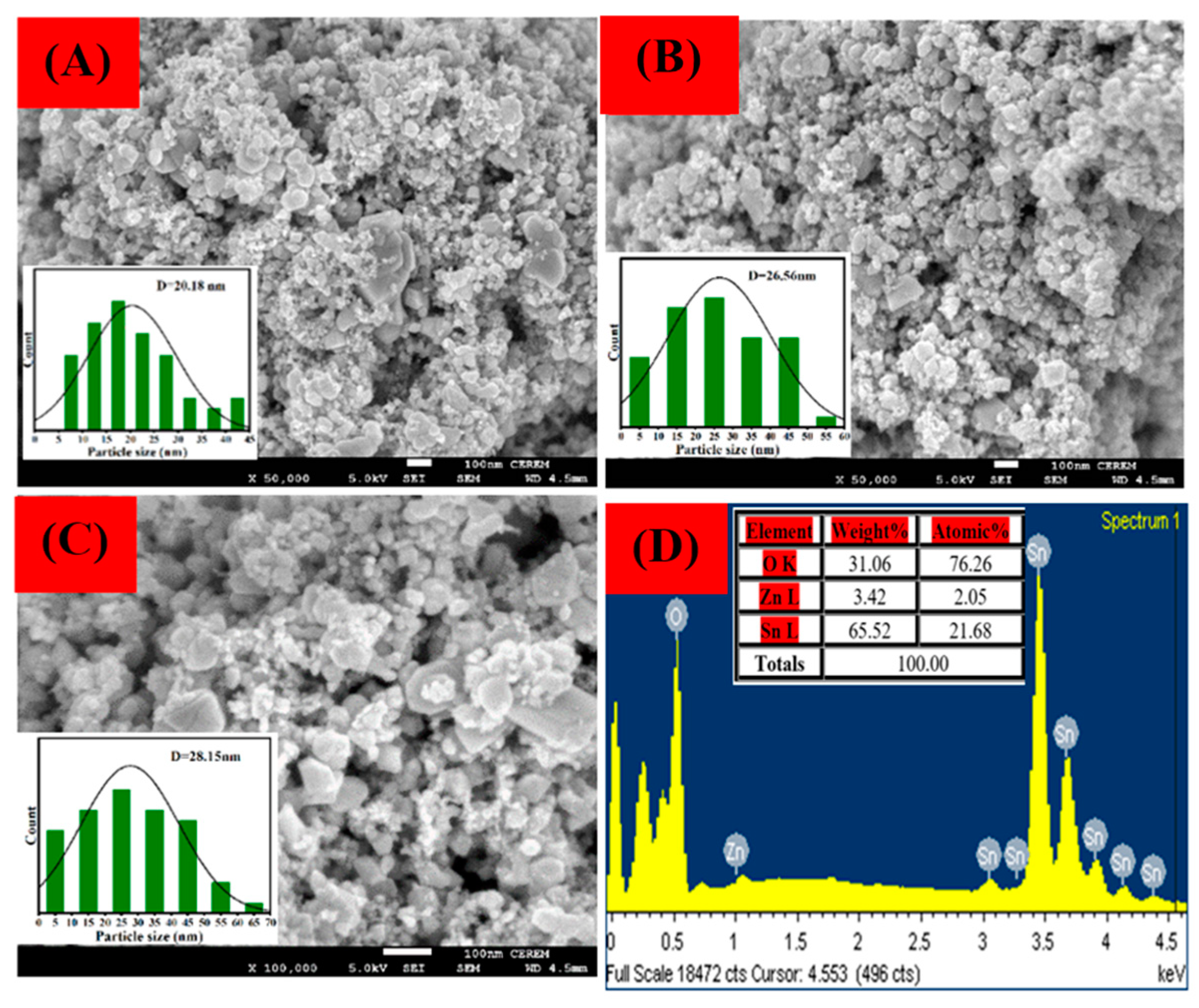
3.3. SEM with EDX Analysis
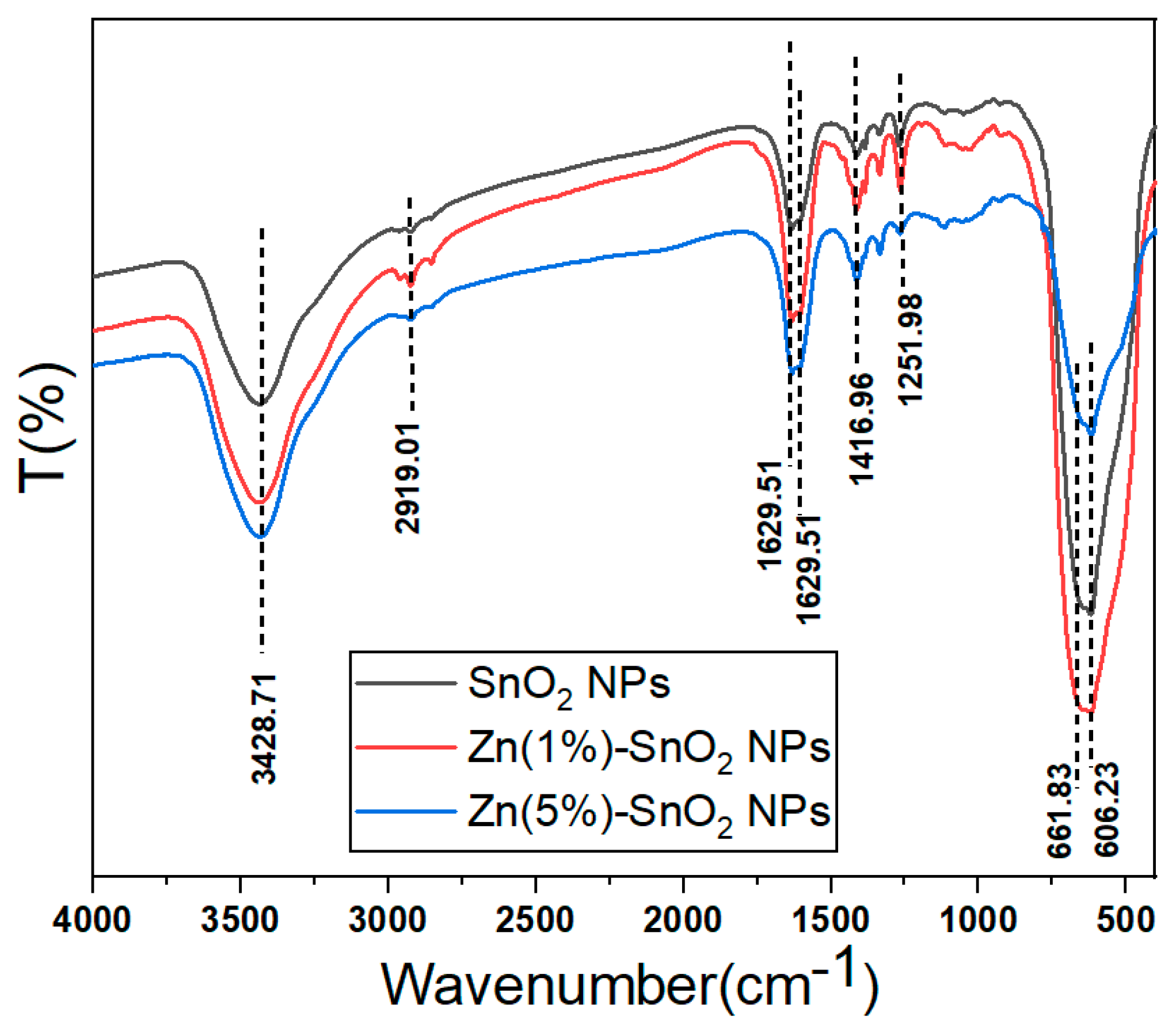
3.4. FTIR Analysis
3.5. UV-Vis Analysis
3.6. PL Analysis
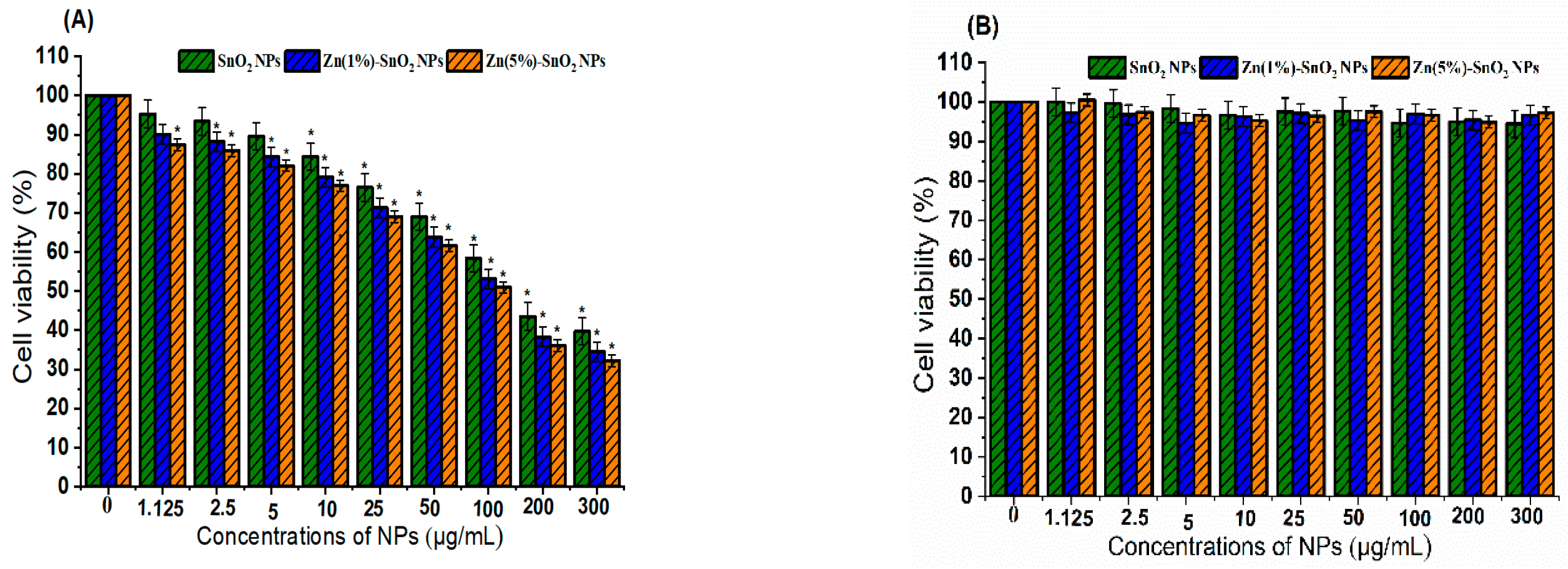
3.7. Anticancer and Biocompatibility Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Altammar, K.A. A Review on Nanoparticles: Characteristics, Synthesis, Applications, and Challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Iversen, T.G.; Sandvig, K. Development of Nanoparticles for Clinical Use. Nanomedicine 2014, 9, 1295–1299. [Google Scholar] [CrossRef]
- Malam, Y.; Lim, E.; Seifalian, A.M. Current Trends in the Application of Nanoparticles in Drug Delivery. Curr. Med. Chem. 2011, 18, 1067–1078. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Li, R.; Li, X.; Gao, J.; Hao, S.; Zhou, W. Study on Sodium Storage Properties of Manganese-doped Sodium Vanadium Phosphate Cathode Materials. Batter. Energy 2023, 2, 20220042. [Google Scholar] [CrossRef]
- Lin, S.; Du, W.; Tong, L.; Ji, T.; Jiao, X. Photocatalytic Degradation of 4-Chlorophenol by Gd-Doped β-Bi2O3 under Visible Light Irradiation. Chem. Res. Chin. Univ. 2019, 35, 120–124. [Google Scholar] [CrossRef]
- Huang, Z.; Zhu, J.; Hu, Y.; Zhu, Y.; Zhu, G.; Hu, L.; Zi, Y.; Huang, W. Tin Oxide (SnO2) Nanoparticles: Facile Fabrication, Characterization, and Application in UV Photodetectors. Nanomaterials 2022, 12, 632. [Google Scholar] [CrossRef]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef] [PubMed]
- Sharker, S.M.; Kim, S.M.; Lee, J.E.; Choi, K.H.; Shin, G.; Lee, S.; Lee, K.D.; Jeong, J.H.; Lee, H.; Park, S.Y. Functionalized Biocompatible WO3 Nanoparticles for Triggered and Targeted In Vitro and In Vivo Photothermal Therapy. J. Control Release 2015, 217, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Thamarai Selvi, E.; Meenakshi Sundar, S. Effect of Mn Doping on Structural, Optical and Magnetic Properties of SnO2 Nanoparticles by Solvothermal Processing. J. Mater. Sci. Mater. Electron. 2017, 28, 15021–15032. [Google Scholar] [CrossRef]
- Venugopal, B.; Nandan, B.; Ayyachamy, A.; Balaji, V.; Amirthapandian, S.; Panigrahi, B.K.; Paramasivam, T. Influence of Manganese Ions in the Band Gap of Tin Oxide Nanoparticles: Structure, Microstructure and Optical Studies. RSC Adv. 2014, 4, 6141–6150. [Google Scholar] [CrossRef]
- Duduman, C.N.; Pérez, M.I.B.; de Salazar, J.M.G.; Carcea, I.; Chicet, D.; Palamarciuc, I. Synthesis of SnO2 by Sol-Gel Method. Solid State Phenom. 2016, 254, 200–206. [Google Scholar] [CrossRef]
- Moghadam, L.N.; Karimabad, A.E.B.; Niasari, M.S.; Safardoust, H. Synthesis and Characterization of SnO2 Nanostructures Prepared by a Facile Precipitation Method. J. Nanostruct. 2015, 5, 47–53. [Google Scholar]
- Janardhan, E.; Reddy, M.M.; Reddy, P.V.; Reddy, M.J. Synthesis of SnO Nanopatricles—A Hydrothermal Approach. World J. Nano Sci. Eng. 2018, 8, 33–37. [Google Scholar] [CrossRef]
- Dadkhah, M.; Ansari, F.; Salavati-Niasari, M. Thermal Treatment Synthesis of SnO2 Nanoparticles and Investigation of Its Light Harvesting Application. Appl. Phys. A 2016, 122, 700. [Google Scholar] [CrossRef]
- Felcia, E.B.; Gnanam, K.D. Synthesis and Characterization of SnO2 Nanoparticle by Microwave Assisted Hydrothermal Method. J. Appl. Phys. 2017, 2278, 89–104. [Google Scholar] [CrossRef]
- Gopal, B.V.; Nandan, B.; Amirthapandian, S.; Panigrahi, B.K.; Thangadurai, P. Structural and Optical Studies of Mn Doped Tin Oxide Nanoparticles. In AIP Conference Proceedings, Proceedings of the National Conference on Physics and Chemistry of Materials: NCPCM2020, Indore, India, 14–16 December 2020; American Institute of Physics: College Park, MD, USA, 2021. [Google Scholar]
- Kar, A.; Patra, A. Optical and Electrical Properties of Eu3+-Doped SnO2 Nanocrystals. J. Phys. Chem. C 2009, 113, 4375–4380. [Google Scholar] [CrossRef]
- Espinosa, A.; Sánchez, N.; Sánchez-Marcos, J.; De Andrés, A.; Muñoz, M.C. Origin of the Magnetism in Undoped and Mn-Doped SnO 2 Thin Films: Sn vs Oxygen Vacancies. J. Phys. Chem. C 2011, 115, 24054–24060. [Google Scholar] [CrossRef]
- Supin, K.K.; George, A.; Kumar, Y. Ranjith Kumar, K.; Thejas, K.; Mandal, G.; Chanda, A.; Vasundhara, M. Structural, Optical and Magnetic Properties of Pure and 3d Metal Dopant-Incorporated SnO2 Nanoparticles. RSC Adv. 2022, 12, 26712–26726. [Google Scholar] [CrossRef]
- Divya, J.; Pramothkumar, A.; Hilary, H.J.L.; Jayanthi, P.; Prabakar, P.C.J. Impact of Copper (Cu) and Iron (Fe) Co-Doping on Structural, Optical, Magnetic and Electrical Properties of Tin Oxide (SnO2) Nanoparticles for Optoelectronics Applications. J. Mater. Sci. Mater. Electron. 2021, 32, 16775–16785. [Google Scholar] [CrossRef]
- Sharma, S.; Ritika; Singh, V.P.; Kumar, S.; Vohra, A. Sol-Gel Synthesis and Properties of Zinc Doped Tin Oxide (Zn-SnO2) Nanostructures. In AIP Conference Proceedings, Proceedings of the National Conference on Physics and Chemistry of Materials: NCPCM2020, Indore, India, 14–16 December 2020; American Institute of Physics: College Park, MD, USA, 2021. [Google Scholar]
- Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alhadlaq, H.A. Oxidative Stress Mediated Cytotoxicity of Tin (IV) Oxide (SnO2) Nanoparticles in Human Breast Cancer (MCF-7) Cells. Colloids Surf. B. Biointerfaces 2018, 172, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Song, L.; Zhang, F.; Wang, D. Comparative Acute Toxicity and Oxidative Stress Responses in Three Aquatic Species Exposed to Stannic Oxide Nanoparticles and Stannic Chloride. Bull. Environ. Contam. Toxicol. 2020, 105, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, Y.; Zhao, X.; Song, S.; Qian, B. Exploring the Anticancer Effects of Tin Oxide Nanoparticles Synthesized by Pulsed Laser Ablation Technique against Breast Cancer Cell Line through Downregulation of PI3K/AKT/MTOR Signaling Pathway. Arab. J. Chem. 2021, 14, 103212. [Google Scholar] [CrossRef]
- Mahjouri, S.; Kosari-Nasab, M.; Mohajel Kazemi, E.; Divband, B.; Movafeghi, A. Effect of Ag-Doping on Cytotoxicity of SnO2 Nanoparticles in Tobacco Cell Cultures. J. Hazard. Mater. 2020, 381, 121012. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Muruganandam, G.; Ravichandran, K.; Jeyaleela, G.D.; Shanthaseelan, K.; Priyadharshini, B. Anticancer Activity of Tin Oxide and Cerium-Doped Tin Oxide Nanoparticles Synthesized from Ipomoea Carnea Flower Extract. Biomed. Biotechnol. Res. J. 2022, 6, 337–340. [Google Scholar] [CrossRef]
- Alaizeri, Z.M.; Alhadlaq, H.A.; Aldawood, S.; Akhtar, M.J.; Ahamed, M. One-Pot Synthesis of SnO2-RGO Nanocomposite for Enhanced Photocatalytic and Anticancer Activity. Polymers 2022, 14, 2036. [Google Scholar] [CrossRef]
- Winyayong, A.; Wongsaprom, K. Nanostructures of Tin Oxide by a Simple Chemical Route: Synthesis and Characterization. J. Phys. Conf. Ser. 2019, 1380, 012002. [Google Scholar] [CrossRef]
- Alaizeri, Z.M.; Alhadlaq, H.A.; Aldawood, S.; Akhtar, M.J.; Ahamed, M. Photodeposition Mediated Synthesis of Silver-Doped Indium Oxide Nanoparticles for Improved Photocatalytic and Anticancer Performance. Environ. Sci. Pollut. Res. Int. 2023, 30, 6055–6067. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Singh, K.; Sharma, J.; Kumar, A.; Vij, A.; Thakur, A. Zn-Doped SnO2 Nanostructures: Structural, Morphological and Spectroscopic Properties. J. Mater. Sci. Mater. Electron. 2017, 28, 18849–18856. [Google Scholar] [CrossRef]
- Minin, I.V.; Minin, O.V. Diffractional Optics of Millimetre Waves, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar] [CrossRef]
- Sivakumar, S.; Manikandan, E. Novel Synthesis of Optical, Photoluminescence Properties and Supercapacitor Application on Zn2+ Doping Sn1-XZnxO2 Nanoparticles. Int. J. Sci. Res. Phys. Appl. Sci. 2018, 6, 1–13. [Google Scholar] [CrossRef]
- Enesca, A.; Andronic, L.; Duta, A. Optimization of Opto-Electrical and Photocatalytic Properties of SnO2 Thin Films Using Zn2+ and W6+ Dopant Ions. Catal. Lett. 2012, 142, 224–230. [Google Scholar] [CrossRef]
- Amutha, A.; Vigneswari, M.; Amirthapandian, S.; Panigrahi, B.K.; Thangadurai, P. Facile Synthesis and Characterization of Microstructure and Optical Properties of Pure and Zn Doped SnO2 Nanorods. J. Clust. Sci. 2021, 33, 1857–1863. [Google Scholar] [CrossRef]
- Baig, A.; Baig, A.; Rathinam, V.; Ramya, V. Facile Fabrication of Zn-Doped SnO2 Nanoparticles for Enhanced Photocatalytic Dye Degradation Performance under Visible Light Exposure. Adv. Compos. Hybrid Mater. 2021, 4, 114–126. [Google Scholar] [CrossRef]
- Yates, H.M.; Evans, P.; Sheel, D.W. The Influence of F-Doping in SnO2 Thin Films. Phys. Procedia 2013, 46, 159–166. [Google Scholar] [CrossRef]
- Jim, W.Y.; Liu, X.; Yiu, W.K.; Leung, Y.H.; Djurišić, A.B.; Chan, W.K.; Liao, C.; Shih, K.; Surya, C. The Effect of Different Dopants on the Performance of SnO2-Based Dye-Sensitized Solar Cells. Phys. Status Solidi Basic Res. 2015, 252, 553–557. [Google Scholar] [CrossRef]
- Suthakaran, S.; Dhanapandian, S.; Krishnakumar, N.; Ponpandian, N. Hydrothermal Synthesis of Surfactant Assisted Zn Doped SnO2 Nanoparticles with Enhanced Photocatalytic Performance and Energy Storage Performance. J. Phys. Chem. Solids 2020, 141, 109407. [Google Scholar] [CrossRef]
- Habte, A.G.; Hone, F.G.; Dejene, F.B. Zn Doping Effect on the Properties of SnO2 Nanostructure by Co-Precipitation Technique. Appl. Phys. A Mater. Sci. Process. 2019, 125, 402. [Google Scholar] [CrossRef]
- Tas Anli, S.; Ebeoglugil, M.F.; Celik, E. Effect of Dopant Elements on Structure and Morphology of SnO2 Nanoparticles. J. Aust. Ceram. Soc. 2020, 56, 403–411. [Google Scholar] [CrossRef]
- Yao, Q.; Xiao, F.; Lin, C.; Xiong, P.; Lai, W.; Zhang, J.; Xue, H.; Sun, X.; Wei, M.; Qian, Q.; et al. Regeneration of Spent Lithium Manganate into Cation-doped and Oxygen-deficient MnO2 Cathodes toward Ultralong Lifespan and Wide-temperature-tolerant Aqueous Zn-ion Batteries. Batter. Energy 2023, 2, 20220065. [Google Scholar] [CrossRef]
- Salah, N.; Habib, S.; Azam, A. The Influence of Transition Metal Doping on the Thermoelectric and Magnetic Properties of Microwave Synthesized SnO2 Nanoparticles. J. Mater. Sci. Mater. Electron. 2017, 28, 435–445. [Google Scholar] [CrossRef]
- Ahmed, A.; Tripathi, P.; Naseem Siddique, M.; Ali, T. Microstructural, Optical and Dielectric Properties of Al-Incorporated SnO2 Nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2017, 225, 012173. [Google Scholar] [CrossRef]
- Davis, E.A.; Mott, N.F. Conduction in Non-Crystalline Systems V. Conductivity, Optical Absorption and Photoconductivity in Amorphous Semiconductors. Philos. Mag. 1970, 22, 903–922. [Google Scholar] [CrossRef]
- Chen, X.; Huang, B.; Zhang, C.; Li, P.; Wang, P. Tunable SnO2 Nanoribbon by Electric Fields and Hydrogen Passivation. J. Nanomater. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Singh, P.; Kaur, G.; Singh, K.; Singh, B.; Kaur, M.; Kaur, M.; Krishnan, U.; Kumar, M.; Bala, R.; Kumar, A. Specially Designed B4C/SnO2 Nanocomposite for Photocatalysis: Traditional Ceramic with Unique Properties. Appl. Nanosci. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Veerasubam, R.; Muthukumaran, S. Tuning of Energy Gap, Structural, FTIR and Photoluminescence Examination of Ni, Sn Dual Doped ZnO Nanoparticles. Mater. Res. Express 2019, 6, 045006. [Google Scholar] [CrossRef]
- Wang, H.; Qi, J.; Yang, N.; Cui, W.; Wang, J.; Li, Q.; Zhang, Q.; Yu, X.; Gu, L.; Li, J.; et al. Dual-Defects Adjusted Crystal-Field Splitting of LaCo1−xNixO3−δ Hollow Multishelled Structures for Efficient Oxygen Evolution. Angew. Chem. Int. Ed. 2020, 59, 19691–19695. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Guo, Y.; Shen, M.; Wang, M.; Li, B.; Shi, J. Multifunctional 2D Porous G-C3N4 Nanosheets Hybridized with 3D Hierarchical TiO2 Microflowers for Selective Dye Adsorption, Antibiotic Degradation and CO2 Reduction. Chem. Eng. J. 2020, 396, 125347. [Google Scholar] [CrossRef]
- Zhou, G.; Xiong, S.; Wu, X.; Liu, L.Z.; Li, T.; Chu, P.K. N-Doped SnO2 Nanocrystals with Green Emission Dependent upon Mutual Effects of Nitrogen Dopant and Oxygen Vacancy. Acta Mater. 2013, 61, 7342–7347. [Google Scholar] [CrossRef]
- Ahamed, M.; Khan, M.A.M.; Akhtar, M.J.; Alhadlaq, H.A.; Alshamsan, A. Role of Zn Doping in Oxidative Stress Mediated Cytotoxicity of TiO2 Nanoparticles in Human Breast Cancer MCF-7 Cells. Sci. Rep. 2016, 6, 30196. [Google Scholar] [CrossRef]



| Synthesized NPs | XRD (nm) | SEM (nm) | TEM (nm) | Bandgap (eV) |
|---|---|---|---|---|
| Pure SnO2 NPs | 21.7 | 20.18 | 15.25 | 3.50 |
| Zn(1%)-SnO2 NPs | 24.5 | 26.56 | 25.92 | 3.42 |
| Zn(5%)-SnO2 NPs | 26.2 | 28.15 | 26.63 | 3.34 |
| Synthesized NPs | MCF-7 Cancer Cells |
|---|---|
| SnO2 NPs | 184.69 ± 0.05 |
| Zn (1%)-SnO2 NPs | 111.68 ± 0.12 |
| Zn (5%)-SnO2 NPs | 90.74 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alanazi, S.; Alaizeri, Z.M.; Lateef, R.; Madkhali, N.; Alharbi, A.; Ahamed, M. Zn Doping Improves the Anticancer Efficacy of SnO2 Nanoparticles. Appl. Sci. 2023, 13, 12456. https://doi.org/10.3390/app132212456
Alanazi S, Alaizeri ZM, Lateef R, Madkhali N, Alharbi A, Ahamed M. Zn Doping Improves the Anticancer Efficacy of SnO2 Nanoparticles. Applied Sciences. 2023; 13(22):12456. https://doi.org/10.3390/app132212456
Chicago/Turabian StyleAlanazi, Sitah, ZabnAllah M. Alaizeri, Rashid Lateef, Nawal Madkhali, Abdullah Alharbi, and Maqusood Ahamed. 2023. "Zn Doping Improves the Anticancer Efficacy of SnO2 Nanoparticles" Applied Sciences 13, no. 22: 12456. https://doi.org/10.3390/app132212456







