Comparative Study on Phytochemical Composition, Antioxidant, and Anti-HSV-2 Activities of Sambucus nigra L. and Sambucus ebulus L. Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. Preparation of Dry Extracts
2.4. Total Polyphenol Content (TPC) Analysis
2.5. High-Performance Liquid Chromatography (HPLC) Analysis of Phenolic Compounds
2.6. High-Performance Liquid Chromatography (HPLC) Analysis of Anthocyanins and Anthocyanidins
2.7. Antioxidant Assays
2.8. Viruses and Cells
2.9. Cytotoxicity Assay
2.10. Effect on the Extracellular Virus (Virucidal Effect)
2.11. Statistical Analysis
3. Results
3.1. Polyphenol Content and Composition
3.1.1. Phenolic Acids and Flavonoids Content and Composition
3.1.2. Anthocyanin and Anthocyanidin Content and Composition
3.2. Antioxidant Activity
3.3. Antiviral Activity
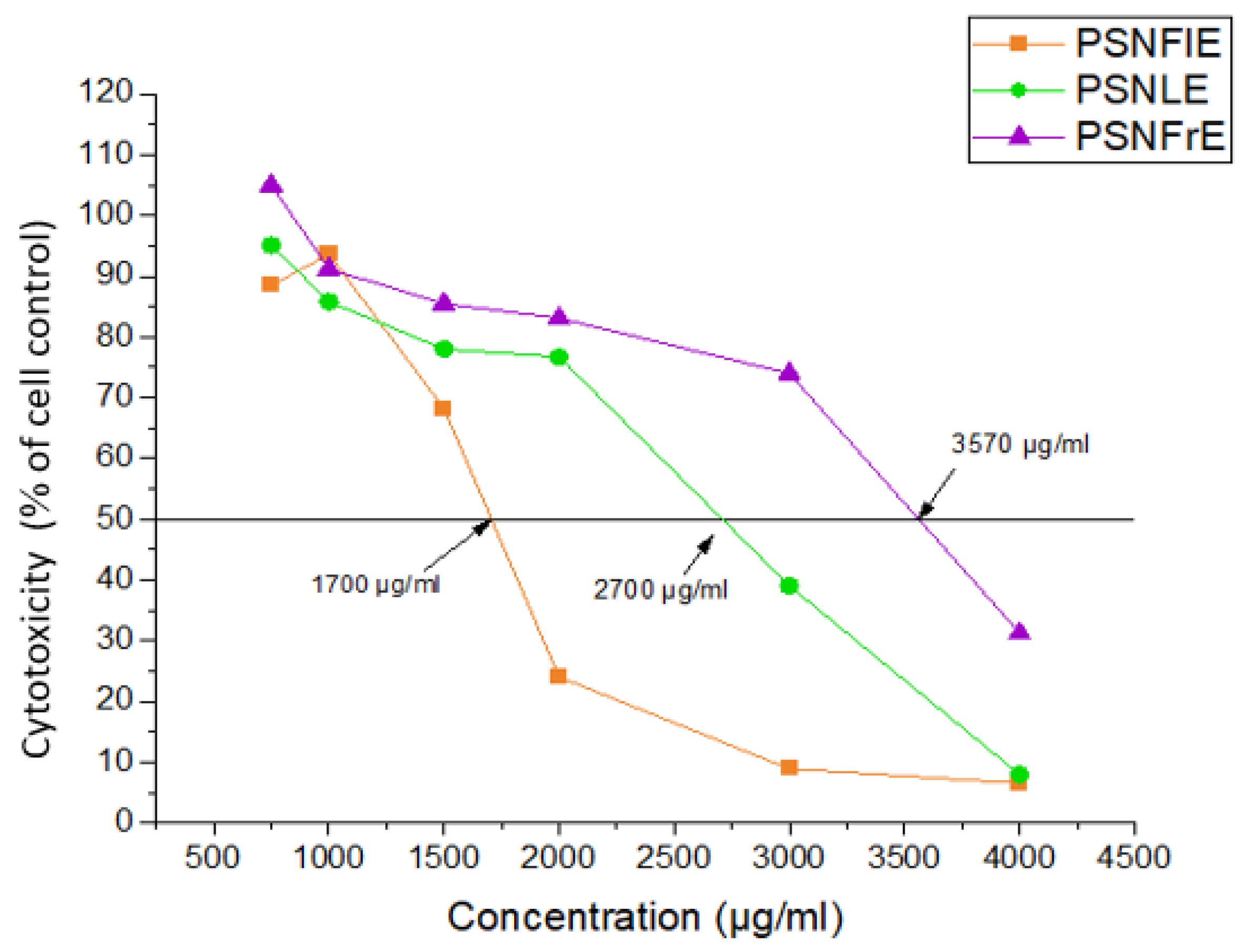
3.3.1. Determination of the Cytotoxic Effect of the Extracts on the MDBK Cell Line
3.3.2. Virucidal Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.; Novellino, E. Resveratrol as a novel anti-herpes Simplex virus nutraceutical agent: An Overview. Viruses 2018, 10, 473. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Rocchetti, G.; Chadha, S.; Zengin, G.; Bungau, S.; Kumar, A.; Mehta, V.; Uddin, M.S.; Khullar, G.; Setia, D.; et al. Phytochemicals from plant foods as potential source of antiviral agents: An Overview. Pharmaceuticals 2021, 14, 381. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines for the Treatment of Genital Herpes Simplex Virus; World Health Organization: Geneva, Switzerland, 2016; ISBN 978-92-4-154987-5.
- Todorov, D.; Hinkov, A.; Dimitrova, M.; Shishkova, K.; Dragolova, D.; Kapchina-Toteva, V.; Shishkov, S. Anti-herpes effects of in vitro and in vivo extracts derived from Lamium album L. Annu. Luniversité Sofia St. Kliment Ohridski 2015, 100, 177–183. [Google Scholar]
- Elion, G.B. Acyclovir: Discovery, mechanism of action, and selectivity. J. Med. Virol. 1993, 41, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Bacon, T.H.; Levin, M.J.; Leary, J.J.; Sarisky, R.T.; Sutton, D. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin. Microbiol. Rev. 2003, 16, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Bostanghadiri, N.; Pormohammad, A.; Chirani, A.S.; Pouriran, R.; Erfanimanesh, S.; Hashemi, A. Comprehensive review on the antimicrobial potency of the plant polyphenol resveratrol. Biomed. Pharmacother. 2017, 95, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, M.; Arshad, M.; Ahmad, M.; Pomerantz, R.J.; Wigdahl, B.; Parveen, Z. Antiviral potentials of medicinal plants. Virus Res. 2008, 131, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Musarra-Pizzo, M.; Ginestra, G.; Smeriglio, A.; Pennisi, R.; Sciortino, M.T.; Mandalari, G. The antimicrobial and antiviral activity of polyphenols from almond (Prunus dulcis L.) skin. Nutrients 2019, 11, 2355. [Google Scholar] [CrossRef]
- Musarra-Pizzo, M.; Pennisi, R.; Ben-Amor, I.; Smeriglio, A.; Mandalari, G.; Sciortino, M.T. In vitro anti-HSV-1 activity of polyphenol-rich extracts and pure polyphenol compounds derived from pistachios Kernels (Pistacia vera L.). Plants 2020, 9, 267. [Google Scholar] [CrossRef]
- Dubey, A.; Kumar, A.; Bankole, M.M.; Khan, M.L. Plants with potent antiviral properties. In Coronavirus Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 155–171. ISBN 978-0-323-95578-2. [Google Scholar]
- Porter, R.S.; Bode, R.F. A review of the antiviral properties of black elder (Sambucus nigra L.) products: Antiviral properties of black elder (Sambucus nigra L.). Phytother. Res. 2017, 31, 533–554. [Google Scholar] [CrossRef]
- Jarić, S.; Popović, Z.; Mačukanović-Jocić, M.; Djurdjević, L.; Mijatović, M.; Karadžić, B.; Mitrović, M.; Pavlović, P. An ethnobotanical study on the usage of wild medicinal herbs from Kopaonik Mountain (central Serbia). J. Ethnopharmacol. 2007, 111, 160–175. [Google Scholar] [CrossRef] [PubMed]
- Shokrzadeh, M.; Saravi, S.S. The chemistry, pharmacology and clinical properties of Sambucus ebulus L.: A Review. J. Med. Plants Res. 2010, 4, 95–103. [Google Scholar]
- Sidor, A.; Gramza-Michałowska, A. Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food—A Review. J. Funct. Foods 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Zahmanov, G.; Alipieva, K.; Denev, P.; Todorov, D.; Hinkov, A.; Shishkov, S.; Simova, S.; Georgiev, M.I. Flavonoid glycosides profiling in dwarf elder fruits (Sambucus ebulus L.) and evaluation of their antioxidant and anti-herpes simplex activities. Ind. Crops Prod. 2015, 63, 58–64. [Google Scholar] [CrossRef]
- Marțiș, G.S.; Mureșan, V.; Marc, R.M.; Mureșan, C.C.; Pop, C.R.; Buzgău, G.; Mureșan, A.E.; Ungur, R.A.; Muste, S. The physicochemical and antioxidant properties of Sambucus nigra L. and Sambucus nigra Haschberg during growth phases: From buds to ripening. Antioxidants 2021, 10, 1093. [Google Scholar] [CrossRef]
- Haș, I.M.; Teleky, B.-E.; Szabo, K.; Simon, E.; Ranga, F.; Diaconeasa, Z.M.; Purza, A.L.; Vodnar, D.C.; Tit, D.M.; Nițescu, M. Bioactive potential of elderberry (Sambucus nigra L.): Antioxidant, antimicrobial activity, bioaccessibility and prebiotic potential. Molecules 2023, 28, 3099. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.G.; Avula, B.; Katragunta, K.; Ali, Z.; Chittiboyina, A.G.; Khan, I.A. Elderberry extracts: Characterization of the polyphenolic chemical composition, quality consistency, safety, adulteration, and attenuation of oxidative stress- and inflammation-induced health disorders. Molecules 2023, 28, 3148. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Martins-Gomes, C.; Nunes, F.M.; Silva, A.M. Elderberry (Sambucus nigra L.) extracts promote anti-inflammatory and cellular antioxidant activity. Food Chem. 2022, 15, 100437. [Google Scholar] [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Staniek, H.; Kidoń, M.; Łysiak, G.P. The content of selected minerals, bioactive compounds, and the antioxidant properties of the flowers and fruit of selected cultivars and wildly growing plants of Sambucus nigra L. Molecules 2020, 25, 876. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, A.M.; Nunes, F.M. Sambucus nigra L. fruits and flowers: Chemical composition and related bioactivities. Food Rev. Int. 2022, 38, 1237–1265. [Google Scholar] [CrossRef]
- Przybylska-Balcerek, A.; Szablewski, T.; Szwajkowska-Michałek, L.; Świerk, D.; Cegielska-Radziejewska, R.; Krejpcio, Z.; Suchowilska, E.; Tomczyk, Ł.; Stuper-Szablewska, K. Sambucus nigra L. extracts–natural antioxidants and antimicrobial compounds. Molecules 2021, 26, 2910. [Google Scholar] [CrossRef] [PubMed]
- Morag, A.; Mumcuoglu, M.; Baybikov, T.; Schelsinger, M.; Zakay-Rones, Z. Inhibition of sensitive and acyclovir-resistant HSV-1 strains by an elderberry extract in vitro. Z Phytother 1997, 25, 97–98. [Google Scholar]
- Sahpira-Nahor, O.; Zakay-Rones, Z.; Mumcuoglu, M. The effects of Sambucol® on HIV infection in vitro. In Proceedings of the Annual Israel Congress of Microbiology, Rehovot, Israel, 14–15 February 1995; pp. 6–7. [Google Scholar]
- Serkedjieva, J.; Manolova, N.; Zgórniak-Nowosielska, I.; Zawilińska, B.; Grzybek, J. Antiviral activity of the infusion (SHS-174) from flowers of Sambucus nigra L., aerial parts of Hypericum perforatum L., and roots of Saponaria officinalis L. against influenza and herpes Simplex viruses. Phytother. Res. 1990, 4, 97–100. [Google Scholar] [CrossRef]
- Serkedjieva, J.; Zgorniak-Nowosielska, I. Combined antiinfluenza activity of a plant preparation SHS-174 and amantadine derivatives. Acta Virol. 1993, 37, 258–264. [Google Scholar] [PubMed]
- Serkedjieva, J. In vitro antiinfluenza virus effect of a plant preparation SHS-174. Fitoter. Milano 1996, 67, 351–358. [Google Scholar]
- Council of Europe. European Pharmacopoeia; Council of Europe: Strasburg, France, 2019; pp. 1421–1423. [Google Scholar]
- Markova, M. Flora of the Republic of Bulgaria, 11th ed.; Academic Publishing House “Prof. Marin Drinov”: Sofia, Bulgaria, 1995. [Google Scholar]
- Denev, P.; Ciz, M.; Ambrozova, G.; Lojek, A.; Yanakieva, I.; Kratchanova, M. Solid-phase extraction of berries’ anthocyanins and evaluation of their antioxidative properties. Food Chem. 2010, 123, 1055–1061. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar] [CrossRef]
- Denev, P.; Kratchanova, M.; Ciz, M.; Lojek, A.; Vasicek, O.; Nedelcheva, P.; Blazheva, D.; Toshkova, R.; Gardeva, E.; Yossifova, L.; et al. Biological activities of selected polyphenol-rich fruits related to immunity and gastrointestinal health. Food Chem. 2014, 157, 37–44. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E.K.; Prior, R.L.; Huang, D. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J. Agric. Food Chem. 2002, 50, 2772–2777. [Google Scholar] [CrossRef] [PubMed]
- Montanha, J.A.; Moellerke, P.; Bordignon, S.A.; Schenkel, E.P.; Roehe, P.M. Antiviral activity of Brazilian plant extracts. Acta Farm. Bonaer. 2004, 23, 183–186. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Hano, C.; Tungmunnithum, D. Plant polyphenols, more than just simple natural antioxidants: Oxidative stress, aging and age-related diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Yarmolinsky, L.; Porat, D.; Dahan, A. Antiviral effect of phytochemicals from medicinal plants: Applications and drug delivery strategies. Drug Deliv. Transl. Res. 2020, 10, 354–367. [Google Scholar] [CrossRef]
- Lee, J.; Finn, C.E. Anthocyanins and other polyphenolics in american elderberry (Sambucus canadensis) and European elderberry (S. nigra) cultivars: Anthocyanins in American and European elderberry cultivars. J. Sci. Food Agric. 2007, 87, 2665–2675. [Google Scholar] [CrossRef]
- Akbulut, M.; Sezai, E.; Murat, T. Physico-chemical characteristics of some wild grown European elderberry (Sambucus nigra L.) genotypes. Pharmacogn. Mag. 2009, 5, 320–323. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, 1064–1070. [Google Scholar] [CrossRef]
- Duymuş, H.G.; Göger, F.; Başer, K.H. In vitro antioxidant properties and anthocyanin compositions of elderberry extracts. Food Chem. 2014, 155, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Mudge, E.; Applequist, W.L.; Finley, J.; Lister, P.; Townesmith, A.K.; Walker, K.M.; Brown, P.N. Variation of select flavonols and chlorogenic acid content of elderberry collected throughout the Eastern United States. J. Food Compos. Anal. 2016, 47, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Tasinov, O.; Dincheva, I.; Badjakov, I.; Kiselova-Kaneva, Y.; Galunska, B.; Nogueiras, R.; Ivanova, D. Phytochemical composition, anti-inflammatory and ER stress-reducing potential of Sambucus ebulus L. fruit extract. Plants 2021, 10, 2446. [Google Scholar] [CrossRef] [PubMed]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Ho, G.T.T.; Kase, E.T.; Wangensteen, H.; Barsett, H. Phenolic elderberry extracts, anthocyanins, procyanidins, and metabolites influence glucose and fatty acid uptake in human skeletal muscle cells. J. Agric. Food Chem. 2017, 65, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk, A.; Olech, M.; Oniszczuk, T.; Wojtunik-Kulesza, K.; Wójtowicz, A. Extraction methods, LC-ESI-MS/MS analysis of phenolic compounds and antiradical properties of functional food enriched with elderberry flowers or fruits. Arab. J. Chem. 2019, 12, 4719–4730. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Gamaleldin Elsadig Karar, M.; Matei, M.-F.; Jaiswal, R.; Illenberger, S.; Kuhnert, N. Neuraminidase inhibition of dietary chlorogenic acids and derivatives—Potential antivirals from dietary sources. Food Funct. 2016, 7, 2052–2059. [Google Scholar] [CrossRef]
- Tamura, H.; Akioka, T.; Ueno, K.; Chujyo, T.; Okazaki, K.; King, P.J.; Robinson, W.E. Anti-human immunodeficiency virus activity of 3,4,5-tricaffeoylquinic acid in cultured cells of lettuce leaves. Mol. Nutr. Food Res. 2006, 50, 396–400. [Google Scholar] [CrossRef]
- Chiang, L.; Chiang, W.; Chang, M.; Ng, L.; Lin, C. Antiviral activity of Plantago major extracts and related compounds in vitro. Antivir. Res. 2002, 55, 53–62. [Google Scholar] [CrossRef]
- Khan, M.T.H.; Ather, A.; Thompson, K.D.; Gambari, R. Extracts and molecules from medicinal plants against herpes simplex viruses. Antivir. Res. 2005, 67, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Luo, T.; Wu, F.; Mei, Y.W.; Peng, J.; Liu, H.; Li, H.R.; Zhang, S.L.; Dong, J.H.; Fang, Y.; et al. Involvement of TLR2 and TLR9 in the anti-inflammatory effects of chlorogenic acid in HSV-1-infected microglia. Life Sci. 2015, 127, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Samoticha, J.; Eler, K.; Stampar, F.; Veberic, R. Traditional elderflower beverages: A rich source of phenolic compounds with high antioxidant activity. J. Agric. Food Chem. 2015, 63, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Anton, A.; Pintea, A.; Rugină, D.; Sconţa, Z.; Hanganu, D.; Vlase, L.; Benedec, D. Preliminary studies on the chemical characterization and antioxidant capacity of polyphenols from Sambucus sp. Dig. J. Nanomater. Biostructures DJNB 2013, 8, 973–980. [Google Scholar]
- Christensen, L.P.; Kaack, K.; Fretté, X.C. Selection of elderberry (Sambucus nigra L.) genotypes best suited for the preparation of elderflower extracts rich in flavonoids and phenolic acids. Eur. Food Res. Technol. 2008, 227, 293–305. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). LWT—Food Sci. Technol. 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Salamon, I.; Şimşek Sezer, E.N.; Kryvtsova, M.; Labun, P. Antiproliferative and antimicrobial activity of anthocyanins from berry fruits after their isolation and freeze-drying. Appl. Sci. 2021, 11, 2096. [Google Scholar] [CrossRef]
- El-Toumy, S.A.; Salib, J.Y.; El-Kashak, W.A.; Marty, C.; Bedoux, G.; Bourgougnon, N. Antiviral effect of polyphenol rich plant extracts on herpes simplex virus type 1. Food Sci. Hum. Wellness 2018, 7, 91–101. [Google Scholar] [CrossRef]
- Committee on Herbal Medicinal Products (HMPC). Assessment Report on Sambucus nigra L., Fructus; European Medicine Agency: London, UK, 2014.
- Chojnacka, K.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Szopa, D.; Witek-Krowiak, A. Antiviral properties of polyphenols from plants. Foods 2021, 10, 2277. [Google Scholar] [CrossRef]
- Bartak, M.; Lange, A.; Słonska, A.; Cymerys, J. Antiviral and healing potential of Sambucus nigra extracts. Bionatura 2020, 5, 1264–1270. [Google Scholar] [CrossRef]
- Vlachojannis, J.; Cameron, M.; Chrubasik, S. A systematic review on the Sambuci fructus effect and efficacy profiles. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2010, 24, 1–8. [Google Scholar] [CrossRef]
- Mocanu, M.L.; Amariei, S. Elderberries—A source of bioactive compounds with antiviral action. Plants 2022, 11, 740. [Google Scholar] [CrossRef]
- Hinkov, A.; Angelova, P.; Marchev, A.; Hodzhev, Y.; Tsvetkov, V.; Dragolova, D.; Todorov, D.; Shishkova, K.; Kapchina-Toteva, V.; Blundell, R.; et al. Nepeta nuda ssp. nuda L. water extract: Inhibition of replication of some strains of Human Alpha herpes virus (genus Simplex virus) in vitro, mode of action and NMR-based metabolomics. J. Herb. Med. 2020, 21, 100334. [Google Scholar] [CrossRef]
- Jassim, S.A.; Naji, M.A. Novel antiviral agents: A medicinal plant perspective. J. Appl. Microbiol. 2003, 95, 412–427. [Google Scholar] [CrossRef]
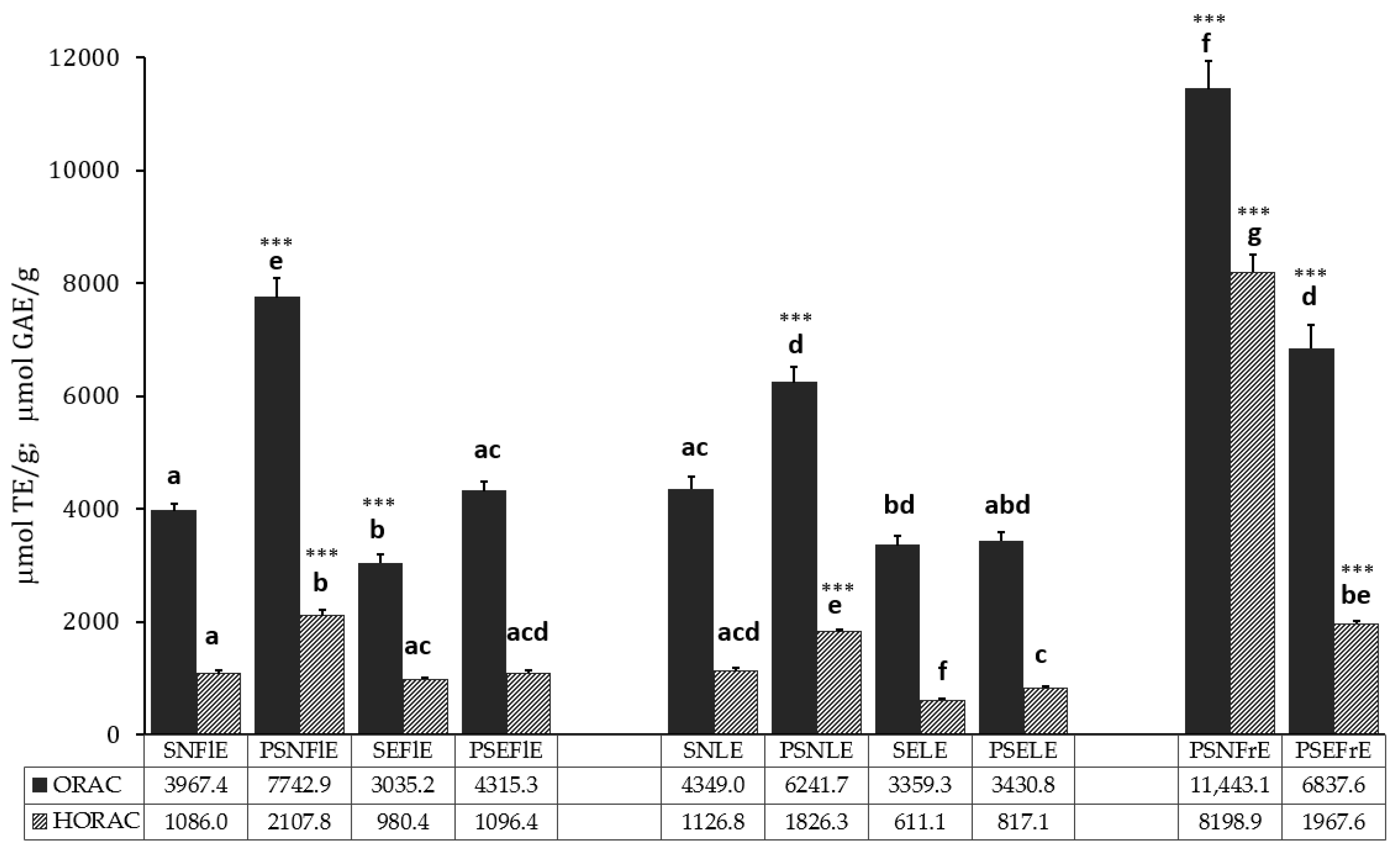

| Compounds | SNFlE | PSNFlE | SEFlE | PSEFlE | SNLE | PSNLE | SELE | PSELE | PSNFrE | PSEFrE |
|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic acids, mg/100 g | ||||||||||
| Chlorogenic acid | 4673.3 b ± 217.6 *** | 7086.7 a ± 312.8 *** | 2307.5 d ± 74.8 ** | 3953.6 c ± 167.6 *** | 2149.1 d ± 97.4 *** | 4810.4 b ± 210.5 *** | 2068.7 d ± 88.3 *** | 3026.6 c ± 154.2 * | 3614.9 c ± 190.1 * | 3214.6 c ± 122.7 * |
| Neochlorogenic acid | 998.5 b ± 53.8 ** | 1342.2 a ± 69.2 ** | 395.6 d ± 19.2 *** | 550.4 c ± 21.8 *** | 485.3 cd ± 19.4 *** | 1082.8 b ± 52.7 * | 583.5 c ± 19.7 *** | 1300.1 a ± 60.0 ** | 417.7 d ± 16.3 *** | 305.1 e ± 18.7 *** |
| Caffeic acid | 131.6 d ± 6.2 * | 234.5 b ± 14.3 * | n.d. | n.d. | 507.9 a ± 20.1 *** | 328.1 b ± 16.7 *** | 80.3 e ± 3.2 *** | 37.6 f ± 1.7 ** | 294.1 bc ± 5.9 *** | 373.8 b ± 14.2 *** |
| p-Coumaricacid | 51.1 d ± 3.1 *** | 103.3 c ± 4.7 * | 62.5 d ± 3.7 *** | 111.7 c ± 4.6 *** | 110.4 c ± 4.0 *** | 366.7 a ± 17.5 *** | 25.6 e ± 0.9 *** | 46.7 d ± 0.8 * | 151.2 b ± 6.9 *** | 164.7 b ± 6.8 *** |
| Rosmarinic acid | 212.4 e ± 5.8 ** | 480.2 d ± 17.9 ** | 915.1 a ± 38.9 *** | 686.7 b ± 28.4 *** | 274.2 e ± 13.6 ** | 734.1 b ± 21.7 *** | 562.8 c ± 22.3 *** | 978.5 a ± 61.3 *** | 237.6 e ± 11.4 *** | 588.3 c ± 24.0 *** |
| Cinnamic acid | 96.9 c ± 3.8 *** | 188.9 b ± 8.7 *** | 133.9 c ± 3.7 * | 254.1 a ± 12.1 *** | 42.1 d ± 1.9 *** | 101.9 c ± 4.9 *** | 11.8 e ± 0.6 *** | 16.8 e ± 0.7 *** | 55.8 d ± 2.3 ** | 41.4 d ± 1.6 *** |
| Vanillic acid | 176.1 e ± 10.7 * | 296.0 d ±13.2 ** | 86.1 f ± 1.8 *** | 180.2 e ± 5.4 ** | 471.0 c ± 19.4 * | 2117.6 a ± 101.6 ** | 237.1 d ± 8.6 ** | 290.7 d ± 10.0 *** | 678.5 b ± 24.7 *** | 286.1 d ± 9.6 *** |
| Benzoic acid | 560.2 e ± 33.6 * | 733.8 d ± 19.7 * | 312.5 f ± 19.5 * | 547.4 e ± 25.2 * | 1048.1 c ± 46.6 * | 1685.5 a ± 76.5 *** | 1237.7 b ± 49.3 * | 1557.5 a ± 61.9 * | 1038.2 c ± 92.6 * | 816.4 d ± 69.1 * |
| Ferulic acid | 128.7 b ± 3.5 *** | 227.7 a ± 8.2 *** | 50.8 d ± 3.0 *** | 155.7 b ± 10.8 *** | 79.7 c ± 2.6 ** | 167.8 b ± 5.5 ** | 27.1 d ± 0.3 *** | 41.8 d ± 0.9 *** | 136.2 b ± 4.5 *** | 218.2 a ± 4.8 *** |
| Gallic acid | 87.2 f ± 3.6 | 91.8 f ± 3.3 *** | 481.5 b ± 17.2 *** | 458.7 b ± 12.3 *** | 206.0 d ± 9.3 ** | 322.1 c ± 6.8 *** | 243.2 d ± 11.0 *** | 147.1 e ± 5.4 | 644.6 a ± 26.5 *** | 512.7 b ± 22.9 *** |
| Flavonoids, mg/100 g | ||||||||||
| Quercetin | 115.9 d ± 4.6 ** | 203.8 b ± 12.2 ** | 163.9 c ± 5.9 * | 227.3 b ± 8.3 *** | 177.3 c ± 7.3 * | 395.1 a ± 15.7 *** | 92.1 d ± 2.7 *** | 142.8 c ± 5.6 *** | 256.1 b ± 11.4 *** | 295.0 b ± 14.5 *** |
| Quercetin-3- glucoside | 1120.8 d ± 35.6 *** | 2963.0 a ± 132.7 *** | 1146.2 d ± 69.1 *** | 2028.2 b ± 94.3 *** | 349.5 g ± 7.7 *** | 717.5 e ± 23.8 * | 248.2 g ± 11.3 *** | 489.2 f ± 23.4 *** | 1513.3 c ± 68.8 * | 1676.5 c ± 33.4 ** |
| Quercetin 3- rutinoside | 8414.2 b ± 250.7 *** | 14,232.1 a ± 648.9 *** | 2010.9 e ± 73.8 *** | 4550.0 c ± 224.5 *** | 4271.1 c ± 178.9 *** | 7004.1 b ± 235.3 *** | 768.9 g ± 47.1 *** | 1092.7 f ± 43.4 *** | 4623.0 c ± 283.7 *** | 3465.6 d ± 144.8 *** |
| Myricetin | 138.5 c ± 6.4 *** | 294.4 a ± 14.8 *** | 167.6 b ± 7.7 | 280.8 a ± 10.3 *** | 119.6 c ± 3.0 *** | 227.4 b ± 9.5 ** | 86.6 d ± 4.7 *** | 178.2 b ± 10.9 ** | 276.8 a ± 16.9 *** | 316.7 a ± 10.1 *** |
| Kaempferol | 38.4 d ± 1.4 ** | 64.6 c ± 2.0 ** | 116.9 b ± 4.8 *** | 210.4 a ± 8.6 *** | 27.3 d ± 0.9 *** | 92.2 b ± 2.4 *** | 24.2 d ± 0.8 ** | 57.0 c ± 3.4 ** | 57.7 c ± 1.5 ** | 86.3 b ± 2.8 *** |
| Apigenin | 125.3 b ± 3.7 *** | 207.1 a ± 8.3 *** | 95.8 c ± 4.8 *** | 137.5 b ± 6.0 *** | 32.6 e ± 1.1 *** | 94.7 c ± 5.7 * | 34.7 e ± 1.2 *** | 47.3 d ± 2.0 *** | 19.8 f ± 0.9 *** | 53.0 d ± 2.0 *** |
| Total polyphenols, mg GAE/100g | 17,944.8 d ± 515.3 *** | 42,647.1 b ± 1331.8 *** | 9803.0 f ± 443.9 *** | 14,617.9 e ± 590.7 ** | 17,842.6 d ± 124.9 ** | 30,120.5 c ± 1748.8 *** | 11,568.5 f ± 644.1 ** | 13,928.7 e ± 63.4 ** | 59,596.6 a ± 1796.3 *** | 32,204.8 c ± 598.4 *** |
| Compounds | PSNFrE | PSEFrE |
|---|---|---|
| Anthocyanins, mg/100 g | ||
| Cyanidin-3-glucoside | 24,341.1 a ± 1017.4 ** | 30.7 b ± 1.1 |
| Cyanidin-3-sambubioside | 21,051.4 a ± 951.5 ** | 44.2 b ± 2.3 |
| Cyanidin-3,5-diglucoside | 4951.2 a ± 197.3 *** | 32.3 b ± 1.6 |
| Cyanidin-3-galactoside | 108.7 b ± 4.4 *** | 1830.1 a± 66.3 |
| Cyanidin-3-rutinoside | 40.5 a ± 1.4 ** | 10.4 b ± 0.5 |
| Cyanidin-3-arabinoside | n.d. | 2.8 ± 0.1 |
| Pelargonidin-3-glucoside | 64.2 ± 2.0 | n.d. |
| Anthocyanidins, mg/100 g | ||
| Cyanidin | 36,500.6 a ± 1868.8 ** | 2063.7 b ± 96.9 |
| Delphinidin | 203.4 ± 9.1 | n.d. |
| Pelargonidin | 112.1 ± 4.8 | n.d. |
| Malvidin | 242.3 ± 12.4 | n.d. |
| Peonidin | n.d. | n.d. |
| Extract | MTC | CC50 |
|---|---|---|
| SNFlE | 2000 μg/mL | 3398 μg/mL |
| PSNFlE | 1000 μg/mL | 1700 μg/mL |
| SEFlE | 1500 μg/mL | 3410 μg/mL |
| PSEFlE | 2000 μg/mL | 3331 μg/mL |
| SNLE | 2000 μg/mL | 3300 μg/mL |
| PSNLE | 2000 μg/mL | 2700 μg/mL |
| SELE | 1500 μg/mL | 3310 μg/mL |
| PSELE | 1500 μg/mL | 3320 μg/mL |
| PSNFrE | 2000 μg/mL | 3570 μg/mL |
| PSEFrE | 2500 μg/mL | 3420 μg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seymenska, D.; Shishkova, K.; Hinkov, A.; Benbassat, N.; Teneva, D.; Denev, P. Comparative Study on Phytochemical Composition, Antioxidant, and Anti-HSV-2 Activities of Sambucus nigra L. and Sambucus ebulus L. Extracts. Appl. Sci. 2023, 13, 12593. https://doi.org/10.3390/app132312593
Seymenska D, Shishkova K, Hinkov A, Benbassat N, Teneva D, Denev P. Comparative Study on Phytochemical Composition, Antioxidant, and Anti-HSV-2 Activities of Sambucus nigra L. and Sambucus ebulus L. Extracts. Applied Sciences. 2023; 13(23):12593. https://doi.org/10.3390/app132312593
Chicago/Turabian StyleSeymenska, Daniela, Kalina Shishkova, Anton Hinkov, Niko Benbassat, Desislava Teneva, and Petko Denev. 2023. "Comparative Study on Phytochemical Composition, Antioxidant, and Anti-HSV-2 Activities of Sambucus nigra L. and Sambucus ebulus L. Extracts" Applied Sciences 13, no. 23: 12593. https://doi.org/10.3390/app132312593
APA StyleSeymenska, D., Shishkova, K., Hinkov, A., Benbassat, N., Teneva, D., & Denev, P. (2023). Comparative Study on Phytochemical Composition, Antioxidant, and Anti-HSV-2 Activities of Sambucus nigra L. and Sambucus ebulus L. Extracts. Applied Sciences, 13(23), 12593. https://doi.org/10.3390/app132312593







