Recent Progress in Applications of Atmospheric Pressure Plasma for Water Organic Contaminants’ Degradation
Abstract
:1. Introduction
2. Types of Plasma Discharge for Water Treatment
3. Plasma Application to Eliminate Organic Pollutants in Water
3.1. Pharmaceuticals
3.1.1. Antibiotics
3.1.2. Recalcitrant Pharmaceuticals
3.1.3. Pesticides
3.2. Organic Chemical Reagents
3.3. Azo Dyes
3.4. Common Industrial Pollutants
3.5. Viruses
4. Effect of Different Discharge Parameters on Wastewater Purification Efficiency
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment—A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef] [PubMed]
- Okpara, C.G.; Oparaku, N.F.; Ibeto, C.N. An Overview of Water Disinfection in Developing Countries and Potentials of Renewable Energy. J. Environ. Sci. Technol. 2011, 4, 18–30. [Google Scholar] [CrossRef]
- Marie, D.; Urs, V.G. Reactions of chlorine with inorganic and organic compounds during water treatment—Kinetics and mechanisms: A critical review. Water Res. 2008, 42, 13–51. [Google Scholar] [CrossRef]
- Stefan, M.I. Advanced Oxidation Processes for Water Treatment—Fundamentals and Applications; Water Intelligence Online 2017; IWA Publishing: London, UK, 2017. [Google Scholar] [CrossRef]
- Mehrjouei, M.; Müller, S.; Möller, D. A review on photocatalytic ozonation used for the treatment of water and wastewater. Chem. Eng. J. 2015, 263, 209–219. [Google Scholar] [CrossRef]
- Ellis, K.V.; Wood, W.E. Slow sand filtration. Crit. Rev. Environ. Control 1985, 15, 315–354. [Google Scholar] [CrossRef]
- Jay, D.B.; Karen, E.T. Water disinfection for developing countries and potential for solar thermal pasteurization. Solar Energy 1998, 64, 87–97. [Google Scholar] [CrossRef]
- Lazar, M.; Varghese, S.; Nair, S. Photocatalytic Water Treatment by Titanium Dioxide: Recent Updates. Catalysts 2012, 2, 572–601. [Google Scholar] [CrossRef]
- Chaplin, B.P. Critical review of electrochemical advanced oxidation processes for water treatment applications. Environ. Sci. Process. Impacts 2014, 16, 1182–1203. [Google Scholar] [CrossRef]
- Gehrke, I.; Geiser, A.; Somborn-Schulz, A. Innovations in nanotechnology for water treatment. Nanotechnol. Sci. Appl. 2015, 8, 1–17. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical treatment technologies for waste-water recycling—An overview. RSC Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Sari, V.; Mika, S. Recent developments in photochemical and chemical AOPs in water treatment: A mini-review. Rev. Environ. Sci. Bio Technol. 2010, 9, 323–330. [Google Scholar] [CrossRef]
- Mijin, D.; Dragana, Z.; Gordana, S.U.; Petar, J. Solvent effects on photodegradation of CI Reactive Orange 16 by simulated solar light. Hem. Ind. 2008, 62, 275–281. [Google Scholar] [CrossRef]
- Mijin, D.; Radulovic, M.; Zlatic, D.; Jovancic, P. Photocatalytic degradation of textile dye RO 16 in TiO2 water suspension by simulated solar light. Chem. Ind. Chem. Eng. Q. 2007, 13, 179–185. [Google Scholar] [CrossRef]
- Khan, M.A.N.; Siddique, M.W.F.; Khan, R. Removal of reactive blue 19 dye by sono, photo and sonophotocatalytic oxidation using visible light. Ultrason. Sonochem. 2015, 26, 370–377. [Google Scholar] [CrossRef]
- Basturk, E.; Karatas, M. Advanced oxidation of Reactive Blue 181 solution: A comparison between Fenton and Sono-Fenton Process. Ultrason. Sonochem. 2014, 21, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Bečelić-Tomin, M.R.; Dalmacija, M.B.; Dalmacija, B.D.; Rajić, L.M.; Tomašević, D.D. Degradation of industrial azo dye in aqueous solution by heterogeneous Fenton process (fly ash/H2O2). Hem. Ind. 2012, 66, 487–496. [Google Scholar] [CrossRef]
- Azadeh, B.Z.D.; Pradeep, L.; Neha, K.; Eun, H.C.; Nagendra, K.K. Recent Progress in Applications of Non-Thermal Plasma for Water Purification, Bio-Sterilization, and Decontamination. Appl. Sci. 2021, 11, 3372. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R.Z. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Arjunan, K.; Sharma, V.; Ptasinska, S. Effects of Atmospheric Pressure Plasmas on Isolated and Cellular DNA—A Review. Int. J. Mol. Sci. 2015, 16, 2971–3016. [Google Scholar] [CrossRef]
- Foster, J.E. Plasma-based water purification: Challenges and prospects for the future. Phys. Plasmas 2017, 24, 055501. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.B.; Ma, R.N.; Zhu, Y.P.; Du, M.R.; Zhang, H.; Jiao, Z. A systematic study of the antimicrobial mechanisms of cold atmospheric-pressure plasma for water disinfection. Sci. Total Environ. 2019, 703, 134965. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.V.; Ho, P.Q.; Pham, T.V.; Nguyen, T.V.; Kim, L. Treatment of surface water using cold plasma for domestic water supply. Environ. Eng. Res. Korean Soc. Environ. Eng. 2018, 24, 412–417. [Google Scholar] [CrossRef]
- Laureano-Anzaldo, C.M.; González-López, M.E.; Pérez-Fonseca, A.A.; Cruz-Barba, L.E.; Robledo-Ortíz, J.R. Plasma-enhanced modification of polysaccharides for wastewater treatment: A review. Carbohydr. Polym. 2020, 252, 117195. [Google Scholar] [CrossRef]
- Gururani, P.; Bhatnagar, P.; Bisht, B.; Kumar, V.; Joshi, N.C.; Tomar, M.S.; Pathak, B. Cold plasma technology: Advanced and sustainable approach for wastewater treatment. Environ. Sci. Pollut. Res. 2021, 28, 65062–65082. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, X.J.; Li, J.R.; Zhang, S.Y. Experimental study on the treatment of dye wastewater by plasma coupled biotechnology. Environ. Sci. Pollut. Res. 2023, 30, 57989–58001. [Google Scholar] [CrossRef]
- Abdallah, D.; Adewale, G.; Ahmed, Y.; Hussein, K.A.; Jamiu, O.E.; Muhammad, R.B.; Oluwadamilola, P. Hazardous and emerging contaminants removal from water by plasma-based treatment: A review of recent advances. Chem. Eng. J. Adv. 2023, 14, 100443. [Google Scholar] [CrossRef]
- Ojha, S.; Frhling, A.; Durek, J.; Ehlbeck, J.; Tiwari, B.K.; Schlüter, O.K.; Bußler, S. Principles and Application of Cold Plasma in Food Processing. In Innovative Food Processing Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 519–540. [Google Scholar] [CrossRef]
- Ozen, E.; Singh, R.K. Atmospheric cold plasma treatment of fruit juices: A review. Trends Food Sci. Technol. 2020, 103, 144–151. [Google Scholar] [CrossRef]
- Cullen, P.J.; Misra, N.N.; Han, L.; Bourke, P.; Keener, K.; O’Donnell, C.; Milosavljević, V. Inducing a dielectric barrier discharge plasma within a package. IEEE Trans. Plasma Sci. 2014, 42, 2368–2369. [Google Scholar] [CrossRef]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta Part B At. Spectrosc. 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Yong, H.I.; Kim, H.J.; Park, S.; Alahakoon, A.U.; Kim, K.; Choe, W.; Jo, C. Evaluation of pathogen inactivation on sliced cheese induced by encapsulated atmospheric pressure dielectric barrier discharge plasma. Food Microbiol. 2015, 46, 46–50. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Babayan, S.E.; Park, J.; Selwyn, G.S.; Hicks, R.F.; Schutze, A.; Sch, A. The atmospheric-pressure plasma jet: A review and comparison to other plasma sources. IEEE Trans. Plasma Sci. 1998, 26, 1685–1694. [Google Scholar] [CrossRef]
- Zhang, S. Atmospheric Pressure RF Plasma Jet: Characterization of Flow and O2 Chemistry. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2015. [Google Scholar]
- Scholtz, V.; Pazlarová, J.; Soušková, H.; Khun, J.; Julák, J. Nonthermal plasma—A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef]
- Krishna, S.; Maslani, A.; Izdebski, T.; Horakova, M.; Klementova, S.; Spatenka, P. Degradation of Verapamil hydrochloride in water by gliding arc discharge. Chemosphere 2016, 152, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Slamani, S.; Abdelmalek, F.; Ghezzar, M.R.; Addou, A. Initiation of Fenton process by plasma gliding arc discharge for the degradation of paracetamol in water. J. Photochem. Photobiol. A Chem. 2018, 359, 1–10. [Google Scholar] [CrossRef]
- Tiya-Djowe, A.; Acayanka, E.; Lontio-Nkouongfo, G.; Laminsi, S.; Gaigneaux, E.M. Enhanced discolouration of methyl violet 10B in a gliding arc plasma reactor by the maghemite nanoparticles used as heterogeneous catalyst. J. Environ. Chem. Eng. 2015, 3, 953–960. [Google Scholar] [CrossRef]
- Taghvaei, H.; Rahimpour, M.R. Upgrading of anisole using in situ generated hydrogen in pin to plate pulsed corona discharge. RSC Adv. 2016, 6, 98369–98380. [Google Scholar] [CrossRef]
- Ajo, P.; Preis, S.; Vornamo, T.; Mänttäri, M.; Kallioinen, M.; Louhi-Kultanen, M. Hospital wastewater treatment with pilot-scale pulsed corona discharge for removal of pharmaceutical residues. J. Environ. Chem. Eng. 2018, 6, 1569–1577. [Google Scholar] [CrossRef]
- García, M.C.; Mora, M.; Esquivel, D.; Foster, J.E.; Rodero, A.; Jiménez-Sanchidrián, C.; Romero-Salguero, F.J. Microwave atmospheric pressure plasma jets for wastewater treatment: Degradation of methylene blue as a model dye. Chemosphere 2017, 180, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.J.; Yin, Y.; Sun, H.; Wang, X.J.; Zhuang, J.; Wang, L.; Ma, R.N.; Jiao, Z. Regulation of cellular redox homeostasis in Arabidopsis thaliana seedling by atmospheric pressure cold plasma-generated reactive oxygen/nitrogen species. Ecotoxicol. Environ. Saf. 2022, 15, 113703. [Google Scholar] [CrossRef] [PubMed]
- Ghezzar, M.R.; Abdelmalek, F.; Belhadj, M.; Benderdouche, N.; Addou, A. Gliding arc plasma assisted photocatalytic degradation of anthraquinonic acid green 25 in solution with TiO2. Appl. Catal. B Environ. 2007, 72, 304–313. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Molina, R.; Bayona, J.M. Assessment of a dielectric barrier discharge plasma reactor at atmospheric pressure for the removal of bisphenol A and tributyltin. Environ. Technol. 2014, 35, 1418–1426. [Google Scholar] [CrossRef]
- Mitrović, T.; Tomić, N.; Djukić-Vuković, A.; Dohčević-Mitrović, Z.; Lazović, S. Atmospheric Plasma Supported by TiO2 Catalyst for Decolourisation of Reactive Orange 16 Dye in Water. Waste Biomass Valoriz. 2020, 11, 6841–6854. [Google Scholar] [CrossRef]
- Judée, F.; Simon, S.; Dufour, T. Plasma-activation of tap water using DBD for agronomy applications: Identification and quantification of long lifetime chemical species and production/consumption mechanisms. Water Res. 2017, 133, 47–59. [Google Scholar] [CrossRef]
- Hu, Y.M.; Bai, Y.; Li, X.; Chen, J. Application of dielectric barrier discharge plasma for degradation and pathways of dimethoate in aqueous solution. Sep. Purif. Technol. 2013, 120, 191–197. [Google Scholar] [CrossRef]
- Attri, P.; Tochikubo, F.; Park, J.H.; Choi, E.H.; Koga, K.; Shiratani, M. Impact of Gamma rays and DBD plasma treatments on wastewater treatment. Sci. Rep. 2018, 8, 2926. [Google Scholar] [CrossRef]
- Bubnov, A.G.; Burova, E.Y.; Grinevich, V.I.; Rybkin, V.V.; Kim, J.K.; Choi, H.S. Plasma-catalytic de- composition of phenols in atmospheric pressure dielectric barrier discharge. Plasma Chem. Plasma Process. 2006, 26, 19–30. [Google Scholar] [CrossRef]
- Sarangapani, C.; Misra, N.N.; Milosavljevic, V.; Bourke, P.; Cullen, P.J. Pesticide degradation in water using atmospheric air cold plasma. J. Water Process Eng. 2016, 9, 225–232. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Palma, V. Enhanced removal of water pollutants by dielectric barrier discharge non-thermal plasma reactor. Sep. Purif. Technol. 2019, 215, 155–162. [Google Scholar] [CrossRef]
- Hanbal, S.E.; Takashima, K.; Miyashita, S.; Ando, S.; Ito, K.; Elsharkawy, M.M.; Kaneko, T.; Takahashi, H. Atmospheric-pressure plasma irradiation can disrupt tobacco mosaic virus particles and RNAs to inactivate their infectivity. Arch. Virol. 2018, 163, 2835–2840. [Google Scholar] [CrossRef] [PubMed]
- Jovicic, V.; Khan, M.; Zbogar-Rasic, A.; Fedorova, N.; Poser, A.; Swoboda, P.; Delgado, A. Degradation of Low Concentrated Perfluorinated Compounds (PFCs) from Water Samples Using Non-Thermal Atmospheric Plasma (NTAP). Energies 2018, 11, 1290. [Google Scholar] [CrossRef]
- Hu, S.; Liu, X.; Xu, Z.; Wang, J.; Li, Y.; Shen, J.; Lan, Y.; Cheng, C. Degradation and mineralization of ciprofloxacin by gas–liquid discharge non-thermal plasma. Plasma Sci. Technol. 2018, 21, 15501. [Google Scholar] [CrossRef]
- Qin, H.; Qiu, H.; He, S.T.; Hong, B.; Liu, K.; Lou, F.; Li, M.; Hu, P.; Kong, X.; Song, Y.; et al. Efficient disinfection of SARS-CoV-2-like coronavirus, pseudotyped SARS-CoV-2 and other coronaviruses using cold plasma induces spike protein damage. J. Hazard. Mater. 2022, 430, 128414. [Google Scholar] [CrossRef] [PubMed]
- Aboubakr, H.A.; Williams, P.; Gangal, U.; Youssef, M.M.; El-Sohaimy, S.A.; Bruggeman, P.J.; Goyal, S.M. Virucidal Effect of Cold Atmospheric Gaseous Plasma on Feline Calicivirus, a Surrogate for Human Norovirus. Appl. Environ. Microbiol. 2015, 81, 3612–3622. [Google Scholar] [CrossRef]
- Filipić, A.; Primc, G.; Zaplotnik, R.; Mehle, N.; Gutierrez-Aguirre, I.; Ravnikar, M.; Mozetič, M.; Žel, J.; Dobnik, D. Cold Atmospheric Plasma as a Novel Method for Inactivation of Potato Virus Y in Water Samples. Food Environ. Virol. 2019, 11, 220–228. [Google Scholar] [CrossRef]
- Lakhian, V.; Dickson-Anderson, S.E. Reduction of bromate and chlorate contaminants in water using aqueous phase corona discharge. Chemosphere 2020, 255, 126864. [Google Scholar] [CrossRef]
- Banaschik, R.; Lukes, P.; Jablonowski, H.; Hammer, M.U.; Weltmann, K.D.; Kolb, J.F. Potential of pulsed corona discharges generated in water for the degradation of persistent pharmaceutical residues. Water Res. 2015, 84, 127–135. [Google Scholar] [CrossRef]
- Panorel, I.; Preis, S.; Kornev, I.; Hatakka, H.; Louhi-Kultanen, M. Oxidation of aqueous paracetamol by pulsed corona discharge. Ozone-Sci. Eng. 2013, 35, 116–124. [Google Scholar] [CrossRef]
- Singh, R.K.; Philip, L.; Ramanujam, S. Continuous flow pulse corona discharge reactor for the tertiary treatment of drinking water: Insights on disinfection and emerging contaminants removal. Chem. Eng. J. 2019, 355, 269–278. [Google Scholar] [CrossRef]
- Lewis, A.J.; Joyce, T.W.; Hadaya, M.; Ebrahimi, F.; Dragiev, I.; Giardetti, N.; Yang, J.; Fridman, G.; Rabinovich, A.; Fridman, A.; et al. Rapid degradation of pfas in aqueous solutions by reverse vortex flow gliding arc plasma. Environ. Sci. Water Res. Technol. 2020, 6, 213959574. [Google Scholar] [CrossRef]
- Sharma, A.K.; Josephson, G.B.; Camaioni, D.M.; Goheen, S.C. Destruction of Pentachlorophenol Using Glow Discharge Plasma Process. Environ. Sci. Technol. 2000, 34, 2267–2272. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, Q.; Wang, T.; Duan, X.; Li, C.; Lei, X. Degradation of organic pollutants using atmospheric pressure glow discharge plasma. Plasma Chem. Plasma Process. 2016, 36, 1011–1020. [Google Scholar] [CrossRef]
- Sugiarto, A.T.; Sato, M. Pulsed plasma processing of organic compounds in aqueous solution. Thin Solid Film. 2001, 386, 295–299. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Alam, D.; Zhang, T.; Li, W.; Xia, Y.; Mai-Prochnow, A.; An, H.; Lovell, E.C.; Masood, H.; et al. Plasmacatalytic bubbles using CeO2 for organic pollutant degradation. Chem. Eng. J. 2021, 403, 126413. [Google Scholar] [CrossRef]
- Hu, Y.M.; Bai, Y.H.; Yu, H.; Zhang, C.H.; Chen, J.R. Degradation of Selected Organophosphate Pesticides in Wastewater by Dielectric Barrier Discharge Plasma. Bull. Environ. Contam. Toxicol. 2013, 91, 314–319. [Google Scholar] [CrossRef]
- Krupez, J.K.; Vesna, V.J.; Milica, R.; Goran, M.N.; Maja, M.K.; Milorad, M.O.; Bratislav, M.D.; Biljana, P. Degradation of nicotine in water solutions using a water falling film dbd plasma reactor: Direct and indirect treatment. J. Phys. D Appl. Phys. A Europhys. J. 2018, 51, 174003. [Google Scholar] [CrossRef]
- Singh, R.K.; Fernando, S.; Baygi, S.F.; Multari, N.; Thagard, S.M.; Holsen, T.M. Breakdown products from perfluorinated alkyl substances (PFAS) degradation in a plasma-based water treatment process. Environ. Sci. Technol. 2019, 53, 2731–2738. [Google Scholar] [CrossRef]
- Singh, R.K.; Philip, L.; Ramanujam, S. Removal of 2,4-dichlorophenoxyacetic acid in aqueous solution by pulsed corona discharge treatment: Effect of different water constituents, degradation pathway and toxicity assay. Chemosphere 2017, 184, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Jiang, B.; Zheng, J.; Liu, Q.; Wu, M. Degradation of azo dye using non-thermal plasma advanced oxidation process in a circulatory airtight reactor system. Chem. Eng. J. 2012, 204, 32–39. [Google Scholar] [CrossRef]
- Magureanu, M.; Mandache, N.B.; Parvulescu, V.I. Degradation of pharmaceutical compounds in water by non-thermal plasma treatment. Water Res. 2015, 81, 124–136. [Google Scholar] [CrossRef]
- Hikmat, H.A.K.; Miessner, H.; Mueller, S.; Kalass, D.; Moeller, D.; Khorshid, I.; Amin, M.R.M. Degradation of pharmaceutical diclofenac and ibuprofen in aqueous solution, a direct comparison of ozonation, photocatalysis, and non-thermal plasma. Chem. Eng. J. 2016, 313, 1033–1041. [Google Scholar] [CrossRef]
- Zhou, R.W.; Zhou, R.S.; Yu, F.; Xi, D.K.; Wang, P.Y.; Li, J.W.; Wang, X.Q.; Zhang, X.H.; Bazaka, K.; Ostrikov, K.K. Removal of organophosphorus pesticide residues from Lycium barbarum by gas phase surface discharge plasma. Chem. Eng. J. 2018, 342, 401–409. [Google Scholar] [CrossRef]
- Zhou, R.W.; Zhou, R.S.; Zhuang, J.X.; Zong, Z.C.; Zhang, X.H.; Liu, D.P.; Bazaka, K.; Ostrikov, K.; Yousfi, M. Interaction of Atmospheric-Pressure Air Microplasmas with Amino Acids as Fundamental Processes in Aqueous Solution. PLoS ONE 2016, 11, e0155584. [Google Scholar] [CrossRef]
- Li, Y.J.; Qu, G.Z.; Zhang, L.Y.; Wang, T.C.; Sun, Q.H.; Liang, D.L.; Hu, S.B. Humic acid removal from micro-polluted source water using gas phase surface discharge plasma at different grounding modes. Sep. Purif. Technol. 2017, 180, 36–43. [Google Scholar] [CrossRef]
- Daniel, G.; Benjamin, D.S.; Rebecca, A.T.; Shane, A.S. An evaluation of a pilot-scale nonthermal plasma advanced oxidation process for trace organic compound degradation. Water Res. 2010, 44, 493–504. [Google Scholar] [CrossRef]
- Krishna, S.; Ceriani, E.; Marotta, E.; Giardina, A.; Špatenka, P.; Paradisi, C. Products and mechanism of verapamil removal in water by air non-thermal plasma treatment. Chem. Eng. J. 2016, 292, 35–41. [Google Scholar] [CrossRef]
- Magureanu, M.; Dobrin, D.; Mandache, N.B.; Bradu, C.; Medvedovici, A.; Parvulescu, V.I. The Mechanism of Plasma Destruction of Enalapril and Related Metabolites in Water. Plasma Process. Polym. 2013, 10, 459–468. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- El Shaer, M.; Eldaly, M.; Heikal, G.; Sharaf, Y.; Diab, H.; Mobasher, M.; Rousseau, A. Antibiotics Degradation and Bacteria Inactivation in Water by Cold Atmospheric Plasma Discharges Above and Below Water Surface. Plasma Chem. Plasma Process. 2020, 40, 971–983. [Google Scholar] [CrossRef]
- Shahsavari, N.; Zhang, X.H. Microbubble-enhanced cold plasma activation for water decontamination: Degradation dynamics and energy yield in relation to pollutant concentration, total volume and flow rate of water. J. Water Process Eng. 2023, 55, 104169. [Google Scholar] [CrossRef]
- Liu, Q.; Ouyang, W.; Yang, X.; He, Y.; Wu, Z.; Ostrikov, K.K. Plasma-microbubble treatment and sustainable agriculture application of diclofenac-contaminated wastewater. Chemosphere 2023, 334, 138998. [Google Scholar] [CrossRef]
- Magureanu, M.; Mandache, N.B.; Bradu, C.; Parvulescu, V.I. High efficiency plasma treatment of water contaminated with organic compounds. Study of the degradation of ibuprofen. Plasma Process. Polym. 2018, 15, 1700201. [Google Scholar] [CrossRef]
- Khetan, S.K.; Collins, T.J. Human Pharmaceuticals in the Aquatic Environment: A Challenge to Green Chemistry. Chem. Rev. 2007, 107, 2319–2364. [Google Scholar] [CrossRef]
- Liu, Y.N.; Mei, S.F.; Djakaou, I.S.; Simeon, C.; Stéphanie, O. Carbamazepine removal from water by dielectric barrier discharge: Comparison of ex situ and in situ discharge on water. Chem. Eng. Process. 2012, 56, 10–18. [Google Scholar] [CrossRef]
- Gao, J.J.; Liu, L.H.; Liu, X.R.; Zhou, H.D.; Lu, J.; Huang, S.B.; Wang, Z.J. The Occurrence and Spatial Distribution of Organophosphorous Pesticides in Chinese Surface Water. Bull. Environ. Contam. Toxicol. 2009, 82, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Z.; Kong, W.J.; Qiu, F.; Wei, J.H.; Yang, S.H.; Zheng, Y.G.; Yang, M.H. One-step extraction for gas chromatography with flame photometric detection of 18 organophosphorus pesticides in Chinese medicine health wines. J. Chromatogr. B 2012, 885, 90–96. [Google Scholar] [CrossRef]
- Foster, J.; Sommers, B.S.; Gucker, S.N.; Blankson, I.M.; Adamovsky, G. Perspectives on the Interaction of Plasmas with Liquid Water for Water Purification. IEEE Trans. Plasma Sci. 2012, 40, 1311–1323. [Google Scholar] [CrossRef]
- Grabowski, L.R.; van Veldhuizen, E.M.; Pemen, A.J.M.; Rutgers, W.R. Corona Above Water Reactor for Systematic Study of Aqueous Phenol Degradation. Plasma Chem. Plasma Process. 2006, 26, 3–17. [Google Scholar] [CrossRef]
- Bobkova, E.S.; Isakina, A.A.; Shishkin, A.I.; Kuznets, N.N.; Morev, A.M. Features of phenol degradation in aqueous solution in dielectric-barrier discharge in oxygen. High Energy Chem. 2015, 49, 68–71. [Google Scholar] [CrossRef]
- Bubnov, A.G.; Burova, E.Y.; Grinevich, V.I.; Rybkin, V.V.; Kim, J.K.; Choi, H.S. Comparative Actions of NiO and TiO2 Catalysts on the Destruction of Phenol and its Derivatives in a Dielectric Barrier Discharge. Plasma Chem. Plasma Process. 2007, 27, 177–187. [Google Scholar] [CrossRef]
- Bobkova, E.S.; Sungurova, A.V.; Rybkin, V.V. Mechanism of phenol degradation processes induced by direct-current atmospheric-pressure discharge in air. High Energy Chem. 2013, 47, 198–200. [Google Scholar] [CrossRef]
- Bobkova, E.S.; Rybkin, V.V. Peculiarities of Energy Efficiency Comparison of Plasma Chemical Reactors for Water Purification from Organic Substances. Plasma Chem. Plasma Process. 2015, 35, 133–142. [Google Scholar] [CrossRef]
- Lukes, P.; Locke, B.R. Plasmachemical oxidation processes in a hybrid gas–liquid electrical discharge reactor. J. Phys. D Appl. Phys. 2005, 38, 4074–4081. [Google Scholar] [CrossRef]
- Bobkova, E.S. Design of Dielectric Barrier Discharge Reactor and Simulation of Purification Processes of Aqueous Solutions. Theor. Found. Chem. Eng. 2020, 54, 500–505. [Google Scholar] [CrossRef]
- Potratz, V.Y. Ground-Water Geochemistry of the Ogallala Aquifer in the Southern High Plains of Texas and New Mexico. Ph.D. Dissertation, Texas Tech University, Lubbock, TX, USA, 1980. [Google Scholar]
- Bubnov, A.G.; Grinevich, V.I.; Kuvykin, N.A.; Maslova, O.N. The Kinetics of Plasma-Induced Degradation of Organic Pollutants in Sewage Water. High Energy Chem. 2004, 38, 41–45. [Google Scholar] [CrossRef]
- Bostick, D.T. Characterization of Soluble Organics in Produced Water; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 2002. [CrossRef]
- Ishiguro, T.; Yasuoka, K. Advanced oxidation process by a combined ozone/plasma system using plasma in bubbles. IEEJ Trans. Fundam. Mater. 2015, 135, 175–181. [Google Scholar] [CrossRef]
- Takeuchi, N.; Ishibashi, N.; Sugiyama, T.; Kim, H.H. Effective utilization of ozone in plasma-based advanced oxidation process. Plasma Sources Sci. Technol. 2018, 27, 055013. [Google Scholar] [CrossRef]
- Qi, Z.H.; Yang, L.; Xia, Y.; Ding, Z.F.; Niu, J.H.; Liu, D.P.; Zhao, Y.; Ji, L.F.; Song, Y.; Lin, X.S. Removal of dimethyl phthalate in water by non-thermal air plasma treatment. Environ. Sci. Water Res. Technol. 2019, 5, 920–930. [Google Scholar] [CrossRef]
- Rodrigo, O.A.d.L.; Ana, P.B.; Daisy MF, S.; Célia, M.R.; de Danielle, P.O.; de Gisela, A.U. Mutagenic and carcinogenic potential of a textile azo dye processing plant effluent that impacts a drinking water source. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2007, 626, 53–60. [Google Scholar] [CrossRef]
- Gao, J.F.; Zhang, Q.; Su, K.; Chen, R.N.; Peng, Y.Z. Biosorption of Acid Yellow 17 from aqueous solution by non-living aerobic granular sludge. J. Hazard. Mater. 2010, 174, 215–225. [Google Scholar] [CrossRef]
- Dutta, S.; Saha, R.; Kalita, H.; Bezbaruah, A.N. Rapid reductive degradation of azo and anthraquinone dyes by nanoscale zero-valent iron. Environ. Technol. Innov. 2016, 5, 176–187. [Google Scholar] [CrossRef]
- Biljana, P.D.; Goran, M.R.; Bratislav, M.O.; Milorad, M.K.; Mirjana, M.K.; Jelena, N.; Dragan, D.M. Decolorization of reactive textile dyes using water falling film dielectric barrier discharge. J. Hazard. Mater. 2011, 192, 763–771. [Google Scholar] [CrossRef]
- Chandanshive, V.V.; Kadam, S.K.; Khandare, R.V.; Kurade, M.B.; Jeon, B.H.; Jadhav, J.P.; Govindwar, S.P. In situ phytoremediation of dyes from textile wastewater using garden ornamental plants, effect on soil quality and plant growth. Chemosphere 2018, 210, 968–976. [Google Scholar] [CrossRef]
- Anjali, P.; Poonam, S.; Leela, I. Bacterial decolorization and degradation of azo dyes. Int. Biodeterior. Biodegrad. 2007, 59, 73–84. [Google Scholar] [CrossRef]
- Puač, N.; Miletić, M.; Mojović, M.; Popović-Bijelić, A.; Vuković, D.; Miličić, B.; Maletić, D.; Lazović, S.; Petrović, Z.L. Sterilization of bacteria suspensions and identification of radicals deposited during plasma treatment. Open Chem. 2014, 13, 332–338. [Google Scholar] [CrossRef]
- Gumuchian, D.; Cavadias, S.; Duten, X.; Tatoulian, M.; Da Costa, P.; Ognier, S. Organic pollutants oxidation by needle/plate plasma discharge: On the influence of the gas nature. Chem. Eng. Process. Process Intensif. 2014, 82, 185–192. [Google Scholar] [CrossRef]
- Yasushi, M.; Kohki, S.; Hidenori, I. Pulsed discharge purification of water containing nondegradable hazardous substances. Electr. Eng. Jpn. 2011, 174, 1–8. [Google Scholar] [CrossRef]
- Shimizu, K.; Masamura, N.; Blajan, M. Water Purification by Using Microplasma Treatment. J. Phys. Conf. Ser. 2013, 441, 012005. [Google Scholar] [CrossRef]
- Manon, V.; Chantal, G.; Jean-Marie, H. Photocatalytic Degradation of Dyes in Water: Case Study of Indigo and of Indigo Carmine. J. Catal. 2001, 201, 46–59. [Google Scholar] [CrossRef]
- Cristina, F.; Salah, A.; Conchita, A.; Enric, B.; Aída, V.V.Z.; Ridha, A. Electro-Fenton and photoelectro-Fenton degradation of indigo carmine in acidic aqueous medium. Appl. Catal. B Environ. 2006, 67, 93–104. [Google Scholar] [CrossRef]
- Attri, P.; Yusupov, M.; Park, J.H.; Lingamdinne, L.P.; Koduru, J.R.; Shiratani, M.; Choi, E.H.; Bogaerts, A. Mechanism and comparison of needle-type non-thermal direct and indirect atmospheric pressure plasma jets on the degradation of dyes. Sci. Rep. 2016, 6, 34419. [Google Scholar] [CrossRef]
- Hamdan, A.; Liu, J.L.; Cha, M.S. Microwave Plasma Jet in Water: Characterization and Feasibility to Wastewater Treatment. Plasma Chem. Plasma Process. 2018, 38, 1003–1020. [Google Scholar] [CrossRef]
- Grabowski, L.R.; Veldhuizen, E.M.; Pemen, A.J.M.; Rutgers, W.R. Breakdown of methylene blue and methyl orange by pulsed corona discharge. Plasma Sources Sci. Technol. 2007, 16, 226–232. [Google Scholar] [CrossRef]
- Hamdan, A.; Gagnon, C.; Aykul, M.; Profili, J. Characterization of a microwave plasma jet (TIAGO) in-contact with water: Application in degradation of methylene blue dye. Plasma Process. Polym. 2019, 17, 1900157. [Google Scholar] [CrossRef]
- Hamdan, A.; Profili, J.; Cha, M.S. Microwave Plasma Jet in Water: Effect of Water Electrical Conductivity on Plasma Characteristics. Plasma Chem. Plasma Process. 2019, 40, 169–185. [Google Scholar] [CrossRef]
- Huang, F.M.; Chen, L.; Wang, H.G.; Yan, Z.C. Analysis of the degradation mechanism of methylene blue by atmospheric pressure dielectric barrier discharge plasma. Chem. Eng. J. 2010, 162, 250–256. [Google Scholar] [CrossRef]
- Xu, L.; Li, H.; Rashid, S.; Shen, C.; Wen, Y.; He, T. Treatment of saline dye wastewater using glow discharge plasma. Fresenius Environ. Bull. 2016, 25, 2466–2472. [Google Scholar]
- Parsons, J.R.; Sáez, M.; Dolfing, J.; de Voogt, P. Biodegradation of perfluorinated compounds. Rev. Environ. Contam. Toxicol. 2008, 196, 53–71. [Google Scholar] [CrossRef]
- Davide, P.; Dimitra, P.; Manuel, L.; Rita, B.; Mohamad, S.; Marco, M.; Claire, R. PFAS Degradation in Ultrapure and Groundwater Using Non-Thermal Plasma. Molecules 2021, 26, 924. [Google Scholar] [CrossRef]
- Blotevogel, J.; Selma, M.T.; Shaily, M. Scaling up water treatment technologies for PFAS destruction: Current status and potential for fit-for-purpose application. Curr. Opin. Chem. Eng. 2023, 41, 100944. [Google Scholar] [CrossRef]
- AMEC. Management of Wastes from Atlantic Seafood Processing Operations; AMEC Earth and Environment Limited: Dartmouth, NS, Canada, 2003. [Google Scholar]
- Cheng, H.H.; Chen, S.S.; Wu, Y.H.; Ho, D.L. Non-thermal plasma technology for degradation of organic compounds in wastewater control: A critical review. J. Environ. Eng. 2007, 17, 427–433. [Google Scholar]
- Malik, M.A.; Ghaffar, A.; Malik, S.A. Water purification by electrical discharges. Plasma Sources Sci. Technol. 2001, 10, 82–91. [Google Scholar] [CrossRef]
- Patange, A.; Boehm, D.; Giltrap, M.; Lu, P.; Cullen, P.J.; Bourke, P. Assessment of the disinfection capacity and eco-toxicological impact of atmospheric cold plasma for treatment of food industry effluents. Sci. Total Environ. 2018, 631, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Naicker, K.I.; Kaweesa, P.; Daramola, M.O.; Iwarere, S.A. Non-Thermal Plasma Review: Assessment and Improvement of Feasibility as a Retrofitted Technology in Tertiary Wastewater Purification. Appl. Sci. 2023, 13, 6243. [Google Scholar] [CrossRef]
- Wang, Z.H.; Bush, R.T.; Sullivan, L.A.; Liu, J.S. Simultaneous Redox Conversion of Chromium(VI) and Arsenic(III) under Acidic Conditions. Environ. Sci. Technol. 2013, 47, 6486–6492. [Google Scholar] [CrossRef]
- Khlyustova, A.; Sirotkin, N.; Titov, V. Plasma-induced precipitation of metal ions in aqueous solutions. J. Chem. Technol. Biotechnol. 2019, 94, 3987–3992. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Venkata Mohan, S.; Lens, P.N.L. Metals removal and recovery in bioelectrochemical systems: A review. Bioresour. Technol. 2015, 195, 102–114. [Google Scholar] [CrossRef]
- Fu, F.L.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Pervez, N.; Rashid, A.; Alam, A.H. Analysis of HV Plasma Corona Reactor Treatment System for Industrial Waste Water. In Proceedings of the 2016 International Conference on Frontiers of Information Technology (FIT), Islamabad, Pakistan, 19–21 December 2016; pp. 269–273. [Google Scholar] [CrossRef]
- Jiang, B.; Guo, J.B.; Wang, Z.H.; Zheng, X.; Zheng, J.T.; Wu, W.T.; Wu, M.B.; Xue, Q.Z. A green approach towards simultaneous remediations of chromium(VI) and arsenic(III) in aqueous solution. Chem. Eng. J. 2015, 262, 1144–1151. [Google Scholar] [CrossRef]
- Munnaf, S.A.; Jang, M.; Choi, E.H. Green iron oxide (GIO) utilized for reductive removal of As(III) and methyl red (MR) with non-thermal plasma via synergistic catalytic study. J. Environ. Chem. Eng. 2023, 11, 109885. [Google Scholar] [CrossRef]
- Nasir, A.; Caetano-Anolles, G. A phylogenomic data-driven exploration of viral origins and evolution. Sci. Adv. 2015, 1, e1500527. [Google Scholar] [CrossRef] [PubMed]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Filipić, A.; Gutierrez-Aguirre, I.; Primc, G.; Mozetič, M.; Dobnik, D. Cold plasma, a new hope in the field of virus inactivation. Trends Biotechnol. 2020, 38, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Shrestha, S.; Shindo, J.; Sherchand, J.B.; Haramoto, E. Virological Quality of Irrigation Water Sources and Pepper Mild Mottle Virus and Tobacco Mosaic Virus as Index of Pathogenic Virus Contamination Level. Food Environ. Virol. 2017, 10, 107–120. [Google Scholar] [CrossRef]
- Rimoldi, S.G.; Stefani, F.; Gigantiello, A.; Polesello, S.; Comandatore, F.; Mileto, D.; Salerno, F. Presence and infectivity of SARSCoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020, 744, 140911. [Google Scholar] [CrossRef]
- Volotskova, O.; Dubrovsky, L.; Keidar, M.; Bukrinsky, M.; Harrich, D. Cold Atmospheric Plasma Inhibits HIV-1 Replication in Macrophages by Targeting Both the Virus and the Cells. PLoS ONE 2016, 11, e0165322. [Google Scholar] [CrossRef]
- Mohamed, H.; Berman, R.; Connors, J.; Haddad, E.K.; Miller, V.; Nonnemacher, M.R.; Dampier, W.; Wigdahl, B.; Krebs, F.C. Immunomodulatory Effects of Non-Thermal Plasma in a Model for Latent HIV-1 Infection: Implications for an HIV-1-Specific Immunotherapy. Biomedicines 2023, 11, 122. [Google Scholar] [CrossRef]
- Sutter, J.; Bruggeman, P.J.; Wigdahl, B.; Krebs, F.C.; Miller, V. Manipulation of Oxidative Stress Responses by Non-Thermal Plasma to Treat Herpes Simplex Virus Type 1 Infection and Disease. Int. J. Mol. Sci. 2023, 24, 4673. [Google Scholar] [CrossRef]
- Ahlfeld, B.; Li, Y.; Boulaaba, A.; Binder, A.; Schotte, U.; Zimmermann, J.L.; Morfill, G.E.; Klein, G. Inactivation of a Foodborne Norovirus Outbreak Strain with Nonthermal Atmospheric Pressure Plasma. mBio 2015, 6, e02300-14. [Google Scholar] [CrossRef]
- Guo, L.; Yao, Z.; Yang, L.; Zhang, H.; Qi, Y.; Gou, L.; Xi, W.; Liu, D.; Zhang, L.; Cheng, Y.; et al. Plasma-activated water: An alternative disinfectant for S protein inactivation to prevent SARS-CoV-2 infection. Chem. Eng. J. 2020, 421, 127742. [Google Scholar] [CrossRef] [PubMed]
- Sahun, M.; Privat-Maldonado, A.; Lin, A.; De Roeck, N.; Van der Heyden, L.; Hillen, M.; Michiels, J.; Steenackers, G.; Smits, E.; Ariën, K.K.; et al. Inactivation of SARS-CoV-2 and Other Enveloped and Non-Enveloped Viruses with Non-Thermal Plasma for Hospital Disinfection. ACS Sustain. Chem. Eng. 2023, 11, 5206–5215. [Google Scholar] [CrossRef]
- Thomas, S.V.; Dienger-Stambaugh, K.; Jordan, M.; Wang, Y.; Hammonds, J.; Spearman, P.; Shi, D. Inactivation of SARS-CoV-2 on Surfaces by Cold-Plasma-Generated Reactive Species. Bioengineering 2023, 10, 280. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.J. Newcastle disease and other avian paramyxoviruses. Rev. Sci. Tech.-Off. Int. Epizoot. 2000, 19, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.M.; Zhu, R.H.; Yang, L.C.; Wang, K.L.; Zhang, Q.; Su, X.; Yang, B.; Zhang, J.; Fang, J. Non-thermal plasma for inactivated-vaccine preparation. Vaccine 2015, 34, 1126–1132. [Google Scholar] [CrossRef]
- Dimitrov, K.M.; Afonso, C.L.; Yu, Q.Z.; Miller, P.J. Newcastle disease vaccines—A solved problem or a continuous challenge? Vet. Microbiol. 2016, 206, 126–136. [Google Scholar] [CrossRef]
- Jang, H.; Elaish, M.; Mahesh, K.C.; Abundo, M.C.; Ghorbani, A.; Ngunjiri, J.M.; Lee, C.; Krammer, F. Efficacy and synergy of live-attenuated and inactivated influenza vaccines in young chickens. PLoS ONE 2018, 13, e0195285. [Google Scholar] [CrossRef]
- Aboubakr, H.A.; Gangal, U.; Youssef, M.M.; Goyal, S.M.; Bruggeman, P.J. Inactivation of virus in solution by cold atmospheric pressure plasma: Identification of chemical inactivation pathways. J. Phys. D Appl. Phys. 2016, 49, 204001. [Google Scholar] [CrossRef]
- Aboubakr, H.A.; Mor, S.K.; Higgins, L.A.; Armien, A.; Youssef, M.M.; Bruggeman, P.J.; Goyal, S.M.; Menéndez-Arias, L. Cold argon-oxygen plasma species oxidize and disintegrate capsid protein of feline calicivirus. PLoS ONE 2018, 13, e0194618. [Google Scholar] [CrossRef] [PubMed]
- Lefeuvre, P.; Martin, D.P.; Elena, S.F.; Shepherd, D.N.; Roumagnac, P.; Varsani, A. Evolution and ecology of plant viruses. Nat. Rev. Microbiol. 2019, 17, 632–644. [Google Scholar] [CrossRef]
- Wetter, C.; Conti, M.; Altschuh, D.; Tabillion, R.; Regenmortel, M.H. Pepper mild mottle virus, a tobamovirus infecting pepper cultivars in Sicily. Phytopathology 1984, 74, 405–410. [Google Scholar] [CrossRef]
- Haramoto, E.; Kitajima, M.; Kishida, N.; Konno, Y.; Katayama, H.; Asami, M.; Akiba, M. Occurrence of pepper mild mottle virus in drinking water sources in Japan. Appl. Environ. Microbiol. 2013, 79, 7413–7418. [Google Scholar] [CrossRef] [PubMed]
- Son, G.; Lee, H.; Gu, J.E.; Lee, S. Decoloration of methylene blue hydrate by submerged plasma irradiation process. Desalination Water Treat. 2015, 54, 1445–1451. [Google Scholar] [CrossRef]
- Takemura, Y.; Yamaguchi, N.; Hara, T. Decomposition of Methylene Blue by using an Atmospheric Plasma Jet with Ar, N2, O2, or Air. Jpn. J. Appl. Phys. 2013, 52, 056102. [Google Scholar] [CrossRef]
- Patinglag, L.; Sawtell, D.; Iles, A.; Melling, L.M.; Shaw, K.J. A Microfluidic Atmospheric-Pressure Plasma Reactor for Water Treatment. Plasma Chem. Plasma Process. 2019, 39, 561–575. [Google Scholar] [CrossRef]
- Magureanu, M.; Bradu, C.; Piroi, D.; Mandache, N.B.; Parvulescu, V. Pulsed Corona Discharge for Degradation of Methylene Blue in Water. Plasma Chem. Plasma Process. 2013, 33, 51–64. [Google Scholar] [CrossRef]
- Magureanu, M.; Piroi, D.; Mandache, N.B.; Parvulescu, V. Decomposition of methylene blue in water using a dielectric barrier discharge: Optimization of the operating parameters. J. Appl. Phys. 2008, 104, 103306. [Google Scholar] [CrossRef]
- Magureanu, M.; Piroi, D.; Mandache, N.B.; David, V.; Medvedovici, A.; Bradu, C.; Parvulescu, V.I. Degradation of antibiotics in water by non-thermal plasma treatment. Water Res. 2011, 45, 3407–3416. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J.; Profili, J.; Hamdan, A. Characterization of Various Air Plasma Discharge Modes in Contact with Water and Their Effect on the Degradation of Reactive Dyes. Plasma Chem. Plasma Process. 2019, 39, 1483–1498. [Google Scholar] [CrossRef]
- Yehia, S.A.; Zarif, M.E.; Bita, B.I.; Teodorescu, M.; Carpen, L.G.; Vizireanu, S.; Petrea, N.; Dinescu, G. Development and Optimization of Single Filament Plasma Jets for Wastewater Decontamination. Plasma Chem. Plasma Process. 2020, 40, 1485–1505. [Google Scholar] [CrossRef]
- Zeghioud, H.; Nguyen-Tri, P.; Khezami, L.; Amrane, A.; Assadi, A.A. Review on discharge Plasma for water treatment: Mechanism, reactor geometries, active species and combined processes. J. Water Process Eng. 2020, 38, 101664. [Google Scholar] [CrossRef]
- Reddy, M.K.P.; Rama Raju, B.; Karuppiah, J.; Linga Reddy, E.; Subrahmanyam, C. Degradation and mineralization of methylene blue by dielectric barrier discharge non-thermal plasma reactor. Chem. Eng. J. 2013, 217, 41–47. [Google Scholar] [CrossRef]
- Kim, K.S.; Yang, C.S.; Mok, Y.S. Degradation of veterinary antibiotics by dielectric barrier discharge plasma. Chem. Eng. J. 2013, 219, 19–27. [Google Scholar] [CrossRef]
- Chen, Y.S.; Zhang, X.S.; Dai, Y.C.; Yuan, W.K. Pulsed high-voltage discharge plasma for degradation of phenol in aqueous solution. Sep. Purif. Technol. 2004, 34, 5–12. [Google Scholar] [CrossRef]
- Sano, N.; Kawashima, T.; Fujikawa, J.; Fujimoto, T.; Kitai, T.; Kanki, T.; Toyoda, A. Decomposition of Organic Compounds in Water by Direct Contact of Gas Corona Discharge: Influence of Discharge Conditions. Ind. Eng. Chem. Res. 2002, 41, 5906–5911. [Google Scholar] [CrossRef]
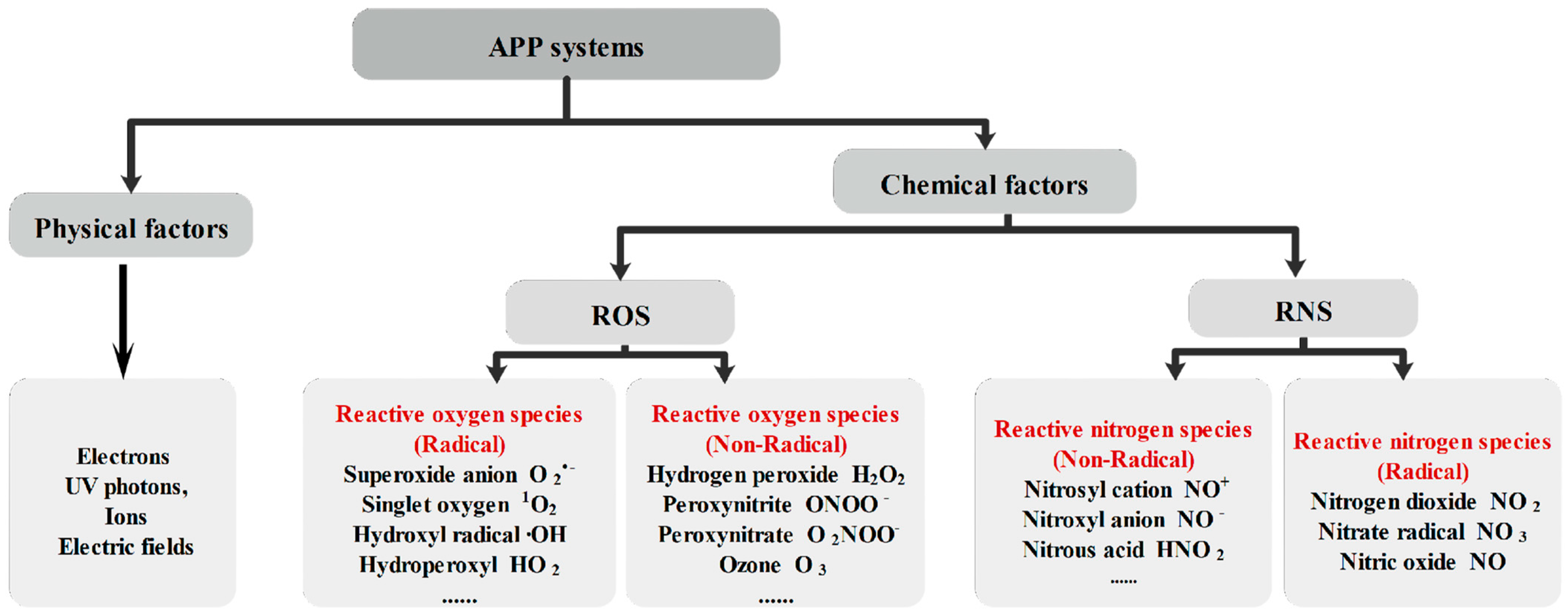

| Water Treatment Technology | Advantages | Disadvantages | Refs. |
|---|---|---|---|
| Conventional methods | |||
| Biodegradation | Eco-friendly and economical | Unrestricted breakdown of products, low biodegradability of some pollutants, requirement of maintenance and management of microorganisms. | [28] |
| Flocculation, coagulation, ion ex-change, filtration | Simple, no chemical reagents and no secondary pollution | High consumption of energy and reagents, low selectivity with high investment and operational costs. | [6,28] |
| Thermal oxidation, boiling | High-efficiency | High running costs, emission of various dioxins and other pollutants into the environment. | [7] |
| Modern oxidation processes | |||
| Photo-Fenton, photocatalysis, ozonation, ultrasonication | Simple, eco-friendly and economical | Lack of a complete oxidation process. | [2,5,14,17] |
| Atmospheric pressure plasma | Does not rely on UV lamps and ex-pensive chemicals to produce reactive substances, emits light and shockwaves; high-efficiency, simple, eco-friendly, economical and easy-to-use technology | Energy output is unclear, small application scale. | [29,30] |
| Plasma Source | Organic Contaminants | Operation Conditions | Degradation | Refs. |
|---|---|---|---|---|
| DBD reactor | Dimethoate | 85 W, 7 min, air | >96% | [51] |
| DBD reactor | Methyl orange, methylene blue | 4.5 W, 10.6 kHz, 10 min, O2 | ~98% | [52] |
| DBD reactor | Phenol | 16 kV, 50 s | 99% | [53] |
| DBD reactor | Dichlorvos | 80 kV, 8 min | 78.9% | [54] |
| DBD reactor | Ceftriaxone | 5 min, O2 | 100% | [55] |
| DBD reactor | Caffeine | 10 min, O2 | 80% | [55] |
| DBD reactor | Tobacco mosaic virus | 20 kV, 10 min, air | Complete inactivation | [56] |
| Plasma jet | PFCs | 300 W, 3–5 min, air | 64–90% | [57] |
| Plasma jet | Ciprofloxacin | 24 min, air | 84.1% | [58] |
| Plasma jet | SARS-CoV-2 | 10.89 W, 300 s, argon (99.999%) | 99.94% | [59] |
| Plasma jet | Swine coronavirus PEDV and SADS-CoV | 10.89 W, 300 s, argon (99.999%) | 99.86%, 99.74% | [59] |
| Plasma jet | Norovirus (feline calicivirus) | 2.5 W, 15 s | 6.0 log inactivation | [60] |
| Plasma jet | Potato virus Y | 3 W, 1 min, Ar/O2 (99:1) | Complete inactivate | [61] |
| Corona plasma reactor | Bromate | 20 kV, 60 min | 95% | [62] |
| Corona plasma reactor | Seven recalcitrant pharmaceuticals | 15–66 min | 45–99% | [63] |
| Corona plasma reactor | Paracetamol | 30 min, air/O2 | 100% | [64] |
| Corona plasma reactor | Carbofuran | 24 min, air | 91% | [65] |
| Arc plasma reactor | Perfluoroalkyl carboxylates | 150 W, 60 min | 98% | [66] |
| Arc plasma reactor | Anthraquinonic acid green 25 | 9 kV, 60 min | 50% | [47] |
| Glow discharge | Pentachlorophenol | 30 min, air/O2 | <0.01 ppm | [67] |
| Glow discharge | Methyl orange | 15 min, air | 93% | [68] |
| Needle-plate reactor | Phenol | 20 kV, 30–60 min | >99% | [69] |
| Organic Contaminants | Degradation Pathway/Intermediates | Products | Refs. |
|---|---|---|---|
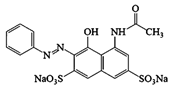 Azophloxine | 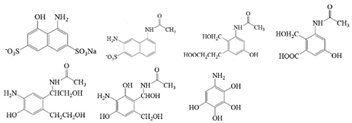 | CO2, H2O | [70] |
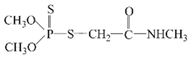 Dimethoate |  | PO43−, SO42−, NO3−, CO2, H2O | [71] |
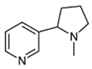 Nicotine | 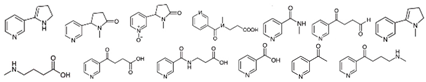 | Nicotinic acid, CO2, H2O | [72] |
 PFOS | 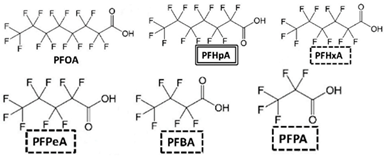 | SO42−, H2O, F−, CO2 | [73] |
 2-4-D | 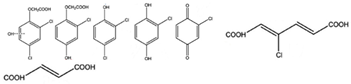 | CO2, H2O | [74] |
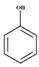 Phenol | 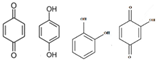 (Hydroxylation) (Hydroxylation) 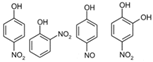 (Nitration/nitrosation) (Nitration/nitrosation) | CO2, H2O | [75] |
 Methyl orange |  | SO42−, NO3−, CO2, H2O, R-COOH | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Xu, H.; Zhu, Y.; Zhuang, J.; Ma, R.; Cui, D.; Jiao, Z. Recent Progress in Applications of Atmospheric Pressure Plasma for Water Organic Contaminants’ Degradation. Appl. Sci. 2023, 13, 12631. https://doi.org/10.3390/app132312631
Yin Y, Xu H, Zhu Y, Zhuang J, Ma R, Cui D, Jiao Z. Recent Progress in Applications of Atmospheric Pressure Plasma for Water Organic Contaminants’ Degradation. Applied Sciences. 2023; 13(23):12631. https://doi.org/10.3390/app132312631
Chicago/Turabian StyleYin, Yue, Hangbo Xu, Yupan Zhu, Jie Zhuang, Ruonan Ma, Dongjie Cui, and Zhen Jiao. 2023. "Recent Progress in Applications of Atmospheric Pressure Plasma for Water Organic Contaminants’ Degradation" Applied Sciences 13, no. 23: 12631. https://doi.org/10.3390/app132312631
APA StyleYin, Y., Xu, H., Zhu, Y., Zhuang, J., Ma, R., Cui, D., & Jiao, Z. (2023). Recent Progress in Applications of Atmospheric Pressure Plasma for Water Organic Contaminants’ Degradation. Applied Sciences, 13(23), 12631. https://doi.org/10.3390/app132312631







