Interleukin-6 Expression of Osteogenic Cell Lines Grown on Laser-Treated and Hydroxyapatite-Coated Titanium Discs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surfaces
2.2. Laser Beam
2.3. Laser Beam Followed by Deposition of Hydroxyapatite Biomimetic Method (LHS)
2.4. Topographic Characterization of Surfaces
2.5. Cell Culture
2.6. Phalloidin Staining
2.7. Viability Assay
2.8. qRT-PCR and Immunoassay Analysis
2.9. Statistical Analysis
3. Results
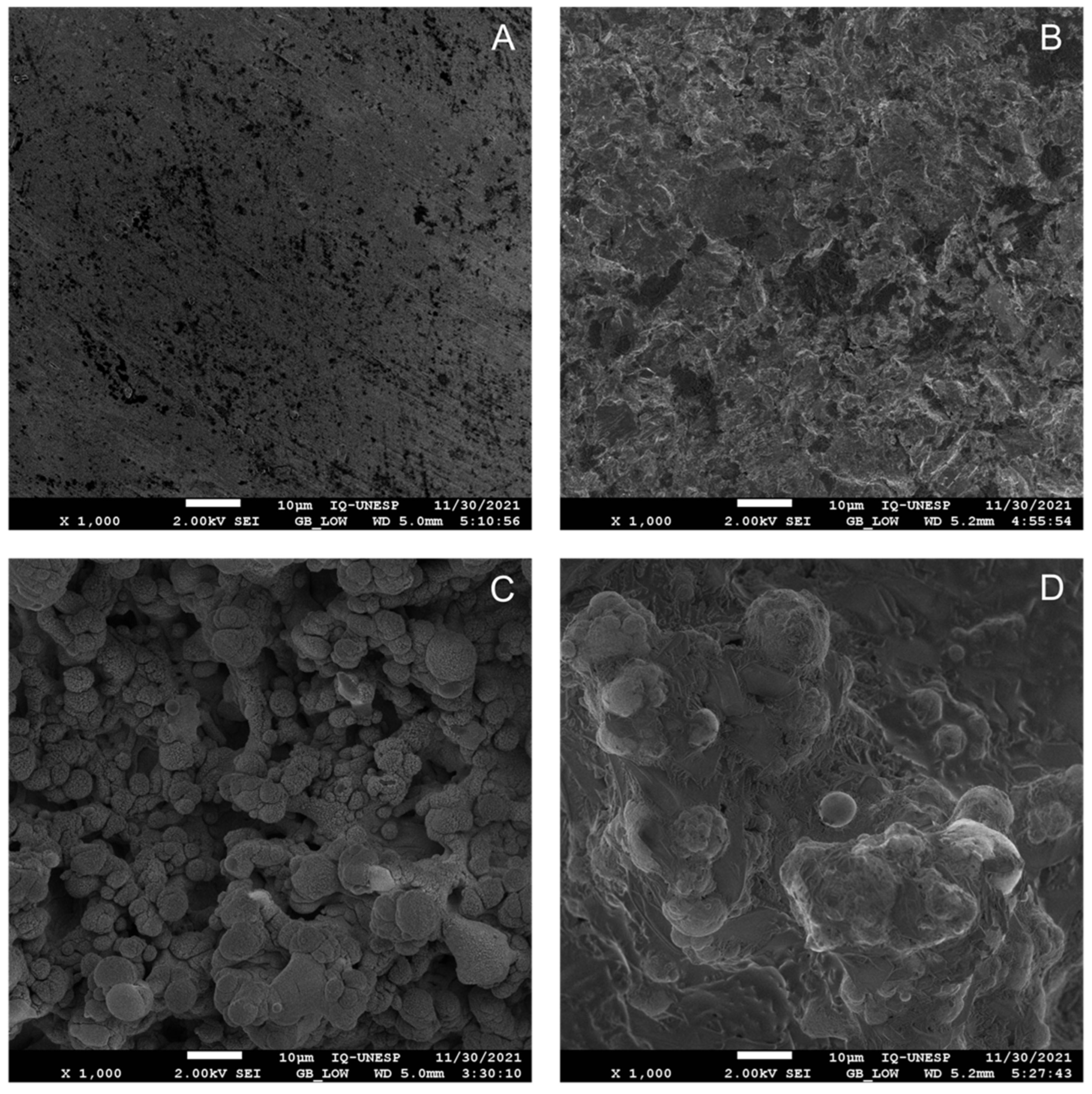
3.1. HA Coating Affects the Surface Morphology Observed with Laser Treatment
3.2. Laser Treatment Enhances the Wettability of Titanium Discs
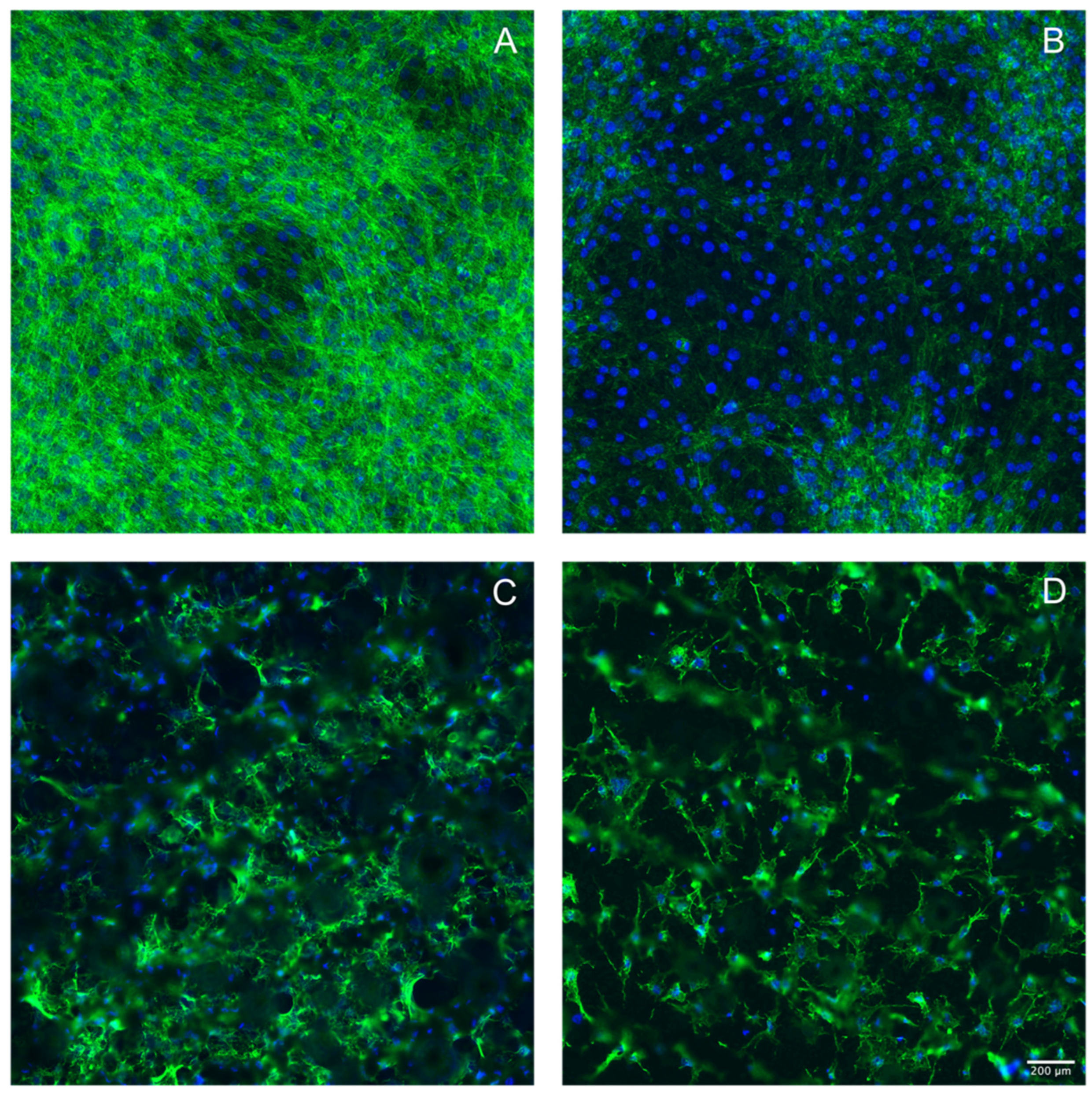
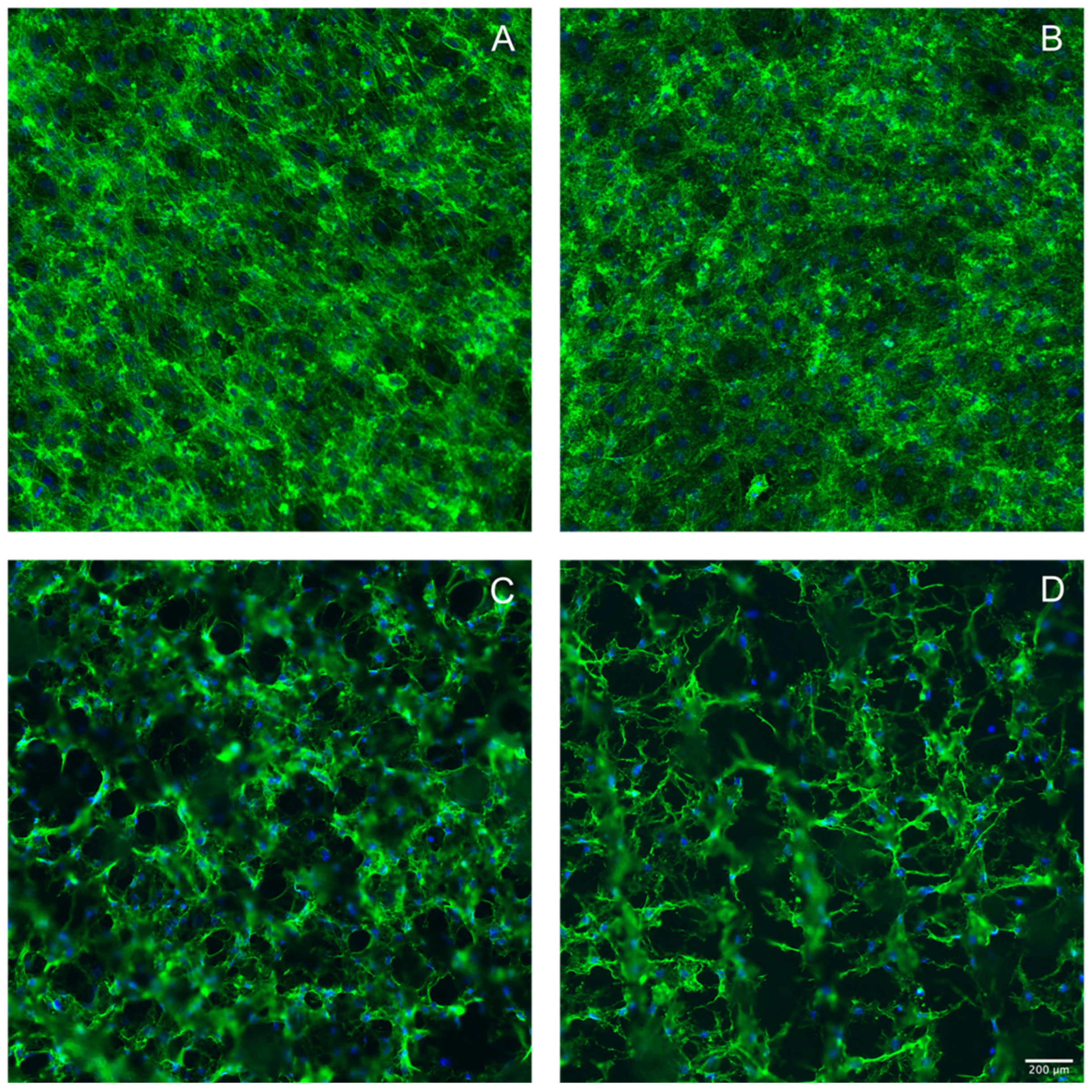
3.3. Laser Treatment of Titanium Discs Altered the Morphology of MC3T3-E1 and ST2 Cells
3.4. Hydroxyapatite Coating Reduced Cell Viability in MC3T3-E1 and ST2 Cells
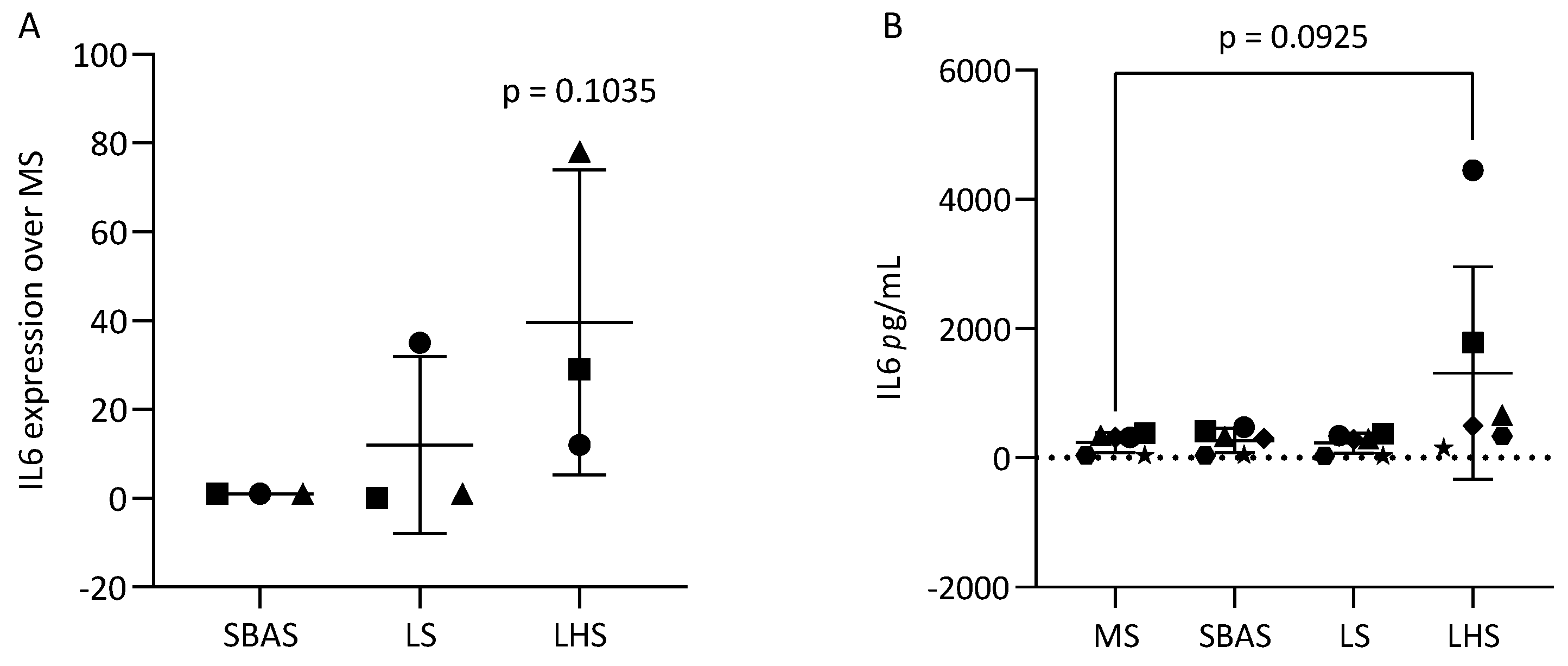
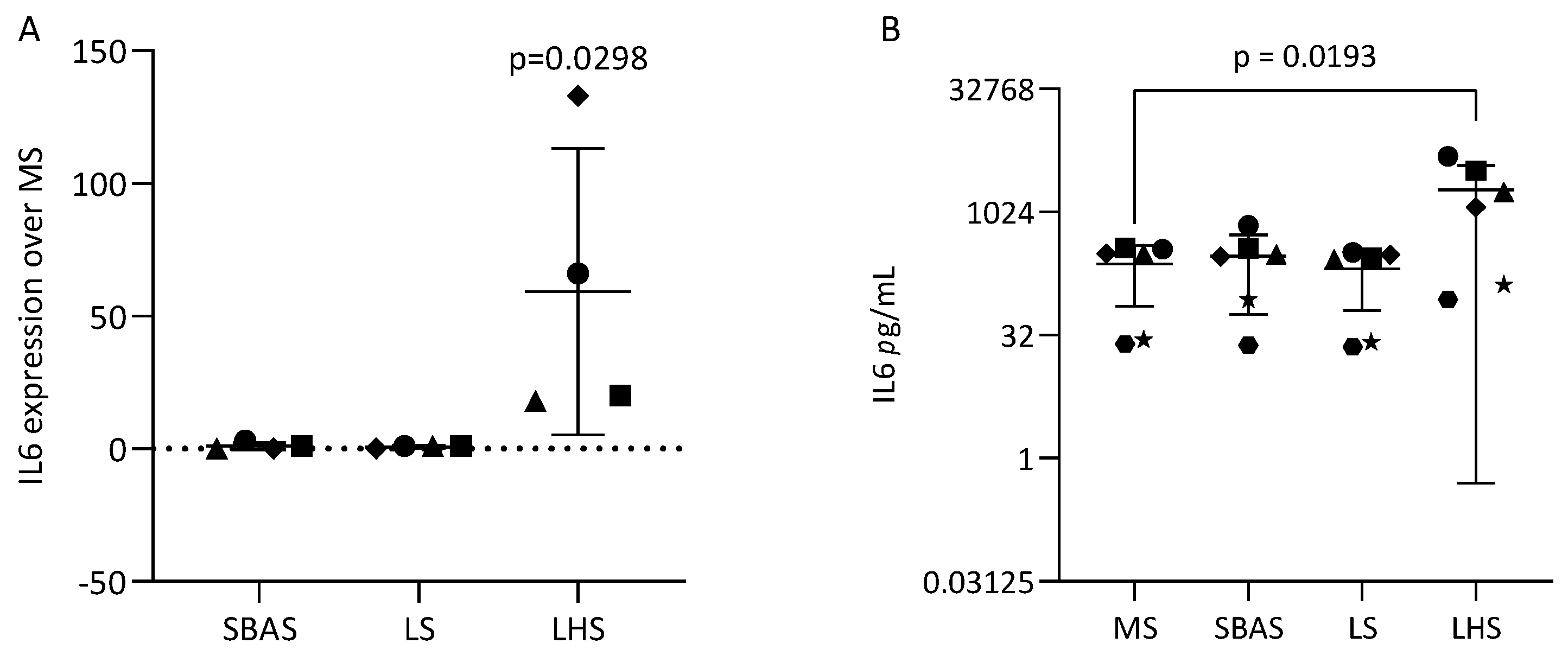
3.5. Hydroxyapatite Coating Increased IL6 in MC3T3-E1 and ST2 Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Junker, R.; Dimakis, A.; Thoneick, M.; Jansen, J.A. Effects of Implant Surface Coatings and Composition on Bone Integration: A Systematic Review. Clin. Oral Implant. Res. 2009, 20 (Suppl. S4), 185–206. [Google Scholar] [CrossRef]
- Albrektsson, T.; Zarb, G.A. Current Interpretations of the Osseointegrated Response: Clinical Significance. Int. J. Prosthodont. 1993, 6, 95–105. [Google Scholar]
- Costa, D.O.; Prowse, P.D.H.; Chrones, T.; Sims, S.M.; Hamilton, D.W.; Rizkalla, A.S.; Dixon, S.J. The Differential Regulation of Osteoblast and Osteoclast Activity by Surface Topography of Hydroxyapatite Coatings. Biomaterials 2013, 34, 7215–7226. [Google Scholar] [CrossRef]
- Faeda, R.S.; Tavares, H.S.; Sartori, R.; Guastaldi, A.C.; Marcantonio, E. Biological Performance of Chemical Hydroxyapatite Coating Associated with Implant Surface Modification by Laser Beam: Biomechanical Study in Rabbit Tibias. J. Oral Maxillofac. Surg. 2009, 67, 1706–1715. [Google Scholar] [CrossRef]
- Sisti, K.E.; de Rossi, R.; Antoniolli, A.M.B.; Aydos, R.D.; Guastaldi, A.C.; Queiroz, T.P.; Garcia, I.R.; Piattelli, A.; Tavares, H.S. Surface and Biomechanical Study of Titanium Implants Modified by Laser with and without Hydroxyapatite Coating, in Rabbits. J. Oral Implantol. 2012, 38, 231–237. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1—Review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar] [PubMed]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef]
- Coelho, P.G.; Jimbo, R.; Tovar, N.; Bonfante, E.A. Osseointegration: Hierarchical Designing Encompassing the Macrometer, Micrometer, and Nanometer Length Scales. Dent. Mater. 2015, 31, 37–52. [Google Scholar] [CrossRef]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of Surface Energy and Roughness on Cell Adhesion and Growth—Facile Surface Modification for Enhanced Cell Culture. RSC Adv. 2021, 11, 15467–15476. [Google Scholar] [CrossRef]
- Oue, H.; Doi, K.; Oki, Y.; Makihara, Y.; Kubo, T.; Perrotti, V.; Piattelli, A.; Akagawa, Y.; Tsuga, K. Influence of Implant Surface Topography on Primary Stability in a Standardized Osteoporosis Rabbit Model Study. J. Funct. Biomater. 2015, 6, 143–152. [Google Scholar] [CrossRef]
- Alghamdi, H.S. Methods to Improve Osseointegration of Dental Implants in Low Quality (Type-IV) Bone: An Overview. J. Funct. Biomater. 2018, 9, 7. [Google Scholar] [CrossRef]
- Gómez-de Diego, R.; del Rocío Mang-de la Rosa, M.; Romero-Pérez, M.-J.; Cutando-Soriano, A.; López-Valverde-Centeno, A. Indications and Contraindications of Dental Implants in Medically Compromised Patients: Update. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e483–e489. [Google Scholar] [CrossRef]
- Zhou, W.; Tangl, S.; Reich, K.M.; Kirchweger, F.; Liu, Z.; Zechner, W.; Ulm, C.; Rausch-Fan, X. The Influence of Type 2 Diabetes Mellitus on the Osseointegration of Titanium Implants with Different Surface Modifications—A Histomorphometric Study in High-Fat Diet/Low-Dose Streptozotocin-Treated Rats. Implant. Dent. 2019, 28, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Hasan, S.M.; Khan, R.S. Bisphosphonate Releasing Dental Implant Surface Coatings and Osseointegration: A Systematic Review. J. Taibah Univ. Med. Sci. 2017, 12, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Joób-Fancsaly, A.; Divinyi, T.; Fazekas, A.; Petó, G.; Karacs, A. Surface Treatment of Dental Implants with High-Energy Laser Beam. Fogorv. Sz. 2000, 93, 169–180. [Google Scholar] [PubMed]
- Bereznai, M.; Pelsöczi, I.; Tóth, Z.; Turzó, K.; Radnai, M.; Bor, Z.; Fazekas, A. Surface Modifications Induced by Ns and Sub-Ps Excimer Laser Pulses on Titanium Implant Material. Biomaterials 2003, 24, 4197–4203. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Johansson, M.L.; Omar, O.; Simonsson, H.; Palmquist, A.; Thomsen, P. Laser-Modified Surface Enhances Osseointegration and Biomechanical Anchorage of Commercially Pure Titanium Implants for Bone-Anchored Hearing Systems. PLoS ONE 2016, 11, e0157504. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Cho, S.A. Comparison of removal torques for laser-treated titanium implants with anodized implants. J. Craniofac. Surg. 2011, 22, 1491–1495. [Google Scholar] [CrossRef]
- Pujari-Palmer, S.; Chen, S.; Rubino, S.; Weng, H.; Xia, W.; Engqvist, H.; Tang, L.; Ott, M.K. In Vivo and In Vitro Evaluation of Hydroxyapatite Nanoparticle Morphology on the Acute Inflammatory Response. Biomaterials 2016, 90, 1–11. [Google Scholar] [CrossRef]
- Lange, T.; Schilling, A.F.; Peters, F.; Haag, F.; Morlock, M.M.; Rueger, J.M.; Amling, M. Proinflammatory and Osteoclastogenic Effects of Beta-Tricalciumphosphate and Hydroxyapatite Particles on Human Mononuclear Cells In Vitro. Biomaterials 2009, 30, 5312–5318. [Google Scholar] [CrossRef]
- Rydén, L.; Omar, O.; Johansson, A.; Jimbo, R.; Palmquist, A.; Thomsen, P. Inflammatory Cell Response to Ultra-Thin Amorphous and Crystalline Hydroxyapatite Surfaces. J. Mater. Sci. Mater. Med. 2017, 28, 9. [Google Scholar] [CrossRef]
- Narducci, P.; Nicolin, V. Differentiation of Activated Monocytes into Osteoclast-like Cells on a Hydroxyapatite Substrate: An In Vitro Study. Ann. Anat. 2009, 191, 349–355. [Google Scholar] [CrossRef]
- Stich, T.; Alagboso, F.; Křenek, T.; Kovářík, T.; Alt, V.; Docheva, D. Implant-Bone-Interface: Reviewing the Impact of Titanium Surface Modifications on Osteogenic Processes In Vitro and In Vivo. Bioeng. Transl. Med. 2022, 7, e10239. [Google Scholar] [CrossRef]
- Lu, R.J.; Wang, X.; He, H.X.; E, L.L.; Li, Y.; Zhang, G.L.; Li, C.J.; Ning, C.Y.; Liu, H.C. Tantalum-incorporated hydroxyapatite coating on titanium implants: Its mechanical and in vitro osteogenic properties. J. Mater. Sci. Mater. Med. 2019, 30, 111. [Google Scholar] [CrossRef]
- Barrère, F.; van der Valk, C.M.; Meijer, G.; Dalmeijer, R.A.J.; de Groot, K.; Layrolle, P. Osteointegration of Biomimetic Apatite Coating Applied onto Dense and Porous Metal Implants in Femurs of Goats. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 67, 655–665. [Google Scholar] [CrossRef]
- Velard, F.; Laurent-Maquin, D.; Braux, J.; Guillaume, C.; Bouthors, S.; Jallot, E.; Nedelec, J.-M.; Belaaouaj, A.; Laquerriere, P. The Effect of Zinc on Hydroxyapatite-Mediated Activation of Human Polymorphonuclear Neutrophils and Bone Implant-Associated Acute Inflammation. Biomaterials 2010, 31, 2001–2009. [Google Scholar] [CrossRef]
- Harada, Y.; Wang, J.T.; Doppalapudi, V.A.; Willis, A.A.; Jasty, M.; Harris, W.H.; Nagase, M.; Goldring, S.R. Differential Effects of Different Forms of Hydroxyapatite and Hydroxyapatite/Tricalcium Phosphate Particulates on Human Monocyte/Macrophages In Vitro. J. Biomed. Mater. Res. 1996, 31, 19–26. [Google Scholar] [CrossRef]
- Velard, F.; Laurent-Maquin, D.; Guillaume, C.; Bouthors, S.; Jallot, E.; Nedelec, J.-M.; Belaaouaj, A.; Laquerriere, P. Polymorphonuclear Neutrophil Response to Hydroxyapatite Particles, Implication in Acute Inflammatory Reaction. Acta Biomater. 2009, 5, 1708–1715. [Google Scholar] [CrossRef]
- Rydén, L.; Molnar, D.; Esposito, M.; Johansson, A.; Suska, F.; Palmquist, A.; Thomsen, P. Early Inflammatory Response in Soft Tissues Induced by Thin Calcium Phosphates. J. Biomed. Mater. Res. A 2013, 101, 2712–2717. [Google Scholar] [CrossRef]
- Ozawa, S.; Kasugai, S. Evaluation of Implant Materials (Hydroxyapatite, Glass-Ceramics, Titanium) in Rat Bone Marrow Stromal Cell Culture. Biomaterials 1996, 17, 23–29. [Google Scholar] [CrossRef]
- Goldberg, A.J.; Liu, Y.; Advincula, M.C.; Gronowicz, G.; Habibovic, P.; Kuhn, L.T. Fabrication and Characterization of Hydroxyapatite-Coated Polystyrene Disks for Use in Osteoprogenitor Cell Culture. J. Biomater. Sci. Polym. Ed. 2010, 21, 1371–1387. [Google Scholar] [CrossRef]
- Xu, W.; Xu, C.; Yi, J.; Dai, H. The Effect of Different Hydroxyapatite Microparticles on the Osteogenic Differentiation of MC3T3-E1 Preosteoblasts. J. Mater. Chem. B 2018, 6, 5234–5242. [Google Scholar] [CrossRef]
- Aparecida, A.H.; Fook, M.V.L.; Guastaldi, A.C. Biomimetic Apatite Formation on Ultra-High Molecular Weight Polyethylene (UHMWPE) Using Modified Biomimetic Solution. J. Mater. Sci. Mater. Med. 2009, 20, 1215–1222. [Google Scholar] [CrossRef]
- Panahipour, L.; Omerbasic, A.; Nasirzade, J.; Gruber, R. TGF-β Activity of a Demineralized Bone Matrix. Int. J. Mol. Sci. 2021, 22, 664. [Google Scholar] [CrossRef]
- Cho, S. A Removal Torque of the Laser-Treated Titanium Implants in Rabbit Tibia. Biomaterials 2003, 24, 4859–4863. [Google Scholar] [CrossRef]
- Gaggl, A.; Schultes, G.; Müller, W.D.; Kärcher, H. Scanning Electron Microscopical Analysis of Laser-Treated Titanium Implant Surfaces—A Comparative Study. Biomaterials 2000, 21, 1067–1073. [Google Scholar] [CrossRef]
- Grandjean-Laquerriere, A.; Laquerriere, P.; Laurent-Maquin, D.; Guenounou, M.; Phillips, T.M. The Effect of the Physical Characteristics of Hydroxyapatite Particles on Human Monocytes IL-18 Production In Vitro. Biomaterials 2004, 25, 5921–5927. [Google Scholar] [CrossRef]
| Surface/Angle | MS | SBAS | LS | LHS |
|---|---|---|---|---|
| 1st review | 68.9° | 48.8° | 0 | 0 |
| 2nd review | 81.2° | 37.5° | 0 | 0 |
| 3rd review | 72.9° | 22.9° | 0 | 0 |
| Average | 74.3° | 36.4° | 0 | 0 |
| MC3T3-E1 | ST2 | |
|---|---|---|
| SBAS | 133.5 ± 43.3 | 122.1 ± 18.7 |
| LS | 95.2 ± 14.1 | 111.3 ± 32.8 |
| LHS | 69.7 ± 13.3 | 53.5 ± 7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, A.F.P.; Cervantes, L.C.C.; Okamoto, R.; Guastaldi, A.C.; Queiroz, T.P.; Panahipour, L.; Gruber, R.; Souza, F.Á. Interleukin-6 Expression of Osteogenic Cell Lines Grown on Laser-Treated and Hydroxyapatite-Coated Titanium Discs. Appl. Sci. 2023, 13, 12646. https://doi.org/10.3390/app132312646
Santos AFP, Cervantes LCC, Okamoto R, Guastaldi AC, Queiroz TP, Panahipour L, Gruber R, Souza FÁ. Interleukin-6 Expression of Osteogenic Cell Lines Grown on Laser-Treated and Hydroxyapatite-Coated Titanium Discs. Applied Sciences. 2023; 13(23):12646. https://doi.org/10.3390/app132312646
Chicago/Turabian StyleSantos, Ana Flávia Piquera, Lara Cristina Cunha Cervantes, Roberta Okamoto, Antonio Carlos Guastaldi, Thallita Pereira Queiroz, Layla Panahipour, Reinhard Gruber, and Francisley Ávila Souza. 2023. "Interleukin-6 Expression of Osteogenic Cell Lines Grown on Laser-Treated and Hydroxyapatite-Coated Titanium Discs" Applied Sciences 13, no. 23: 12646. https://doi.org/10.3390/app132312646
APA StyleSantos, A. F. P., Cervantes, L. C. C., Okamoto, R., Guastaldi, A. C., Queiroz, T. P., Panahipour, L., Gruber, R., & Souza, F. Á. (2023). Interleukin-6 Expression of Osteogenic Cell Lines Grown on Laser-Treated and Hydroxyapatite-Coated Titanium Discs. Applied Sciences, 13(23), 12646. https://doi.org/10.3390/app132312646









