An Overview of Transcranial Magnetic Stimulation and Its Application in Multiple Sclerosis
Abstract
:1. Introduction
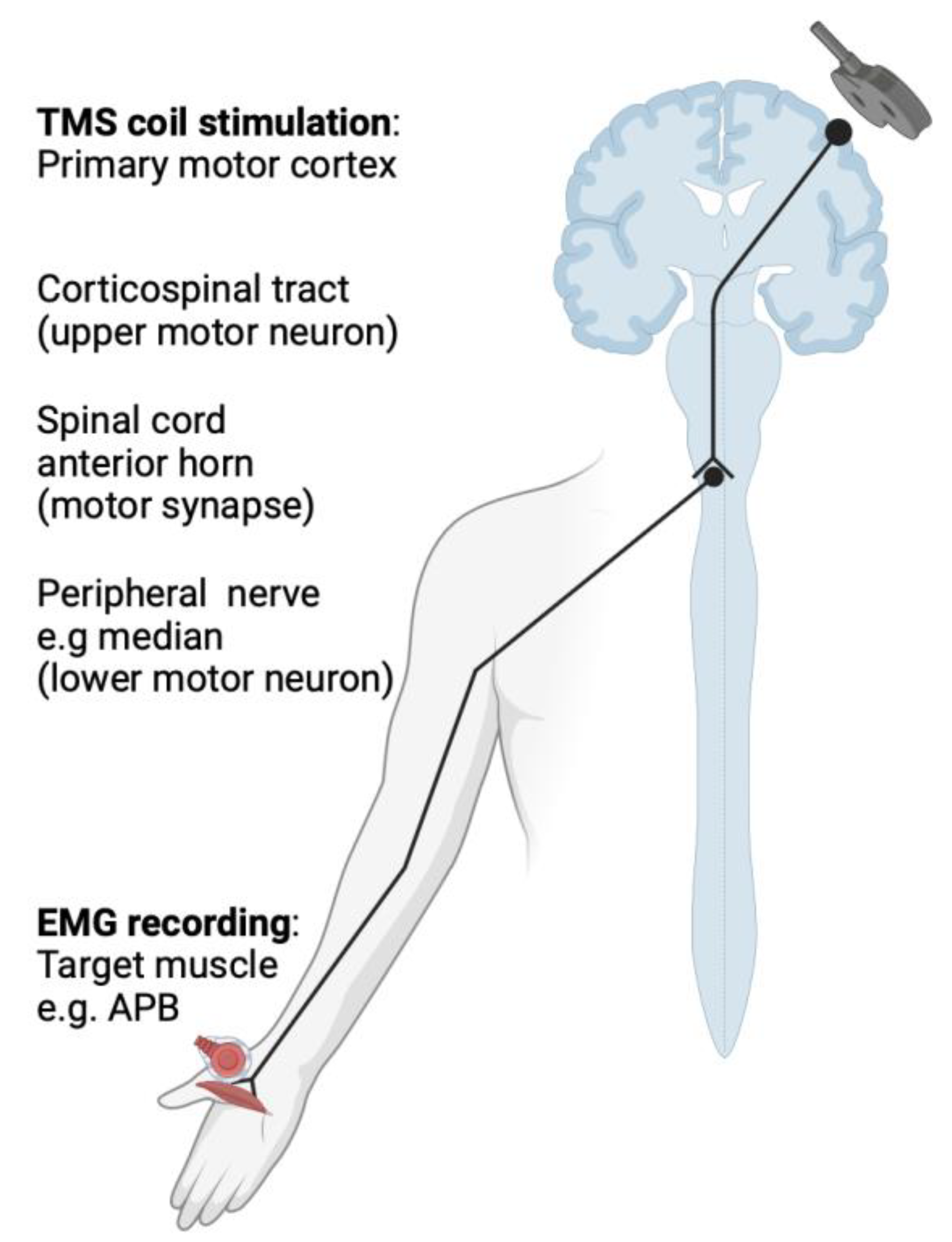
2. Basic Principles of Transcranial Magnetic Stimulation (TMS)
Safety Profile
3. TMS Measures
3.1. Motor Threshold
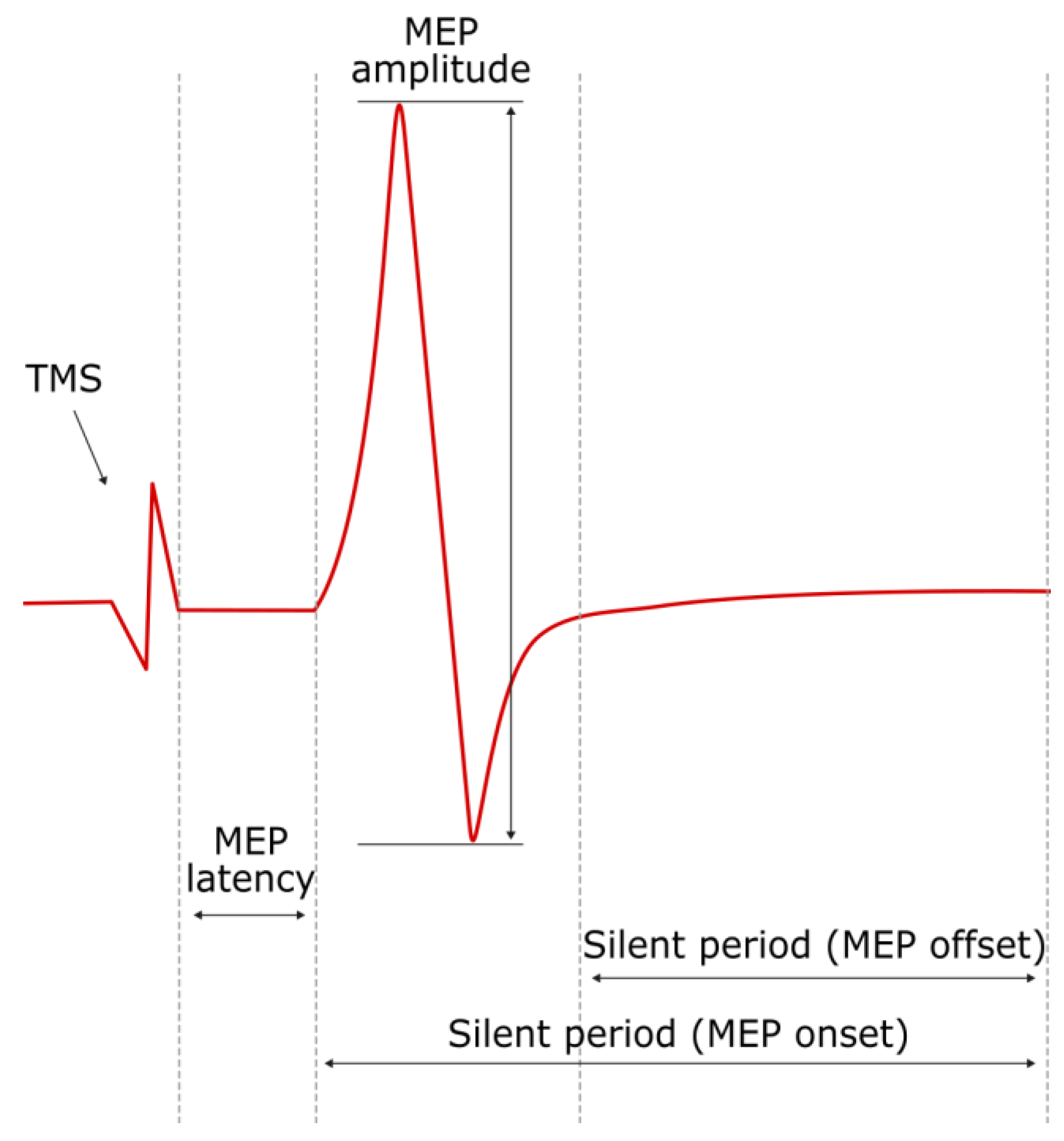
3.2. MEP Latency and Amplitude
3.3. Central Motor Conduction Time
3.4. Silent Period
3.5. Short-Interval Intracortical Inhibition and Facilitation
4. Overview of Multiple Sclerosis
The Use of TMS Measures in MS
5. TMS Measures in the Diagnosis and Evaluation of MS
5.1. TMS Measures and the Association with MRI Markers
5.2. TMS Measures in Longitudinal Assessment of MS
5.3. rTMS Applications in MS
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef]
- Howard, L.; Zwibel, M.D.; Jennifer Smrtka, M.S.N. Improving Quality of Life in Multiple Sclerosis: An Unmet Need. Am. J. Manag. Care 2011, 17, S139. [Google Scholar]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising Prevalence of Multiple Sclerosis Worldwide: Insights from the Atlas of MS, Third Edition. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Klomjai, W.; Katz, R.; Lackmy-Vallée, A. Basic Principles of Transcranial Magnetic Stimulation (TMS) and Repetitive TMS (rTMS). Ann. Phys. Rehabil. Med. 2015, 58, 208–213. [Google Scholar] [CrossRef]
- Rothwell, J. Transcranial Brain Stimulation: Past and Future. Brain Neurosci. Adv. 2018, 2. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.-P. Chapter 37—Transcranial Magnetic Stimulation. In Handbook of Clinical Neurology; Levin, K.H., Chauvel, P., Eds.; Clinical Neurophysiology: Basis and Technical Aspects; Elsevier: Amsterdam, The Netherlands, 2019; Volume 160, pp. 559–580. [Google Scholar]
- Simpson, M.; Macdonell, R. The Use of Transcranial Magnetic Stimulation in Diagnosis, Prognostication and Treatment Evaluation in Multiple Sclerosis. Mult. Scler. Relat. Disord. 2015, 4, 430–436. [Google Scholar] [CrossRef]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A. Safety, Ethical Considerations, and Application Guidelines for the Use of Transcranial Magnetic Stimulation in Clinical Practice and Research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef]
- Lerner, A.J.; Wassermann, E.M.; Tamir, D.I. Seizures from Transcranial Magnetic Stimulation 2012–2016: Results of a Survey of Active Laboratories and Clinics. Clin. Neurophysiol. 2019, 130, 1409–1416. [Google Scholar] [CrossRef]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmöller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and Recommendations for TMS Use in Healthy Subjects and Patient Populations, with Updates on Training, Ethical and Regulatory Issues: Expert Guidelines. Clin. Neurophysiol. 2021, 132, 269–306. [Google Scholar] [CrossRef]
- Fernández, V. The Use of Motor-Evoked Potentials in Clinical Trials in Multiple Sclerosis. J. Clin. Neurophysiol. 2021, 38, 166–170. [Google Scholar] [CrossRef]
- Groppa, S.; Oliviero, A.; Eisen, A.; Quartarone, A.; Cohen, L.G.; Mall, V.; Kaelin-Lang, A.; Mima, T.; Rossi, S.; Thickbroom, G.W.; et al. A Practical Guide to Diagnostic Transcranial Magnetic Stimulation: Report of an IFCN Committee. Clin. Neurophysiol. 2012, 123, 858–882. [Google Scholar] [CrossRef]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-Invasive Electrical and Magnetic Stimulation of the Brain, Spinal Cord, Roots and Peripheral Nerves: Basic Principles and Procedures for Routine Clinical and Research Application. An Updated Report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef] [PubMed]
- Stampanoni Bassi, M.; Buttari, F.; Gilio, L.; De Paolis, N.; Fresegna, D.; Centonze, D.; Iezzi, E. Inflammation and Corticospinal Functioning in Multiple Sclerosis: A TMS Perspective. Front. Neurol. 2020, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, U.; Reis, J.; Schwenkreis, P.; Rosanova, M.; Strafella, A.; Badawy, R.; Müller-Dahlhaus, F. TMS and Drugs Revisited 2014. Clin. Neurophysiol. 2015, 126, 1847–1868. [Google Scholar] [CrossRef] [PubMed]
- Neva, J.L.; Lakhani, B.; Brown, K.E.; Wadden, K.P.; Mang, C.S.; Ledwell, N.H.M.; Borich, M.R.; Vavasour, I.M.; Laule, C.; Traboulsee, A.L.; et al. Multiple Measures of Corticospinal Excitability Are Associated with Clinical Features of Multiple Sclerosis. Behav. Brain Res. 2016, 297, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Brum, M.; Cabib, C.; Valls-Solé, J. Clinical Value of the Assessment of Changes in MEP Duration with Voluntary Contraction. Front. Neurosci. 2016, 9, 505. [Google Scholar] [CrossRef]
- Ammann, C.; Guida, P.; Caballero-Insaurriaga, J.; Pineda-Pardo, J.A.; Oliviero, A.; Foffani, G. A Framework to Assess the Impact of Number of Trials on the Amplitude of Motor Evoked Potentials. Sci. Rep. 2020, 10, 21422. [Google Scholar] [CrossRef] [PubMed]
- Bastani, A.; Jaberzadeh, S. A Higher Number of TMS-Elicited MEP from a Combined Hotspot Improves Intra- and Inter-Session Reliability of the Upper Limb Muscles in Healthy Individuals. PLoS ONE 2012, 7, e47582. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, K.; Thijs, H.; Meesen, R.L.J. Optimization of the Transcranial Magnetic Stimulation Protocol by Defining a Reliable Estimate for Corticospinal Excitability. PLoS ONE 2014, 9, e86380. [Google Scholar] [CrossRef]
- Goldsworthy, M.R.; Hordacre, B.; Ridding, M.C. Minimum Number of Trials Required for within- and between-Session Reliability of TMS Measures of Corticospinal Excitability. Neuroscience 2016, 320, 205–209. [Google Scholar] [CrossRef]
- Jaiser, S.R.; Barnes, J.D.; Baker, S.N.; Baker, M.R. A Multiple Regression Model of Normal Central and Peripheral Motor Conduction Times. Muscle Nerve 2015, 51, 706–712. [Google Scholar] [CrossRef]
- Imajo, Y.; Kanchiku, T.; Suzuki, H.; Yoshida, Y.; Funaba, M.; Nishida, N.; Fujimoto, K.; Taguchi, T. Effects of Differences in Age and Body Height on Normal Values of Central Motor Conduction Time Determined by F-Waves. J. Spinal Cord Med. 2017, 40, 181–187. [Google Scholar] [CrossRef]
- Claus, D. Central Motor Conduction: Method and Normal Results. Muscle Nerve 1990, 13, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Fernández, V.; Valls-Sole, J.; Relova, J.L.; Raguer, N.; Miralles, F.; Dinca, L.; Taramundi, S.; Costa-Frossard, L.; Ferrandiz, M.; Ramió-Torrentà, L.; et al. Recommendations for the Clinical Use of Motor Evoked Potentials in Multiple Sclerosis. Neurología 2013, 28, 408–416. [Google Scholar] [CrossRef]
- Škarabot, J.; Mesquita, R.N.O.; Brownstein, C.G.; Ansdell, P. Myths and Methodologies: How Loud Is the Story Told by the Transcranial Magnetic Stimulation-Evoked Silent Period? Exp. Physiol. 2019, 104, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Zeugin, D.; Ionta, S. Anatomo-Functional Origins of the Cortical Silent Period: Spotlight on the Basal Ganglia. Brain Sci. 2021, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Hübers, A.; Kassubek, J.; Müller, H.-P.; Broc, N.; Dreyhaupt, J.; Ludolph, A.C. The Ipsilateral Silent Period: An Early Diagnostic Marker of Callosal Disconnection in ALS. Ther. Adv. Chronic Dis. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Cash, R.F.H.; Noda, Y.; Zomorrodi, R.; Radhu, N.; Farzan, F.; Rajji, T.K.; Fitzgerald, P.B.; Chen, R.; Daskalakis, Z.J.; Blumberger, D.M. Characterization of Glutamatergic and GABAA-Mediated Neurotransmission in Motor and Dorsolateral Prefrontal Cortex Using Paired-Pulse TMS–EEG. Neuropsychopharmacology 2017, 42, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Conte, A.; Lenzi, D.; Frasca, V.; Gilio, F.; Giacomelli, E.; Gabriele, M.; Marini Bettolo, C.; Iacovelli, E.; Pantano, P.; Pozzilli, C.; et al. Intracortical Excitability in Patients with Relapsing–Remitting and Secondary Progressive Multiple Sclerosis. J. Neurol. 2009, 256, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Vucic, S.; Burke, T.; Lenton, K.; Ramanathan, S.; Gomes, L.; Yannikas, C.; Kiernan, M.C. Cortical Dysfunction Underlies Disability in Multiple Sclerosis. Mult. Scler. J. 2012, 18, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Liepert, J.; Mingers, D.; Heesen, C.; Bäumer, T.; Weiller, C. Motor Cortex Excitability and Fatigue in Multiple Sclerosis: A Transcranial Magnetic Stimulation Study. Mult. Scler. 2005, 11, 316–321. [Google Scholar] [CrossRef]
- Ni, Z.; Bahl, N.; Gunraj, C.A.; Mazzella, F.; Chen, R. Increased Motor Cortical Facilitation and Decreased Inhibition in Parkinson Disease. Neurology 2013, 80, 1746–1753. [Google Scholar] [CrossRef]
- Mori, F.; Kusayanagi, H.; Monteleone, F.; Moscatelli, A.; Nicoletti, C.G.; Bernardi, G.; Centonze, D. Short Interval Intracortical Facilitation Correlates with the Degree of Disability in Multiple Sclerosis. Brain Stimul. 2013, 6, 67–71. [Google Scholar] [CrossRef]
- Nantes, J.C.; Zhong, J.; Holmes, S.A.; Narayanan, S.; Lapierre, Y.; Koski, L. Cortical Damage and Disability in Multiple Sclerosis: Relation to Intracortical Inhibition and Facilitation. Brain Stimul. 2016, 9, 566–573. [Google Scholar] [CrossRef]
- Howard, J.; Trevick, S.; Younger, D.S. Epidemiology of Multiple Sclerosis. Neurol. Clin. 2016, 34, 919–939. [Google Scholar] [CrossRef]
- Pellegrino, L.; Coscia, M.; Muller, M.; Solaro, C.; Casadio, M. Evaluating Upper Limb Impairments in Multiple Sclerosis by Exposure to Different Mechanical Environments. Sci. Rep. 2018, 8, 2110. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.A.; Thompson, A.J.; Swash, M. Central Motor Conduction in Multiple Sclerosis: Evaluation of Abnormalities Revealed by Transcutaneous Magnetic Stimulation of the Brain. J. Neurol. Neurosurg. Psychiatry 1988, 51, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Streletz, L.J.; Raab, V.E.; Knobler, R.L.; Lublin, F.D. Lower Extremity Motor Evoked Potentials in Multiple Sclerosis. Arch. Neurol. 1991, 48, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Sue, C.M.; Yiannikas, C.; Clouston, P.D.; Lim, C.L.; Graham, S. Transcranial Cortical Stimulation in Disorders of the Central Motor Pathways. J. Clin. Neurosci. 1997, 4, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kidd, D.; Thompson, P.D.; Day, B.L.; Rothwell, J.C.; Kendall, B.E.; Thompson, A.J.; Marsden, C.D.; McDonald, W.I. Central Motor Conduction Time in Progressive Multiple Sclerosis. Correlations with MRI and Disease Activity. Brain 1998, 121, 1109–1116. [Google Scholar] [CrossRef]
- Petajan, J.H.; White, A.T. Motor-Evoked Potentials in Response to Fatiguing Grip Exercise in Multiple Sclerosis Patients. Clin. Neurophysiol. 2000, 111, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Schmierer, K.; Niehaus, L.; Roricht, S.; Meyer, B. Conduction Deficits of Callosal Fibres in Early Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry 2000, 68, 633–638. [Google Scholar] [CrossRef]
- Tataroglu, C.; Genc, A.; Idiman, E.; Cakmur, R.; Idiman, F. Cortical Silent Period and Motor Evoked Potentials in Patients with Multiple Sclerosis. Clin. Neurol. Neurosurg. 2003, 105, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Tataroglu, C.; Genc, A.; Idiman, E.; Cakmur, R.; Idiman, F. Cortical Relay Time for Long Latency Reflexes in Patients with Definite Multiple Sclerosis. Can. J. Neurol. Sci. 2004, 31, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Sahota, P.; Prabhakar, S.; Lal, V.; Khurana, D.; Das, C.P.; Singh, P. Transcranial Magnetic Stimulation: Role in the Evaluation of Disability in Multiple Sclerosis. Neurol. India 2005, 53, 197. [Google Scholar] [CrossRef] [PubMed]
- Thickbroom, G.W.; Byrnes, M.L.; Archer, S.A.; Kermode, A.G.; Mastaglia, F.L. Corticomotor Organisation and Motor Function in Multiple Sclerosis. J. Neurol. 2005, 252, 765–771. [Google Scholar] [CrossRef]
- Jørgensen, L.M.; Nielsen, J.E.; Ravnborg, M. MEP Recruitment Curves in Multiple Sclerosis and Hereditary Spastic Paraplegia. J. Neurol. Sci. 2005, 237, 25–29. [Google Scholar] [CrossRef]
- Gagliardo, A.; Galli, F.; Grippo, A.; Amantini, A.; Martinelli, C.; Amato, M.P.; Borsini, W. Motor Evoked Potentials in Multiple Sclerosis Patients without Walking Limitation: Amplitude vs. Conduction Time Abnormalities. J. Neurol. 2007, 254, 220–227. [Google Scholar] [CrossRef]
- Kalkers, N.F.; Strijers, R.L.M.; Jasperse, M.M.S.; Neacsu, V.; Geurts, J.J.G.; Barkhof, F.; Polman, C.H.; Stam, C.J. Motor Evoked Potential: A Reliable and Objective Measure to Document the Functional Consequences of Multiple Sclerosis? Relation to Disability and MRI. Clin. Neurophysiol. 2007, 118, 1332–1340. [Google Scholar] [CrossRef]
- Kale, N.; Agaoglu, J.; Onder, G.; Tanik, O. Correlation between Disability and Transcranial Magnetic Stimulation Abnormalities in Patients with Multiple Sclerosis. J. Clin. Neurosci. 2009, 16, 1439–1442. [Google Scholar] [CrossRef]
- Llufriu, S.; Blanco, Y.; Martinez-Heras, E.; Casanova-Molla, J.; Gabilondo, I.; Sepulveda, M.; Falcon, C.; Berenguer, J.; Bargallo, N.; Villoslada, P.; et al. Influence of Corpus Callosum Damage on Cognition and Physical Disability in Multiple Sclerosis: A Multimodal Study. PLoS ONE 2012, 7, e37167. [Google Scholar] [CrossRef] [PubMed]
- Di Sapio, A.; Bertolotto, A.; Melillo, F.; Sperli, F.; Malucchi, S.; Troni, W. A New Neurophysiological Approach to Assess Central Motor Conduction Damage to Proximal and Distal Muscles of Lower Limbs. Clin. Neurophysiol. 2014, 125, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Manogaran, P.; Vavasour, I.; Borich, M.; Kolind, S.H.; Lange, A.P.; Rauscher, A.; Boyd, L.; Li, D.K.; Traboulsee, A. Corticospinal Tract Integrity Measured Using Transcranial Magnetic Stimulation and Magnetic Resonance Imaging in Neuromyelitis Optica and Multiple Sclerosis. Mult. Scler. J. 2016, 22, 43–50. [Google Scholar] [CrossRef]
- Ayache, S.S.; Créange, A.; Farhat, W.H.; Zouari, H.G.; Lesage, C.; Palm, U.; Abdellaoui, M.; Lefaucheur, J.-P. Cortical Excitability Changes over Time in Progressive Multiple Sclerosis. Funct. Neurol. 2016, 30, 257–263. [Google Scholar] [CrossRef]
- Coates, K.D.; Aboodarda, S.J.; Krüger, R.L.; Martin, T.; Metz, L.M.; Jarvis, S.E.; Millet, G.Y. Multiple Sclerosis-Related Fatigue: The Role of Impaired Corticospinal Responses and Heightened Exercise Fatigability. J. Neurophysiol. 2020, 124, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Pisa, M.; Chieffo, R.; Congiu, M.; Dalla Costa, G.; Esposito, F.; Romeo, M.; Comola, M.; Comi, G.; Leocani, L. Intracortical Motor Conduction Is Associated with Hand Dexterity in Progressive Multiple Sclerosis. Mult. Scler. J. 2021, 27, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Alusi, S.H.; Macerollo, A.; MacKinnon, C.D.; Rothwell, J.C.; Bain, P.G. Tremor and Dysmetria in Multiple Sclerosis: A Neurophysiological Study. Tremor Other Hyperkinetic Mov. 2021, 11, 30. [Google Scholar] [CrossRef]
- Tremblay, F.; Ansari, Y.; Remaud, A.; Freedman, M.S. Neurophysiological Outcomes Following Mesenchymal Stem Cell Therapy in Multiple Sclerosis. Clin. Neurophysiol. 2022, 136, 69–81. [Google Scholar] [CrossRef]
- Meyer-Moock, S.; Feng, Y.-S.; Maeurer, M.; Dippel, F.-W.; Kohlmann, T. Systematic Literature Review and Validity Evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in Patients with Multiple Sclerosis. BMC Neurol. 2014, 14, 58. [Google Scholar] [CrossRef]
- van Munster, C.E.P.; Uitdehaag, B.M.J. Outcome Measures in Clinical Trials for Multiple Sclerosis. CNS Drugs 2017, 31, 217–236. [Google Scholar] [CrossRef]
- Ömerhoca, S.; Akkaş, S.Y.; İçen, N.K. Multiple Sclerosis: Diagnosis and Differential Diagnosis. Arch. Neuropsychiatry 2018, 55, S1–S9. [Google Scholar] [CrossRef]
- Zhao, E.Y.; Vavasour, I.M.; Zakeri, M.; Borich, M.R.; Laule, C.; Rauscher, A.; Traboulsee, A.; Li, D.K.B.; Boyd, L.A.; MacKay, A.L. Myelin Water Imaging and Transcranial Magnetic Stimulation Suggest Structure-Function Relationships in Multiple Sclerosis. Front. Phys. 2019, 7, 141. [Google Scholar] [CrossRef]
- Kandler, R.H.; Jarratt, J.A.; Davies-Jones, G.A.B.; Gumpert, E.J.W.; Venables, G.S.; Sagar, H.J.; Zeman, A. The Role of Magnetic Stimulation as a Quantifier of Motor Disability in Patients with Multiple Sclerosis. J. Neurol. Sci. 1991, 106, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Salle, J.Y.; Hugon, J.; Tabaraud, F.; Boulesteix, J.M.; Vallat, J.M.; Dumas, M.; Poser, C.M. Improvement in Motor Evoked Potentials and Clinical Course Post-Steroid Therapy in Multiple Sclerosis. J. Neurol. Sci. 1992, 108, 184–188. [Google Scholar] [CrossRef]
- Aloizou, A.-M.; Pateraki, G.; Anargyros, K.; Siokas, V.; Bakirtzis, C.; Liampas, I.; Nousia, A.; Nasios, G.; Sgantzos, M.; Peristeri, E.; et al. Transcranial Magnetic Stimulation (TMS) and Repetitive TMS in Multiple Sclerosis. Rev. Neurosci. 2021, 32, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.-P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-Based Guidelines on the Therapeutic Use of Repetitive Transcranial Magnetic Stimulation (rTMS): An Update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
| Relapsing Remitting MS (RRMS) | RRMS is the most common clinical phenotype of MS that affects approximately 85% of patients. RRMS is dominated by unpredictable waves of focal inflammatory injury and demyelination, reflected by clinical relapses and the emergence of new lesions via MRI. Subsequent to this injury, varying degrees of repair and remission follow [36]. |
| Primary Progressive MS (PPMS) | PPMS affects approximately 10% of patients with MS. Thought to be reflective of more CNS compartmentalized injury, it is clinically characterized by worsening disability from initial onset, without clear clinical relapses, along with occasional plateaus and temporary improvements [36]. MRI scans may show silent or minimally symptomatic accumulation of white matter lesions. |
| Secondary Progressive MS (SPMS) | With conventional therapies, approximately one half of patients with RRMS convert to SPMS after around a decade with the illness. SPMS is characterized by an initial relapsing disease course, followed by progression with (active) or without (non-active) occasional relapses and plateaus [36]. |
| Author et al. | Year | TMS and MRI Measures | Clinical Assessment Measures | Relationship between Measures |
|---|---|---|---|---|
| Ingram et al. [38] | 1988 | CMCT | EDSS, AI | Prolonged CMCT was correlated with greater impairment in EDSS and AI scores. |
| Jones et al. [39] | 1991 | CMCT, MEP amplitude and latency | EDSS, SNRS | Prolonged CMCT was correlated with impairment in EDSS and SNRS scores. |
| Sue et al. [40] | 1997 | CMCT, MEP amplitude | None | Prolonged CMCT and decreased MEP amplitude observed in patients. |
| Kidd et al. [41] | 1998 | CMCT Brain lesion volumes (T1 and T2) and spinal cord lesion load | EDSS, FS | Upper limb CMCT unchanged over study period (12 months). CMCT to tibialis anterior, weakly associated with EDSS scores. Lesion load in the cervical cord correlated with CMCT to upper limb, but no correlation with lower limb. Increase in CMCT to tibialis anterior in 4/19 patients who developed new cord lesions. |
| Petajan et al. [42] | 2000 | CMCT, MEP amplitude, grip force | None | Prolonged CMCT after fatiguing exercise as compared to control. Higher MEP amplitude following fatiguing exercise in controls and MS patients without weakness, but not MS patients with weakness. |
| Schmierer et al. [43] | 2000 | CMCT, MEP amplitude | None | Prolonged CMCT and decreased MEP amplitude observed in upper and lower limbs. |
| Tataroglu et al. [44] | 2003 | CMCT, SP, MEP amplitude and latency | EDSS | Prolonged CMCT and SP. Prolonged MEP latency and decreased amplitude. However, isolated amplitude reduction, i.e., without prolonged CMCT or MEP latency, was not found. CMCT and decreased MEP amplitude were correlated with EDSS, but no correlation was observed between SP and EDSS. |
| Tataroglu et al. [45] | 2004 | MEP amplitude and latency, CMCT | EDSS, FSS | Prolonged CMCT. Prolonged MEP latency correlated with EDSS and FSS. |
| Sahota et al. [46] | 2005 | CMCT, MEP amplitude and latency, MT | EDSS | Prolonged CMCT, but no correlation found with EDSS. Decreased MEP amplitude and increased MT. |
| Thickbroom et al. [47] | 2005 | CMCT, MEP amplitude and latency, MT | EDSS, Purdue pegboard score | Increased MEP latency, increased MT, and decreased amplitude correlated with Purdue score impairment. Only increased MEP latency was correlated with EDSS. |
| Jørgensen et al. [48] | 2005 | CMCT, MEP amplitude, MT | MSIS | Prolonged CMCT, decreased amplitude, and increased MT. No measure was correlated with MSIS. |
| Gagliardo et al. [49] | 2007 | CMCT, MEP amplitude and area, MT | EDSS | Prolonged CMCT, often with MEP amplitude and area abnormalities (decreases). In patients with normal CMCT observations, many still had MEP amplitude and area abnormalities. |
| Kalkers et al. [50] | 2007 | CMCT Brain lesion volumes (T1, T2), brain volume, spinal cord lesions, and volume | EDSS, MSFC | Increased CMCT is correlated with worsened EDSS scores and worsened performance in terms of MSFC. T1- and (to a lesser extent) T2-weighted brain lesions, and volume of the spinal cord lesions that are correlated with CMCT. |
| Conte et al. [30] | 2009 | CMCT, MEP amplitude, MT, ICI, ICF Brain lesion volumes (T1 and T2) | EDSS | EDSS scores were significantly higher in SPMS than in RRMS. CMCT, MEP amplitude, ICI, and MT were correlated with EDSS. No correlation between TMS measures and MRI lesion load observed. |
| Kale et al. [51] | 2009 | CMCT, MEP amplitude and latency | EDSS | Prolonged CMCT, prolonged MEP latency, and decreased MEP amplitude in participants with MS. Only CMCT is correlated with EDSS. |
| Vucic et al. [31] | 2012 | CMCT, MEP amplitude, SP, MT, SICI, ICF Brain lesion volumes (T1 and T2); number of gadolinium-enhanced lesions | EDSS, MFIS | Prolonged CMCT for participants with SPMS and RRMS. For SPMS: decreased MEP amplitude, increased motor threshold, reduction in SICI. Each measure was correlated with EDSS. Reduced SP in SPMS was observed, but not in RRMS—not statistically significant. CMCT was correlated with the T2 lesion load. Weak correlation between the EDSS and the T2 lesion load. |
| Llufriu et al. [52] | 2012 | iSP latency, CMCT | EDSS, MSFC | Prolonged CMCT and iSP onset latency. Only iSP latency was correlated with EDSS and MSFC. |
| Di Sapio et al. [53] | 2014 | CMCT, MEP amplitude and area, MT | BMRC scale for muscle strength | Prolonged CMCT and decreased MEP area. Both measures were correlated with decreased muscle strength. |
| Neva et al. [16] | 2016 | CMCT, MEP amplitude and latency, MT, SP, SICI | EDSS | Increased MT, increased MEP latency, delayed SP onset, prolonged MEP duration. |
| Manogaran et al. [54] | 2016 | MEP recruitment curve slope, resting and active MT Myelin water fraction | EDSS | Higher resting MT and active MT. No correlations between TMS or MRI measures and EDSS reported after correction. |
| Ayache et al. [55] | 2015 | Resting and active MT, MEP amplitude and latency, SICI, ICF | EDSS | At baseline, prolonged MEP and increased resting and active MT were correlated with worsened EDSS scores. Over 1 year, EDSS scores increased, resting MT increased, and maximal SICI decreased only in untreated patients. |
| Nantes et al. [35] | 2016 | MEP amplitude, MT, ICF, SICI, SP Magnetization transfer ratio | EDSS, 9HPT | Magnetization transfer ratio was correlated with total MSFC score. |
| Coates et al. [56] | 2020 | MEP amplitude and latency | EDSS, FSS | Prolonged MEP latency, decreased MEP amplitude only in highly fatigued MS group after exercise. |
| Pisa et al. [57] | 2021 | MEP amplitude and latency | EDSS, various dexterity tests | Latency of upper limb MEP is correlated with strength, but poorly detects dexterity impairment. |
| Alusi et al. [58] | 2021 | CMCT | FTSS | Prolonged CMCT across all four groups (MS controls, MS tremor, MS with pure dysmetria, MS with mixed tremor and dysmetria). |
| Tremblay et al. [59] | 2022 | CMCT and MEP latency | 9HPT | Changes in 9HPT performance were found to be correlated with changes in CMCT and MEP latency, but only on the left side. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sy, A.; Thebault, S.; Aviv, R.I.; Auriat, A.M. An Overview of Transcranial Magnetic Stimulation and Its Application in Multiple Sclerosis. Appl. Sci. 2023, 13, 12679. https://doi.org/10.3390/app132312679
Sy A, Thebault S, Aviv RI, Auriat AM. An Overview of Transcranial Magnetic Stimulation and Its Application in Multiple Sclerosis. Applied Sciences. 2023; 13(23):12679. https://doi.org/10.3390/app132312679
Chicago/Turabian StyleSy, Alex, Simon Thebault, Richard I. Aviv, and Angela M. Auriat. 2023. "An Overview of Transcranial Magnetic Stimulation and Its Application in Multiple Sclerosis" Applied Sciences 13, no. 23: 12679. https://doi.org/10.3390/app132312679






