Enhanced Degradation of Decabromodiphenyl Ether via Synergetic Assisted Mechanochemical Process with Lithium Cobalt Oxide and Iron
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. MC Treatments
2.3. Analysis
3. Results and Discussion
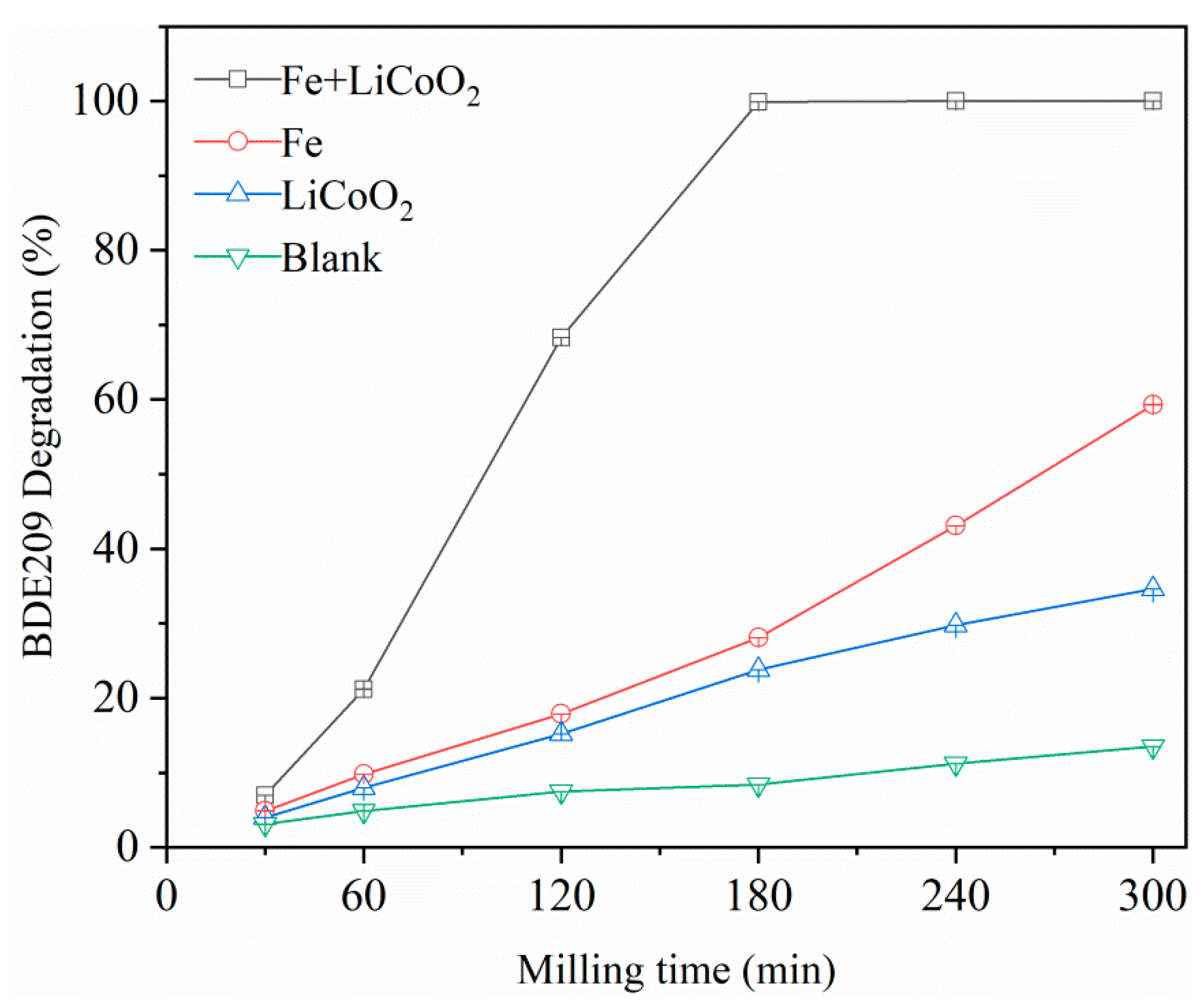
3.1. Performance of BDE 209 Degradation
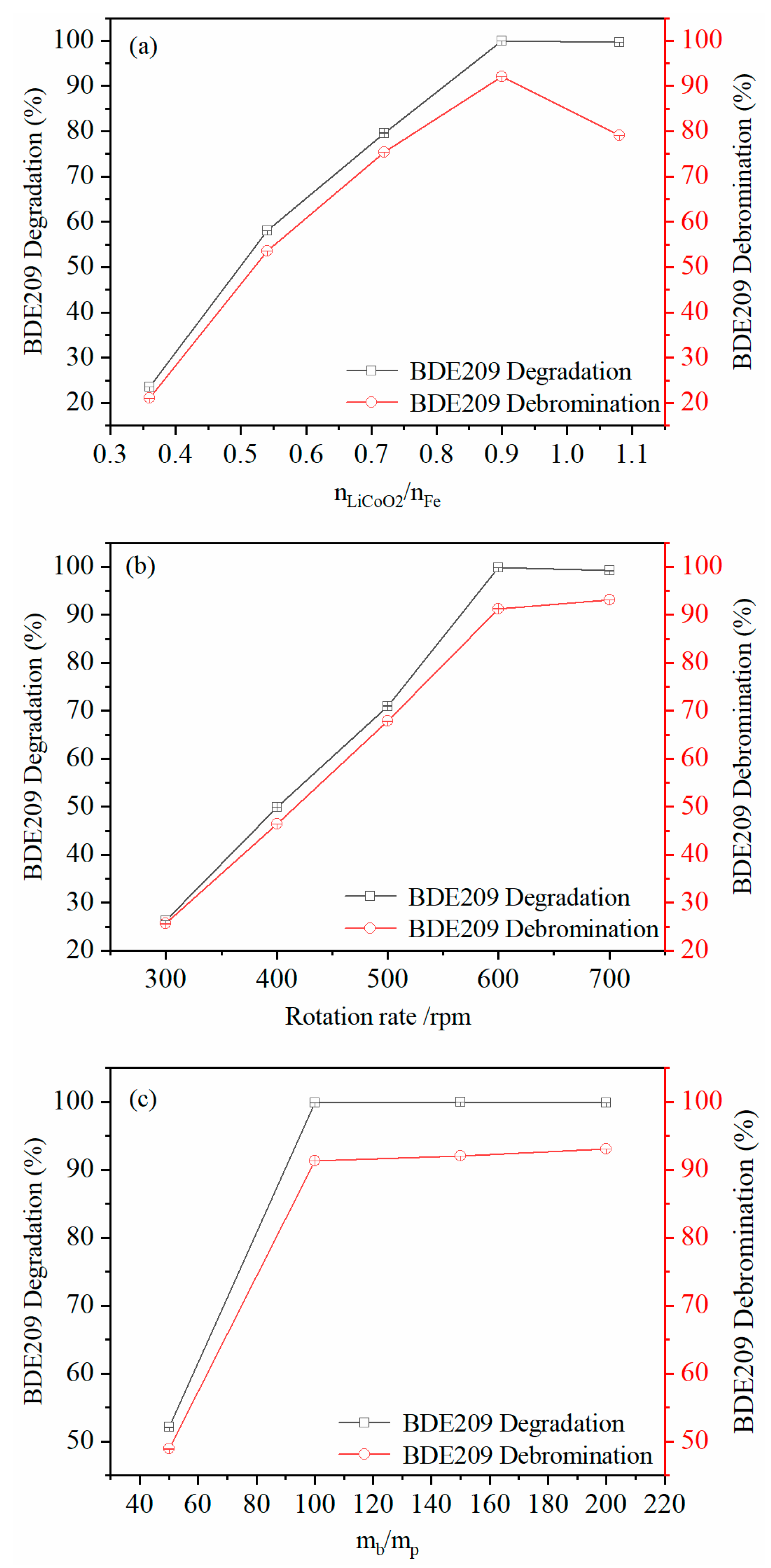
3.2. Effect of Milling Conditions on BDE 209 Degradation and Debromination
3.3. Characterization Analysis and Mechanism
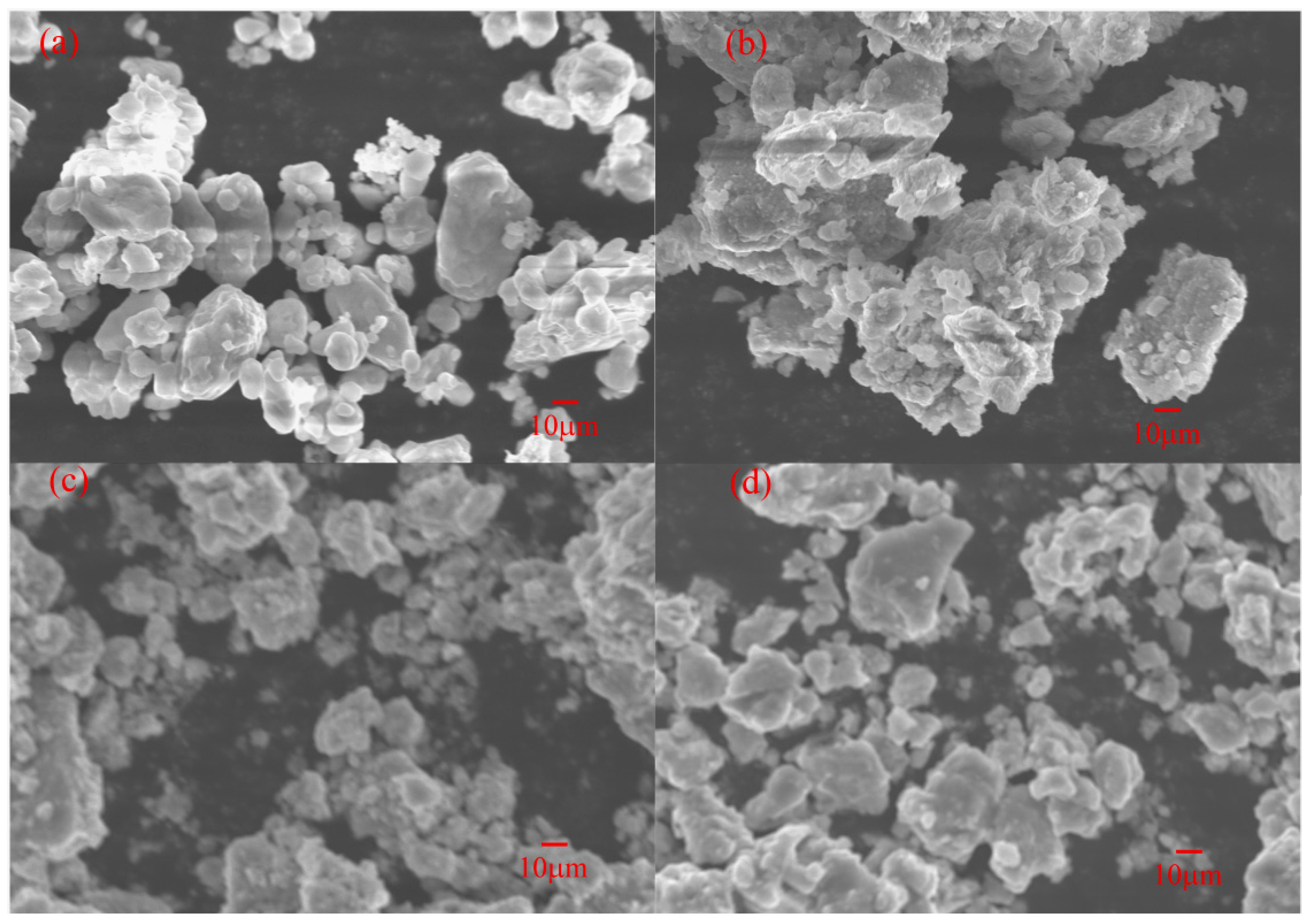
3.3.1. SEM Analysis
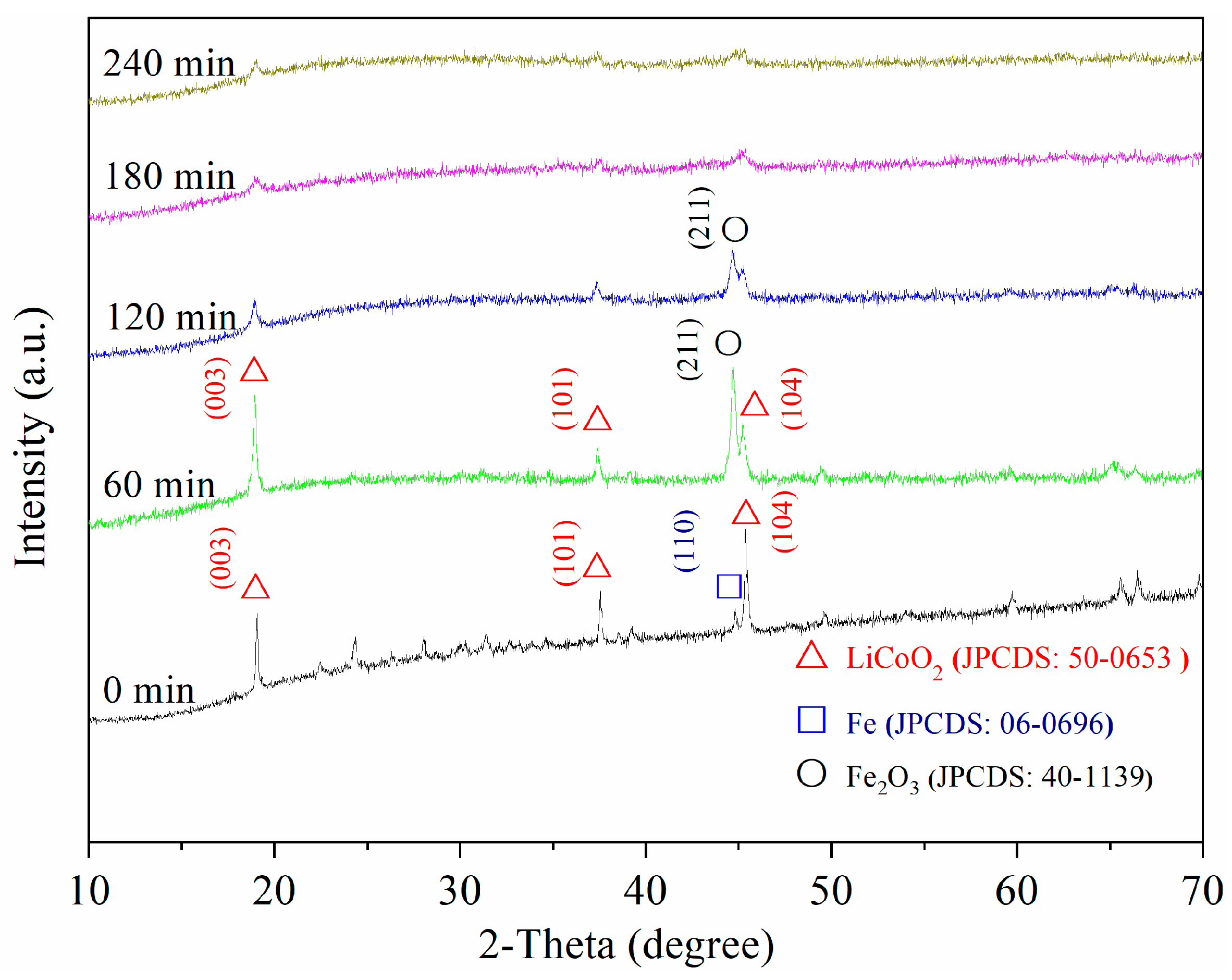
3.3.2. XRD Analysis
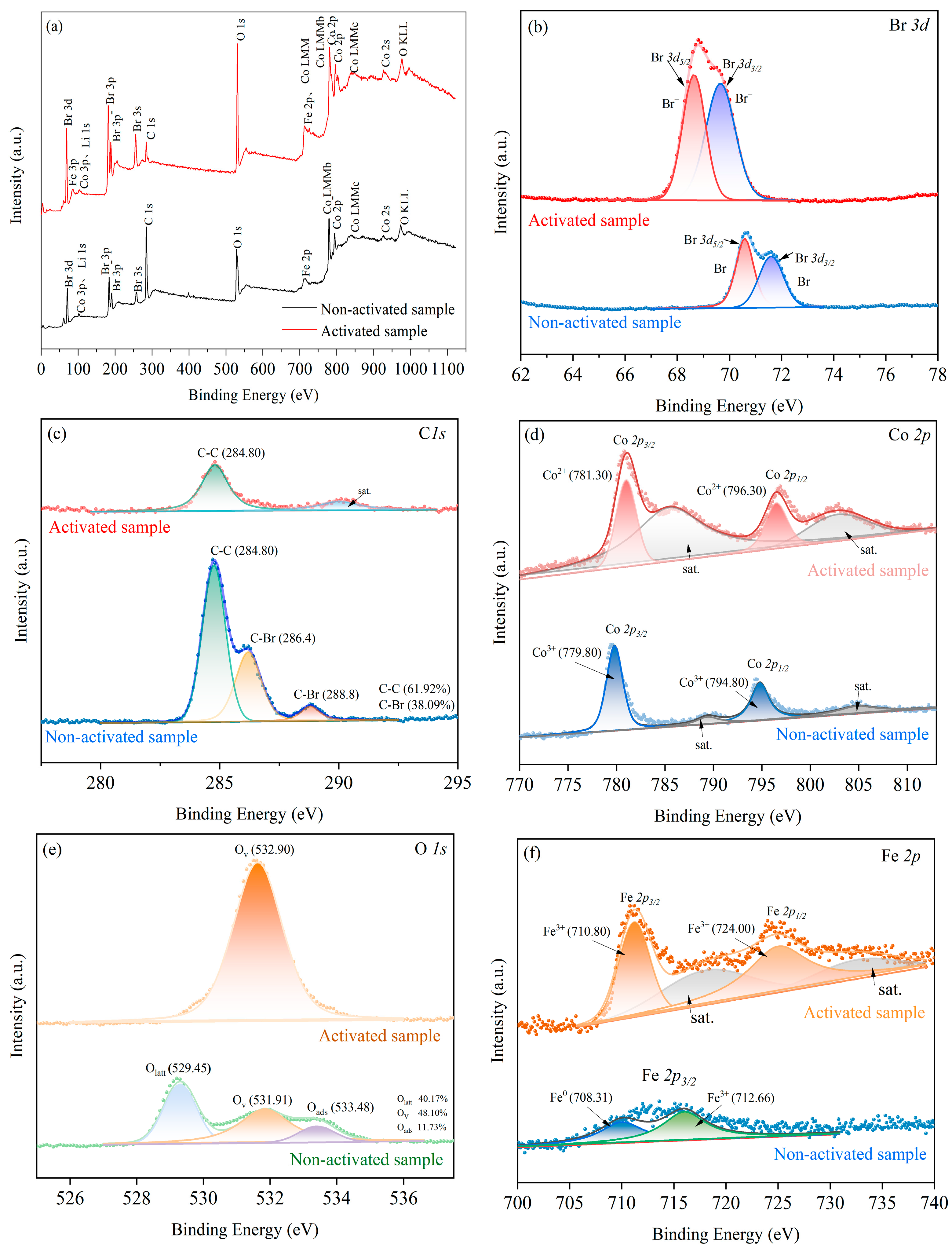
3.3.3. XPS Analysis
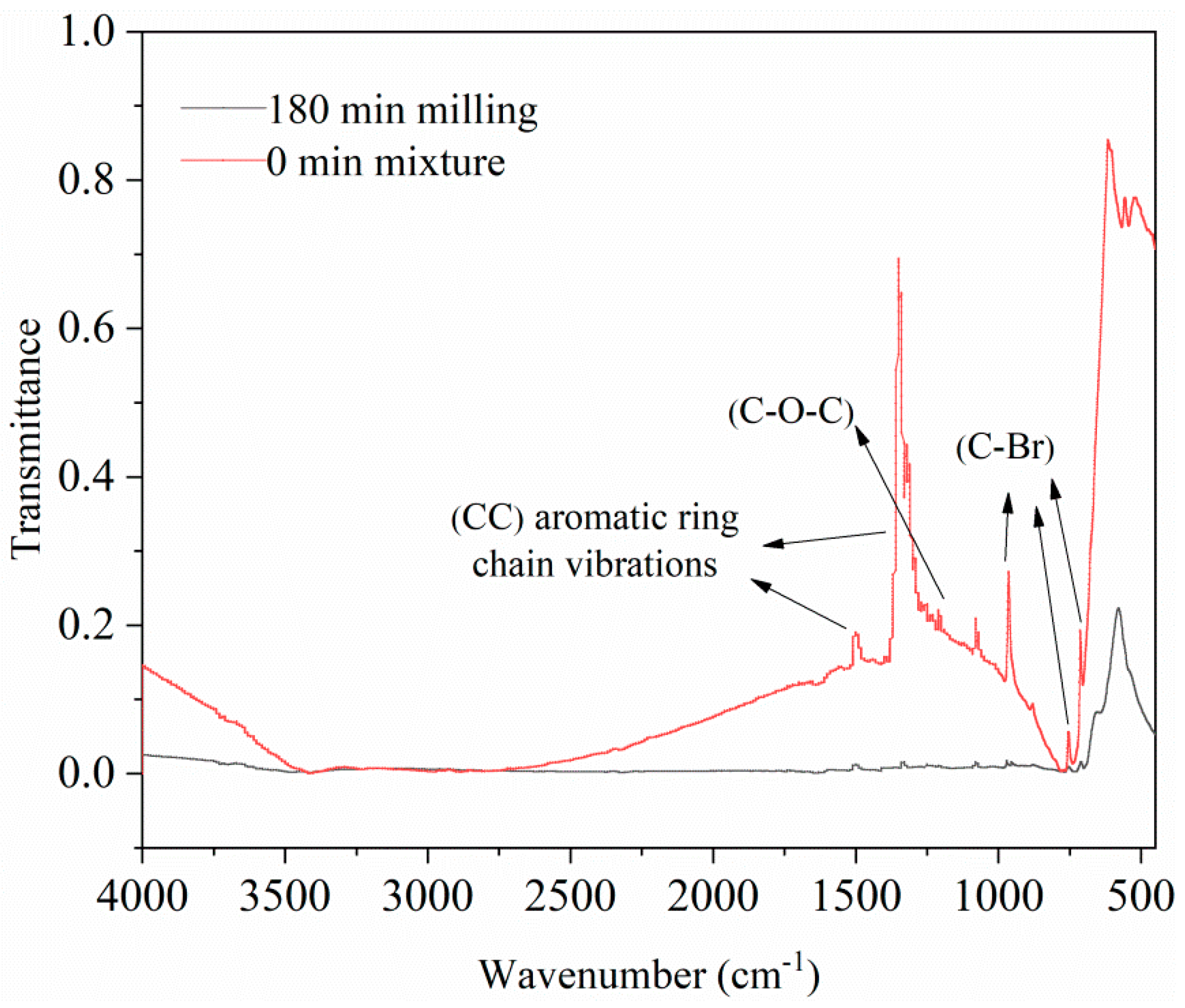
3.3.4. FT-IR Analysis
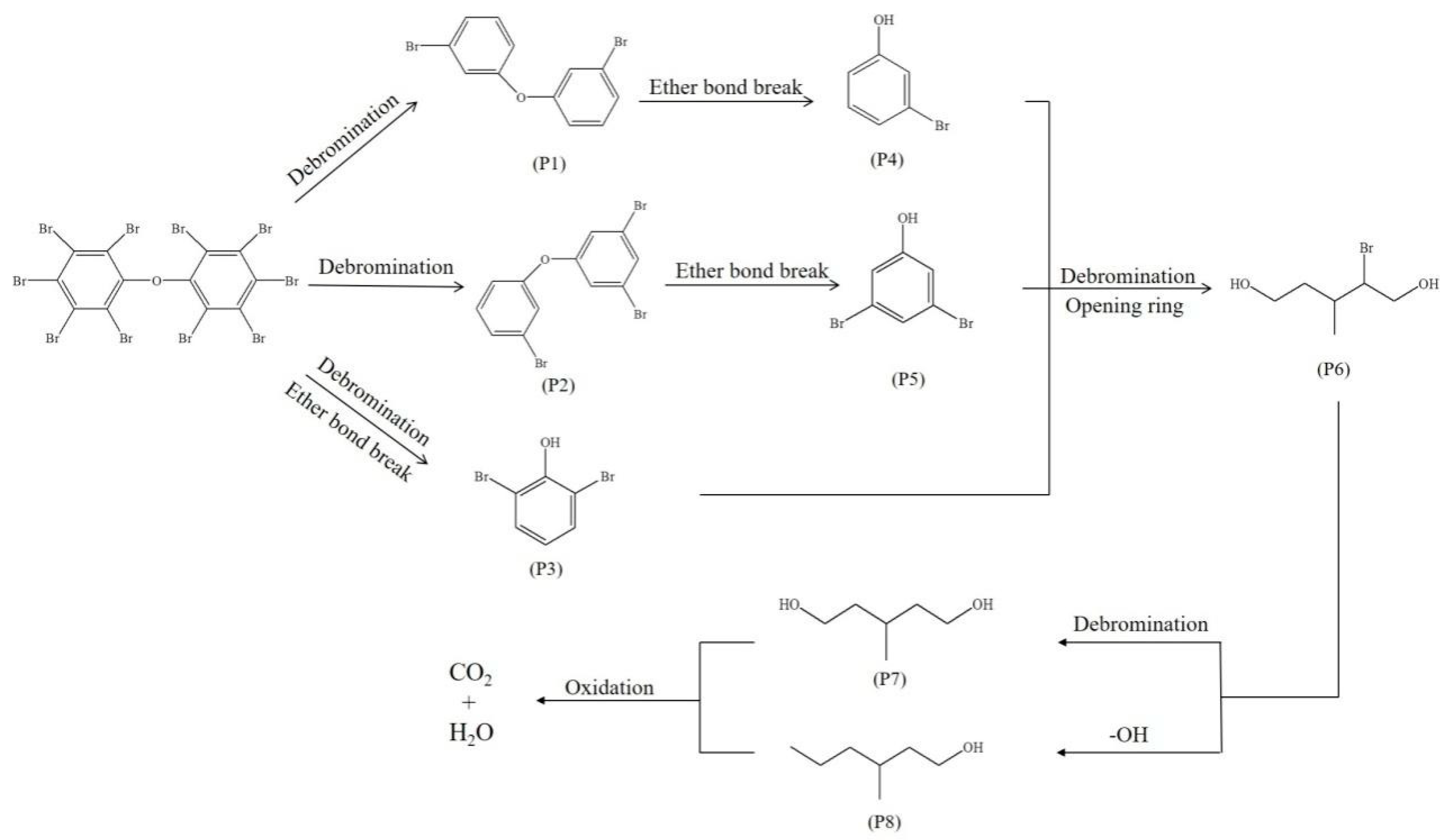
3.3.5. Degradation Pathway of BDE 209
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, N.; Lai, C.; Xu, F.; Huang, D.; Zhang, M.; Zhou, X.; Xu, M.; Li, Y.; Li, L.; Liu, S.; et al. A review of polybrominated diphenyl ethers and novel brominated flame retardants in Chinese aquatic environment: Source, occurrence, distribution, and ecological risk assessment. Sci. Total Environ. 2023, 904, 166180. [Google Scholar] [CrossRef] [PubMed]
- MJin, M.; Xu, Z.; Zhang, S.; Sun, L.; Wu, J. Pollution status, particle-size distribution and health impacts (people at different ages) of polybrominated diphenyl ethers in bedrooms. J. Environ. Chem. Eng. 2023, 11, 111289. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Tang, Y.; Dang, X.; Zhou, K.; Liu, B.; Bian, B. Optimal emission reductions pathway for polybrominated diphenyl ethers in typical household e-waste dismantling products. Sci. Total Environ. 2023, 883, 163697. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Lin, L.; Li, H.; Liu, S.; Xu, X.; Xu, Y.; Jin, X.; Yuan, Y.; Wang, Z. Occurrence, bioaccumulation, fate, and risk assessment of novel brominated flame retardants (NBFRs) in aquatic environments—A critical review. Water Res. 2021, 198, 117168. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Chen, J.; Wang, C.; Wang, P.; Wang, R.; Feng, B. Regulatory mechanisms of submerged macrophyte on bacterial community recovery in decabromodiphenyl ether contaminated sediment: Microbiological and metabolomic perspectives. Environ. Pollut. 2023, 337, 122616. [Google Scholar] [CrossRef]
- Zhang, Y.; Xi, B.; Tan, W. Release, transformation, and risk factors of polybrominated diphenyl ethers from landfills to the surrounding environments: A review. Environ. Int. 2021, 157, 106780. [Google Scholar] [CrossRef]
- Shi, C.; Hu, Y.; Kobayashi, T.; Zhang, N.; Kuramochi, H.; Zhang, Z.; Lei, Z.; Xu, K.-Q. Comparison of decabromodiphenyl ether degradation in long-term operated anaerobic bioreactors under thermophilic and mesophilic conditions and the pathways involved. J. Environ. Manag. 2021, 294, 113009. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Tan, Q. Trends of production, consumption and environmental emissions of Decabromodiphenyl ether in mainland China. Environ. Pollut. 2020, 260, 114022. [Google Scholar] [CrossRef]
- Hoang, A.Q.; Tran, T.M.; Tu, M.B.; Takahashi, S. Polybrominated diphenyl ethers in indoor and outdoor dust from Southeast Asia: An updated review on contamination status, human exposure, and future perspectives. Environ. Pollut. 2021, 272, 116012. [Google Scholar] [CrossRef]
- Donaldson, D.L.; Jayaweera, D. Effective solar prosumer identification using net smart meter data. Int. J. Elect. Power Energy Syst. 2021, 118, 105823. [Google Scholar] [CrossRef]
- Motamedi, M.; Yerushalmi, L.; Haghighat, F.; Chen, Z. A critical review of water contamination by polybrominated diphenyl ethers (PBDE) and main degradation techniques. J. Environ. Chem. Eng. 2022, 10, 108196. [Google Scholar] [CrossRef]
- Xiong, P.; Yan, X.; Zhu, Q.; Qu, G.; Shi, J.; Liao, C.; Jiang, G. A review of environmental occurrence, fate, and toxicity of novel brominated flame retardants. Environ. Sci. Technol. 2019, 53, 13551–13569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, X.; Liu, H.; Huang, X.; Chen, Q.; Guo, X.; Zhang, J. A systematic review of microplastics in the environment: Sampling, separation, characterization and coexistence mechanisms with pollutants. Sci. Total Environ. 2023, 859, 160151. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yang, J.; Rao, Q.; Liu, Z.; Song, W.; Guan, S.; Zhao, Z.; Song, W. Toxic effects of Decabromodiphenyl ether (BDE-209) on thyroid of broiler chicks by transcriptome profile analysis. Ecotoxicol. Environ. Saf. 2021, 219, 112305. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Wang, C.; Zhao, C.; Jiang, R.; Song, J. Effects of maternal exposure to BDE 209 on neuronal development and transcription of iodothyronine deiodinase in offspring mice. Toxicol. Mech. Methods 2019, 29, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; He, J.; Li, C.; Lu, Y.; Zhang, Y.-N.; Qu, J. Photo-induced degradation and toxicity change of decabromobiphenyl ethers (BDE-209) in water: Effects of dissolved organic matter and halide ions. J. Hazard. Mater. 2021, 416, 125842. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, X.; Zhang, J.; Huang, X. Distribution and migration characteristics of microplastics in farmland soils, surface water and sediments in Caohai Lake, southwestern plateau of China. J. Clean. Prod. 2022, 366, 132912. [Google Scholar] [CrossRef]
- Lei, M.; Wang, N.; Zhu, L.; Zhou, Q.; Nie, G.; Tang, H. Photocatalytic reductive degradation of polybrominated diphenyl ethers on CuO/TiO2 nanocomposites: A mechanism based on the switching of photocatalytic reduction potential being controlled by the valence state of copper. Appl. Catal. B Environ. 2016, 182, 414–423. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Smith, S.M.; Wantala, K.; Kajitvichyanukul, P. Photocatalytic remediation of persistent organic pollutants (POPs): A review. Arabian J. Chem. 2020, 13, 8309–8337. [Google Scholar] [CrossRef]
- Dong, Y.; Li, Y.; Zhao, C.; Feng, Y.; Chen, S.; Dong, Y. Mechanism of the rapid mechanochemical degradation of hexachlorobenzene with silicon carbide as an additive. J. Hazard. Mater. 2019, 379, 120653. [Google Scholar] [CrossRef]
- Wang, N.; Lv, H.; Zhou, Y.; Zhu, L.; Hu, Y.; Majima, T.; Tang, H. Complete Defluorination and Mineralization of Perfluorooctanoic Acid by a Mechanochemical Method Using Alumina and Persulfate. Environ. Sci. Technol. 2019, 53, 8302–8313. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Min, X.; Chai, L.; Wang, M.; Liyang, W.; Pan, Q.; Okido, M. Stabilization of arsenic sludge with mechanochemically modified zero valent iron. Chemosphere 2017, 168, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Kang, S.; Feng, N.; Zhu, J.; Yu, B.; Xie, X.; Chen, J. Mechanochemical mechanism of rapid dechlorination of hexachlorobenzene. J. Hazard. Mater. 2017, 333, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, M.; Li, G.; Wang, P. Constructing MnO2 alpha/amorphous heterophase junction by mechanochemically induced phase transformation for formaldehyde oxidation. Appl. Surf. Sci. 2022, 589, 152855. [Google Scholar] [CrossRef]
- Huang, A.; Zhang, Z.; Wang, N.; Zhu, L.; Zou, J. Green mechanochemical oxidative decomposition of powdery decabromodiphenyl ether with persulfate. J. Hazard. Mater. 2016, 302, 158–165. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, J.; Xu, F.; Deng, S.; Zhu, W.; Yu, G. Mechanochemical destruction of pentachloronitrobenzene with reactive iron powder. J. Hazard. Mater. 2011, 198, 275–281. [Google Scholar] [CrossRef]
- Nasser, A.; Mingelgrin, U. Birnessite-induced mechanochemical degradation of 2,4-dichlorophenol. Chemosphere 2014, 107, 175–179. [Google Scholar] [CrossRef]
- Deng, S.; Bao, Y.; Cagnetta, G.; Huang, J.; Yu, G. Mechanochemical degradation of perfluorohexane sulfonate: Synergistic effect of ferrate(VI) and zero-valent iron. Environ. Pollut. 2020, 264, 114789. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Jun, H.; Yu, M.; Wang, F.; Zhou, L.; Yu, G. Acceleration and mechanistic studies of the mechanochemical dechlorination of HCB with iron powder and quartz sand. Chem. Eng. J. 2014, 239, 185–191. [Google Scholar] [CrossRef]
- Li, Q.; Yang, S.; Wu, S.; Fan, D. Mechanochemically synthesized Al–Fe (oxide) composite with superior reductive performance: Solid-state kinetic processes during ball milling. Chemosphere 2022, 298, 134280. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, N.; Zhu, L.; Lv, H.; Dong, X.; Chai, H.; Tang, H. Synergistic effect between Fe and Bi 2 O 3 on enhanced mechanochemical treatment of decabromodiphenyl ether. J. Environ. Chem. Eng. 2017, 5, 915–923. [Google Scholar] [CrossRef]
- Saeki, S.; Lee, J.; Zhang, Q.; Saito, F. Co-grinding LiCoO2 with PVC and water leaching of metal chlorides formed in ground product. Int. J. Miner. Process. 2004, 74, S373–S378. [Google Scholar] [CrossRef]
- Guan, J.; Li, Y.; Guo, Y.; Su, R.; Gao, G.; Song, H.; Yuan, H.; Liang, B.; Guo, Z. Mechanochemical process enhanced cobalt and lithium recycling from wasted lithium-ion batteries. ACS Sustain. Chem. Eng. 2016, 5, 1026–1032. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, S.; Mao, Q.; Buekens, A.; Wang, Y.; Yan, J. Energy transfer and kinetics in mechanochemistry. Environ. Sci. Pollut. Res. 2017, 24, 24562–24571. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.; Lou, X.; Guan, J.; Li, Y.; Mai, X.; Liu, H.; Zhao, C.X.; Wang, N.; Yan, C.; et al. Improved extraction of cobalt and lithium by reductive acid from spent lithium-ion batteries via mechanical activation process. J. Mater. Sci. 2018, 53, 13790–13800. [Google Scholar] [CrossRef]
- Chai, H.; Zhang, Z.; Zhou, Y.; Zhu, L.; Lv, H.; Wang, N. Roles of intrinsic Mn3+ sites and lattice oxygen in mechanochemical debromination and mineralization of decabromodiphenyl ether with manganese dioxide. Chemosphere 2018, 207, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhu, Z.; Tan, S.; Guo, J.; Xu, Z. Mechanochemical degradation of brominated flame retardants in waste printed circuit boards by Ball Milling. J. Hazard. Mater. 2020, 385, 121509. [Google Scholar] [CrossRef] [PubMed]
- Mio, H.; Saeki, S.; Kano, J.; Saito, F. Estimation of Mechanochemical Dechlorination Rate of Poly(vinyl chloride). Environ. Sci. Technol. 2002, 36, 1344–1348. [Google Scholar] [CrossRef]
- Yi, Y.; Kou, F.; Tsang, P.E.; Fang, Z. Highly efficient remediation of decabromodiphenyl ether-contaminated soil using mechanochemistry in the presence of additive and its mechanism. J. Environ. Manag. 2021, 299, 113595. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, X.; Jiang, L.; Li, X.; Zhang, J.; Wang, H. Reutilization of cathode material from spent batteries as a heterogeneous catalyst to remove antibiotics in wastewater via peroxymonosulfate activation. Chem. Eng. J. 2020, 400, 125903. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, Y.; Dong, P. Use of electrochemical cathode-reduction method for leaching of cobalt from spent lithium-ion batteries. J. Clean. Prod. 2018, 180, 64–70. [Google Scholar] [CrossRef]
- Nie, M.; Li, Y.; Dong, Y.; Song, Z.; Zhao, C.; Chen, S. Mechanochemical degradation of hexachlorobenzene with a combined additive of SiC and Fe. Chem. Eng. Res. Des. 2022, 177, 167–173. [Google Scholar] [CrossRef]
- Li, H.; Zhu, F.; He, S. The degradation of decabromodiphenyl ether in the e-waste site by biochar supported nanoscale zero-valent iron/persulfate. Ecotoxicol. Environ. Saf. 2019, 183, 109540. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, X.; Sui, Y.; Zhang, Q.; Fang, C.; Tang, Y.; Zhang, X.; Yang, G.; Shi, Y.; Huang, D.; Guan, J.; et al. Enhanced Degradation of Decabromodiphenyl Ether via Synergetic Assisted Mechanochemical Process with Lithium Cobalt Oxide and Iron. Appl. Sci. 2023, 13, 12924. https://doi.org/10.3390/app132312924
Lou X, Sui Y, Zhang Q, Fang C, Tang Y, Zhang X, Yang G, Shi Y, Huang D, Guan J, et al. Enhanced Degradation of Decabromodiphenyl Ether via Synergetic Assisted Mechanochemical Process with Lithium Cobalt Oxide and Iron. Applied Sciences. 2023; 13(23):12924. https://doi.org/10.3390/app132312924
Chicago/Turabian StyleLou, Xiaoyi, Yifan Sui, Qichao Zhang, Changling Fang, Yunyu Tang, Xuan Zhang, Guangxin Yang, Yongfu Shi, Dongmei Huang, Jie Guan, and et al. 2023. "Enhanced Degradation of Decabromodiphenyl Ether via Synergetic Assisted Mechanochemical Process with Lithium Cobalt Oxide and Iron" Applied Sciences 13, no. 23: 12924. https://doi.org/10.3390/app132312924
APA StyleLou, X., Sui, Y., Zhang, Q., Fang, C., Tang, Y., Zhang, X., Yang, G., Shi, Y., Huang, D., Guan, J., & Guo, Y. (2023). Enhanced Degradation of Decabromodiphenyl Ether via Synergetic Assisted Mechanochemical Process with Lithium Cobalt Oxide and Iron. Applied Sciences, 13(23), 12924. https://doi.org/10.3390/app132312924





