Abstract
The yeast Yarrowia lipolytica degrades petroleum compounds, including alkanes, via the monoterminal oxidation pathway, the hydrophobic carbon substrate assimilation is mediated by biosurfactants, and extracellular amphiphilic molecules are produced by the yeast cell. This study focuses on the ability of the strain Y. lipolytica CMGB32 to degrade n-hexadecane by producing biosurfactants with high potential for bioremediation. The hydrocarbon-degrading potential of the yeast strain was observed via a 2,6-dichlorophenolindophenol (DCPIP) test in Bushnell–Hass medium with 1% n-hexadecane, and cell hydrophobicity was expressed as microbial adhesion to hydrocarbons (MATH). Biosurfactant production on yeast peptone (YP) with 1% n-hexadecane was estimated after 72 h using the emulsification index (E24%) against toluene. Crude biosurfactant (cell-free broth) stability tests were performed at different temperatures (4 °C, 70 °C) and NaCl concentrations (2–10%). The effects of a biosurfactant on synthetic wastewater remediation comprised the growth curves (OD measurements) of natural heavy metal degrader Rhodotorula mucilaginosa, determination of nutrients (spectrophotometrically), physico-chemical parameters, and removal capacity of lead and cadmium ions (via inductively coupled plasma mass spectrometry—ICP-MS). The antimicrobial and anti-adherence activities of 20 mg/mL and 40 mg/mL of the biosurfactant against pathogenic Candida krusei strains involved growth observations and the crystal violet microtiter method. The DCPIP decolorization occurred after six days, corresponding to the maximum growth phase of the Y. lipolytica culture. After 72 h, the cells presented high hydrophobicity (82.61% MATH) and stable biosurfactant production (E24% 47%). The crude biosurfactant (5%) increased the growth of R. mucilaginosa strains cultivated on synthetic wastewater cultures contaminated with Pb2+ and Cd2+, increased the conductivity and COD (86%) of the samples, and determined Pb2+ (66%) and Cd2+ (42%) ions reduction. The concentrated biosurfactant inhibited C. krusei growth (70%) and biofilm adherence. In conclusion, Y. lipolytica CMGB32 shows important potential for development of biosurfactant-based technologies for the remediation of heavy-metal- and emerging pathogen-contaminated wastewaters.
Keywords:
Yarrowia lipolytica; n-hexadecane; wastewater; lead; cadmium; biosurfactant; antimicrobial activity 1. Introduction
n-alkanes are components of crude oil (petroleum) and diesel oil that are rising high concerns regarding environmental preservation and protection in the context of the massive contamination of soil and water due to accidental oil spills, industrial development, and the extension of population habitats. Oil spills can affect the entire ecosystem in a certain geographic region and directly impair the quality of human life. When reaching agricultural soil, it affects its texture and its mineral/heavy metal composition, and determines the inhibition of the crop plant root development [1], affects chlorophyll synthesis, and reduces the light reaction efficiency of photosynthesis [2,3]. In case of short time exposure of humans/animals to petroleum hydrocarbons by direct contact through breathing, skin contact, or consumption of contaminated foods, it causes nasal discharge, burning in the eye area, respiratory problems, vomiting, nausea, confusion, chronic fatigue, and dizziness. Long time exposure is associated with severe hemocytotoxicity, cytotoxicity, neurotoxicity, and immunotoxicity [4]. Among the most studied hydrocarbons, the hexadecane (C16H34), a medium carbon-chain alkane, is frequently used as a model carbon substrate for evaluation of biodegradation abilities of various microorganisms [5,6]. The effect of hexadecane exposure of animal and human organisms ranges from primary skin and mild eye and mucous membrane irritations to persistent intoxications of the pancreas during the weeks after exposure [7].
Many microorganisms, such as bacteria (e.g., Pseudomonas Sphingomonas, Mycobacterium, Bacillus, Rhodococcus, Burkholderia, Stenotrophomonas genera [8,9,10]), filamentous fungi (e.g., Aspergillus, Penicillium, Mucor, Cephalosporium [11,12,13]), and yeast species (e.g., Candida maltosa, Candida tropicalis, Rhodotorula sp., Starmerella bombicola, Candida antarctica, Meyerozima guilliermondii, Rhodorosporidium toruloides, Wickerhamomyces anomalus, Sporobolomyces salmonicolor [14,15,16,17]), present strains able to degrade a wide range of alkanes. Among these, Yarrowia lipolytica is one of the most versatile species, comprising strains isolated from natural and industrial habitats, with a wide range of practical applications in industry, bioremediation, and biomedicine [18]. The scientific interest for this species is still growing, especially due to its ability to assimilate non-conventional carbon substrates including crude oil and oil derivatives by converting them into beneficial biocompounds (e.g., lipases, lipids, and single-cell oils). The morphology, genetics, and metabolism of Y. lipolytica has been extensively studied over the years [19,20]. Also, the knowledge concerning the main genes involved in the monoterminal oxidation pathway, particularly of medium and high carbon-chain alkanes, has been completed by genome sequencing and genome-scale metabolic studies [21,22].
Numerous Y. lipolytica strains, including some isolated from aquatic environments, have been described as having high aliphatic and aromatic hydrocarbon degrading abilities, with rates of hexadecane degradation ranging from 39.9% to 78% over a maximum of 7 days of incubation [20,23]. Furthermore, the alkanes, including hexadecane, can be used as the sole carbon substrate for the production of biosurfactants in Y. lipolytica cells.
Biosurfactants are surfactants produced and excreted by numerous microorganisms (bacteria, yeasts, and fungi). Due to their amphiphilic structure comprising a hydrophobic moiety (consisting of long-chain fatty acids) and a hydrophilic moiety (represented mainly by carbohydrates, alcohols, and proteins), biosurfactants can decrease the surface tension between the hydrophobic (hydrocarbons) and hydrophilic (microbial cells) phases. This process determines the formation of hydrophobic micro-droplets, which are easily assimilated into the microbial cell via specific cell wall structures [19]. Moreover, the high biodegrability, specificity, and stability of biosurfactants make them one of the most promising strategies that are developed for the bioremediation of polluted soil and water. This includes the removal of heavy metals by trapping them within micelles, from which they are recovered via precipitation or membrane separation [24]. Different Y. lipolytica strains synthesize lipoproteic biosurfactants in the presence of alkanes, which are able to emulsify plant oils (Liposan) or aromatic hydrocarbons (Yasan) [20]. Also, the crude biosurfactant produced by Candida lipolytica UCP 0988 was used for removing 96% of Zn and Cu and reducing the concentration of Pb, Cd, and Fe from barrier silty soil [25], and for removing about 30–40% of Pb and Cu from sand samples [26], respectively.
This study focuses, on the one hand, on the ability of the strain Y. lipolytica CMGB32 to assimilate n-hexadecane for the production of stable biosurfactants and, on the other hand, on the complex characterization of the produced biosurfactants as potential highly efficient agents for removing heavy metals (Pb and Cd) and combating emerging pathogenic microbial strains from wastewaters.
2. Materials and Methods
2.1. Yeast Strains
The yeast strains Yarrowia lipolytica CMGB32, Rhodotorula mucilaginosa CMGB188, Rhodotorula mucilaginosa CMGB-G1, Candida krusei CMGB94, and C. krusei CMGB-Y8 (the Collection of Microorganisms of the Department of Genetics, Faculty of Biology, University of Bucharest, Bucharest, Romania) were maintained at −70 °C, on yeast peptone glucose (YPG) medium (g/L: yeast extract 5, peptone 10, glucose 2) supplemented with 20% glycerol, and grown for 20 h on YPGA (YPG with 1% agar-agar) before each experiment.
2.2. n-Hexadecane Biodegradability Assay Using 2,6-Dichlorophenolindophenol (DCPIP)
The biodegradability of n-hexadecane was assessed using the redox indicator 2,6-dichlorophenolindophenol (DCPIP) [27]. In yeast cells, C15-C36 hydrocarbons (including the n-hexadecane) are first hydroxylated to fatty alcohols by cytochrome P450 monooxygenase (based on nicotinamide adenine dinucleotide phosphate—NADPH and flavin adenine dinucleotide—FAD system). Fatty alcohols are further oxidized to corresponding fatty aldehydes and fatty acids in the endoplasmic reticulum, thus forming triacylglycerols or, in the peroxisomes, forming acetyl coenzyme A (Ac-CoA) molecules, which enter the glyoxylate and citrate cycles. The process comprises redox reactions and electron transfer to acceptors (O2, nitrates or sulphates) [19]. The DCPIP added to a mineral medium, in which a hydrocarbon (i.e., n-hexadecane) represents the sole carbon substrate, acts as an electron receptor during the oxidation process, changing from the oxidized form (blue) to the reduced form (colorless) [28].
The strain Y. lipolytica CMGB32 was cultivated for 72 h at 28 °C, 150 rpm in two flasks: on a YP medium supplemented with 1% n-hexadecane (FLUKA) and on a YPG medium, respectively. The inoculum of Y. lipolytica CMGB32 (0.3 × 108 cells/mL, determined using the Thoma chamber) obtained from each flask was used in a total volume of 10 mL Bushnell–Hass (BS) mineral medium (g/L: MgSO4 ∙ 7H2O 0.2, CaCl2 ∙ 2 H2O 0.02, KH2PO4 1.0, K2HPO4 1.0, NH4NO3 1.0, FeCl3 0.05) (pH 7.0 ± 0.2) with 1% n-hexadecane and 30 µg/mL DCPIP. The test tubes (duplicates) were incubated for 9 days at 120 rpm and 28 °C ± 1 °C and observed every day. The substrate control tubes did not contain inoculum, and the inoculum controls did not contain n-hexadecane.
2.3. Assessment of Microbial Adhesion to n-Hexadecane
The hydrophobicity of Y. lipolytica CMGB32 cells to n-hexadecane was assessed using the microbial adhesion to hydrocarbons (MATH) [29]. The yeast culture (starting from approximately 0.5 McFarland inoculum) was grown for three days on yeast peptone (YP) medium (g/L: Yeast extract 10, peptone 10) supplemented with 1% n-hexadecane. Then, 1.5 mL culture (with cell density equivalent to 3 McFarland) was centrifuged for 10 min at 10.000 rpm and 4 °C, and the cell pellet (containing both cells with a normal cell wall and cells with a cell wall whose degree of hydrophobicity is increased as a result of the changes induced by cultivation in the presence of non-conventional carbon sources) was washed three times with 1.5 mL phosphate-urea-magnesium buffer (PUM buffer) (g/L: KH2PO4 7.26; K2HPO4 19.7; urea 1.8; Mg2SO4 ∙ 7H2O 0.2; pH 7.0) and then diluted in order to obtain an OD570nm of approximately 1 (A0). A volume of 1.5 mL of cell suspension was mixed with 120 µL n-hexadecane, vortexed, and incubated for 15 min at room temperature. Moreover, the OD was determined using 200 µL of the lower phase (represented by cell suspension in the PUM buffer containing only cells with no modification of the cell wall hydrophobicity degree and which did not attach to the n-hexadecane layer after vortexing) (A1). All the samples were performed in duplicates. The MATH value was obtained using the mean of the two values calculated using the following equation:
MATH% = [(A0 − A1)/A0] × 100
2.4. Interactions between Yeast Cells and n-Hexadecane
The interactions between Y. lipolytica CMGB32 cells and n-hexadecane droplets were observed using an optic microscope (40×) (MICROS, St. Veit an der Glan, Austria) after three days of incubation at 28 °C, 150 rpm, in YP medium with 1% n-hexadecane.
2.5. Biosurfactant Production and Characterization
2.5.1. Determination of the Emulsification Index
The strain Y. lipolytica CMGB32 was grown on YP medium (1% yeast extract, 1% peptone) supplemented with 1% n-hexadecane (Sigma-Aldrich, St. Louis, MO, USA), for 72 h at 150 rpm. The cell-free broth was mixed (3:2 v:v) with toluene, vortexed for 2 min at 2500 rpm, and maintained at room temperature (~22 °C) for 24 h, and the production of the biosurfactant was evaluated using the emulsification index (E24%) [30].
2.5.2. Stability Tests on Emulsifying Properties of the Biosurfactants
The effects of temperature and sodium chloride (NaCl) variations on biosurfactant stability were determined by calculating the emulsification index (E24%) of cell-free broth towards toluene after 24 h of incubation at room temperature (22 °C). The effect of temperature was determined during two parallel experiments. In the first experiment, the cell-free broth (crude biosurfactant) was incubated at 4 °C for 20 min, brought to room temperature, and then mixed with toluene for the determination of E24%, as described before (Section 2.5.1). The second experiment followed the same protocol after the incubation of the crude biosurfactant at 70 °C. The effect of salinity was assayed during five parallel experiments (NaCl—crude biosurfactant mixtures) using NaCl solution in final concentrations of 2%, 4%, 6%, 8%, and 10%, under the same condition as Section 2.5.1. The results were compared to those obtained from Section 2.5.1.
2.6. Biosurfactant Effect on Removal of Some Pollutants from Synthetic Wastewater
2.6.1. Growth Curves in Contaminated and Uncontaminated Synthetic Wastewater
The biosurfactant was obtained using a protocol described previously by Nicula et al. [31]. The cell-free broth obtained after filtering the supernatant was further used as a crude biosurfactant [32,33].
The growth curve of the yeast strains (Rhodotorula mucilaginosa CMGB-G1 and R. mucilaginosa CMGB 188—previously isolated from oil polluted environments and included in the CMGB collection of microbial strains) was determined in synthetic wastewater contaminated and uncontaminated with Pb2+ and Cd2+ ions, both in the presence or absence of a biosurfactant. This was achieved using 96-well plates containing 400 µL of sample inoculated with 1% of each yeast strain with an initial concentration of 2 McFarland (for Sw, Pb and Cd with P1, and P2, respectively) supplemented with 5% (v/v) biosurfactant (for Bio Y, BioY + Pb, Bio Y + Cd). The experiments were conducted for 24 h at a temperature of 28 ± 1 °C (i.e., controlled conditions), and optical density values were recorded hour by hour at 570 nm using a Synergy HTX Multimode reader (BioTEK, Winooski, VT, USA). Optical density (OD) measurements were also used to determine the degree of yeast development during the 120 h of the experiment (i.e., experimental conditions).
Synthetic wastewater was prepared by adapting the formulations outlined by [31,34] comprising the following components: 200 mg/L D-glucose, 200 mg/L sucrose, 70 mg/L yeast extract, 66.73 mg/L (NH4)2SO4, 10.91 mg/L NH4Cl, 4.43 mg/L KH2PO4, 21 mg/L MgSO4·7H2O, 2.68 mg/L MnSO4·H2O, 30 mg/L NaHCO3, 19.74 mg/L CaCl2, 0.14 mg/L FeCl3·6H2O, 330 mg/L NaNO3, and 390 mg/L NaNO2. For experiments regarding the removal efficiency of heavy metal ions, synthetic wastewater was contaminated with Pb2+ (32 mg/L, (CH3COO)2Pb) and Cd2+ (30 mg/L, Cd(NO3)2) ions. The presented heavy metal ion concentrations were determined using ICP-MS analysis.
The coding of the samples used in this paper is shown in Table 1.

Table 1.
Sample coding and description.
2.6.2. Determination of Physico-Chemical Parameters of Synthetic Municipal Wastewater
The physico-chemical parameters of the inoculated synthetic wastewater were determined initially and after 24, 48, 96, and 120 h by evaluating the temperature, pH (using a pH meter HI10832, Hanna Instruments, Inc., Woonsocket, RI, USA), conductivity (using a Multiparameter HI2020-01 Edge, Hanna Instruments, Inc., Woonsocket, RI, USA), and dissolved oxygen (Multiparameter Oxi340i, Weiheim, Germany).
2.6.3. Removal of Pollutants
For the experiments, 450 mL concentrations of uncontaminated and contaminated synthetic wastewater with Pb2+ and Cd2+, respectively, were inoculated with 1% of each yeast strain having an initial concentration of 2 McFarland. To prepare the samples with a biosurfactant, 5% (v/v) biosurfactant was introduced in inoculated synthetic wastewater. Subsequently, all synthetic wastewater samples were incubated at 28 ± 2 °C for 120 h. The removal efficiency of nutrients and heavy metals was determined initially and at the end of the experiments.
The capacity to remove organic substances was evaluated by determining the chemical oxygen demand (COD), according to the ISO 6060 standard [35], using the Gerhardt Chemical Oxygen Analyzer equipment (C. Gerhardt GmbH & Co., Königswinter, Germany).
The ability to remove nitrate, nitrite, ammonium, phosphate, and sulfate ions was evaluated spectrophotometrically by using specific methods and reagents [36], and using the Hanna HI83300 Multiparameter Photometer (Hanna Instruments, Inc., Woonsocket, RI, USA).
ELAN DRC-e ICP-MS (PerkinElmer, Waltham, MA, USA) equipment was employed to assess the efficiency of removing Pb2+ and Cd2+ ions. A quantitative evaluation was conducted for each element individually using the calibration curve method.
2.6.4. Determination of the Biomass Amount
The amount of obtained yeast biomass was determined gravimetrically, at the end of the experiment, by filtering the samples in a vacuum tube through filter paper with pores of 0.45 µm, followed by drying at room temperature [31].
2.7. Antimicrobial and Anti-Adhesion Activity of the Biosurfactant
2.7.1. Growth Inhibition Assays
Aliquots of 20 mL crude biosurfactant were placed on watch glass and incubated in a lab oven at 40 °C until total evaporation of the liquid. The dried biosurfactant was recovered in sterile Eppendorf tubes and used for obtaining 20 mg/mL and 40 mg/mL concentrated biosurfactants in sterile distilled water, which were then sterilized via filtration using 0.24 μm syringe filters.
Standard suspensions of Candida krusei strains, CMGB94 and CMGB-Y8, respectively, were prepared using fresh cultures grown for 18 h on YPGA medium at 37 °C, which were dissolved in sterile distilled water. A volume of 10 µL of the suspensions (0.3 × 107 cells/mL determined using a Thoma chamber) was mixed with 100 µL of concentrated biosurfactants (final concentrations of 20 mg/mL and 40 mg/mL, prepared in YPG broth) and then placed in microtiter wells, resulting in a final volume of 110 µL. The mixtures were then incubated at 37 °C. Cell growth was assessed hourly up to 21 h by measuring the OD at 570 nm using an automatic plate reader (Multi-mode reader Synergy HTX, BioTek) [21,22]. The positive control for growth consisted of strains cultivated in the presence of YPG broth, while sterility control consisted of YPG broth supplemented with 20 mg/mL or 40 mg/mL of concentrated biosurfactant.
2.7.2. Anti-Adhesion Activity Tests
To determine the influence of the tested biosurfactant on the microbial adherence to inert substratum (96-well plate, untreated polystyrene), the crystal violet microtiter method was used. C. krusei CMGB94 and C. krusei CMGB-Y8 were cultured in the presence of 20 mg/mL and 40 mg/mL biosurfactants using a protocol similar to the one described above. After 24 h of incubation at 37 °C, the unattached cells were removed using sterile distilled water. After stabilization of the adherent cells to the well using 110 µL methanol solution (99%), the wells were emptied and dried at room temperature for the fixation step. The number of attached cells was determined using 110 µL crystal violet solution (1%) added to each well, followed by incubation for 15 min. The plates were washed with running tap water to remove the excess stain, and the adherent cells were solubilized using 110 µL of 33% acetic acid (v/v), followed by absorbance reading (OD = 595 nm) [37,38]. All the samples were tested in triplicate, and the control wells contained only YPG broth (with no inhibitory compound added).
The microbial adherence inhibition percentages (PICA%) in the presence of concentrated biosurfactants were calculated using Equation (2), as described by [37,38].
where As is the absorbance of the sample (adherence in the presence of biosurfactants), and Ac is the absorbance of the control well (adherence in absence of any inhibitory compound).
PICA% = (As/Ac) × 100
2.8. Statistical Analysis
The experiments regarding synthetic wastewater treatment were conducted in triplicates, and the results were expressed as the mean ± standard deviation (SD) derived from three replicates. Statistical significance was assessed using Student’s t-test, considering a significance level of p < 0.05. Statistical analysis was carried out using Microsoft Excel 2021 software.
For the antimicrobial properties of the biosurfactant triplicates, data were expressed as mean ± SD, and statistical analysis was performed using GraphPad Prism v9. Data were analyzed using ordinary two-way ANOVA using post hoc tests (Tukey’s correction for the growth inhibition percentage and Dunnett’s test for the PICA%) for multiple comparisons, with individual variances calculated for a comparison between the biological activities of the biosurfactant and the control strain. The significance level was set at p < 0.05.
3. Results and Discussion
3.1. n-Hexadecane Assimilation
Previous studies showed that the strain Y. lipolytica CMGB32 has high ability to assimilate n-hexadecane when grown on a minimal BS medium, with a plateau between the 6th and the 10th day of growth [39]. This period corresponds to the time required for the DCPIP color change observed on the 6th and 9th days of the experiment using the YPG- and YP-grown inoculum, respectively. The longer period needed for DCPIP decolorization by the YP-hexadecane-grown inoculum can be related to a slower cell metabolism caused by the changes induced in the expression of genes associated with the assimilation of hydrophobic substrates, compared to the cells growing on glucose, in the case of YPG medium inoculum. Although the expression of genes associated with non-conventional carbon sources (such as n-alkanes) is essential for the n-hexadecane assimilation of the metabolic shift, in the case of yeasts, it is time dependent and requires prolonged exposure to support an exponential growth in the first hours of cultivation. Meanwhile, no decolorization was observed for the inoculum and substrate control tubes.
The information on the DCPIP method used for biodegradability tests on yeasts is rather poor. However, ref. [40] described a DCPIP decolorization time ranging from 8 to 29 h for a Candida vishwanathii strain able to degrade various biodiesel blends. Also, ref. [41] reported two strains—Rhodotorula aurantiaca UFPEDA 845 and Candida ernobii UFPEDA 862—as inducing a positive response in the presence of DCPIP and diesel oil within 16 and 24 h, respectively. The shorted DCPIP decolorization periods mentioned in the cited literature can be most probably related to the composition of the carbon substrate, which is more accessible for the yeast cells compared to our experiment, where the sole carbon source is represented by the n-hexadecane.
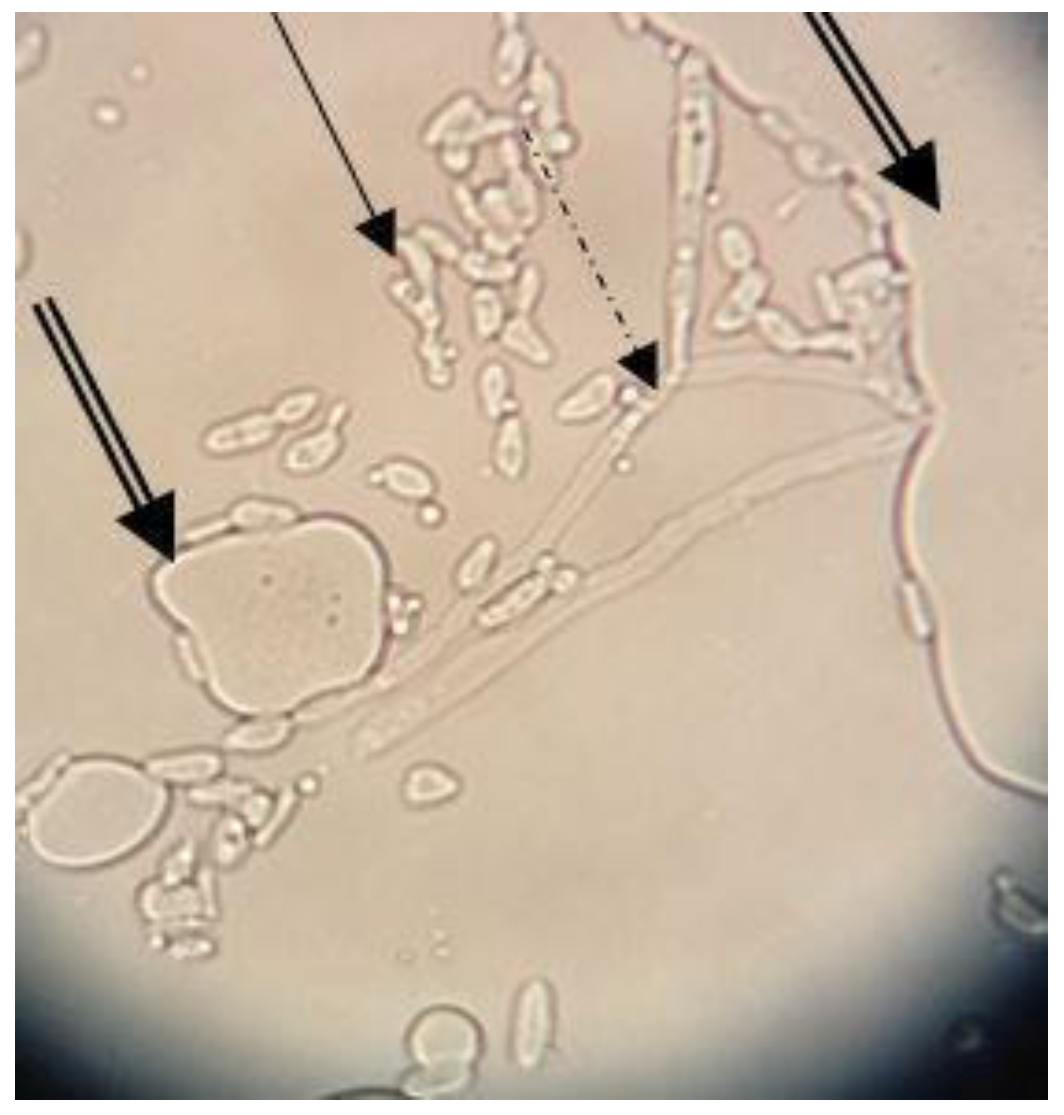
The MATH value (82.61%) determined after three days in the presence of n-hexadecane revealed the high hydrophobicity of the Y. lipolytica CMGB32 cells. The result corresponds to other studies [29] in which Y. lipolytica cells showed 76.5–80.2% hydrophobicity when grown on a mixture of C12-C16 alkanes, but after seven days of incubation. Our results suggest a higher ability of Y. lipolytica CMGB32 cells to adapt to hydrocarbons substrates, such as n-hexadecane. The ability of yeast cells to attach to the alkane droplets was also evaluated using an optic microscope. Yeast cells and large pseudohyphae corresponding to morphological modifications related to the adaptation to hydrocarbons substrate adhere to n-hexadecane droplets (Figure 1).
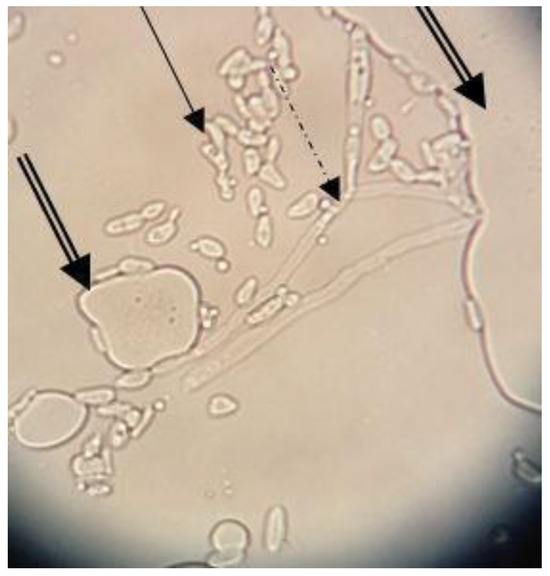
Figure 1.
Y. lipolytica CMGB32 cell interactions with n-hexadecane droplets after three days of incubation (40×) (plain arrow—yeast cells; dashed arrow—pseudohyphae; double arrow—n-hexadecane droplets).
3.2. Stability of the Emulsifying Properties of the Biosurfactant
Biosurfactant production was evaluated using the emulsification index (E24%), and the value obtained against toluene was 47% (Figure S1). The biosurfactant showed high thermal stability (50% at 4 °C and 41% at 70 °C), with the E24% values being also stable at NaCl concentrations between 2 and 6% (53% for NaCl 2% and 4%, 47% for NaCl 6%), decreasing for higher 8% and 10% concentrations (31% and 28%, respectively). In our previous study, it was shown that the biosurfactant produced by the Y. lipolytica CMGB 32 strain is essentially a lipoprotein composed of a high amount of aromatic amino acids [31]. The aromatic amino acid residues, such as tyrosine and tryptophan, are involved in establishing energetically favorable interactions with molecules from the surrounding environment (interaction strongly influenced by temperature and ionic strength) [42,43]. Thus, the stability of the biosurfactant at the tested temperature and the NaCl concentrations might indicate that the sequences of aromatic amino acids interfere in a limited way in the stabilization of the lipoprotein biosurfactant’s functional structure. Moreover, the applicability of microbial biosurfactants in several fields is strongly dependent on their stability at different temperatures and salinity values. The high thermostability suggests that the biosurfactant produced by Y. lipolytica CMGB32 can be used in food, pharmaceutical, and cosmetic industries, where heating is an important step in the manufacture process [44]. Additionally, it can be used in the bioremediation of habitats within a wide range of temperatures. Also, stability at high salinity concentrations is an important parameter when determining the applicability of biosurfactants in the bioremediation of spills in marine water [45]. As recorded previously [39], the biosurfactant produced by Y. lipolytica CMGB32 in the presence of n-hexadecane had an E24% of 52% against petroleum. In addition, we showed that the strain Y. lipolytica CMGB32 was able to synthesize stable biosurfactants also on a YP medium supplemented with 1% petroleum, with an E24% value of 52% against toluene [32]. Meanwhile, ref. [46] described the strain Y. lipolytica IMUFRJ 50682 as producing biosurfactants with an emulsification index against hexadecane that reached a maximum of only 17.5% after 100 h of incubation on crude oil as a sole carbon substrate. Other studies reported an E24% of 62.4% for toluene emulsification using the bacterial strain Streptomyces sp. MOE6 [47], and reported values ranging between 33.3 ± 0.0 and 75.0 ± 1.7 for various strains belonging to Marinobacter genus [48].
Toluene is one of the hydrocarbons found in crude oil’s composition and represents one of the main industrial solvents used for paints, glues, cleaning products, etc. Long exposure to accidental oil spills and work conditions in the presence of toluene leads to renal and hepatocellular injuries, rhabdomyolysis, and altered nervous system functions [49]. Moreover, toluene contamination can occur also in the presence of heavy metals, influencing the efficiency of bioremediation technologies and the composition of microbial communities with biodegrading abilities [50]. Therefore, the good ability of the Y. lipolytica CMGB32 biosurfactant to emulsify toluene, and its high stability, might be considered as a solid base for its use as an additive for toluene removal from contaminated areas.
3.3. Growth Curves of Yeast Strains
The yeast R. mucilaginosa has been described as one of the species present in municipal wastewater treatment plants polluted with Cr, Cd, and nitrates [51]; in olive mill wastewater, where is able to assimilate and degrade phenolic compounds [52]; or in textile wastewater, having the ability to remove Cu2+, Ni2+, and Cr6+ ions with an efficiency of 30.6%, 38%, and 94.5%, respectively [53]. Grujić et al. [54] reported that R. mucilaginosa planktonic cells were resistant to cadmium concentrations up to 10 mM and nickel concentrations up to 3.200 mg/L. Also, two R. mucilaginosa strains isolated from wetlands from Ventanilla (Callao, Peru) showed intensive growth in the presence of 10 mg/L of Pb2+ at pH 5.0 and pH 6.0, and an ability to tolerate up to 1 g/L Pb2+ at pH 6.0 [55].
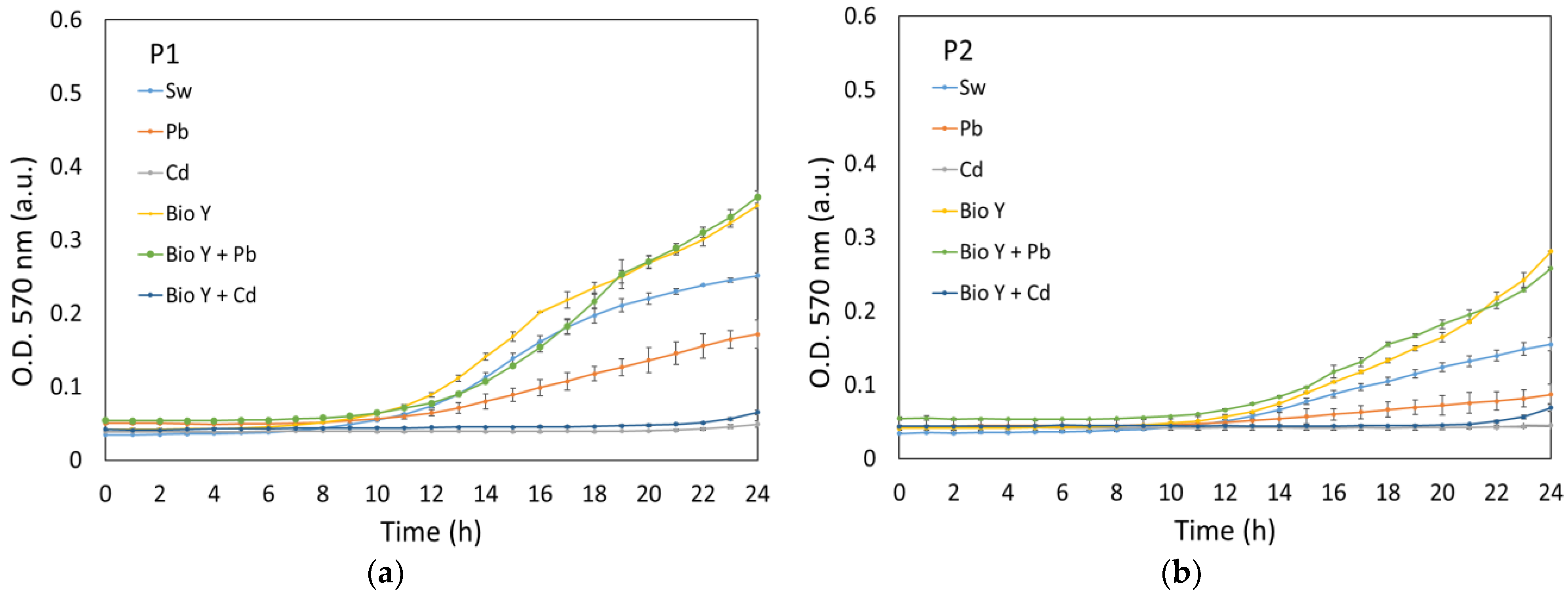
In our study, the growth of the strains R. mucilaginosa CMGB-G1 (P1) and R. mucilaginosa CMGB188 (P2) was monitored and recorded through optical density measurements, both in controlled (24 h, Figure 2) and experimental conditions (120 h, Figure 3). Under controlled conditions, the development of yeasts was observed for a shorter duration of 24 h in order to understand their normal growth pattern without any external variables (i.e., temperature, day/night alternation).
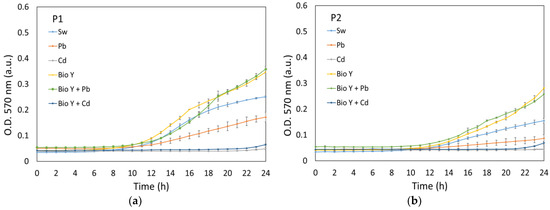
Figure 2.
Growth curves of P1 (a) and P2 (b) recorded in controlled conditions.
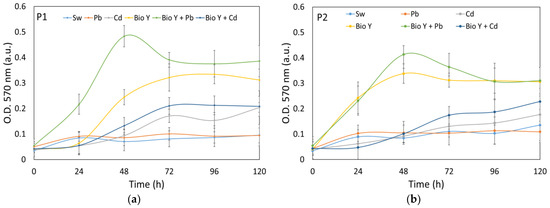
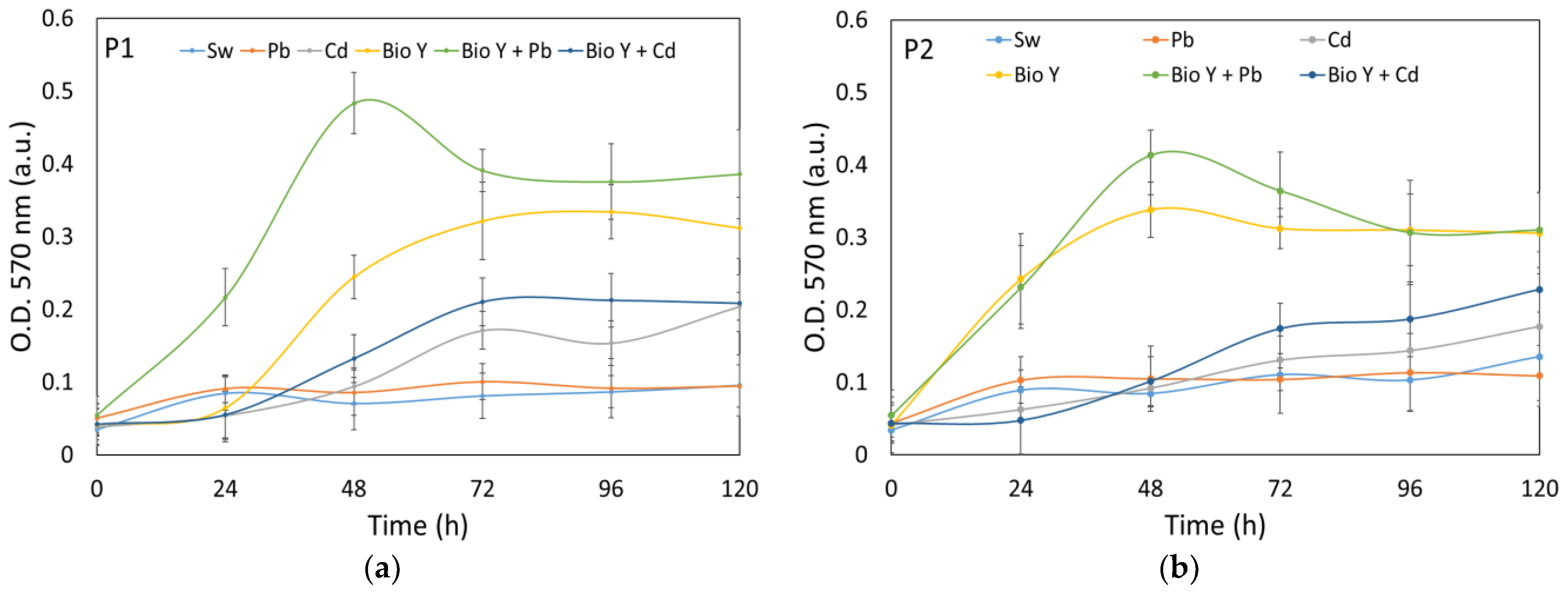
Figure 3.
Growth curves of P1 (a) and P2 (b) recorded in experimental conditions.
The data from Figure 2a,b reveal that all the tested samples showed the same development pattern over the 24 h test period, with only the lag and exponential development phases being visible. Both yeast strains showed a lag period between 9 and 14 h for most tested synthetic wastewaters, with the order of growth being generally in the direction of Pb < Sw < Bio Y + Pb ≤ Bio Y. This trend demonstrates the positive influence of a biosurfactant on the faster growth of R. mucilaginosa cultures. However, a particular case was observed regarding the water contaminated with cadmium ions, where the lag period was 22 h, which was probably due to the more pronounced toxic effect of Cd2+ ions on the tested strains [31].
On the other hand, under experimental conditions (Figure 3a,b), the yeast strains were subjected to a more extended observation period of 120 h. This allowed the examination of the long-term growth behavior of the yeast strains and their responses to specific experimental variables.
The data reflect a steady increase in OD values throughout the experiment. In the case of the biosurfactant-supplemented sample (Bio Y), a maximum OD was observed for both yeast strains within the 48–96 h period of the experiment. At the end of the 120 h experiment, it becoma evident that the water samples enriched with the biosurfactant yielded the highest optical density (OD) values for both types of yeast strains. Notably, there existed a discernible decreasing trend in OD values, with the samples treated with BioY + Pb exhibiting the highest values, followed by BioY and then by BioY + Cd samples. In contrast, samples with Cd2+, Pb2+, and initial synthetic wastewater, as well as those without the biosurfactant, showed lower OD values. These findings underline the effectiveness of a biosurfactant in stimulating yeast cell growth, highlighting its importance in the context of the experiment.
3.4. Wastewater Physico-Chemical Parameters
The efficiency of wastewater treatment with yeast cultures and biosurfactants could be influenced by several parameters, such as temperature, pH, conductivity, dissolved oxygen, etc. [56,57,58,59]. Temperature is an important parameter which affects the metabolic processes of yeast, while pH could influence heavy metal solubility and yeast cell surface charge, thereby impacting the biosorption capacity of the yeast cells. Additionally, the conductivity, reflecting the ionic content of the wastewater, can influence the treatment efficiency by indicating potential competition between the ions and the heavy metals for the yeast binding sites. On the other hand, yeasts use dissolved oxygen to effectively metabolize and reduce heavy metals in wastewater.
Table 2 presents the evolution of pH and conductivity of synthetic wastewater during the experiments. Temperature was kept quasi-constant at about 26 ± 1 °C. The samples were aerated daily using a wood air diffuser for about 15 min in order to increase the amount of dissolved oxygen at about 5 mg/L (the initial amount of dissolved oxygen was 7.5 ± 0.5 mg/L).

Table 2.
Variations in the physico-chemical parameters of inoculated synthetic wastewater.
A general increase in the conductivity of the samples was observed during the experiments for both yeast strains, with the maximum conductivity values being observed in the case of samples with biosurfactants and heavy metals. The growth of yeasts involves the release of ions, such as ammonium ions derived from the assimilation of nitrogen, which contribute to the increase in the ionic strength of the water and, consequently, to the overall conductivity of the water [60,61]. Also, the pH values showed a decrease in the first 96 h of the experiment, followed by a slight increase until 120 h, regardless of the tested sample, with the lowest pH values being observed in the case of synthetic wastewater with Pb2+ at 96 h from the beginning of the experiment for both R. mucilaginosa CMGB-G1 (P1, pH = 6.12) and R. mucilaginosa CMGB188 (P2, pH = 6.14) strains. The decrease in pH during yeast development in the presence of Pb2+ and Cd2+ can be influenced by various factors, including the release of ions and the metabolic activity of the yeast strains, with the optimal pH for yeast growth being in the range of 4.0–8.5 [62].
These results are consistent with those reported by our group in a previous study [31]. While not the primary focus of this paper, it is essential to acknowledge several potential factors that could impact the removal efficiency of cadmium and lead ions by yeast strains [63]. However, it is important to note that the removal mechanism is very complex. It involves intricate interactions between the yeast cells and the metal ions, including adsorption, ion exchange, surface attachment, and intracellular sequestration. Additionally, factors such as cell wall composition, metabolic activity, and genetic variability among yeast strains can further influence the removal process [64]. For example, variations in the pH of wastewater solutions could significantly affect the adsorption capacity of heavy metals via yeasts. At lower pH levels, the abundance of H+ ions prevails, overpowering the adsorption sites restricting the access of cations to these sites [65]. This phenomenon is particularly notable for Cd2+ ions, indicating a greater susceptibility to pH fluctuations compared to Pb2+ [66,67]. Moreover, the toxicity of cadmium and lead ions can significantly impact the efficacy of yeast strains in removing them from wastewater. Our results suggest that the toxicity of cadmium on yeast strains tends to be higher than that of lead, as evidenced by the observations of growth rate inhibition in yeast exposed to these metal ions (Figure 2). These results are consistent with those reported in other studies, which have also highlighted the higher toxicity of cadmium compared to lead on yeast strains [31,68,69].
3.5. Removal Efficiency of Micropollutants from Water
3.5.1. Chemical Oxygen Demand (COD)
COD is a measure of the amount of oxygen required to break down organic matter in water and is an important parameter in water quality analysis because it can be a measure of the wastewater treatment efficiency [35].
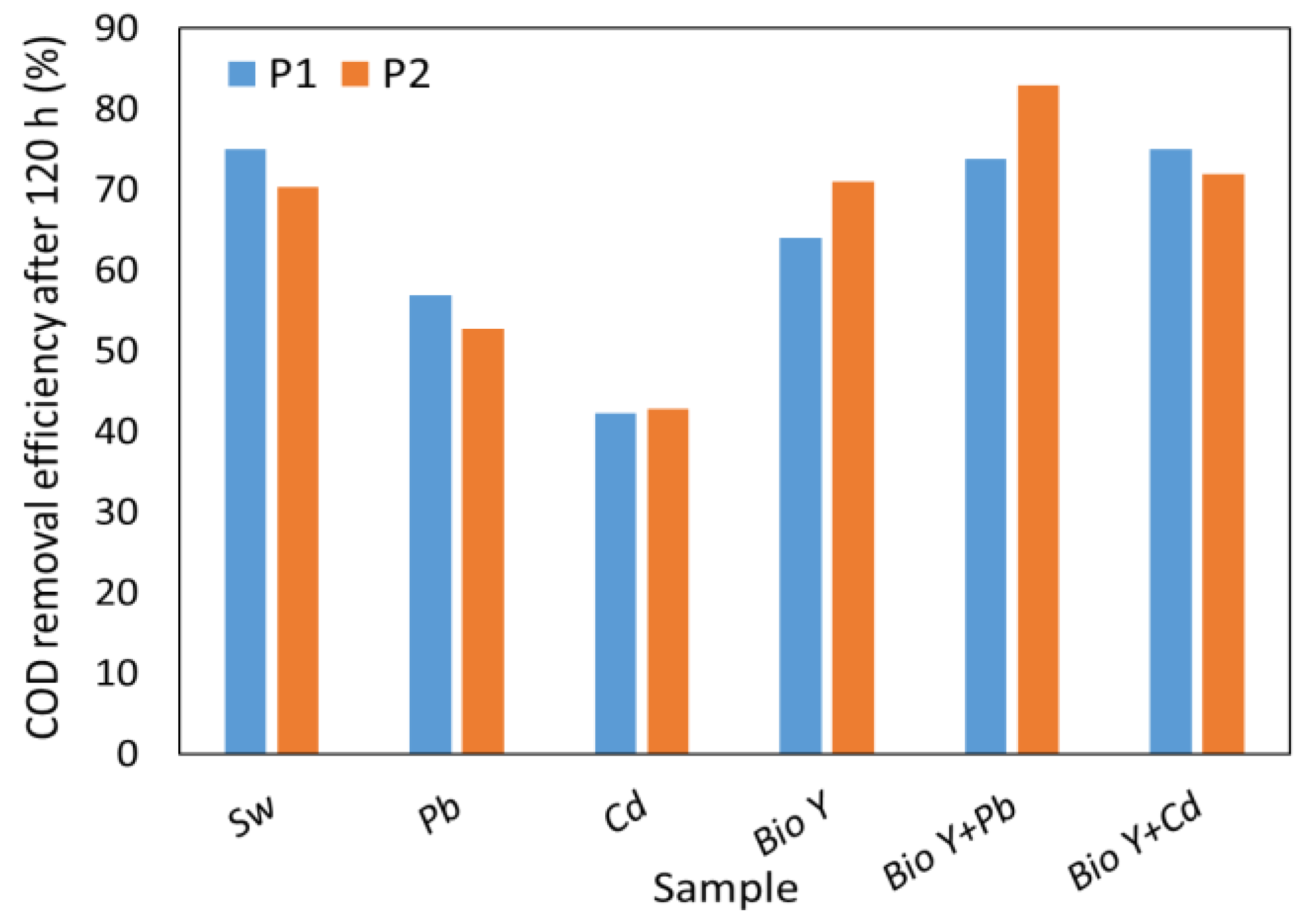
The results obtained in this study (Figure 4) show a significant reduction in COD by up to 86% compared to the reference (508 ± 9.6 mg O2/L) value. It is important to emphasize that the synthetic wastewater samples containing a biosurfactant showed COD values comparable to or even higher than the synthetic wastewater without Pb2+ and Cd2+ ions. In the presence of metal ions, without biosurfactants, COD reduction ranged from 42% for Cd2+ to 57% for Pb2+. It is interesting to note that R. mucilaginosa CMGB 188 (P2) showed, on average, a higher COD reduction capacity than R. mucilaginosa CMGB-G1 (P1), considering all types of samples tested. Moreover, the results obtained in this study are comparable with a previous study [31] conducted by our group, where the efficiency of removing COD from synthetic wastewater was tested using five different yeast strains: Kluyveromyces marxianus CMGBP16, Saccharomyces cerevisiae S228C, Saccharomyces cerevisiae CM6B70, Saccharomyces cerevisiae CMGB234, and Pichia anomala CMGB88. The results showed a COD removal efficiency of up to 70%. In a different investigation, a reduction in COD ranging from 50% (Candida krusei) to 71.6% (Candida albicans) was observed when using a 1% (v/v) inoculum rate, but after 25 days of the experiment [70].
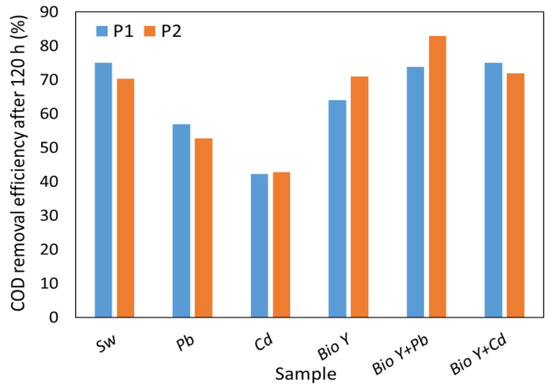
Figure 4.
COD removal efficiency by R. mucilaginosa CMGB-G1 (P1) and R. mucilaginosa CMGB 188 (P2).
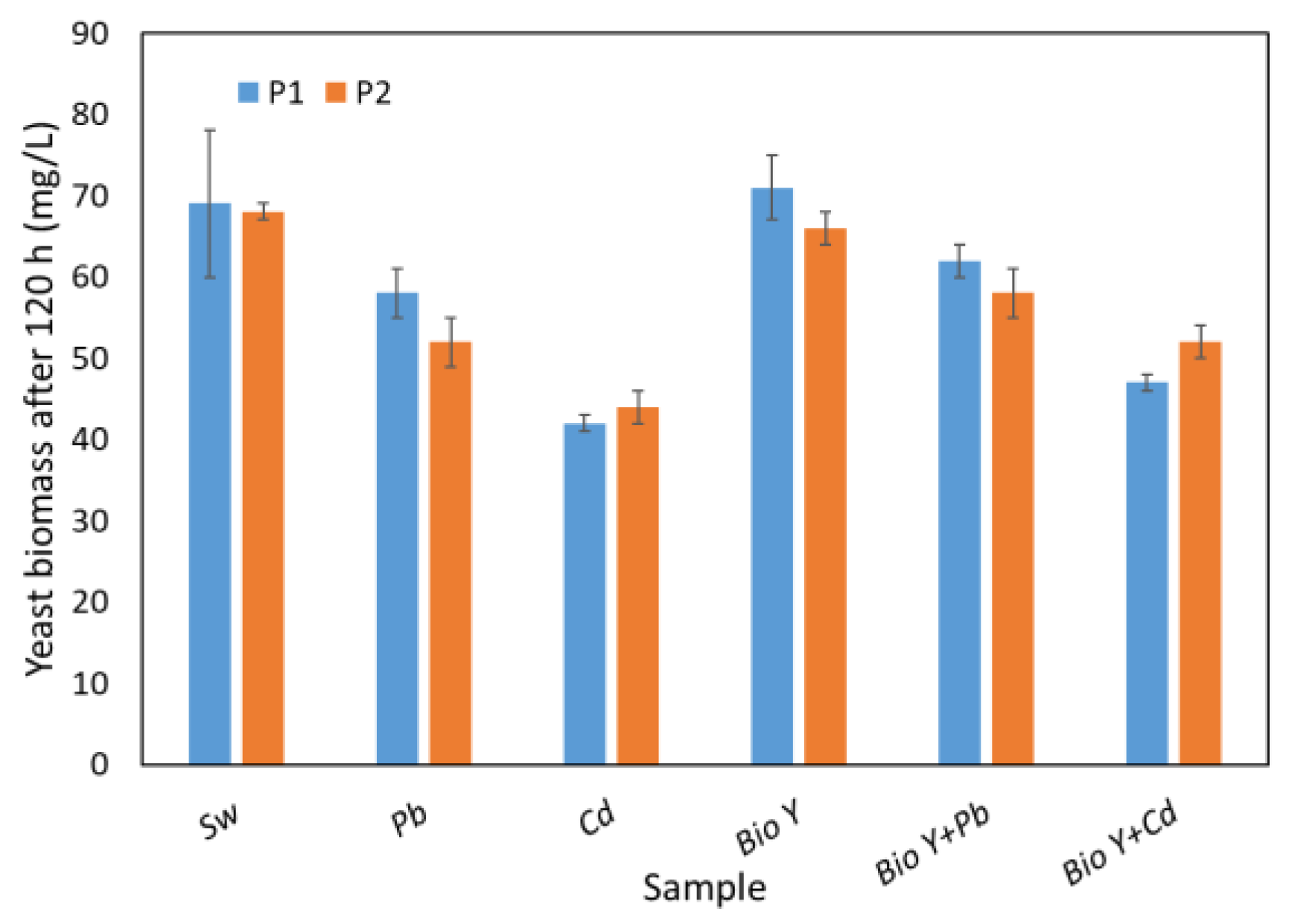
The capacity for COD removal appears to be affected by the quantity of yeast biomass obtained at the end of the 120 h experiment (Figure 5). This is contingent upon the experimental conditions, including the presence or absence of heavy metal ions and the biosurfactant in the water sample. By analyzing Figure 5, it is evident that changing the biomass amount leads to a similar behavior as in the COD analysis. Consequently, the maximum biomass (66–76 mg/L) was observed in the case of synthetic wastewater with biosurfactants. It is noteworthy to mention that slightly elevated values of the biomass amount were obtained in the case of R. mucilaginosa CMGB-G1 (P1).
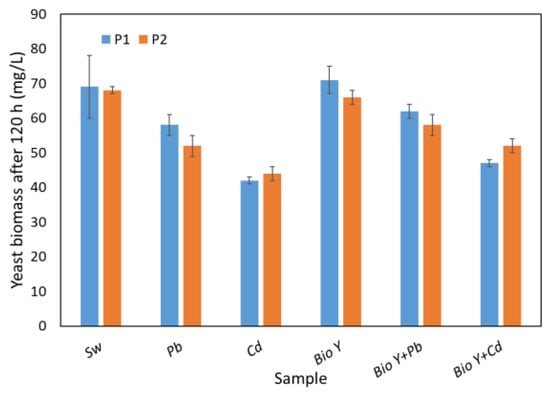
Figure 5.
Yeasts biomass amount at the end of the experiment.
In wastewater samples without biosurfactants, the amount of biomass decreased from ca. 70 mg/L (synthetic wastewater (Sw)) to 52 mg/L (R. mucilaginosa CMGB188 (P2)) and 58 mg/L (R. mucilaginosa CMGB-G1(P1)) in the presence of Pb2+, respectively, and to 42 mg/L (R. mucilaginosa CMGB-G1 (P1)) and 44 mg/L (R. mucilaginosa CMGB188(P2)) in the presence of Cd2+, respectively. In the combined presence of metal ions and biosurfactants in wastewater, the highest amount of biomass of about 62 mg/L was obtained in the presence of Pb2+ for the R. mucilaginosa CMGB-G1 (P1) strain, followed by 58 mg/L for the R. mucilaginosa CMGB188 (P2) strain. The lowest values in the presence of biosurfactants were obtained for Cd2+ (between 47 and 52 mg/L), and the highest values were obtained for the R. mucilaginosa CMGB188 (P2) strain.
3.5.2. Nutrient Removal
The removal of excess nutrients, particularly nitrogen compounds (nitrate, nitrite, and ammonium) and phosphate ions, from wastewater is important to prevent eutrophication. Eutrophication is the excessive growth of algae and other aquatic plants that is fueled by these nutrients. This growth can lead to oxygen depletion, causing significant harm to aquatic life [71]. The removal of these nutrients from wastewater can be achieved through various methods, including active metal reduction, electrochemical catalytic reduction, chemical precipitation, adsorption, and biological treatment [31,72,73,74,75].
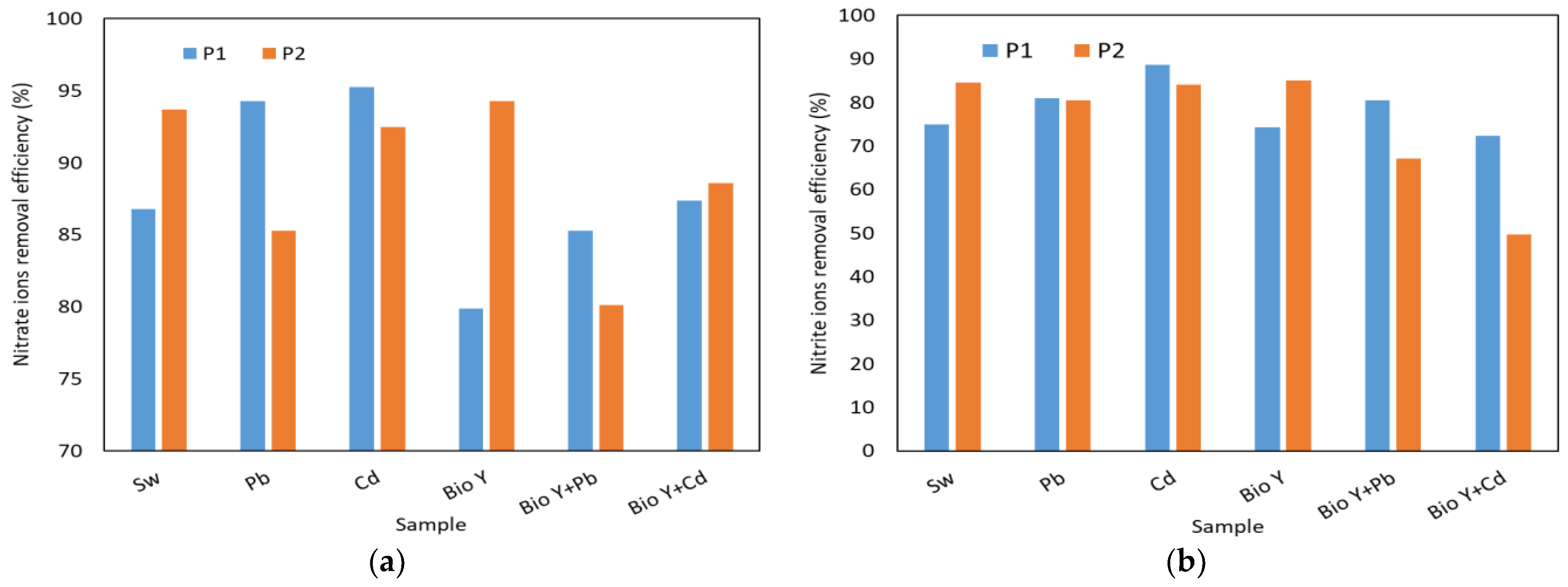
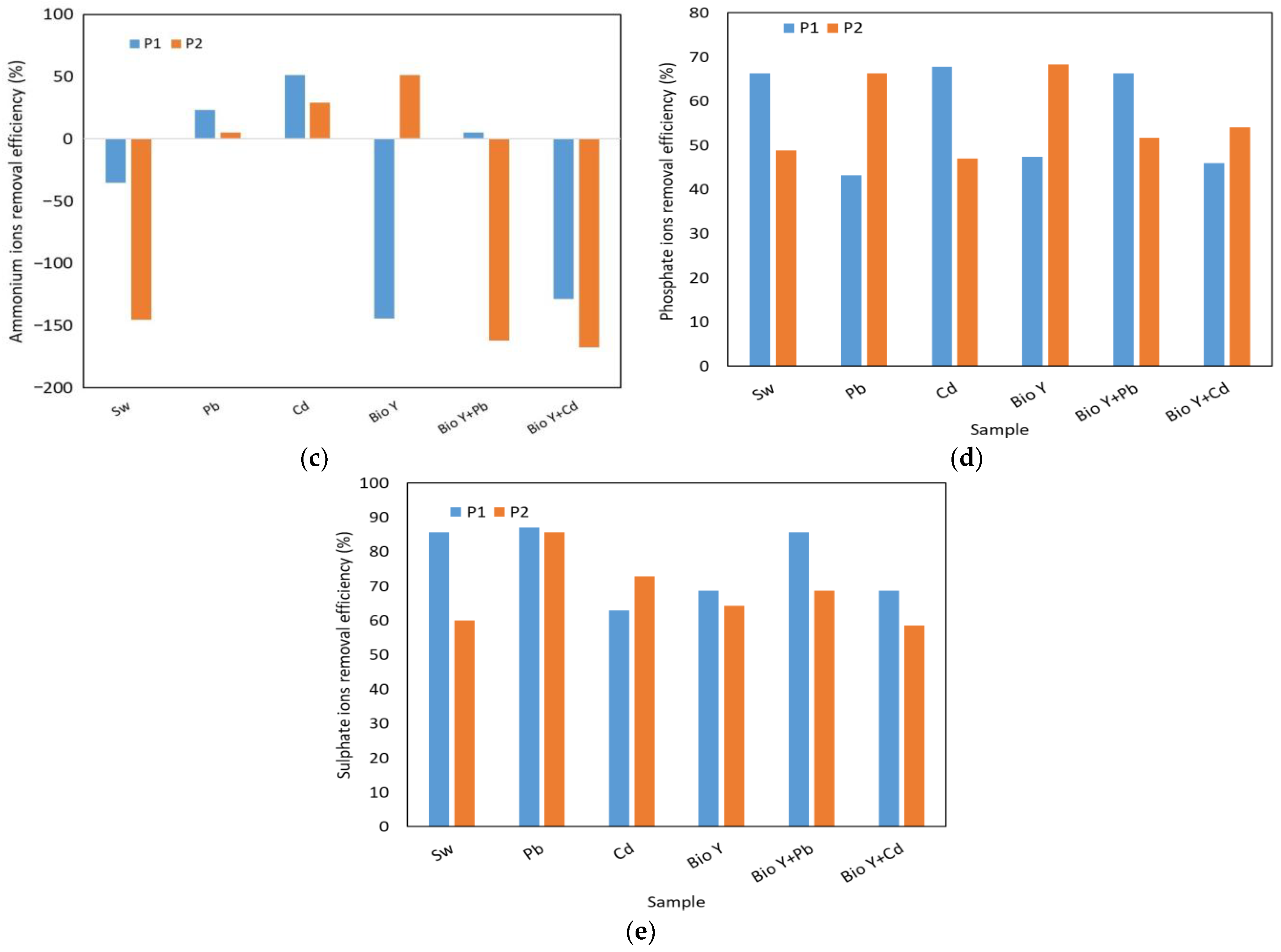
In Figure 6, the results obtained for the determination of the reduction efficiency of nitrate, nitrite, ammonium, phosphate, and sulfate ions by the two yeast strains are presented based on the type of sample tested. The key factors that influenced the removal capacity of these ions were the yeast strain used and the type of heavy metal.
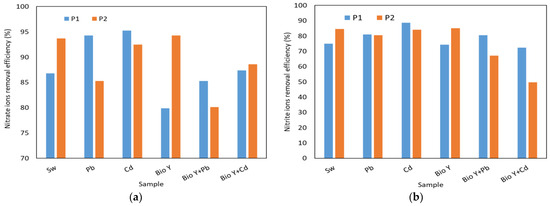
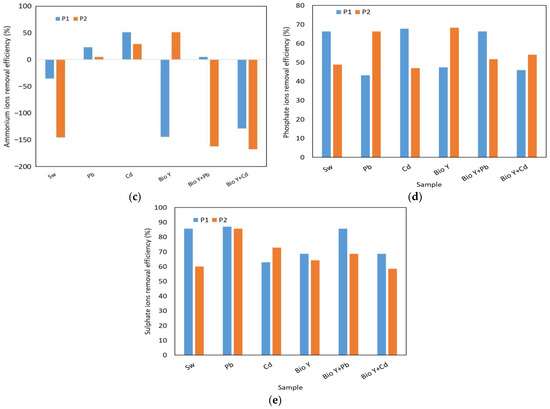
Figure 6.
Yeast nutrient removal efficiency at the end of the experiment of: (a) nitrate ions; (b) nitrite ions; (c) ammonium ions: (d) phosphate ions; (e) sulphate ions.
For nitrate ions (Figure 6a), a reduction ranging from 80 to 95% was observed. Also, no clear trend was observed in the behavior of the two yeast strains in the presence of nitrate ions, each having a higher or lower removal efficiency depending on the type of wastewater tested. When the biosurfactant was absent, R. mucilaginosa CMGB-G1 (P1) exhibited the highest reduction efficiencies (94–95%) for nitrate ions in the presence of Pb and Cd ions, compared to 87% in synthetic wastewater (Sw). R. mucilaginosa CMGB188 (P2) also displayed a reduction efficiency ranging from 85% (Pb) to 94% (Sw). In the presence of the biosurfactant, notably lower efficiencies were observed, generally falling between 80 and 89%, with a peak of 94% for R. mucilaginosa CMGB188 (P2) in the absence of metal ions. Regarding nitrite ions (Figure 6b), the greatest reduction of 89% was observed for the same yeast strain, but in the presence of synthetic wastewater contaminated with Cd2+ ions. The strain of R. mucilaginosa CMGB188 (P2) exhibited reduction efficiencies of up to 85% for synthetic wastewater and water containing Bio Y, as well as for water contaminated with Pb2+ and Cd2+. Similar behaviors were observed in the reduction of phosphate ions (Figure 6d) (between 43 and 66%, generally higher values for R. mucilaginosa CMGB188 (P2) and sulfate ions (between 59 and 86% but for R. mucilaginosa CMGB-G1 (P1).
A particular case was observed in the reduction of ammonium ions (Figure 6c), where the majority of tested samples exhibited an increase in their ion concentrations throughout the experiment. Reductions of up to 51% were observed in synthetic wastewater with Cd2+ (R. mucilaginosa CMGB-G1 (P1) and synthetic wastewater Bio Y (R. mucilaginosa CMGB188 (P2). Smaller reductions were noted for R. mucilaginosa CMGB-G1 (P1) in the presence of Pb2+ (29%) and in the presence of Pb2+ and Bio Y (5%). R. mucilaginosa CMGB188 (P2) exhibited similar reductions, but in water contaminated with Cd2+ and Pb2+. All the other samples showed increases in ammonium ion concentrations ranging from +35% (R. mucilaginosa CMGB-G1 (P1) in Sw) up to + 145 and + 168% for the rest of the samples. A possible explanation for the accumulation of ammonium ions could be that during the development of yeast strains, nitrogen is metabolized and transformed into ammonium ions, which correlates with an increase in conductivity (see Table 2) [60].
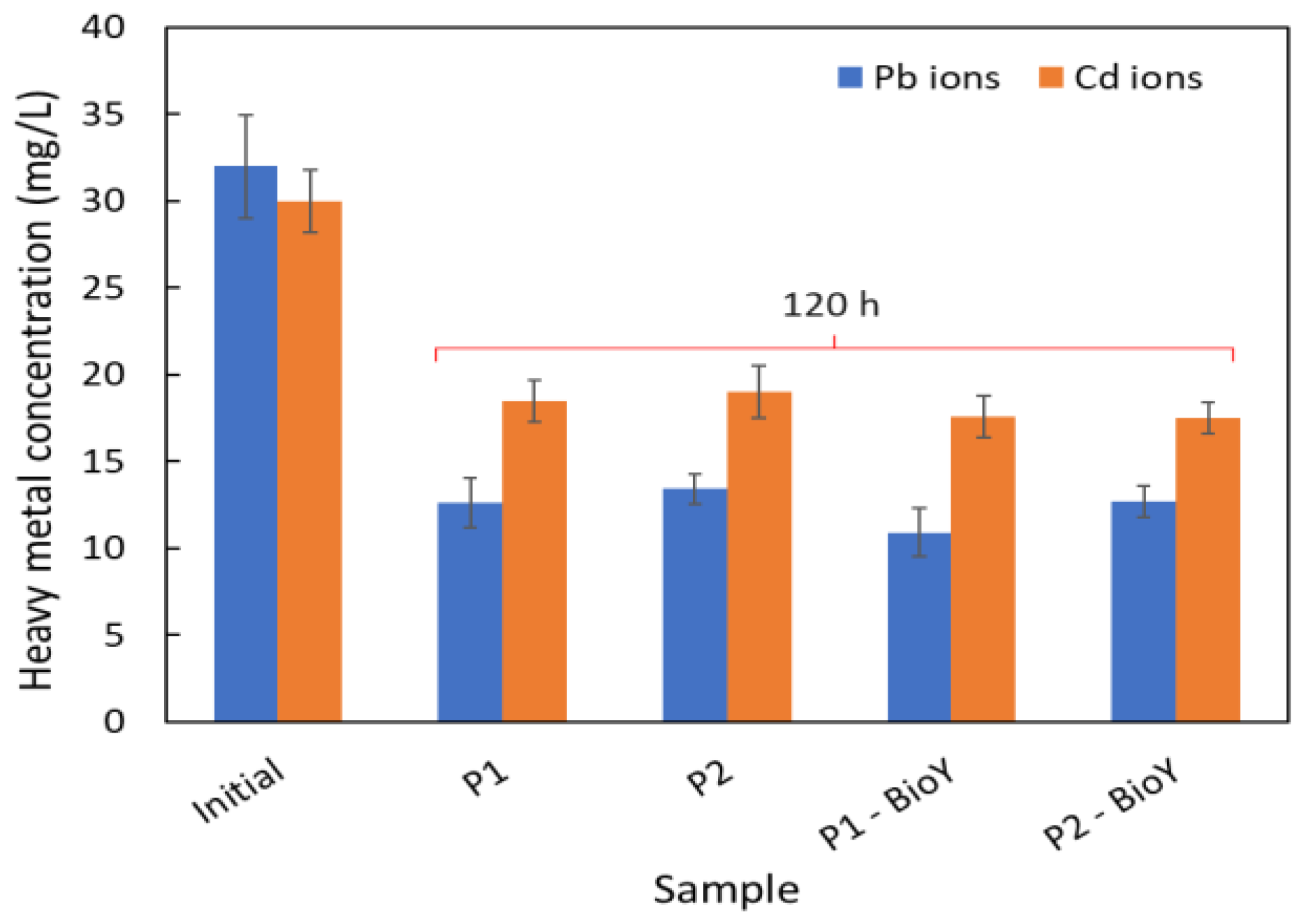
3.5.3. Removal of Pb2+ and Cd2+
A comparison of the efficiency of Pb and Cd ions for all tested samples, after 120 h (Figure 7), suggested that the sample type influenced the removal efficiency. Thus, both R. mucilaginosa strains presented higher Pb2+ removal efficiencies compared to the samples without biosurfactants: R. mucilaginosa CMGB-G1 (P1)—66% in Bio Y vs. 61%, respectively; R. mucilaginosa CMGB188 (P2) 59% vs. 58%, respectively. In the case of synthetic wastewater with Cd2+, the removal efficiency was lower compared to Pb2+, ranging between 41 and 42% for all the samples.
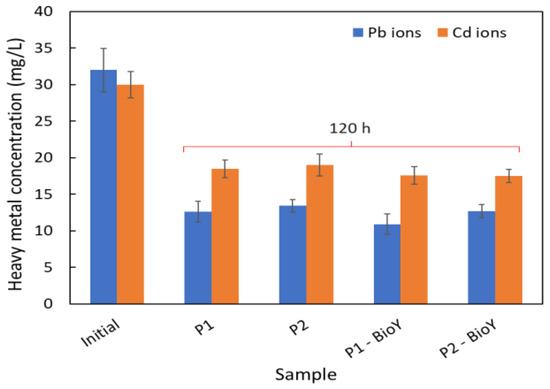
Figure 7.
Cd and Pb ions’ removal efficiency at the end of the experiment.
In our previous study [31], we determined the removal efficiency of heavy metals form wastewater in the presence of a crude biosurfactant from Y. lipolytica CMGB32, but using different yeast strains. The results showed a maximum reduction of 99% in Pb2+ with the Saccharomyces cerevisiae CMGB234 strain, and a reduction of 56% in Cd2+ ions with Kluyveromyces marxianus CMGBP16, being a proof that the removal efficiency is also dependent on the yeast strain used.
3.6. Antimicrobial Activity against Candida Strains
Biosurfactants have multiple roles in the biotechnological field, being able to be used both for the bioremediation of polluted environments and in the food, cosmetic, textile, chemical, and biomedical industries. Different types of biosurfactants were already tested for their ability to inhibit the growth of pathogenic and potential pathogenic yeast and bacterial strains. They have demonstrated fungicidal, bactericidal, and antiviral properties, along with the potential to serve as anti-adhesive inhibitors [24]. In this study, we tested the antimicrobial activity of a biosurfactant secreted by Y. lipolytica against two C. krusei strains: the C. krusei CMGB94 lab strain and C. krusei CMGB-Y8 isolated from vulvo-vaginal infections [76]. C. krusei is one of the main Candida species present in wastewaters and sewage sludges [77,78].
3.6.1. Growth Inhibition Properties of Y. lipolytica CMGB32 Biosurfactant
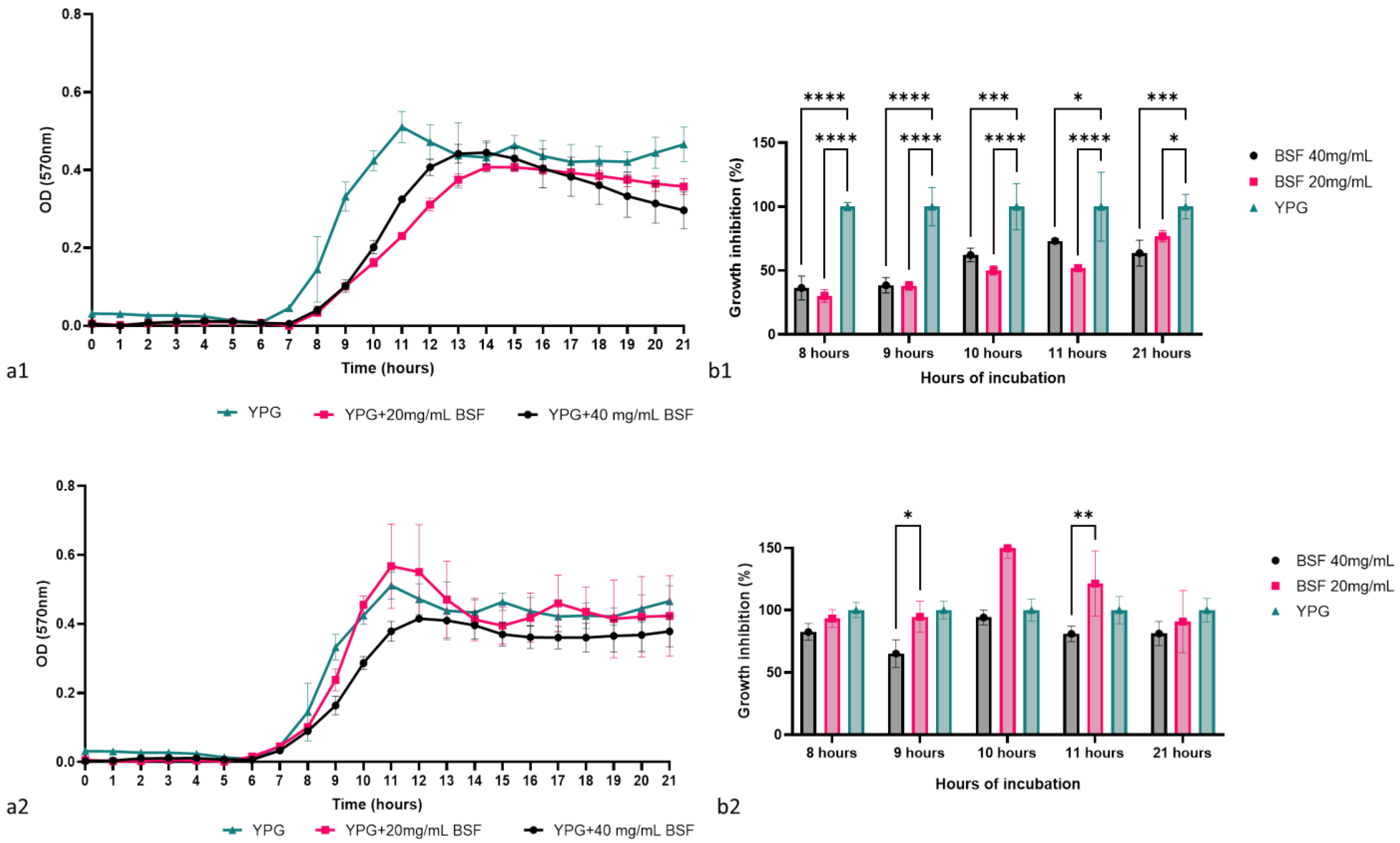
The antimicrobial activity of the biosurfactant was determined by monitoring the growth dynamics of the two C. krusei strains in the presence of the biosurfactant in concentrations of 40 mg/mL and 20 mg/mL, followed by determining the percentage of microbial growth at different key moments of the growth curve. According to Figure 8(a1,a2), the concentrated biosurfactant successfully inhibited the growth of the laboratory strain C. krusei CMGB94 and had a limited effect on the pathogenic strain C. krusei CMGB-Y8. During the logarithmic growth phase, the presence of the biosurfactant in the culture medium reduced the microbial growth of C. krusei CMGB94 by more than 50%. Moreover, after 8 h of incubation, the percentage of microbial growth recorded for the two biosurfactant concentrations was 30.14% and 36.41%, respectively, which indicates that the biosurfactant delays the entry of yeast into the logarithmic phase of growth (Figure 8(b1)). During the 21 h of incubation, the inhibitory effect of the biosurfactant was constant, but the upward trajectory of the growth curve indicated that it most likely intervenes in the basal metabolism of the cells without having an obvious fungicidal/fungistatic effect. In the case of the pathogenic strain, the antimicrobial effect was not obvious; the biosurfactant had no significant effect on the growth of the pathogenic strain regardless of the key moment analyzed. This result is not surprising, considering that the pathogenic C. krusei strains are described as having additional mechanisms for dealing with stress conditions, which most likely allows them to quickly adapt to the stress represented by the presence of biosurfactants in low concentrations in the culture medium [79]. In general terms, it is considered that a biosurfactant can destabilize microbial membranes or interfere with their cell cycles. Moreover, it seems that its effect is directly linked to the chemical composition of the cell wall and membrane, since many studies reported that Gram-positive bacteria and yeasts are more sensitive to the biosurfactants than Gram-negative bacteria or filamentous fungi [80,81,82]. According to a study conducted by Rufino et al. [25], the biosurfactant, rufisan, produced by C. lipolytica UCP0988 (currently known as Y. lipolytica), was effective against a wide range of microbial pathogens, including Streptococcus mutans, S. oralis, and S. sanguis, and it had a rather limited effect on pathogenic C. albicans strains.
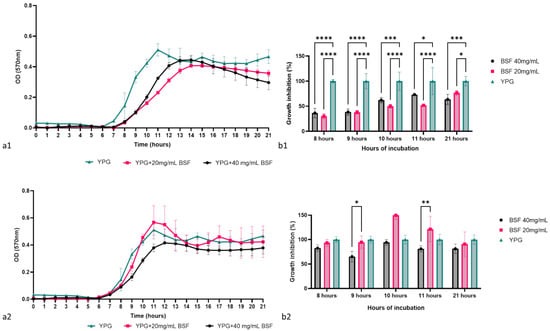
Figure 8.
The antimicrobial effect of the biosurfactant produced by Y. lipolytica CMGB32: (a1)—the impact on C. krusei CMGB94 growth behavior; (a2)—the impact on C. krusei CMGB-Y8 growth behavior; (b1)—the growth inhibition percentage determined for C. krusei CMGB94 at specific points of time; (b2)—the growth inhibition percentage determined for C. krusei CMGB-Y8 at specific points of time (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001) (Tukey’s method).
3.6.2. Anti-Adherence Properties of Y. lipolytica CMGB32 Biosurfactant
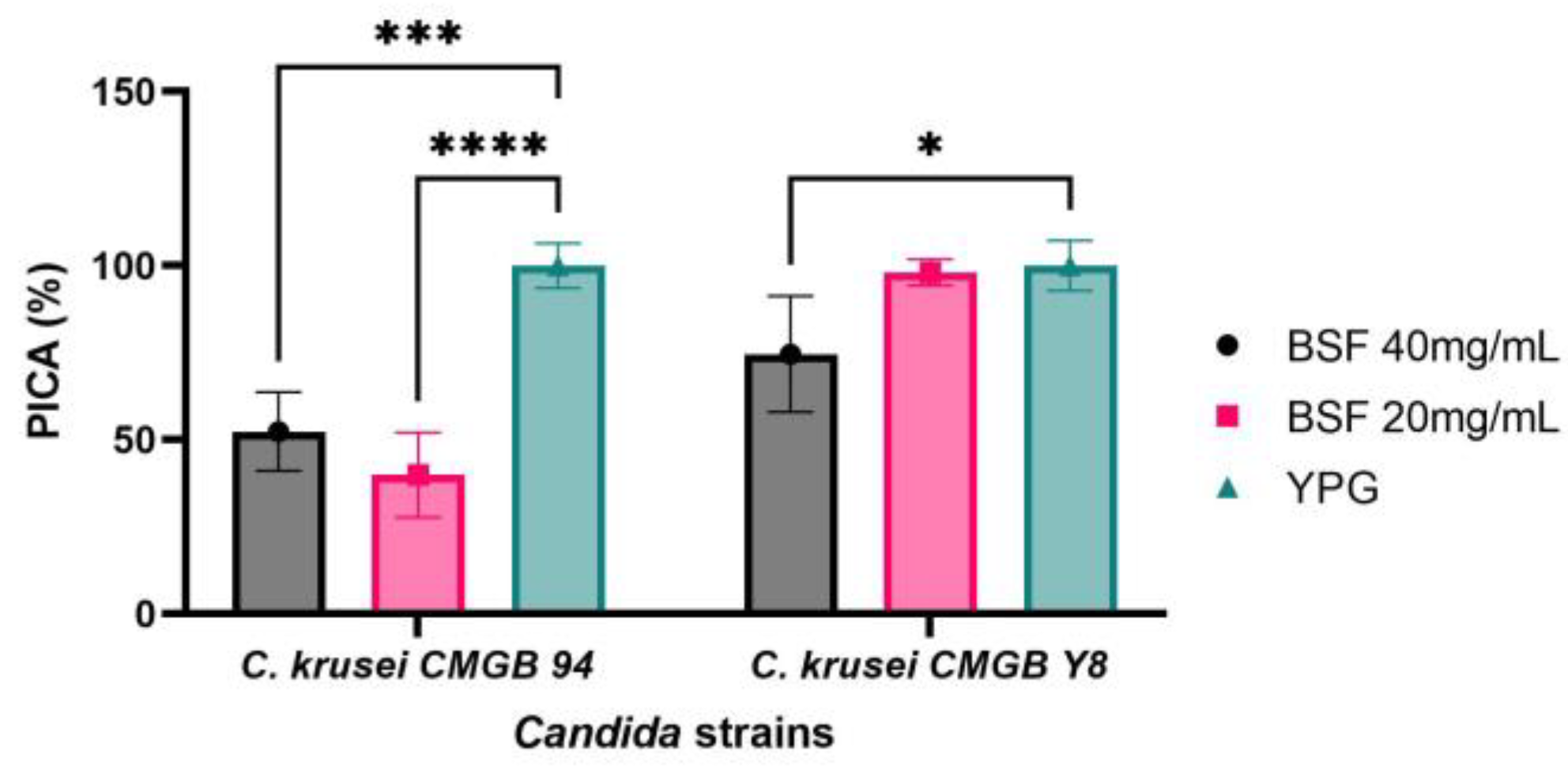
Microbial capacity of forming biofilms represents a difficult challenge for both the food industry and biomedical field, since the biofilms are characterized by low permeability to antimicrobial agents due to the matrix barriers containing viscous and compact exopolisaccharide structures associated with highly negatively charged surfaces. Hence, developing novel therapeutic strategies to prevent biofilm formation or to enhance biofilm penetrability is crucial. Biofilm colony formation is a complex process involving, as a first step, microbial adherence to the inert substratum [83,84]. Since C. krusei is a complex pathogen with intrinsic resistance to azole antifungals and is currently described as the pathogenic microorganism responsible for life-threatening infections in immunocompromised patients, especially for those using prolonged azole prophylaxis [85,86], we evaluated the ability of biosurfactants to inhibit C. krusei strains adherence to polypropylene substratum. According to Figure 9, the biosurfactant produced by Y. lipolytica CMGB32 is highly efficient in preventing both C. krusei strains’ adherence to inert substratum. The efficiency of anti-adherence properties of the biosurfactant is not directly linked to its concentration, but rather depends on the characteristics of the microbial strains used in this study. The lab strain C. krusei CMGB94′s ability to adhere to inert substratum is strongly inhibited in the presence of the biosurfactant, with the PICA% values obtained being 52.37% for the 40 mg/mL concentration and 39.91% for the 20 mg/mL concentration, respectively. This result can probably be explained by the fact that, at higher concentrations, the biosurfactant has stronger antimicrobial activity, thus limiting the total number of cells that are able to adhere. In the case of the pathogenic strain C. krusei CMGB-Y8, higher concentrations of biosurfactants are required to inhibit adherence (PICA% 74.61%).
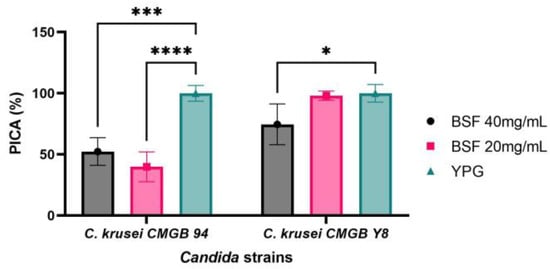
Figure 9.
Graphical representation of the inhibitory effect on the adhesion capacity of C. krusei CMGB94 and C. krusei CMGB-Y8 strains treated with different concentrations of the biosurfactant produced by Y. lipolytica CMGB32 (* p < 0.05, *** p < 0.001, **** p < 0.0001) (Dunnett’s test).
Although many studies regarding biosurfactants have been conducted, their role as anti-adherence agents and the mechanisms involved in the process are not well described in the literature. The proposed mechanism of action relies on their amphiphilic characteristics, and the adsorption of biosurfactants onto specific surface modifies their hydrophobicity, thus altering the adhesion process [87,88]. Also, although unproved yet, biosurfactants might modify the expression profile of specific Candida genes involved in genetic dimorphism or ones that are directly linked to microbial adherence. This hypothesis is supported by a study conducted by [89], in which it was demonstrated that the biosurfactant produced by Lactobacillus reuteri alters the expression profile of gtfB, gtfC, and ftf S. mutans genes, which are involved in the initial adherence of cells to dental surfaces. Nevertheless, the results published so far suggest that these molecules are a viable strategy for combating microbial infections. Sophorolipids, which are one of the types of biosurfactants produced by Starmerella bombicola strains, were investigated for their antimicrobial properties and biofilm disruption against Cupriavidus necator, B. subtilis, and S. aureus reference strains [87]. Similarly, a mixture of biosurfactants produced by Y. lipolytica showed high efficiency in preventing S. enterica biofilm development [90]. So far, there are no significant studies concerning the applicability of biosurfactants produced by Y. lipolytica or other yeasts as agents aimed to prevent the formation of polyspecific biofilms developed by multi-resistant microbial strains, including both pathogenic bacteria and antifungal resistant Candida strains. Thus, the results obtained in this study represent a promising basis for obtaining active biocompounds for preventing polyspecific biofilm formation.
4. Conclusions
The extensive pollution caused by petroleum, heavy metals, and emerging pathogens represents a growing threat to the ecological balance and health of different habitats. During the recent four decades, the development of bioremediation technologies gained attention as a promising ecological solution for the removal of a wide range of contaminants from polluted environments. The yeast Y. lipolytica is able to produce eco-friendly biocompounds, such as biosurfactants, by degrading petroleum and petroleum compounds (including alkanes), and it is considered as one of the most versatile non-conventional yeast species with a high biotechological potential. Our study showed that the strain Y. lipolytica CMGB32 has a high ability to assimilate n-hexadecane by producing stable biosurfactants. The addition of a crude biosurfactant in synthetic wastewaters contaminated with Pb2+ and Cd2+ promoted the growth of natural heavy metal degraders, such as R. mucilaginosa, and enhanced their ability to remove heavy metals ions. Also, it positively influenced the physico-chemical parameters of synthetic wastewater and determined the reduction of lead and cadmium ions. Moreover, the concentrated biosurfactants inhibited cell growth and biofilm formation with human pathogenic C. krusei strains. In conclusion, Y. lipolytica CMGB32 can be considered a promising strain for the further development of biological agents aimed to bioremediate heavy-metal- and emerging pathogen-polluted environments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app14073048/s1, Figure S1: Emulsification capacity of Y. lipolytica CMGB32 crude biosurfactant against toluene.
Author Contributions
Conceptualization, O.E.C. and V.M.C.; methodology, O.E.C., V.M.C., N.-O.N., E.-M.L. and V.M.; software, V.M.C. and N.-O.N.; validation, O.E.C., V.M.C. and V.M.; formal analysis, O.E.C., V.M.C. and N.-O.N.; investigation, O.E.C., V.M.C., N.-O.N., E.-M.L. and V.M.; resources, O.E.C. and E.-M.L.; data curation, O.E.C.; writing—original draft preparation, O.E.C., V.M.C. and N.-O.N.; writing—review and editing, O.E.C. and V.M.C.; visualization, O.E.C. and V.M.C.; supervision, O.E.C.; project administration, O.E.C., V.M.C. and N.-O.N.; funding acquisition, O.E.C., V.M.C., N.-O.N. and E.-M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the ICUB Senior Grant, contract no. 7423/04.07.2023 financed by the Research Institute of the University of Bucharest and the ICUB Young Research Grant, contract no. 7412/04.07.2023 financed by the Research Institute of the University of Bucharest. N.O.N. and E.M.L. also acknowledge the support provided by the Ministry of Research, Innovation and Digitization through contract no. PN 23140201-42N/2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available upon request to the corresponding author. A sample of the biosurfactant is available at the Department of Genetics, Faculty of Biology, University of Bucharest.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Hreniuc, M.; Coman, M.; Cioruţa, B. Considerations Regarding the Soil Pollution with Oil Products in Săcel-Maramureș. In Proceedings of the International Conference of Scientific Paper AFASES, Brasov, Romania, 28–30 May 2015; pp. 28–30. [Google Scholar]
- Rusin, M.; Gospodarek, J.; Nadgórska-Socha, A. The Effect of Petroleum-Derived Substances on the Growth and Chemical Composition of Vicia faba L. Pol. J. Environ. Stud. 2015, 24, 2157–2166. [Google Scholar] [CrossRef] [PubMed]
- Rusin, M.; Gospodarek, J.; Nadgórska-Socha, A. Soil Pollution by Petroleum-Derived Substances and Its Bioremediation: The Effect on Aphis fabae Scop. Infestation and Antioxidant Response in Vicia faba L. Agronomy 2020, 10, 147. [Google Scholar] [CrossRef]
- Jung, J.; Park, W. Acinetobacter species as model microorganisms in environmental microbiology: Current state and perspectives. Appl. Microbiol. Biotechnol. 2015, 99, 2533–2548. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.; Taatizadeh, S.B.; Samaei, M.R. Biodegradation of n-hexadecane in Acinetobacter radioresistens liquid culture. Health Scope 2013, 2, 162–167. [Google Scholar] [CrossRef]
- Morovati, R.; Abbasi, F.; Samaei, M.R.; Mehrazmay, H.; Lari, A.R. Modelling of n-Hexadecane Bioremediation from Soil by Slurry Bioreactors Using Artificial Neural Network Method. Sci. Rep. 2022, 12, 19662. [Google Scholar] [CrossRef]
- Eula, B.; Barbara, C.; Charles, P. Patty’s Toxicology, 5th ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Ali Khan, A.H.; Tanveer, S.; Alia, S.; Anees, M.; Sultan, A.; Iqbal, M.; Yousaf, S. Role of Nutrients in Bacterial Biosurfactant Production and Effect of Biosurfactant Production on Petroleum Hydrocarbon Biodegradation. Ecol. Eng. 2017, 104, 158–164. [Google Scholar] [CrossRef]
- Pandolfo, E.; Barra Caracciolo, A.; Rolando, L. Recent Advances in Bacterial Degradation of Hydrocarbons. Water 2023, 15, 375. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Tian, S.; Wang, W.; Qi, Q.; Jiang, P.; Gao, X.; Li, F.; Li, H.; Yu, H. Petroleum Hydrocarbon-Degrading Bacteria for the Remediation of Oil Pollution Under Aerobic Conditions: A Perspective Analysis. Front. Microbiol. 2018, 9, 2885. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.A.; Tanveer, S.; Kiyani, A.; Barros, R.; Iqbal, M.; Yousaf, S. Biosurfactant-Producing Aspergillus, Penicillium, and Candida Performed Higher Biodegradation of Diesel Oil than a Non-Producing Fungal Strain. Appl. Biochem. Microbiol. 2023, 59, 282–289. [Google Scholar] [CrossRef]
- Asemoloye, M.D.; Tosi, S.; Daccò, C.; Wang, X.; Xu, S.; Marchisio, M.A.; Gao, W.; Jonathan, S.G.; Pecoraro, L. Hydrocarbon Degradation and Enzyme Activities of Aspergillus oryzae and Mucor irregularis Isolated from Nigerian Crude Oil-Polluted Sites. Microorganisms 2020, 8, 1912. [Google Scholar] [CrossRef]
- Daccò, C.; Girometta, C.; Asemoloye, M.D.; Carpani, G.; Picco, A.M.; Tosi, S. Key Fungal Degradation Patterns, Enzymes and Their Applications for the Removal of Aliphatic Hydrocarbons in Polluted Soils: A Review. Int. Biodeterior. Biodegrad. 2020, 147, 104866. [Google Scholar] [CrossRef]
- Pan, F.; Yang, Q.; Zhang, Y.; Zhang, S.; Yang, M. Biodegradation of Polycyclic Aromatic Hydrocarbons by Pichia anomala. Biotechnol. Lett. 2004, 26, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, R.; Schauer, F. Biotransformation and Detoxification of Environmental Pollutants with Aromatic Structures by Yeasts. In Yeast Diversity in Human Welfare; Satyanarayana, T., Kunze, G., Eds.; Springer: Singapore, 2017; pp. 323–369. ISBN 978-981-10-2621-8. [Google Scholar]
- Krallish, I.; Gonta, S.; Savenkova, L.; Bergauer, P.; Margesin, R. Phenol Degradation by Immobilized Cold-Adapted Yeast Strains of Cryptococcus terreus and Rhodotorula creatinivora. Extremophiles 2006, 10, 441–449. [Google Scholar] [CrossRef]
- Chen, W.; Lee, M.K.; Jefcoate, C.; Kim, S.C.; Chen, F.; Yu, J.H. Fungal Cytochrome P450 Monooxygenases: Their Distribution, Structure, Functions, Family Expansion, and Evolutionary Origin. Genome Biol. Evol. 2014, 6, 1620–1634. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T. (Eds.) The Yeasts: A Taxonomic Study, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2010; ISBN 978-0-444-52149-1. [Google Scholar]
- Fickers, P.; Benetti, P.H.; Waché, Y.; Marty, A.; Mauersberger, S.; Smit, M.S.; Nicaud, J.M. Hydrophobic Substrate Utilisation by the Yeast Yarrowia lipolytica, and Its Potential Applications. FEMS Yeast Res. 2005, 5, 527–543. [Google Scholar] [CrossRef]
- Harzevili, F.D. Biotechnological Applications of the Yeast Yarrowia lipolytica; Springer: New York, NY, USA, 2014; Volume 74. [Google Scholar] [CrossRef]
- Loira, N.; Dulermo, T.; Nicaud, J.M.; Sherman, D.J. A Genome-Scale Metabolic Model of the Lipid-Accumulating Yeast Yarrowia lipolytica. BMC Syst. Biol. 2012, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Devillers, H.; Neuvéglise, C. Genome Sequence of the Oleaginous Yeast Yarrowia lipolytica H222. Microbiol. Resour. Announc. 2019, 8, 10–1128. [Google Scholar] [CrossRef]
- Coelho, M.A.Z.; Amaral, P.F.F.; Belo, I. Yarrowia lipolytica: An Industrial Workhorse; Formatex Research Center: Badajoz, Spain, 2010. [Google Scholar]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional Biomolecules of the 21st Century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef] [PubMed]
- Rufino, R.D.; Luna, J.M.; Campos-Takaki, G.M.; Ferreira, S.R.M.; Sarubbo, L.A. Application of the Biosurfactant Produced by Candida lipolytica in the Remediation of Heavy Metals. Chem. Eng. Trans. 2012, 27, 61–66. [Google Scholar] [CrossRef]
- Fernandes, N.; Simoes, L.A.; Dias, D.R. Biosurfactants Produced by Yeasts: Fermentation, Screening, Recovery, Purification, Characterization, and Applications. Fermentation 2023, 9, 207. [Google Scholar] [CrossRef]
- Hanson, K.G.; Desai, J.D.; Desai, A.J. A Rapid and Simple Screening Technique for Potential Crude Oil Degrading Microorganisms. Biotechnol. Tech. 1993, 7, 745–748. [Google Scholar] [CrossRef]
- Lopes, P.; Bidoia, E.; Montagnolli, R. Microbial Biodegradation Potential of Hydrocarbons Evaluated by Colorimetric Technique: A Case Study. Appl. Microbiol. Biotechnol. 2010, 7, 1277–1288. [Google Scholar]
- Chrzanowski, Ł.; Bielicka-Daszkiewicz, K.; Owsianiak, M.; Aurich, A.; Kaczorek, E.; Olszanowski, A. Phenol and n-Alkanes (C 12 and C 16) Utilization: Influence on Yeast Cell Surface Hydrophobicity. World J. Microbiol. Biotechnol. 2008, 24, 1943–1949. [Google Scholar] [CrossRef]
- Cooper, D.G.; Goldenberg, B.G. Surface-Active Agents from Two Bacillus Species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Nicula, N.-O.; Lungulescu, E.-M.; Rîmbu, G.A.; Marinescu, V.; Corbu, V.M.; Csutak, O. Bioremediation of Wastewater Using Yeast Strains: An Assessment of Contaminant Removal Efficiency. Int. J. Environ. Res. Public Health 2023, 20, 4795. [Google Scholar] [CrossRef]
- Csutak, O.; Corbu, V.; Stoica, I.; Ionescu, R.; Vassu, T. Biotechnological Applications of Yarrowia Lipolytica CMGB32. Agric. Agric. Sci. Procedia 2015, 6, 545–553. [Google Scholar] [CrossRef]
- Csutak, O.; Sarbu, I.; Rusu, E.; Vassu, T.; Corbu, V. Antimicrobial and antiadhesion activity of biosurfactants from Rhodotorula glutinis grown on n-dodecane. Rev. Chim. 2020, 71, 99–105. [Google Scholar] [CrossRef]
- Corpuz, M.V.A.; Borea, L.; Senatore, V.; Castrogiovanni, F.; Buonerba, A.; Oliva, G.; Ballesteros, F.; Zarra, T.; Belgiorno, V.; Choo, K.-H.; et al. Wastewater Treatment and Fouling Control in an Electro Algae-Activated Sludge Membrane Bioreactor. Sci. Total Environ. 2021, 786, 147475. [Google Scholar] [CrossRef] [PubMed]
- ISO 6060:1989; Water Quality—Determination of the Chemical Oxygen Demand. International Organization for Standardization: Geneva, Switzerland, 1989.
- Hanna Hanna Instruments HI83300 Instruction Manual. Available online: https://www.haines.com.au/mwdownloads/download/link/id/222/ (accessed on 19 March 2024).
- Corbu, V.M.; Gheorghe, I.; Marinaș, I.C.; Geanǎ, E.I.; Moza, M.I.; Csutak, O.; Chifiriuc, M.C. Demonstration of Allium sativum Extract Inhibitory Effect on Biodeteriogenic Microbial Strain Growth, Biofilm Development, and Enzymatic and Organic Acid Production. Molecules 2021, 26, 7195. [Google Scholar] [CrossRef]
- Luna, J.M.; Rufino, R.D.; Sarubbo, L.A.; Rodrigues, L.R.M.; Teixeira, J.A.C.; De Campos-Takaki, G.M. Evaluation Antimicrobial and Antiadhesive Properties of the Biosurfactant Lunasan Produced by Candida sphaerica UCP 0995. Curr. Microbiol. 2011, 62, 1527–1534. [Google Scholar] [CrossRef]
- Csutak, O.; Simon-Gruiţă, A.; Corbu, V.; Constantin, N.; Pojoga, D.; Vassu, T.; Duţă-Cornescu, G. Preliminary studies on yeast-plant systems with applications in phytoremediation. Sci. Bull. Ser. F Biotechnol. 2017, 21, 183–189. [Google Scholar]
- Junior, J.S.; Mariano, A.P.; Angelis, D. Biodegradation of biodiesel/diesel blends by Candida viswanathii. Afr. J. Biotechnol. 2009, 8, 2774–2778. [Google Scholar]
- Miranda, R.D.C.; Souza, C.S.D.; Gomes, E.D.B.; Lovaglio, R.B.; Lopes, C.E.; Sousa, M.D.F.V.D.Q. Biodegradation of Diesel Oil by Yeasts Isolated from the Vicinity of Suape Port in the State of Pernambuco-Brazil. Braz. Arch. Biol. Technol. 2007, 50, 147–152. [Google Scholar] [CrossRef]
- Bogan, A.A.; Thorn, K.S. Anatomy of Hot Spots in Protein Interfaces. J. Mol. Biol. 1998, 280, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moreira, I.S.; Martins, J.M.; Ramos, R.M.; Fernandes, P.A.; Ramos, M.J. Understanding the Importance of the Aromatic Amino-Acid Residues as Hot-Spots. Biochim. Et Biophys. Acta BBA Proteins Proteom. 2013, 1834, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Khopade, A.; Biao, R.; Liu, X.; Mahadik, K.; Zhang, L.; Kokare, C. Production and Stability Studies of the Biosurfactant Isolated from Marine Nocardiopsis sp. B4. Desalination 2012, 285, 198–204. [Google Scholar] [CrossRef]
- Ilori, M.O.; Amobi, C.J.; Odocha, A.C. Factors Affecting Biosurfactant Production by Oil Degrading Aeromonas Spp. Isolated from a Tropical Environment. Chemosphere 2005, 61, 985–992. [Google Scholar] [CrossRef]
- Ferreira, T.F.; Martins, F.F.; Cayres, C.A.; Amaral, P.F.; Azevedo, D.D.A.; Coelho, M.A.Z. Biosurfactant Production from the Biodegradation of n-Paraffins, Isoprenoids and Aromatic Hydrocarbons from Crude Petroleum by Yarrowia lipolytica IMUFRJ 50682. Fermentation 2022, 9, 21. [Google Scholar] [CrossRef]
- Elnahas, M.O.; Hou, L.; Wall, J.D.; Majumder, E.L.W. Bioremediation Potential of Streptomyces sp. MOE6 for Toxic Metals and Oil. Polysaccharides 2021, 2, 47–68. [Google Scholar] [CrossRef]
- Raddadi, N.; Giacomucci, L.; Totaro, G.; Fava, F. Marinobacter sp. from Marine Sediments Produce Highly Stable Surface-Active Agents for Combatting Marine Oil Spills. Microb. Cell Factories 2017, 16, 186. [Google Scholar] [CrossRef]
- Camara-Lemarroy, C.R.; Rodríguez-Gutiérrez, R.; Monreal-Robles, R.; González-González, J.G. Acute Toluene Intoxication–Clinical Presentation, Management and Prognosis: A Prospective Observational Study. BMC Emerg. Med. 2015, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Konya, A.; Fiddler, B.A.; Bunch, O.; Hess, K.Z.; Ferguson, C.; Krzmarzick, M.J. Lead or Cadmium Co-Contamination Alters Benzene and Toluene Degrading Bacterial Communities. Biodegradation 2023, 34, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Caicedo-Bejarano, L.D.; Osorio-Vanegas, L.S.; Ramírez-Castrillón, M.; Castillo, J.E.; Martínez-Garay, C.A.; Chávez-Vivas, M. Water Quality, Heavy Metals, and Antifungal Susceptibility to Fluconazole of Yeasts from Water Systems. Int. J. Environ. Res. Public Health 2023, 20, 3428. [Google Scholar] [CrossRef] [PubMed]
- Jarboui, R.; Baati, H.; Fetoui, F.; Gargouri, A.; Gharsallah, N.; Ammar, E. Yeast Performance in Wastewater Treatment: Case Study of Rhodotorula mucilaginosa. Environ. Technol. 2012, 33, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Ertuđrul, S.; San, N.O.; Dönmez, G. Treatment of Dye (Remazol Blue) and Heavy Metals Using Yeast Cells with the Purpose of Managing Polluted Textile Wastewaters. Ecol. Eng. 2009, 35, 128–134. [Google Scholar] [CrossRef]
- Grujić, S.; Radojevic, I.; Vasić, S.; Čomić, L.; Ostojić, A. Heavy Metal Tolerance and Removal Efficiency of the Rhodotorula mucilaginosa and Saccharomyces boulardii Planktonic Cells and Biofilm. Kragujev. J. Sci. 2018, 40, 217–226. [Google Scholar] [CrossRef]
- Vidal, N.F.; Dávila, J.W. Lead and Cadmium Removal with Native Yeast from Coastal Wetlands. Open Chem. 2022, 20, 1096–1109. [Google Scholar] [CrossRef]
- Alisawi, H.A.O. Performance of Wastewater Treatment during Variable Temperature. Appl. Water Sci. 2020, 10, 89. [Google Scholar] [CrossRef]
- Olabode, G.S.; Olorundare, O.F.; Somerset, V.S. Physicochemical Properties of Wastewater Effluent from Two Selected Wastewater Treatment Plants (Cape Town) for Water Quality Improvement. Int. J. Environ. Sci. Technol. 2020, 17, 4745–4758. [Google Scholar] [CrossRef]
- Sahu, O.; Mazumdar, B.; Chaudhari, P.K. Treatment of Wastewater by Electrocoagulation: A Review. Environ. Sci. Pollut. Res. 2014, 21, 2397–2413. [Google Scholar] [CrossRef]
- Li, D.; Zou, M.; Jiang, L. Dissolved Oxygen Control Strategies for Water Treatment: A Review. Water Sci. Technol. 2022, 86, 1444–1466. [Google Scholar] [CrossRef] [PubMed]
- Paquet, J.; Lacroix, C.; Audet, P.; Thibault, J. Electrical Conductivity as a Tool for Analysing Fermentation Processes for Production of Cheese Starters. Int. Dairy J. 2000, 10, 391–399. [Google Scholar] [CrossRef]
- Zikánová, B.; Kuthan, M.; Řičicová, M.; Forstová, J.; Palková, Z. Amino Acids Control Ammonia Pulses in Yeast Colonies. Biochem. Biophys. Res. Commun. 2002, 294, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Corbu, V.; Petrut, S.; Vassu, T.; Pelinescu, D.; Sarbu, I.; Rusu, E.; Csutak, O. Environmental Stress Responses in Yeasts and Lactic Acid Bacteria Strains Isolated from Dairy Traditional Romanian Fermented Products. Rom. Biotechnol. Lett. 2021, 26, 2548–2559. [Google Scholar] [CrossRef]
- Hu, X.; Cao, J.; Yang, H.; Li, D.; Qiao, Y.; Zhao, J.; Zhang, Z.; Huang, L. Pb2+ biosorption from aqueous solutions by live and dead biosorbents of the hydrocarbon-degrading strain Rhodococcus sp. HX-2. PLoS ONE 2020, 15, e0226557. [Google Scholar] [CrossRef] [PubMed]
- Wifak, B.; Nezha Tahri, J.; Meryem, A.; Hanane, S.; Nabil, T.; Naïma, E.G. Yeast Biomass: An Alternative for Bioremediation of Heavy Metals. In Yeast; Antonio, M., Iris, L., Eds.; IntechOpen: Rijeka, Croatia, 2017; Chapter 12. [Google Scholar]
- Khorram Abadi, V.; Habibi, D.; Heydari, S.; Ariannezhad, M. The effective removal of Ni2+, Cd2+, and Pb2+ from aqueous solution by adenine-based nano-adsorbent. RSC Adv. 2023, 13, 5970–5982. [Google Scholar] [CrossRef] [PubMed]
- Sandifer, R.D.; Hopkin, S.P. Effects of pH on the toxicity of cadmium, copper, lead and zinc to Folsomia candida Willem, 1902 (Collembola) in a standard laboratory test system. Chemosphere 1996, 33, 2475–2486. [Google Scholar] [CrossRef]
- Naeem, A.; Woertz, J.R.; Fein, J.B. Experimental Measurement of Proton, Cd, Pb, Sr, and Zn Adsorption Onto the Fungal Species Saccharomyces cerevisiae. Environ. Sci. Technol. 2006, 40, 5724–5729. [Google Scholar] [CrossRef]
- El-Shimi, N.M.; Ihab, A.M.; Saad, M.M.; Abdel-Rhaman, G.N. Effects of Cu, Mn, Pb and Cd on the technological properties of baker’s yeast (in vitro study). J. Food Dairy Sci. 2009, 34, 8891–8901. [Google Scholar] [CrossRef]
- Wierzbicka, M.H.; Przedpełska, E.; Ruzik, R.; Ouerdane, L.; Połeć-Pawlak, K.; Jarosz, M.; Szpunar, J.; Szakiel, A. Comparison of the toxicity and distribution of cadmium and lead in plant cells. Protoplasma 2007, 231, 99–111. [Google Scholar] [CrossRef]
- Keffala, C.; Zouhir, F.; Abdallah, B.H.; Kammoun, S. Use of Bacteria and Yeast Strains for Dairy Wastewater Treatment. Int. J. Res. Eng. Technol. 2017, 6, 108–113. [Google Scholar]
- Gizaw, A.; Zewge, F.; Kumar, A.; Mekonnen, A.; Tesfaye, M. A Comprehensive Review on Nitrate and Phosphate Removal and Recovery from Aqueous Solutions by Adsorption. AQUA Water Infrastruct. Ecosyst. Soc. 2021, 70, 921–947. [Google Scholar] [CrossRef]
- Nicula, N.O.; Lungulescu, E.M.; Ieropoulos, I.A.; Rimbu, G.A.; Csutak, O. Nutrients Removal from Aquaculture Wastewater by Biofilter/Antibiotic-Resistant Bacteria Systems. Water 2022, 14, 607. [Google Scholar] [CrossRef]
- Nicula, N.O.; Lungulescu, E.M.; Rimbu, G.A.; Culcea, A.; Csutak, O. Nutrient and Organic Pollutants Removal in Synthetic Wastewater by Pseudomonas aeruginosa and Chryseobacterium sp./Biofilter Systems. Environ. Monit. Assess. 2022, 194, 881. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jin, Q.; Liang, Y.; Geng, J.; Xia, J.; Chen, H.; Yun, M. Highly Efficient Removal of Nitrate and Phosphate to Control Eutrophication by the Dielectrophoresis-Assisted Adsorption Method. Int. J. Environ. Res. Public Health 2022, 19, 1890. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhou, Y.; Yang, S.-T. Production of Polymalic Acid and Malic Acid by Aureobasidium Pullulans Fermentation and Acid Hydrolysis. Biotechnol. Bioeng. 2013, 110, 2105–2113. [Google Scholar] [CrossRef]
- Avram, I.; Pelinescu, D.; Stoica, I.; Marutescu, L.; Vassu, T. Phenotypic Profiles of Virulence in Different Candida Species Isolated from Vulvovaginal Infections. Roum. Arch. Microbiol. Immunol. 2013, 72, 225–233. [Google Scholar]
- Kacprzak, M.; Neczaj, E.; Okoniewska, E. The Comparative Mycological Analysis of Wastewater and Sewage Sludges from Selected Wastewater Treatment Plants. Desalination 2005, 185, 363–370. [Google Scholar] [CrossRef]
- Hamed, S.Z. Isolation and Identification of Yeasts along Wastewater Treatment Lines at Zagazig Plant. Ann. Agric. Sci. Moshtohor 2016, 54, 77–84. [Google Scholar] [CrossRef]
- Gómez-Gaviria, M.; Mora-Montes, H.M. Current Aspects in the Biology, Pathogeny, and Treatment of Candida krusei, a Neglected Fungal Pathogen. Infect. Drug Resist. 2020, 13, 1673–1689. [Google Scholar] [CrossRef]
- Rufino, R.D.; de Luna, J.M.; Sarubbo, L.A.; Rodrigues, L.R.M.; Teixeira, J.A.C.; de Campos-Takaki, G.M. Antimicrobial and Anti-Adhesive Potential of a Biosurfactants Produced by Candida Species. In Practical Applications in Biomedical Engineering; IntechOpen: Rijeka, Croatia, 2013; ISBN 978-953-51-0924-2. [Google Scholar]
- Carrillo, C.; Teruel, J.A.; Aranda, F.J.; Ortiz, A. Molecular Mechanism of Membrane Permeabilization by the Peptide Antibiotic Surfactin. Biochim. Biophys. Acta 2003, 1611, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, K. Molecular Biology of the Koji Molds. In Advances in Applied Microbiology; Laskin, A.I., Bennett, J.W., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2002; pp. 129–153. [Google Scholar]
- Zhao, A.; Sun, J.; Liu, Y. Understanding Bacterial Biofilms: From Definition to Treatment Strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-Associated Infections, Medical Devices and Biofilms: Risk, Tolerance and Control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Jamiu, A.T.; Albertyn, J.; Sebolai, O.M.; Pohl, C.H. Update on Candida krusei, a Potential Multidrug-Resistant Pathogen. Med. Mycol. 2021, 59, 14–30. [Google Scholar] [CrossRef]
- Sachivkina, N.; Podoprigora, I.; Bokov, D. Morphological Characteristics of Candida albicans, Candida krusei, Candida guilliermondii, and Candida glabrata Biofilms, and Response to Farnesol. Vet. World 2021, 14, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Díaz De Rienzo, M.A.; Banat, I.M.; Dolman, B.; Winterburn, J.; Martin, P.J. Sophorolipid Biosurfactants: Possible Uses as Antibacterial and Antibiofilm Agent. New Biotechnol. 2015, 32, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Jurjevic, M.; Badia, D. Utilization of Restaurant Waste Oil as a Precursor for Sophorolipid Production. Biotechnol. Progress. 2007, 23, 512–515. [Google Scholar] [CrossRef]
- Salehi, R.; Savabi, O.; Kazemi, M.; Kamali, S.; Salehi, A.R.; Eslami, G.; Tahmourespour, A. Effects of Lactobacillus reuteri-Derived Biosurfactant on the Gene Expression Profile of Essential Adhesion Genes (gtfB, gtfC and Ftf) of Streptococcus Mutans. Adv. Biomed. Res. 2014, 3, 169. [Google Scholar] [CrossRef]
- Shatila, F.; Uyar, E.; Yalçın, H.T. Screening of Biosurfactant Production by Yarrowia lipolytica Strains and Evaluation of Their Antibiofilm and Anti-Adhesive Activities against Salmonella enterica Ser. enteritidis Biofilms. Microbiology 2021, 90, 839–847. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).