The Order of Limiting Amino Acids in a Wheat–Sorghum-Based Reduced-Protein Diet for Laying Hens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Birds and Animal Husbandry
2.2. Experimental Design and Dietary Treatment
2.3. Data Collection
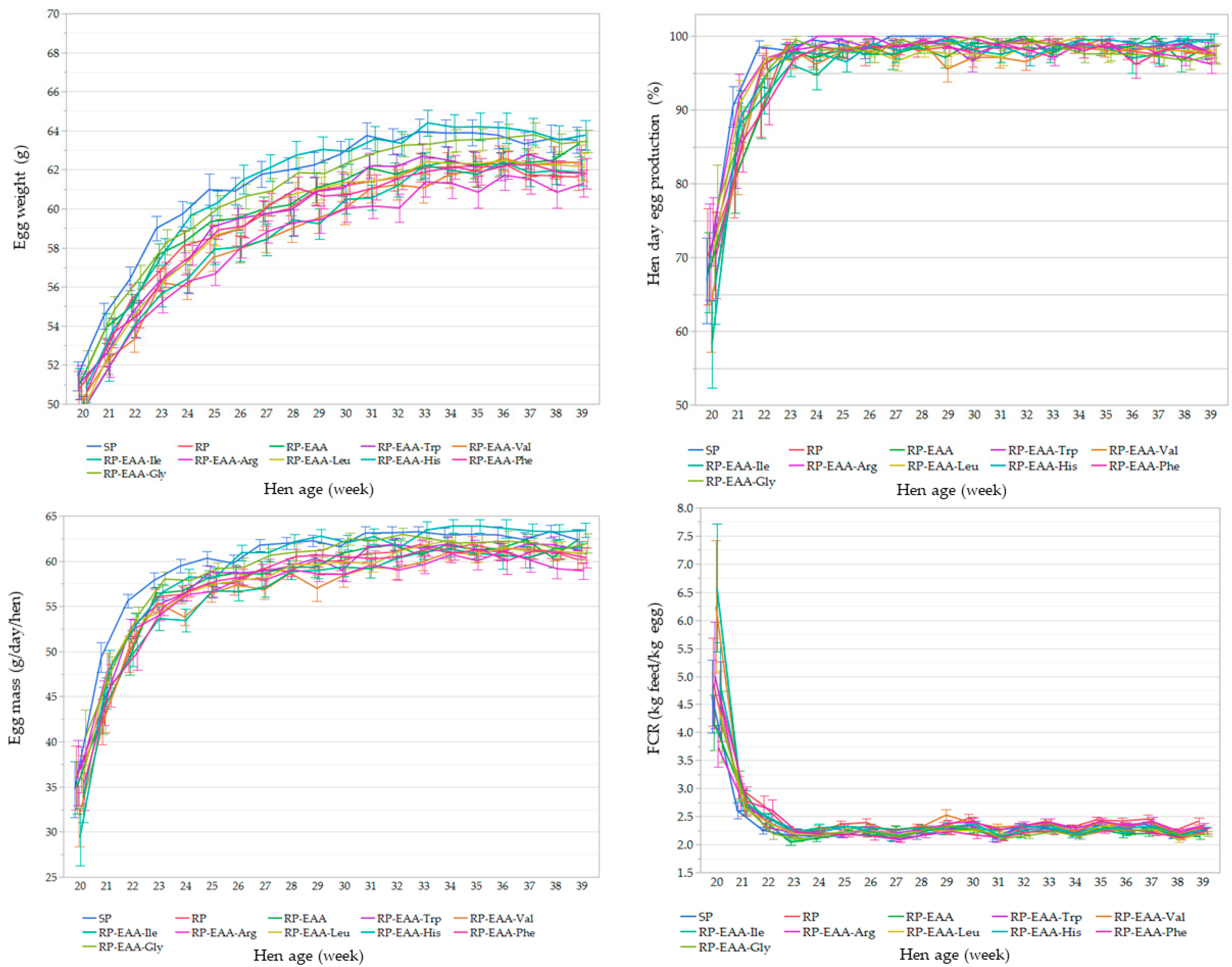
2.4. Laying Performance
2.5. Egg Quality
2.6. Gross Energy and Protein Digestibility
2.7. Serum Uric Acid
2.8. Bone Parameters
2.9. Caecal Microbiome Profile
2.10. Statistical Analysis
3. Results
3.1. Housing Environment, Hen Weight, and Mortality
3.2. Laying Performance and Egg Quality
3.3. Apparent Protein and Gross Energy Digestibility, and Serum Uric Acid Levels
3.4. Bone Parameters
3.5. Caecal Microbiome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, S.; Naz, S.; Sultan, A.; Alhidary, I.; Abdelrahman, M.; Khan, R.; Khan, N.; Khan, M.; Ahmad, S. Worm meal: A potential source of alternative protein in poultry feed. World’s Poult. Sci. J. 2016, 72, 93–102. [Google Scholar] [CrossRef]
- McGill, J.; Moss, A.; Swick, R.; Jackson, D.; Todd, M. The future protein decade: Perspectives on global pressure to agriculture. Anim. Prod. Sci. 2019, 59, 1951–1956. [Google Scholar] [CrossRef]
- Kidd, M.; Maynard, C.; Mullenix, G. Progress of amino acid nutrition for diet protein reduction in poultry. J. Anim. Sci. Biotechnol. 2021, 12, 45. [Google Scholar] [CrossRef]
- Corzo, A.; Loar, R., II; Kidd, M. Limitations of dietary isoleucine and valine in broiler chick diets. Poult. Sci. 2009, 88, 1934–1938. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Rojas, I.; Murakami, A.; Eyng, C.; Nunes, R.; Duarte, C.; Vargas, M. Commercially available amino acid supplementation of low-protein diets for broiler chickens with different ratios of digestible glycine+ serine: Lysine. Poult. Sci. 2012, 91, 3148–3155. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, S.; Chrystal, P.; Selle, P.; Liu, S. Reduced-crude protein diets in chicken-meat production: Justification for an imperative. World’s Poult. Sci. J. 2020, 76, 537–548. [Google Scholar] [CrossRef]
- Liu, S.Y.; Macelline, S.P.; Chrystal, P.V.; Selle, P.H. Progress towards reduced-crude protein diets for broiler chickens and sustainable chicken-meat production. J. Anim. Sci. Biotechnol. 2021, 12, 20. [Google Scholar] [CrossRef]
- Selle, P.H.; de Paula Dorigam, J.C.; Lemme, A.; Chrystal, P.V.; Liu, S.Y. Synthetic and crystalline amino acids: Alternatives to soybean meal in chicken-meat production. Animals 2020, 10, 729. [Google Scholar] [CrossRef]
- Swick, R.A.; Creswell, D.C. Economics of low protein broiler diets: A formulation exercise. In Proceedings of the 30th Australian Poultry Science Symposium, Sydney, Australia, 17–20 February 2019; University of Sydney: Sydney, Australia, 2019. [Google Scholar]
- Mohamed, E.A.A.; Loh, T.C.; Hossain, M. Effect of low-protein diet, gender and age on the apparent ileal amino acid digestibility in broiler chickens raised under. Indian J. Anim. Sci. 2016, 86, 696–701. [Google Scholar]
- Hilliar, M.; Hargreave, G.; Girish, C.; Barekatain, R.; Wu, S.-B.; Swick, R. Using crystalline amino acids to supplement broiler chicken requirements in reduced protein diets. Poult. Sci. 2020, 99, 1551–1563. [Google Scholar] [CrossRef]
- Chrystal, P.V.; Moss, A.F.; Khoddami, A.; Naranjo, V.D.; Selle, P.H.; Liu, S.Y. Impacts of reduced-crude protein diets on key parameters in male broiler chickens offered maize-based diets. Poult. Sci. 2020, 99, 505–516. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, R.; Brooker, J.; Acamovic, T.; Sparks, N. Necrotic enteritis; a continuing challenge for the poultry industry. World’s Poult. Sci. J. 2006, 62, 221–247. [Google Scholar] [CrossRef]
- Hilliar, M.; Keerqin, C.; Girish, C.; Barekatain, R.; Wu, S.-B.; Swick, R. Reducing protein and supplementing crystalline amino acids, to alter dietary amino acid profiles in birds challenged for subclinical necrotic enteritis. Poult. Sci. 2020, 99, 2048–2060. [Google Scholar] [CrossRef] [PubMed]
- Bunchasak, C.; Silapasorn, T. Effects of adding methionine in low-protein diet on production performance, reproductive organs and chemical liver composition of laying hens under tropical conditions. Int. J. Poult. Sci. 2005, 4, 301–308. [Google Scholar]
- Roberts, S.A.; Xin, H.; Kerr, B.J.; Russell, J.R.; Bregendahl, K. Effects of dietary fiber and reduced crude protein on nitrogen balance and egg production in laying hens. Poult. Sci. 2007, 86, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Hernández, F.; Megias, M.; Orengo, J.; Martinez, S.; Lopez, M.; Madrid, J. Effect of dietary protein level on retention of nutrients, growth performance, litter composition and NH3 emission using a multi-phase feeding programme in broilers. Span. J. Agric. Res. 2013, 11, 736–746. [Google Scholar] [CrossRef]
- Boisen, S.; Hvelplund, T.; Weisbjerg, M. Ideal amino acid profiles as a basis for feed protein evaluation. Livest. Prod. Sci. 2000, 64, 239–251. [Google Scholar] [CrossRef]
- Fernandez, S.R.; Aoyagi, S.; Han, Y.; Parsons, C.M.; Baker, D.H. Limiting order of amino acids in corn and soybean meal for growth of the chick. Poult. Sci. 1994, 73, 1887–1896. [Google Scholar] [CrossRef]
- Peter, C.; Han, Y.; Boling-Frankenbach, S.; Parsons, C.; Baker, D. Limiting order of amino acids and the effects of phytase on protein quality in corn gluten meal fed to young chicks. J. Anim. Sci. 2000, 78, 2150–2156. [Google Scholar] [CrossRef]
- Wang, X.; Parsons, C.M. Order of amino acid limitation in poultry by-product meal. Br. Poult. Sci. 1998, 39, 113–116. [Google Scholar] [CrossRef]
- Kidd, M.; Hackenhaar, L. Dietary threonine for broilers: Dietary interactions and feed additive supplement use. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2006, 2006, 6. [Google Scholar] [CrossRef]
- Corzo, A.; Kidd, M.; Dozier, W., III; Vieira, S. Marginality and needs of dietary valine for broilers fed certain all-vegetable diets. J. Appl. Poult. Res. 2007, 16, 546–554. [Google Scholar] [CrossRef]
- Rehman, A.U.; Arif, M.; Husnain, M.M.; Alagawany, M.; El-Hack, A.; Mohamed, E.; Taha, A.E.; Elnesr, S.S.; Abdel-Latif, M.A.; Othman, S.I. Growth performance of broilers as influenced by different levels and sources of methionine plus cysteine. Animals 2019, 9, 1056. [Google Scholar] [CrossRef]
- Macelline, S.P.; Toghyani, M.; Chrystal, P.V.; Selle, P.H.; Liu, S.Y. Amino acid requirements for laying hens: A comprehensive review. Poult. Sci. 2021, 100, 101036. [Google Scholar] [CrossRef] [PubMed]
- Siegert, W.; Rodehutscord, M. The relevance of glycine and serine in poultry nutrition: A review. Br. Poult. Sci. 2019, 60, 579–588. [Google Scholar] [CrossRef]
- Parenteau, I. Performance and Metabolic Responses to Dietary Levels of Isoleucine in Laying Hens Fed Low Crude Protein Diets Fortified with Synthetic Amino Acids. Master’s Thesis, University of Guelph, Guelph, ON, Canada, 2019. [Google Scholar]
- Vieira, D.V.G.; de Sousa Melo, T.; da Silva, J.H.V.; Costa, F.G.P.; Cavalcante, D.T.; de Lima, M.R.; Bonaparte, T.P.; de Vargas Júnior, J.G.; Sousa, M.S.; Conti, A.C.M. Order of amino acid inclusion in the diet of DeKalb White laying hens. Semin. Ciências Agrárias 2016, 37, 1539–1550. [Google Scholar] [CrossRef]
- Maynard, C.; Liu, S.; Lee, J.; Caldas, J.; Diehl, E.; Rochell, S.; Kidd, M. Determining the 4th limiting amino acid in low crude protein diets for male and female cobb mv× 500 broilers. Br. Poult. Sci. 2020, 61, 695–702. [Google Scholar] [CrossRef]
- Farkhoy, M.; Modirsanei, M.; Ghavidel, O.; Sadegh, M.; Jafarnejad, S. Evaluation of protein concentration and limiting amino acids including lysine and Met+ Cys in prestarter diet on performance of broilers. Vet. Med. Int. 2012, 2012, 394189. [Google Scholar] [CrossRef]
- Bray, D. Studies with Corn-Soya Laying Diets: 8. Requirements for Limiting Amino Acids—The Basal Diet and the Requirements for Isoleucine, Lysine and Tryptophan. Poult. Sci. 1969, 48, 674–684. [Google Scholar] [CrossRef]
- Dong, X.; Azzam, M.; Zou, X. Effects of dietary L-isoleucine on laying performance and immunomodulation of laying hens. Poult. Sci. 2016, 95, 2297–2305. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Poultry; National Academy of Sciences Press: Washington, DC, USA, 1994. [Google Scholar]
- NHMRC. Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, 8th ed.; The National Health and Medical Research Council: Canberra, Australia, 2013.
- Hy-Line International. Hy-Line Brown Commercial Layers Managmenet Guide; Hy-Line-International: West Des Moines, IA, USA, 2016. [Google Scholar]
- Novak, C.; Yakout, H.; Scheideler, S. The effect of dietary protein level and total sulfur amino acid: Lysine ratio on egg production parameters and egg yield in Hy-Line W-98 hens. Poult. Sci. 2006, 85, 2195–2206. [Google Scholar] [CrossRef]
- Dumas, J. Procédés de l’analyse organique. Ann. Chim. Phys 1831, 47, 198–205. [Google Scholar]
- Kong, C.; Adeola, O. Evaluation of amino acid and energy utilization in feedstuff for swine and poultry diets. Asian-Australas. J. Anim. Sci. 2014, 27, 917. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Zhang, Z.; Chen, X.; Liu, J.; Yu, H.; Han, L.; Jin, L.; Zhang, Y.; Wang, T. An improved UPLC method for determining uric acid in rat serum and comparison study with commercial colorimetric kits. Acta Chromatogr. 2019, 31, 201–205. [Google Scholar] [CrossRef]
- Seedor, J.G.; Quartuccio, H.A.; Thompson, D.D. The bisphosphonate alendronate (MK-217) inhibits bone loss due to ovariectomy in rats. J. Bone Miner. Res. 1991, 6, 339–346. [Google Scholar] [CrossRef]
- Kheravii, S.K.; Swick, R.A.; Choct, M.; Wu, S.-B. Coarse particle inclusion and lignocellulose-rich fiber addition in feed benefit performance and health of broiler chickens. Poult. Sci. 2017, 96, 3272–3281. [Google Scholar] [CrossRef] [PubMed]
- Standard 4.2.5; Primary Production and Processing Standard for Eggs and Egg Product. Australian Federal Register of Legislation: Canberra, Australia, 2014. Available online: https://www.legislation.gov.au/Details/F2014C00965 (accessed on 15 October 2023).
- Abou-Elkhair, R.; Ahmed, H.; Ketkat, S.; Selim, S. Supplementation of a low-protein diet with tryptophan, threonine, and valine and its impact on growth performance, blood biochemical constituents, immune parameters, and carcass traits in broiler chickens. Vet. World 2020, 13, 1234. [Google Scholar] [CrossRef] [PubMed]
- Layton, A.; McKay, L.; Williams, D.; Garrett, V.; Gentry, R.; Sayler, G. Development of Bacteroides 16S rRNA gene TaqMan-based real-time PCR assays for estimation of total, human, and bovine fecal pollution in water. Appl. Environ. Microbiol. 2006, 72, 4214–4224. [Google Scholar] [CrossRef] [PubMed]
- Requena, T.; Burton, J.; Matsuki, T.; Munro, K.; Simon, M.A.; Tanaka, R.; Watanabe, K.; Tannock, G.W. Identification, detection, and enumeration of human Bifidobacterium species by PCR targeting the transaldolase gene. Appl. Environ. Microbiol. 2002, 68, 2420–2427. [Google Scholar] [CrossRef]
- Bartosch, S.; Fite, A.; Macfarlane, G.T.; McMurdo, M.E. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl. Environ. Microbiol. 2004, 70, 3575–3581. [Google Scholar] [CrossRef]
- Wise, M.; Siragusa, G. Quantitative analysis of the intestinal bacterial community in one-to three-week-old commercially reared broiler chickens fed conventional or antibiotic-free vegetable-based diets. J. Appl. Microbiol. 2007, 102, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of inulin on the human gut microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br. J. Nutr. 2008, 101, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Zo, Y.-G.; Kim, S.-J. Nonradioactive method to study genetic profiles of natural bacterial communities by PCR-single-strand-conformation polymorphism. Appl. Environ. Microbiol. 1996, 62, 3112–3120. [Google Scholar] [CrossRef] [PubMed]
- Lemme, A. The “Ideal Protein Concept” in broiler nutrition 1. Methodological aspects-opportunities and limitations. Degussa AG Amino News 2003, 4, 7–14. [Google Scholar]
- Dao, H.T.; Sharma, N.K.; Bradbury, E.J.; Swick, R.A. Response of laying hens to l-arginine, l-citrulline and guanidinoacetic acid supplementation in reduced protein diet. Anim. Nutr. 2021, 7, 460–471. [Google Scholar] [CrossRef]
- Alagawany, M.; El-Hindawy, M.M.; EL-HACK, M.E.; Arif, M.; EL-SAYED, S.A. Influence of low-protein diet with different levels of amino acids on laying hen performance, quality and egg composition. An. Da Acad. Bras. De Ciências 2020, 92, e20180230. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.; Song, E.; Billard, L.; Aggrey, S.; Pesti, G.; Sodsee, P. Effects of balanced dietary protein levels on egg production and egg quality parameters of individual commercial layers. Poult. Sci. 2013, 92, 2687–2696. [Google Scholar] [CrossRef]
- Azzam, M.; Dong, X.; Zou, X. Effect of dietary threonine on laying performance and intestinal immunity of laying hens fed low-crude-protein diets during the peak production period. J. Anim. Physiol. Anim. Nutr. 2017, 101, e55–e66. [Google Scholar] [CrossRef]
- Da Silva, J.H.V.; Melo, T.S.; Vieira, D.V.G.; De Lacerda, P.B.; Filho, J.J.; Brito, J.M.F.; Cruz, E.Y.G.S.; Da Silva, A.N.; Dantas, G.M. A determination of order of limiting amino acids in a low crude protein diet for laying hens. Poult. Sci. 2012, 91, 113. [Google Scholar]
- Khajali, F.; Khoshouie, E.; Dehkordi, S.; Hematian, M. Production performance and egg quality of Hy-line W36 laying hens fed reduced-protein diets at a constant total sulfur amino acid: Lysine ratio. J. Appl. Poult. Res. 2008, 17, 390–397. [Google Scholar] [CrossRef]
- Lee, J.T.; Rochell, S.J.; Kriseldi, R.; Kim, W.K.; Mitchell, R.D. Functional properties of amino acids: Improve health status and sustainability. Poult. Sci. 2023, 102, 102288. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.L.d.S.; Kim, W.K. Secondary functions of arginine and sulfur amino acids in poultry health. Animals 2020, 10, 2106. [Google Scholar] [CrossRef] [PubMed]
- Harms, R.; Ivey, F. Performance of commercial laying hens fed various supplemental amino acids in a corn-soybean meal diet. J. Appl. Poult. Res. 1993, 2, 273–282. [Google Scholar] [CrossRef]
- Bai, J.; Greene, E.; Li, W.; Kidd, M.T.; Dridi, S. Branched-chain amino acids modulate the expression of hepatic fatty acid metabolism-related genes in female broiler chickens. Mol. Nutr. Food Res. 2015, 59, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, R.; Zangeronimo, M.; Pereira, L.; Rodrigues, P.; Gomide, E. Lipoprotein metabolism in poultry. World’s Poult. Sci. J. 2011, 67, 431–440. [Google Scholar] [CrossRef]
- Harms, R.; Russell, G. Evaluation of the isoleucine requirement of the commercial layer in a corn-soybean meal diet. Poult. Sci. 2000, 79, 1154–1157. [Google Scholar] [CrossRef]
- Peganova, S.; Eder, K. Interactions of various supplies of isoleucine, valine, leucine and tryptophan on the performance of laying hens. Poult. Sci. 2003, 82, 100–105. [Google Scholar] [CrossRef]
- Ospina-Rojas, I.; Pozza, P.; Rodrigueiro, R.; Gasparino, E.; Khatlab, A.; Murakami, A. High leucine levels affecting valine and isoleucine recommendations in low-protein diets for broiler chickens. Poult. Sci. 2020, 99, 5946–5959. [Google Scholar] [CrossRef]
- Shivazad, M.; Harms, R.; Russell, G.; Faria, D.E.d.; Antar, R. Re-evaluation of the isoleucine requirement of the commercial layer. Poult. Sci. 2002, 81, 1869–1872. [Google Scholar] [CrossRef]
- Parenteau, I.A.; Stevenson, M.; Kiarie, E.G. Egg production and quality responses to increasing isoleucine supplementation in Shaver white hens fed a low crude protein corn-soybean meal diet fortified with synthetic amino acids between 20 and 46 weeks of age. Poult. Sci. 2020, 99, 1444–1453. [Google Scholar] [CrossRef]
- Youssef, S.; Shaban, S.; Inas, I.I. Effect of l-arginine supplementation on productive, reproductive performance, immune response and gene expression in two local chicken strains: 1-egg production, reproduction performance and immune response. Egypt. Poult. Sci 2015, 35, 573–590. [Google Scholar]
- Le Floc’h, N.; Otten, W.; Merlot, E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids 2011, 41, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J. Nutr. 2007, 137, 1539S–1547S. [Google Scholar] [CrossRef] [PubMed]
- Birkl, P.; Chow, J.; McBride, P.; Kjaer, J.B.; Kunze, W.; Forsythe, P.; Harlander-Matauschek, A. Effects of acute tryptophan depletion on repetitive behavior in laying hens. Front. Vet. Sci. 2019, 6, 230. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Gou, Z.; Lin, X.; Li, L. Effects of dietary tryptophan levels on performance and biochemical variables of plasma and intestinal mucosa in yellow-feathered broiler breeders. J. Anim. Physiol. Anim. Nutr. 2018, 102, e387–e394. [Google Scholar] [CrossRef] [PubMed]
- Moro, J.; Tomé, D.; Schmidely, P.; Demersay, T.-C.; Azzout-Marniche, D. Histidine: A systematic review on metabolism and physiological effects in human and different animal species. Nutrients 2020, 12, 1414. [Google Scholar] [CrossRef]
- Namgung, N.; Shin, D.; Park, S.; Paik, I. Effects of supplementary blood meal on carnosine content in the breast meat and laying performance of old hens. Asian-Australas. J. Anim. Sci. 2010, 23, 946–951. [Google Scholar] [CrossRef]
- Han, Y.-K.; Thacker, P.A. Influence of energy level and glycine supplementation on performance, nutrient digestibility and egg quality in laying hens. Asian-Australas. J. Anim. Sci. 2011, 24, 1447–1455. [Google Scholar] [CrossRef]
- Xie, C.; Elwan, H.; Elnesr, S.; Dong, X.; Zou, X. Effect of iron glycine chelate supplementation on egg quality and egg iron enrichment in laying hens. Poult. Sci. 2019, 98, 7101–7109. [Google Scholar] [CrossRef]
- El-Atty, A.; Hanaa, K.; Attia, K.; Salim, I.; Yassein, D.M.; El-Slamony, A. Effect of Glycine Supplementation of Mandarah Laying Hens Diets on Production Performance and Egg Quality. J. Anim. Poult. Prod. 2020, 11, 583–589. [Google Scholar] [CrossRef]
- Lelis, G.; Albino, L.; Tavernari, F.; Calderano, A.; Rostagno, H.; Barros, V.; Maia, R. Digestible valine-to-digestible lysine ratios in brown commercial layer diets. J. Appl. Poult. Res. 2014, 23, 683–690. [Google Scholar] [CrossRef]
- Jian, H.; Miao, S.; Liu, Y.; Li, H.; Zhou, W.; Wang, X.; Dong, X.; Zou, X. Effects of dietary valine levels on production performance, egg quality, antioxidant capacity, immunity, and intestinal amino acid absorption of laying hens during the peak lay period. Animals 2021, 11, 1972. [Google Scholar] [CrossRef]
- Harms, R.; Russell, G. Evaluation of valine requirement of the commercial layer using a corn-soybean meal basal diet. Poult. Sci. 2001, 80, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Helmbrecht, A.; Elliot, M.; Thomson, J.; Persia, M.E. Evaluation of the Valine requirement of small-framed first cycle laying hens. Poult. Sci. 2019, 98, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Kumari, K.N.R.; Reddy, V.R.; Preetham, V.C.; Kumar, D.S.; Sen, A.R.; Rao, S.V.R. Effect of level of protein and concentrations of amino acids on egg quality parameters in WL layers. J. Anim. Feed Sci. Technol. 2017, 5, 9–14. [Google Scholar] [CrossRef]
- Heo, Y.-J.; Park, J.; Kim, Y.-B.; Kwon, B.-Y.; Kim, D.-H.; Song, J.-Y.; Lee, K.-W. Effects of dietary protein levels on performance, nitrogen excretion, and odor emission of growing pullets and laying hens. Poult. Sci. 2023, 102, 102798. [Google Scholar] [CrossRef]
- Hilliar, M.; Swick, R. Nutritional implications of feeding reduced-protein diets to meat chickens. Anim. Prod. Sci. 2019, 59, 2069–2081. [Google Scholar] [CrossRef]
- Donsbough, A.; Powell, S.; Waguespack, A.; Bidner, T.; Southern, L. Uric acid, urea, and ammonia concentrations in serum and uric acid concentration in excreta as indicators of amino acid utilization in diets for broilers. Poult. Sci. 2010, 89, 287–294. [Google Scholar] [CrossRef]
- Geng, S.; Huang, S.; Ma, Q.; Li, F.; Gao, Y.; Zhao, L.; Zhang, J. Alterations and correlations of the gut microbiome, performance, egg quality, and serum biochemical indexes in laying hens with low-protein amino acid-deficient diets. ACS Omega 2021, 6, 13094–13104. [Google Scholar] [CrossRef]
- Xin, Q.; Ma, N.; Jiao, H.; Wang, X.; Li, H.; Zhou, Y.; Zhao, J.; Lin, H. Dietary energy and protein levels during the prelay period on production performance, egg quality, expression of genes in hypothalamus-pituitary-ovary axis, and bone parameters in aged laying hens. Front. Physiol. 2022, 13, 887381. [Google Scholar] [CrossRef]
- Hassan, M.R.; Choe, H.S.; Jeong, Y.D.; Hwangbo, J.; Ryu, K.S. Effect of dietary energy and protein on the performance, egg quality, bone mineral density, blood properties and yolk fatty acid composition of organic laying hens. Ital. J. Anim. Sci. 2013, 12, e10. [Google Scholar] [CrossRef]
- Dai, Z.-L.; Wu, G.; Zhu, W.-Y. Amino acid metabolism in intestinal bacteria: Links between gut ecology and host health. Front. Biosci.-Landmark 2011, 16, 1768–1786. [Google Scholar] [CrossRef] [PubMed]
- Apajalahti, J.; Vienola, K. Interaction between chicken intestinal microbiota and protein digestion. Anim. Feed Sci. Technol. 2016, 221, 323–330. [Google Scholar] [CrossRef]
- Dao, H.T.; Sharma, N.K.; Barekatain, R.; Kheravii, S.K.; Bradbury, E.J.; Wu, S.-B.; Swick, R.A. Supplementation of reduced protein diets with l-arginine and l-citrulline for broilers challenged with subclinical necrotic enteritis. 2. Intestinal permeability, microbiota, and short-chain fatty acid production. Anim. Prod. Sci. 2022, 62, 1250–1265. [Google Scholar] [CrossRef]
- Dong, X.; Azzam, M.; Zou, X. Effects of dietary threonine supplementation on intestinal barrier function and gut microbiota of laying hens. Poult. Sci. 2017, 96, 3654–3663. [Google Scholar] [CrossRef]
- Shazali, N.; Loh, T.C.; Foo, H.L.; Samsudin, A.A. Gut microflora and intestinal morphology changes of broiler chickens fed reducing dietary protein supplemented with lysine, methionine, and threonine in tropical environment. Rev. Bras. De Zootec. 2019, 48, e20170265. [Google Scholar] [CrossRef]

| Treatment Number | Treatment Code | Treatment Description |
|---|---|---|
| 1 | SP | A standard-protein diet with sufficient levels of all essential amino acids |
| 2 | RP | A reduced-protein diet with sufficient levels of Lys, Met, and Thr but deficient in Trp, Arg, Ile, Val, Leu, His, Phe, and Gly |
| 3 | RP-EAA | A reduced-protein diet with sufficient levels of all essential amino acids, including Lys, Met, Thr, Trp, Arg, Ile, Val, Leu, His, Phe, and Gly |
| 4 | RP-EAA-Trp | Treatment 3 deficient in Trp |
| 5 | RP-EAA-Val | Treatment 3 deficient in Val |
| 6 | RP-EAA-Ile | Treatment 3 deficient in Ile |
| 7 | RP-EAA-Arg | Treatment 3 deficient in Arg |
| 8 | RP-EAA-Leu | Treatment 3 deficient in Leu |
| 9 | RP-EAA-His | Treatment 3 deficient in His |
| 10 | RP-EAA-Phe | Treatment 3 deficient in Phe |
| 11 | RP-EAA-Glycineequivalent | Treatment 3 deficient in Gly |
| Ingredients (%, Otherwise as Indicated) | SP | RP |
|---|---|---|
| Wheat | 38.44 | 49.52 |
| Barley | 4.00 | 4.00 |
| Sorghum | 20.00 | 20.00 |
| Soybean meal | 13.77 | 2.68 |
| Canola meal | 10.00 | 9.66 |
| Canola oil | 2.59 | 0.80 |
| Limestone (fine) | 4.93 | 4.94 |
| Limestone (coarse) | 4.93 | 4.94 |
| Monocalcium phosphate | 0.549 | 0.623 |
| Salt | 0.232 | 0.159 |
| Sodium bicarbonate | 0.200 | 0.300 |
| Choline Cl 60% | 0.000 | 0.000 |
| L-lysine HCl 78.5% | 0.078 | 0.410 |
| DL-methionine 99% | 0.163 | 0.245 |
| L-threonine 99% | 0.016 | 0.159 |
| L-tryptophan 99% | - | 0.018 |
| L-valine 99% | - | 0.137 |
| L-isoleucine 99% | - | 0.175 |
| L-arginine 99% | - | 0.237 |
| L-leucine 99% | - | 0.287 |
| L-histidine 99% | - | 0.101 |
| L-phenylalanine 99% | - | 0.194 |
| L-glycine 99% | - | 0.276 |
| Xylanase 1 | 0.010 | 0.010 |
| Phytase 2 | 0.006 | 0.006 |
| Pigment jabiru red | 0.004 | 0.004 |
| Pigment jabiru yellow | 0.003 | 0.003 |
| Vitamin–mineral premix 3 | 0.100 | 0.100 |
| Calculated nutrient composition (%, otherwise as indicated) | ||
| AMEn 4, kcal/kg | 2740 | 2740 |
| Crude protein | 17.24 | 15.00 |
| Crude fat | 4.60 | 2.90 |
| Crude fibre | 3.19 | 3.00 |
| SID 5 arginine | 0.893 | 0.780 |
| SID lysine | 0.740 | 0.740 |
| SID methionine | 0.400 | 0.435 |
| SID cysteine | 0.269 | 0.233 |
| SID methionine + cysteine | 0.670 | 0.670 |
| SID tryptophan | 0.192 | 0.160 |
| SID histidine | 0.370 | 0.370 |
| SID phenylalanine | 0.706 | 0.706 |
| SID leucine | 1.152 | 1.152 |
| SID isoleucine | 0.590 | 0.590 |
| SID threonine | 0.520 | 0.520 |
| SID valine | 0.692 | 0.650 |
| SID glycine equivalent | 0.971 | 0.971 |
| Calcium | 4.10 | 4.10 |
| Available phosphorus | 0.40 | 0.40 |
| Sodium | 0.17 | 0.17 |
| Potassium | 0.64 | 0.46 |
| Chloride | 0.22 | 0.24 |
| Choline, mg/kg | 1557 | 1333 |
| Linoleic acid | 1.55 | 1.12 |
| Nutrient Composition | SP | RP | RP-EAA | RP-EAA-Trp | RP-EAA-Val | RP-EAA-Ile | RP-EAA-Arg | RP-EAA-Leu | RP-EAA-His | RP-EAA-Phe | RP-EAA-Gly |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dry matter | 91.46 | 91.34 | 91.66 | 91.76 | 91.25 | 91.06 | 91.08 | 91.34 | 91.35 | 91.33 | 91.26 |
| Gross energy, kcal/kg | 3712 | 3523 | 3638 | 3589 | 3584 | 3569 | 3571 | 3536 | 3571 | 3583 | 3509 |
| Crude protein | 18.15 | 14.37 | 15.82 | 15.66 | 15.84 | 15.25 | 15.44 | 15.30 | 15.20 | 14.90 | 14.77 |
| Ash | 15.07 | 15.04 | 13.64 | 14.70 | 14.27 | 14.53 | 14.52 | 14.77 | 14.40 | 13.75 | 15.73 |
| Calcium | 5.00 | 5.27 | 4.81 | 4.37 | 4.60 | 4.33 | 4.84 | 4.45 | 4.22 | 4.48 | 5.53 |
| Total phosphorus | 0.60 | 0.58 | 0.63 | 0.57 | 0.58 | 0.57 | 0.61 | 0.57 | 0.58 | 0.54 | 0.58 |
| Aspartic acid | 1.073 | 0.645 | 0.671 | 0.688 | 0.687 | 0.691 | 0.723 | 0.723 | 0.750 | 0.696 | 0.692 |
| Serine | 0.836 | 0.483 | 0.517 | 0.525 | 0.572 | 0.555 | 0.560 | 0.560 | 0.550 | 0.548 | 0.527 |
| Glutamic acid | 2.897 | 2.323 | 2.506 | 2.501 | 2.547 | 2.512 | 2.607 | 2.607 | 2.656 | 2.543 | 2.512 |
| Glycine | 0.723 | 0.406 | 0.671 | 0.684 | 0.778 | 0.721 | 0.715 | 0.715 | 0.686 | 0.701 | 0.463 |
| Histidine | 0.447 | 0.212 | 0.342 | 0.345 | 0.383 | 0.353 | 0.350 | 0.350 | 0.253 | 0.349 | 0.329 |
| Arginine | 0.922 | 0.536 | 0.742 | 0.824 | 0.888 | 0.806 | 0.560 | 0.859 | 0.754 | 0.838 | 0.828 |
| Threonine | 0.637 | 0.433 | 0.510 | 0.514 | 0.557 | 0.528 | 0.525 | 0.530 | 0.502 | 0.540 | 0.499 |
| Alanine | 0.649 | 0.465 | 0.496 | 0.512 | 0.533 | 0.522 | 0.528 | 0.537 | 0.546 | 0.521 | 0.508 |
| Proline | 1.265 | 0.768 | 0.885 | 0.809 | 0.937 | 0.914 | 0.916 | 0.914 | 0.937 | 0.960 | 0.944 |
| Cysteine | 0.621 | 0.528 | 0.317 | 0.430 | 0.611 | 0.575 | 0.571 | 0.563 | 0.612 | 0.623 | 0.569 |
| Tyrosine | 0.578 | 0.268 | 0.213 | 0.328 | 0.359 | 0.313 | 0.325 | 0.314 | 0.318 | 0.344 | 0.321 |
| Valine | 0.575 | 0.307 | 0.515 | 0.473 | 0.359 | 0.500 | 0.499 | 0.499 | 0.456 | 0.484 | 0.499 |
| Methionine | 0.460 | 0.315 | 0.565 | 0.324 | 0.413 | 0.432 | 0.448 | 0.349 | 0.397 | 0.376 | 0.328 |
| Lysine | 0.708 | 0.746 | 0.795 | 0.740 | 0.853 | 0.823 | 0.864 | 0.844 | 0.911 | 0.858 | 0.790 |
| Isoleucine | 0.491 | 0.298 | 0.387 | 0.487 | 0.516 | 0.366 | 0.507 | 0.504 | 0.482 | 0.527 | 0.493 |
| Leucine | 1.197 | 0.698 | 0.844 | 1.072 | 1.119 | 1.125 | 1.094 | 0.852 | 1.054 | 1.088 | 1.058 |
| Phenylalanine | 0.847 | 0.396 | 0.669 | 0.707 | 0.726 | 0.706 | 0.648 | 0.668 | 0.623 | 0.504 | 0.699 |
| Target Group or Organism | Primer Sequence (5′–3′) | Annealing Temperature (°C) | Reference |
|---|---|---|---|
| Bacillus spp. | F-GCA ACG AGC GCA ACC CTT GA | 63 | [43] |
| R-TCA TCC CCA CCT TCC TCC GGT | |||
| Bacteroides spp. | F-GAG AGG AAG GTC CCC CAC | 63 | [44] |
| R-CGC TAC TTG GCT GGT TCA G | |||
| Bifidobacterium spp. | F-GCG TCC GCT GTG GGC | 63 | [45] |
| R-CTT CTC CGG CAT GGT GTT G | |||
| Enterobacteriaceae | F-CAT TGA CGT TAC CCG CAG AAG AAG C | 63 | [46] |
| R-CTC TAC GAG ACT CAA GCT TGC | |||
| Lactobacillus spp. | F-CAC CGC TAC ACA TGG AG | 63 | [47] |
| R-AGC AGT AGG GAA TCT TCC A | |||
| Ruminococcus spp. | F-GGC GGC YTR CTG GGC TTT | 63 | [48] |
| R-CCA GGT GGA TWA CTT ATT GTG TTA A | |||
| Total bacteria | F-CGG YCC AGA CTC CTA CGG G | 63 | [49] |
| R-TTA CCG CGG CTG CTG GCA C |
| Hen Age (Week) | Nutrient Composition | SP | RP | RP-EAA | RP-EAA-Trp | RP-EAA-Val | RP-EAA-Ile | RP-EAA-Arg | RP-EAA-Leu | RP-EAA-His | RP-EAA-Phe | RP-EAA-Gly | SEM | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20–29 | Hen-day egg production (%) | 95.51 | 93.34 | 92.76 | 94.84 | 93.21 | 92.52 | 95.8 | 94.52 | 93.99 | 94.14 | 95.25 | 0.82 | 0.051 |
| Egg weight (g) | 59.02 a | 57.25 bcd | 57.78 abc | 57.32 bcd | 56.13 d | 56.20 cd | 56.06 d | 57.17 bcd | 58.93 a | 57.49 abcd | 58.63 ab | 0.57 | <0.001 | |
| Egg mass (g) | 56.65 b | 53.76 cde | 53.80 cde | 54.61 bc | 52.55 de | 52.30 e | 53.92 cde | 54.27 bcd | 55.64 abc | 54.34 bcd | 56.06 ab | 0.67 | <0.0001 | |
| Feed intake (g) | 129 abc | 131 ab | 122 d | 126 bcd | 127 abcd | 126 bcd | 126 bcd | 125 bcd | 132 a | 124 cd | 128 abc | 2.13 | 0.039 | |
| FCR (kg feed/kg egg) | 2.456 | 2.616 | 2.451 | 2.515 | 2.696 | 2.740 | 2.452 | 2.478 | 2.574 | 2.490 | 2.430 | 0.08 | 0.089 | |
| 30–39 | Hen-day egg production (%) | 98.74 | 98.60 | 98.70 | 98.47 | 97.72 | 98.27 | 97.95 | 98.33 | 98.91 | 98.28 | 98.14 | 0.38 | 0.551 |
| Egg weight (g) | 63.51 ab | 61.81 bcd | 62.28 abcd | 62.22 abcd | 61.55 cd | 61.62 cd | 60.91 d | 62.06 abcd | 63.80 a | 61.76 bcd | 63.31 abc | 0.64 | 0.032 | |
| Egg mass (g) | 62.70 ab | 60.95 bcd | 61.45 abcd | 61.27 abcd | 60.14 d | 60.54 cd | 59.68 d | 61.00 bcd | 63.11 a | 60.72 cd | 62.16 abc | 0.69 | 0.012 | |
| Feed intake (g) | 144 ab | 144 a | 135 d | 140 abcd | 137 abcd | 140 bcd | 140 abcd | 136 cd | 142 abc | 133 d | 137 cd | 2.38 | 0.018 | |
| FCR (kg feed/kg egg) | 2.300 abcd | 2.377 a | 2.201 d | 2.296 abcd | 2.334 abc | 2.271 abcd | 2.347 ab | 2.228 cd | 2.252 bcd | 2.203 d | 2.209 d | 0.04 | 0.007 | |
| 20–39 | Hen-day egg production (%) | 95.4 | 92.8 | 94.7 | 94.6 | 93.3 | 93.4 | 95.8 | 95.6 | 95.2 | 94.8 | 96.2 | 0.25 | 0.098 |
| Egg weight (g) | 60.2 abc | 57.7 d | 59.1 abcd | 58.5 cd | 57.7 d | 57.6 d | 57.8 d | 59.1 abcd | 60.5 ab | 58.7 bcd | 60.7 a | 0.21 | 0.001 | |
| Egg mass (g) | 57.5 ab | 53.7 d | 56.0 abcd | 55.4 bcd | 54.0 cd | 53.9 d | 55.4 bcd | 56.5 abc | 57.7 ab | 55.7 bcd | 58.4 a | 0.28 | <0.001 | |
| Feed intake (g) | 128 | 127 | 123 | 125 | 125 | 124 | 125 | 125 | 131 | 122 | 128 | 0.7 | 0.281 | |
| FCR (kg feed/kg egg) | 2.238 bc | 2.378 a | 2.203 c | 2.262 abc | 2.334 ab | 2.305 abc | 2.263 abc | 2.212 c | 2.278 abc | 2.205 c | 2.193 c | 0.013 | 0.046 |
| Treatment | Protein Intake (g/day) | Protein Excreted (g/day) | Retained Protein (g/day) | Protein Digestibility (%) | Energy Intake (Kcal/day) | Energy Excreted (Kcal/day) | Retained Energy (Kcal/day) | Energy Digestibility (%) | Serum Uric Acid Level ((mg/dl)) |
|---|---|---|---|---|---|---|---|---|---|
| SP | 27.74 a | 13.05 a | 14.68 | 51.72 | 567.25 | 109.8 | 457.45 | 80.05 | 5.58 |
| RP | 20.97 b | 10.33 ab | 10.64 | 49.99 | 514.19 | 102.48 | 411.7 | 79.82 | 4.41 |
| RP-EAA | 21.28 b | 10.87 ab | 10.4 | 49.05 | 489.38 | 95.24 | 394.14 | 80.65 | 5.20 |
| RP-EAA-Trp | 21.75 b | 10.52 ab | 11.23 | 51.53 | 498.58 | 96.07 | 402.51 | 80.65 | 4.63 |
| RP-EAA-Val | 22.62 ab | 10.04 ab | 12.58 | 54.72 | 511.92 | 93.84 | 418.07 | 81.37 | 4.94 |
| RP-EAA-Ile | 21.63 b | 11.38 ab | 10.25 | 46.93 | 506.36 | 102.27 | 404.09 | 79.65 | 5.31 |
| RP-EAA-Arg | 20.58 b | 9.7 b | 10.87 | 52.57 | 476.09 | 86.83 | 389.26 | 81.72 | 4.67 |
| RP-EAA-Leu | 19.64 b | 9.61 b | 10.03 | 50.79 | 454.01 | 89.07 | 364.94 | 80.25 | 4.98 |
| RP-EAA-His | 20.8 b | 10.74 ab | 10.11 | 48.64 | 490.05 | 96.26 | 393.78 | 80.42 | 5.00 |
| RP-EAA-Phe | 20.4 b | 10.19 ab | 10.25 | 48.56 | 491.73 | 94 | 397.72 | 80.46 | 4.37 |
| RP-EAA-Gly | 20.9 b | 9.7 b | 11.28 | 53.44 | 498.73 | 97.36 | 401.37 | 80.46 | 4.88 |
| SEM | 1.18 | 0.64 | 1.13 | 3.18 | 26.66 | 6.01 | 24.82 | 1.16 | 0.32 |
| p-value | 0.001 | 0.018 | 0.155 | 0.854 | 0.367 | 0.347 | 0.603 | 0.984 | 0.234 |
| Treatments | Fresh Weight (g) | Air-Dry Weight (g) | Length (mm) | Width (mm) | Seedor Index | Bone Breaking Strength (Kgf) | Ash Content (g) | Ash (%) |
|---|---|---|---|---|---|---|---|---|
| SP | 12.38 | 9.73 | 125.04 | 8.70 | 0.070 | 158.76 | 3.23 | 37.15 |
| RP | 12.01 | 9.55 | 124.27 | 8.63 | 0.069 | 134.64 | 3.29 | 38.64 |
| RP-EAA | 11.90 | 9.25 | 123.39 | 8.78 | 0.067 | 150.54 | 3.20 | 38.70 |
| RP-EAA-Trp | 12.10 | 9.45 | 124.84 | 8.70 | 0.068 | 138.54 | 3.21 | 38.02 |
| RP-EAA-Val | 12.16 | 9.53 | 125.40 | 8.67 | 0.068 | 126.26 | 3.20 | 37.66 |
| RP-EAA-Ile | 12.25 | 9.71 | 123.55 | 8.72 | 0.070 | 146.58 | 3.35 | 38.70 |
| RP-EAA-Arg | 27.83 | 9.72 | 124.54 | 9.04 | 0.070 | 127.01 | 3.17 | 36.58 |
| RP-EAA-Leu | 12.17 | 9.66 | 123.45 | 8.69 | 0.071 | 134.71 | 3.28 | 37.65 |
| RP-EAA-His | 12.60 | 10.02 | 126.48 | 8.76 | 0.071 | 134.43 | 3.40 | 37.87 |
| RP-EAA-Phe | 12.48 | 9.78 | 123.73 | 8.80 | 0.071 | 151.79 | 3.24 | 37.03 |
| RP-EAA-Gly | 11.98 | 9.45 | 124.09 | 8.77 | 0.069 | 134.96 | 3.14 | 36.67 |
| SEM | 4.709 | 0.230 | 1.18 | 0.172 | 0.002 | 16.89 | 0.120 | 1.004 |
| p-value | 0.448 | 0.626 | 0.761 | 0.943 | 0.741 | 0.947 | 0.926 | 0.804 |
| Treatment | Lactobacillus | Ruminococcus | Bacteroides | Bacillus | Bifidobacteria | Enterobacteria | Total Bacteria |
|---|---|---|---|---|---|---|---|
| SP | 8.98 | 9.45 | 10.88 | 7.84 | 9.65 | 7.27 | 12.59 |
| RP | 8.70 | 9.34 | 11.05 | 7.58 | 9.76 | 7.61 | 12.48 |
| RP-EAA | 8.65 | 9.53 | 10.95 | 7.85 | 9.85 | 7.40 | 12.52 |
| RP-EAA-Trp | 8.67 | 9.41 | 11.01 | 7.84 | 9.80 | 8.07 | 12.55 |
| RP-EAA-Val | 8.86 | 9.42 | 11.04 | 7.68 | 9.79 | 7.18 | 12.51 |
| RP-EAA-Ile | 8.69 | 9.40 | 11.09 | 7.77 | 9.83 | 7.30 | 12.52 |
| RP-EAA-Arg | 8.72 | 9.59 | 10.96 | 7.86 | 9.91 | 7.32 | 12.57 |
| RP-EAA-Leu | 8.71 | 9.50 | 10.94 | 7.77 | 9.79 | 7.14 | 12.59 |
| RP-EAA-His | 8.69 | 9.48 | 10.95 | 7.84 | 9.83 | 7.60 | 12.53 |
| RP-EAA-Phe | 8.68 | 9.52 | 11.10 | 7.93 | 9.78 | 7.52 | 12.56 |
| RP-EAA-Gly | 8.81 | 9.50 | 11.06 | 7.76 | 9.80 | 7.88 | 12.56 |
| SEM | 0.11 | 0.05 | 0.05 | 0.14 | 0.06 | 0.59 | 0.05 |
| p-value | 0.744 | 0.082 | 0.067 | 0.908 | 0.373 | 0.991 | 0.903 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahan, A.A.; Dao, T.H.; Akter, N.; Sukirno; Swick, R.A.; Morgan, N.K.; Crowley, T.M.; Moss, A.F. The Order of Limiting Amino Acids in a Wheat–Sorghum-Based Reduced-Protein Diet for Laying Hens. Appl. Sci. 2023, 13, 12934. https://doi.org/10.3390/app132312934
Jahan AA, Dao TH, Akter N, Sukirno, Swick RA, Morgan NK, Crowley TM, Moss AF. The Order of Limiting Amino Acids in a Wheat–Sorghum-Based Reduced-Protein Diet for Laying Hens. Applied Sciences. 2023; 13(23):12934. https://doi.org/10.3390/app132312934
Chicago/Turabian StyleJahan, Afsana A., Thi Hiep Dao, Nasima Akter, Sukirno, Robert A. Swick, Natalie K. Morgan, Tamsyn M. Crowley, and Amy F. Moss. 2023. "The Order of Limiting Amino Acids in a Wheat–Sorghum-Based Reduced-Protein Diet for Laying Hens" Applied Sciences 13, no. 23: 12934. https://doi.org/10.3390/app132312934
APA StyleJahan, A. A., Dao, T. H., Akter, N., Sukirno, Swick, R. A., Morgan, N. K., Crowley, T. M., & Moss, A. F. (2023). The Order of Limiting Amino Acids in a Wheat–Sorghum-Based Reduced-Protein Diet for Laying Hens. Applied Sciences, 13(23), 12934. https://doi.org/10.3390/app132312934






