Rapid Induction of Astaxanthin in Haematococcus lacustris by Mild Electric Stimulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalga and Culture Conditions
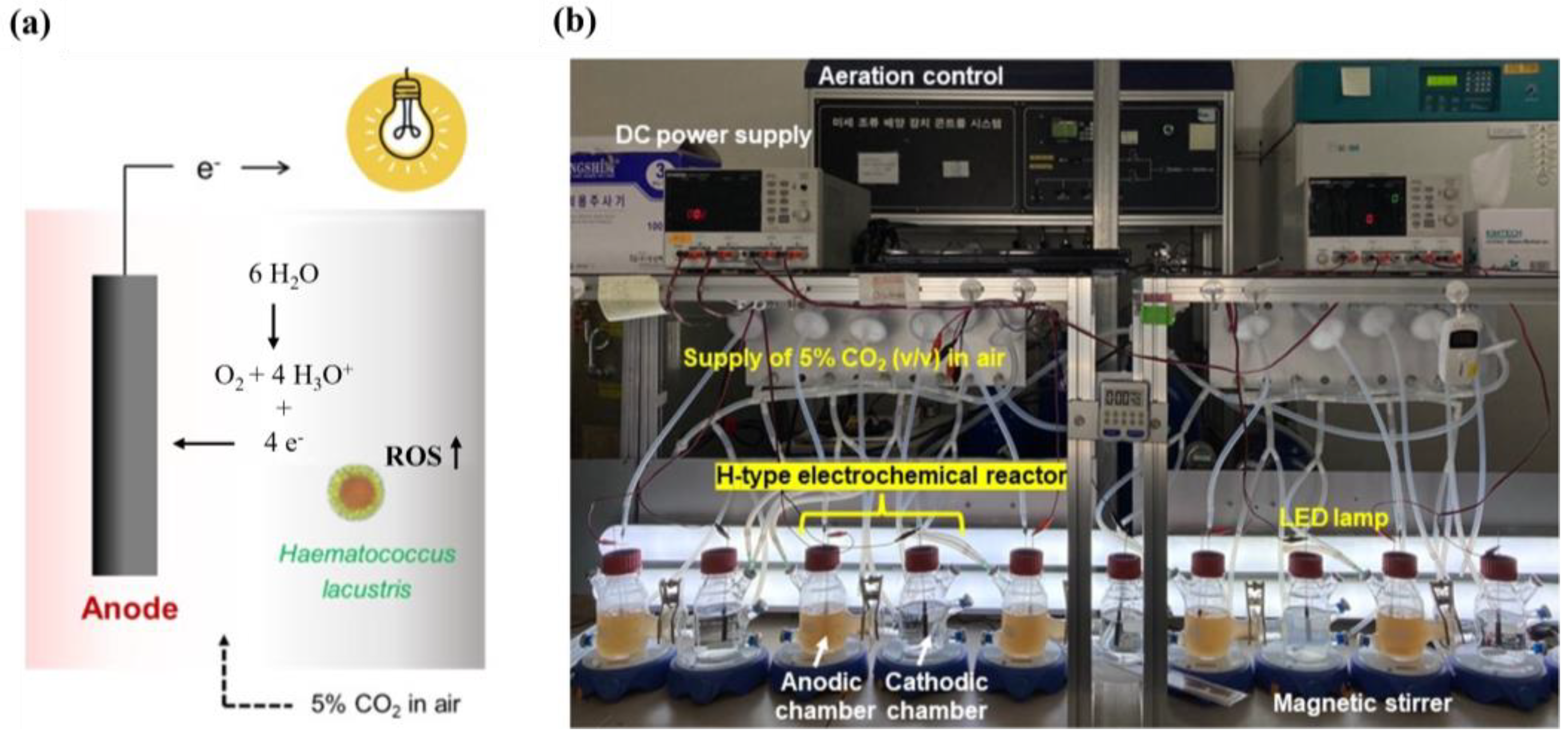
2.2. Electric Stimulation of AXT Biosynthesis
2.3. Cell Morphology and Viability Analyses
2.4. ROS Measurement
2.5. Carotenoid Quantification
2.6. Other Analytical Methods
2.7. Statistical Analysis
3. Results and Discussion
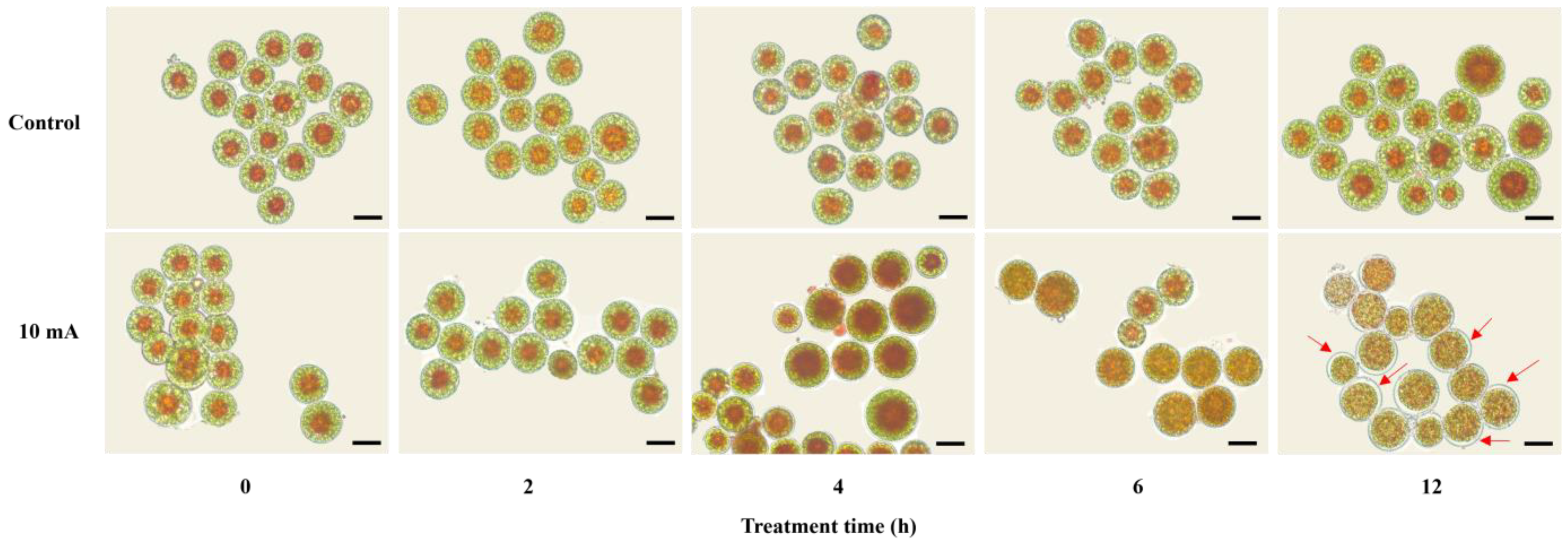
3.1. Morphological Changes during 10 mA Electrotreatment for 12 h
3.2. AXT Accumulation and Cell Viability during 10 mA Electrotreatment for 12 h
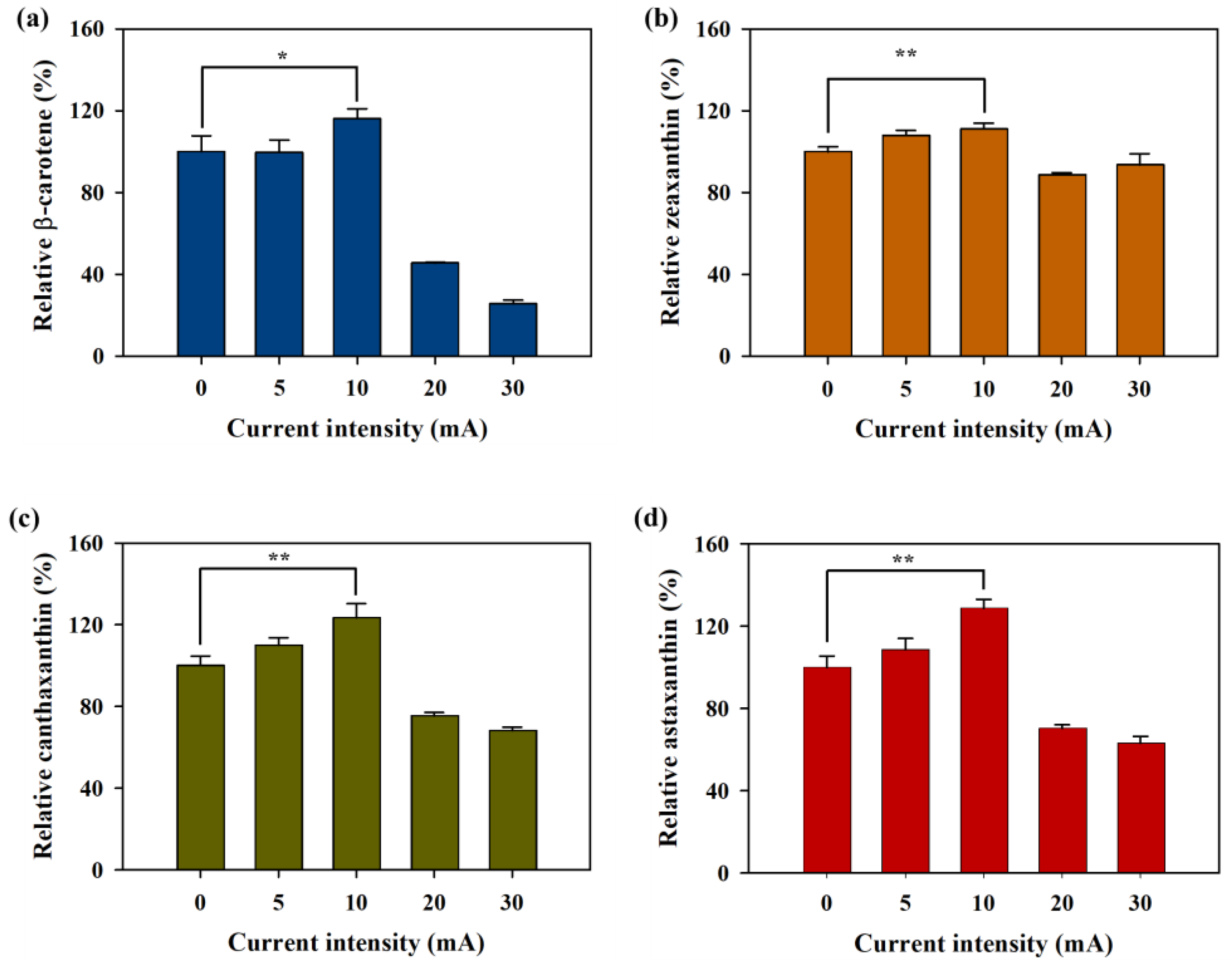
3.3. Effect of Current Intensity on AXT and Other Carotenoids
3.4. Effect of Current Intensity on Cell Morphology, Viability, and ROS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hong, J.S.; Shin, W.; Nam, H.; Yun, J.-H.; Kim, H.-S.; Ahn, K.H. Sedimentation and rheological study of microalgal cell (Chlorella sp. HS2) suspension. Biotechnol. Bioprocess Eng. 2022, 27, 451–460. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Jacob, A.; Nader, C.; Oliveira, C.D.L.; Matos, Â.P.; Araújo, E.S.; Shabnam, N.; Ashok, B.; Gálvez, A.O. An overview on microalgae as renewable resources for meeting sustainable development goals. J. Environ. Manag. 2022, 320, 115897. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, L.; Zhang, Y.; Gao, H.; Zhao, Y.; Yu, X. Chemical inducers regulate ROS signalling to stimulate astaxanthin production in Haematococcus pluvialis under environmental stresses: A review. Trends Food Sci. Technol. 2023, 136, 181–193. [Google Scholar] [CrossRef]
- Yao, Q.; Ma, J.; Chen, X.; Zhao, G.; Zang, J. A natural strategy for astaxanthin stabilization and color regulation: Interaction with proteins. Food Chem. 2023, 402, 134343. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, R.; Diksha; Kumari, A.; Panwar, A. Astaxanthin: A super antioxidant from microalgae and its therapeutic potential. J. Basic Microbiol. 2022, 62, 1064–1082. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, S.Y.; Narasimhan, A.L.; Kim, S.; Oh, Y.-K. Cell disruption and astaxanthin extraction from Haematococcus pluvialis: Recent advances. Bioresour. Technol. 2022, 343, 126124. [Google Scholar] [CrossRef]
- Udayan, A.; Arumugam, M.; Pandey, A. Nutraceuticals from algae and cyanobacteria. In Algal Green Chemistry. Recent Progress in Biotechnology, 1st ed.; Rastogi, R.P., Madamwar, D., Pandey, A., Eds.; Elsevier: Cambridge, MA, USA, 2017; pp. 65–89. [Google Scholar]
- Nakada, T.; Ota, S. What is the correct name for the type of Haematococcus Flot.(Volvocales, Chlorophyceae)? Taxon 2016, 65, 343–348. [Google Scholar] [CrossRef]
- Mota, G.C.P.; Moraes, L.B.S.D.; Oliveira, C.Y.B.; Oliveira, D.W.S.; Abreu, J.L.D.; Dantas, D.M.M.; Gálvez, A.O. Astaxanthin from Haematococcus pluvialis: Processes, applications, and market. Prep. Biochem. Biotechnol. 2022, 52, 598–609. [Google Scholar] [CrossRef]
- Kim, Y.-E.; Matter, I.A.; Lee, N.; Jung, M.; Lee, Y.-C.; Choi, S.-A.; Lee, S.Y.; Kim, J.R.; Oh, Y.-K. Enhancement of astaxanthin production by Haematococcus pluvialis using magnesium aminoclay nanoparticles. Bioresour. Technol. 2020, 307, 123270. [Google Scholar] [CrossRef]
- Hu, C.; Cui, D.; Sun, X.; Shi, J.; Xu, N. Primary metabolism is associated with the astaxanthin biosynthesis in the green algae Haematococcus pluvialis under light stress. Algal Res. 2020, 46, 101768. [Google Scholar] [CrossRef]
- Wang, X.; Miao, X.; Chen, G.; Cui, Y.; Sun, F.; Fan, J.; Gao, Z.; Meng, C. Identification of microRNAs involved in astaxanthin accumulation responding to high light and high sodium acetate (NaAC) stresses in Haematococcus pluvialis. Algal Res. 2021, 54, 102179. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Y.; Ding, W.; Han, B.; Geng, S.; Ning, D.; Ma, T.; Yu, X. Gamma-aminobutyric acid facilitates the simultaneous production of biomass, astaxanthin, and lipids in Haematococcus pluvialis under salinity and high-light stress conditions. Bioresour. Technol. 2021, 320, 124418. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Sung, Y.J.; Lee, J.S.; Yu, B.S.; Sim, S.J. Robust cyst germination induction in Haematococcus pluvialis to enhance astaxanthin productivity in a semi-continuous outdoor culture system using power plant flue gas. Bioresour. Technol. 2021, 338, 125533. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.-E.; Hwang, S.K.; Chang, W.S.; Kim, B.W.; Lee, J.; Sim, S.J. Enhanced autotrophic astaxanthin production from Haematococcus pluvialis under high temperature via heat stress-driven Haber–Weiss reaction. Appl. Microbiol. Biotechnol. 2015, 99, 5203–5215. [Google Scholar] [CrossRef] [PubMed]
- Mahadi, R.; Vahisan, L.P.S.; Ilhamsyah, D.P.A.; Kim, S.; Kim, B.; Lee, N.; Oh, Y.-K. Enhancement of astaxanthin and fatty acid production in Haematococcus pluvialis using strigolactone. Appl. Sci. 2022, 12, 1791. [Google Scholar] [CrossRef]
- Yao, J.; Kim, H.S.; Kim, J.Y.; Choi, Y.-E.; Park, J. Mechanical stress induced astaxanthin accumulation of H. pluvialis on a chip. Lab Chip. 2020, 20, 647–654. [Google Scholar] [CrossRef]
- Mahadi, R.; Kim, S.; Ilhamsyah, D.P.A.; Vahisan, L.P.S.; Narasimhan, L.A.; Park, G.W.; Lee, S.Y.; Oh, Y.-K. Rapid accumulation of astaxanthin in Haematococcus pluvialis induced by mild hydrostatic pressure. Biotechnol. Bioprocess Eng. 2023, 28, 345–351. [Google Scholar] [CrossRef]
- Lee, S.Y.; Oh, Y.-K.; Lee, S.; Fitriana, H.N.; Moon, M.; Kim, M.-S.; Lee, J.; Min, K.; Park, G.W.; Lee, J.-P.; et al. Recent developments and key barriers to microbial CO2 electrobiorefinery. Bioresour. Technol. 2021, 320, 124350. [Google Scholar] [CrossRef]
- Singh, N.K.; Mathuriya, A.S.; Mehrotra, S.; Pandit, S.; Singh, A.; Jadhav, D. Advances in bioelectrochemical systems for bio-products recovery. Environ. Technol. 2023, 44, 1–24. [Google Scholar] [CrossRef]
- ElSayed, M.; Aghahosseini, A.; Caldera, U.; Breyer, C. Analysing the techno-economic impact of e-fuels and e-chemicals production for exports and carbon dioxide removal on the energy system of sunbelt countries—Case of Egypt. Appl. Energy. 2023, 343, 121216. [Google Scholar] [CrossRef]
- Bauer, A.; Minceva, M. Examination of photo-, mixo-, and heterotrophic cultivation conditions on Haematococcus pluvialis cyst cell germination. Appl. Sci. 2021, 11, 7201. [Google Scholar] [CrossRef]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, C.; Jeon, M.S.; Park, J.; Choi, Y.-E. Enhancement of microalga Haematococcus pluvialis growth and astaxanthin production by electrical treatment. Bioresour. Technol. 2018, 268, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Fitriana, H.N.; Lee, S.-Y.; Choi, S.-A.; Lee, J.-Y.; Kim, B.-L.; Lee, J.-S.; Oh, Y.-K. Electric stimulation of astaxanthin biosynthesis in Haematococcus pluvialis. Appl. Sci. 2021, 11, 3348. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.Y.; Yoon, J. The role of reactive oxygen species in the electrochemical inactivation of microorganisms. Environ. Sci. Technol. 2006, 40, 6117–6122. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.-E.; Park, M.; Song, Y.E.; Seol, E.; Kim, J.R.; Oh, Y.-K. Microbial enrichment and community analysis for bioelectrochemical acetate production from carbon dioxide. New Renew. Energy. 2020, 16, 58–67. [Google Scholar] [CrossRef]
- Choi, S.-A.; Lee, S.Y.; Lee, J.; Cho, J.M.; Lee, J.-S.; Kim, S.W.; Kim, D.-Y.; Park, S.-K.; Jin, C.-S.; Oh, Y.-K. Rapid induction of edible lipids in Chlorella by mild electric stimulation. Bioresour. Technol. 2019, 292, 121950. [Google Scholar] [CrossRef]
- Peled, E.; Pick, U.; Zarka, A.; Shimoni, E.; Leu, S.; Boussiba, S. Light-induced oil globule migration in Haematococcus pluvialis (Chlorophyceae). J. Phycol. 2012, 48, 1209–1219. [Google Scholar] [CrossRef]
- Elisabeth, B.; Rayen, F.; Behnam, T. Microalgae culture quality indicators: A Review. Crit. Rev. Biotechnol. 2021, 41, 457–473. [Google Scholar] [CrossRef]
- Delran, P.; Frances, C.; Peydecastaing, J.; Pontalier, P.Y.; Guihéneuf, F.; Barthe, L. Cell destruction level and metabolites green-extraction of Tetraselmis suecica by low and intermediate frequency ultrasound. Ultrason. Sonochem. 2023, 98, 106492. [Google Scholar] [CrossRef]
- Li, D.; Zhao, Y.; Ding, W.; Zhao, P.; Xu, J.-W.; Li, T.; Ma, H.; Yu, X. A strategy for promoting lipid production in green microalgae Monoraphidium sp. QLY-1 by combined melatonin and photoinduction. Bioresour. Technol. 2017, 235, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Che, R.; Huang, L.; Xu, J.W.; Zhao, P.; Li, T.; Ma, H.; Yu, X. Effect of fulvic acid induction on the physiology, metabolism, and lipid biosynthesis-related gene transcription of Monoraphidium sp. FXY-10. Bioresour. Technol. 2017, 227, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.S.; do Nascimento, T.C.; Jacob-Lopes, E.; De Rosso, V.V.; Zepka, L.Q. Introductory chapter: Carotenoids: A brief overview on its structure, biosynthesis, synthesis, and applications. In Progress in Carotenoid Research; Zepka, L.Q., Jacob-Lopes, E., De Rosso, V.V., Eds.; BoD-Books on Demand: Norderstedt, Germany, 2018; pp. 1–17. [Google Scholar]
- Chekanov, K. Diversity and distribution of carotenogenic algae in Europe: A review. Mar. Drugs 2023, 21, 108. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Li, Y.; Hu, Q. Astaxanthin in microalgae: Pathways, functions, and biotechnological implications. Algae 2013, 28, 131–147. [Google Scholar] [CrossRef]
- Yu, X.; Chen, L.; Zhang, W. Chemicals to enhance microalgal growth and accumulation of high-value bioproducts. Front. Microbiol. 2015, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, Z.; Xu, X.; Cheng, J.; Chen, S.; Tian, J.; Yang, W.; Crocker, M. Simultaneous promotion of photosynthesis and astaxanthin accumulation during two stages of Haematococcus pluvialis with ammonium ferric citrate. Sci. Total Environ. 2021, 750, 141689. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; You, J.; Qiao, T.; Zhong, D.-B.; Yu, X. Sodium chloride stimulates the biomass and astaxanthin production by Haematococcus pluvialis via a two-stage cultivation strategy. Bioresour. Technol. 2022, 344, 126214. [Google Scholar] [CrossRef]
- Han, D.; Wang, J.; Sommerfeld, M.; Hu, Q. Susceptibility and protective mechanisms of motile and non motile cells of Haematococcus pluvialis (Chlorophyceae) to photooxidative stress. J. Phycol. 2012, 48, 693–705. [Google Scholar] [CrossRef]
- Pan, X.; Li, T.; Wang, B.; Qi, S.; Yang, D.; Huang, Z.; Gao, R.; Li, J.; Ling, X.; Lu, Y. Metabolic mechanism of astaxanthin biosynthesis in Xanthophyllomyces dendrorhous in response to sodium citrate treatment. Bioresour. Bioprocess. 2023, 10, 29. [Google Scholar] [CrossRef]
- Guermazi, W.; Masmoudi, S.; Trabelsi, N.A.; Gammoudi, S.; Ayadi, H.; Morant-Manceau, A.; Hotos, G.N. Physiological and biochemical responses in microalgae Dunaliella salina, Cylindrotheca closterium and Phormidium versicolor NCC466 exposed to high salinity and irradiation. Life 2023, 13, 313. [Google Scholar] [CrossRef]
- Choi, S.-A.; Jeong, Y.; Lee, J.; Huh, Y.H.; Choi, S.H.; Kim, H.-S.; Cho, D.-H.; Lee, J.-S.; Kim, H.; An, H.-R.; et al. Biocompatible liquid-type carbon nanodots (C-paints) as light delivery materials for cell growth and astaxanthin induction of Haematococcus pluvialis. Mater. Sci. Eng. C 2020, 109, 110500. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.V.; Derner, R.B.; Lemos-Senna, E. Preparation and characterization of Haematococcus pluvialis carotenoid-loaded PLGA nanocapsules in a gel system with antioxidant properties for topical application. J. Drug Deliv. Sci. Technol. 2021, 61, 102099. [Google Scholar] [CrossRef]
- Yilancioglu, K.; Cokol, M.; Pastirmaci, I.; Erman, B.; Cetiner, S. Oxidative stress is a mediator for increased lipid accumulation in a newly isolated Dunaliella salina strain. PLoS ONE 2014, 9, e91957. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sathiyavahisan, L.P.; Lakshmi Narasimhan, A.; Mahadi, R.; Kim, S.; Christabel, C.; Yu, H.; Kim, Y.-E.; Oh, Y.-K. Rapid Induction of Astaxanthin in Haematococcus lacustris by Mild Electric Stimulation. Appl. Sci. 2023, 13, 12959. https://doi.org/10.3390/app132312959
Sathiyavahisan LP, Lakshmi Narasimhan A, Mahadi R, Kim S, Christabel C, Yu H, Kim Y-E, Oh Y-K. Rapid Induction of Astaxanthin in Haematococcus lacustris by Mild Electric Stimulation. Applied Sciences. 2023; 13(23):12959. https://doi.org/10.3390/app132312959
Chicago/Turabian StyleSathiyavahisan, Laxmi Priya, Aditya Lakshmi Narasimhan, Rendi Mahadi, Sangui Kim, Catherine Christabel, Hyoji Yu, Young-Eun Kim, and You-Kwan Oh. 2023. "Rapid Induction of Astaxanthin in Haematococcus lacustris by Mild Electric Stimulation" Applied Sciences 13, no. 23: 12959. https://doi.org/10.3390/app132312959
APA StyleSathiyavahisan, L. P., Lakshmi Narasimhan, A., Mahadi, R., Kim, S., Christabel, C., Yu, H., Kim, Y. -E., & Oh, Y. -K. (2023). Rapid Induction of Astaxanthin in Haematococcus lacustris by Mild Electric Stimulation. Applied Sciences, 13(23), 12959. https://doi.org/10.3390/app132312959






