Experimental Study of Discharging Magnesium-Dissolved Oxygen Seawater Batteries with Various Binder Ratios
Abstract
:1. Introduction
2. Experimental Methods
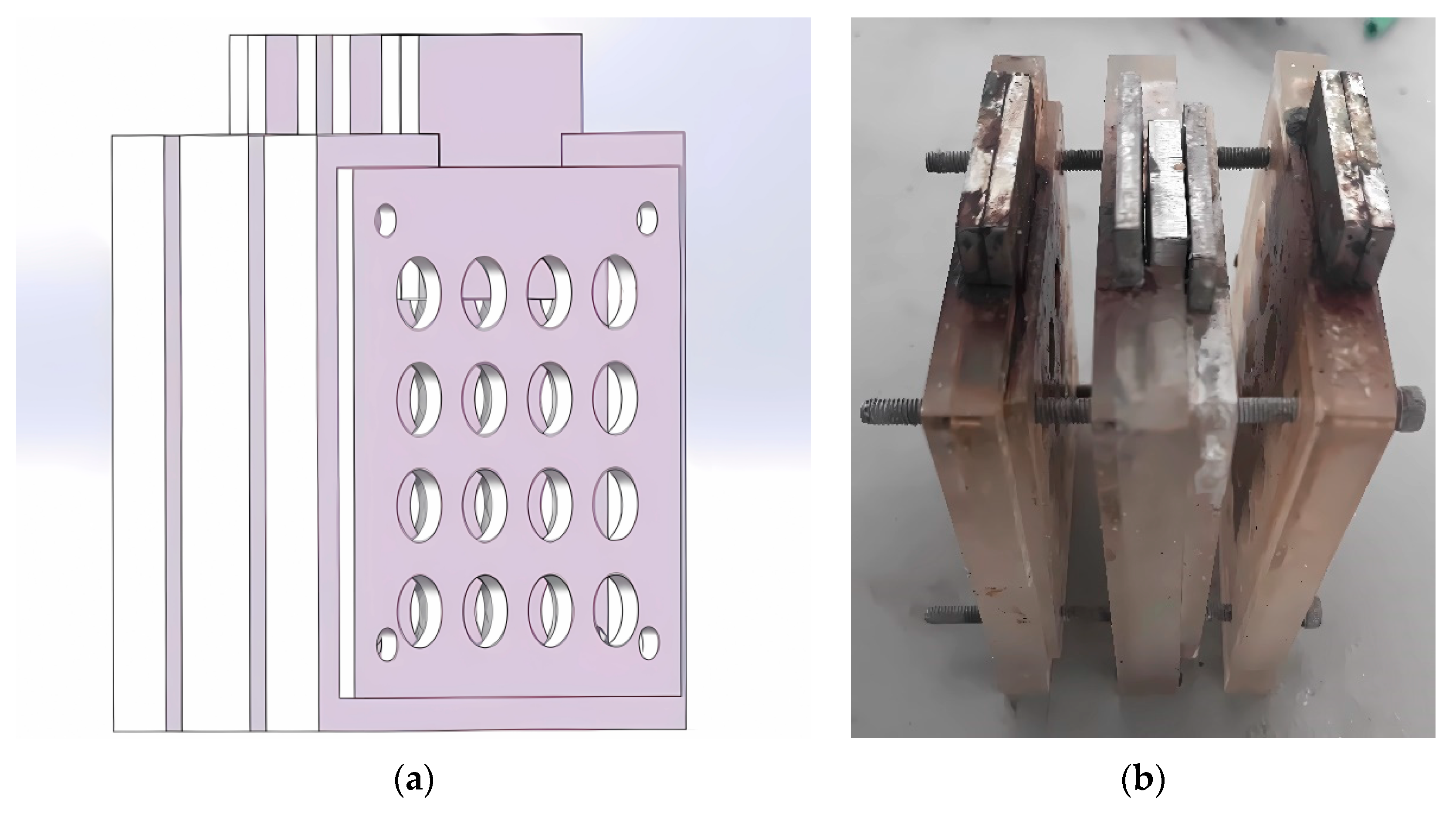
2.1. Electrode Preparation
2.2. Cell Assembly and Testing
2.3. Nitrogen Adsorption and Desorption
3. Results and Discussion
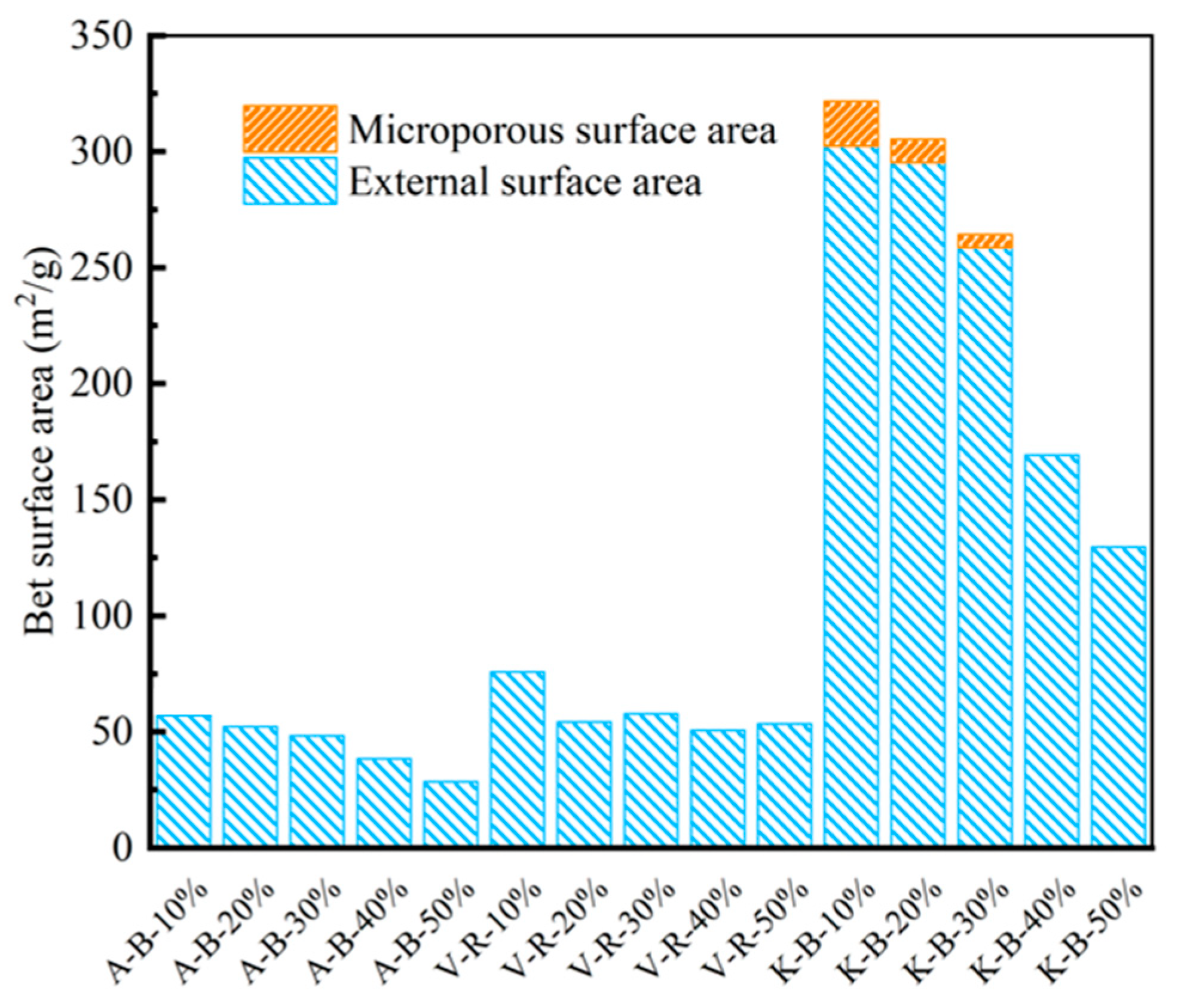
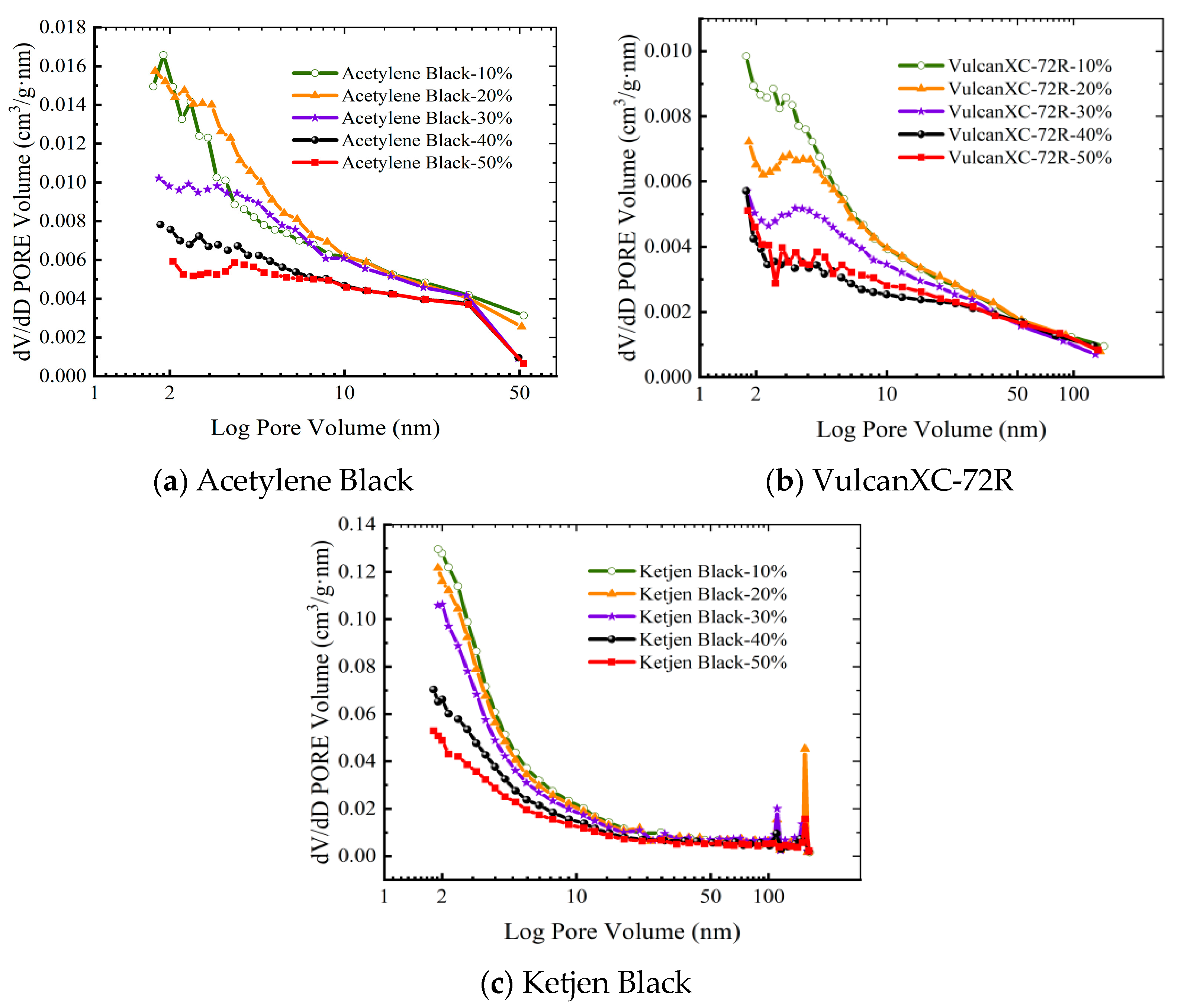
3.1. Porous Structure Characterization
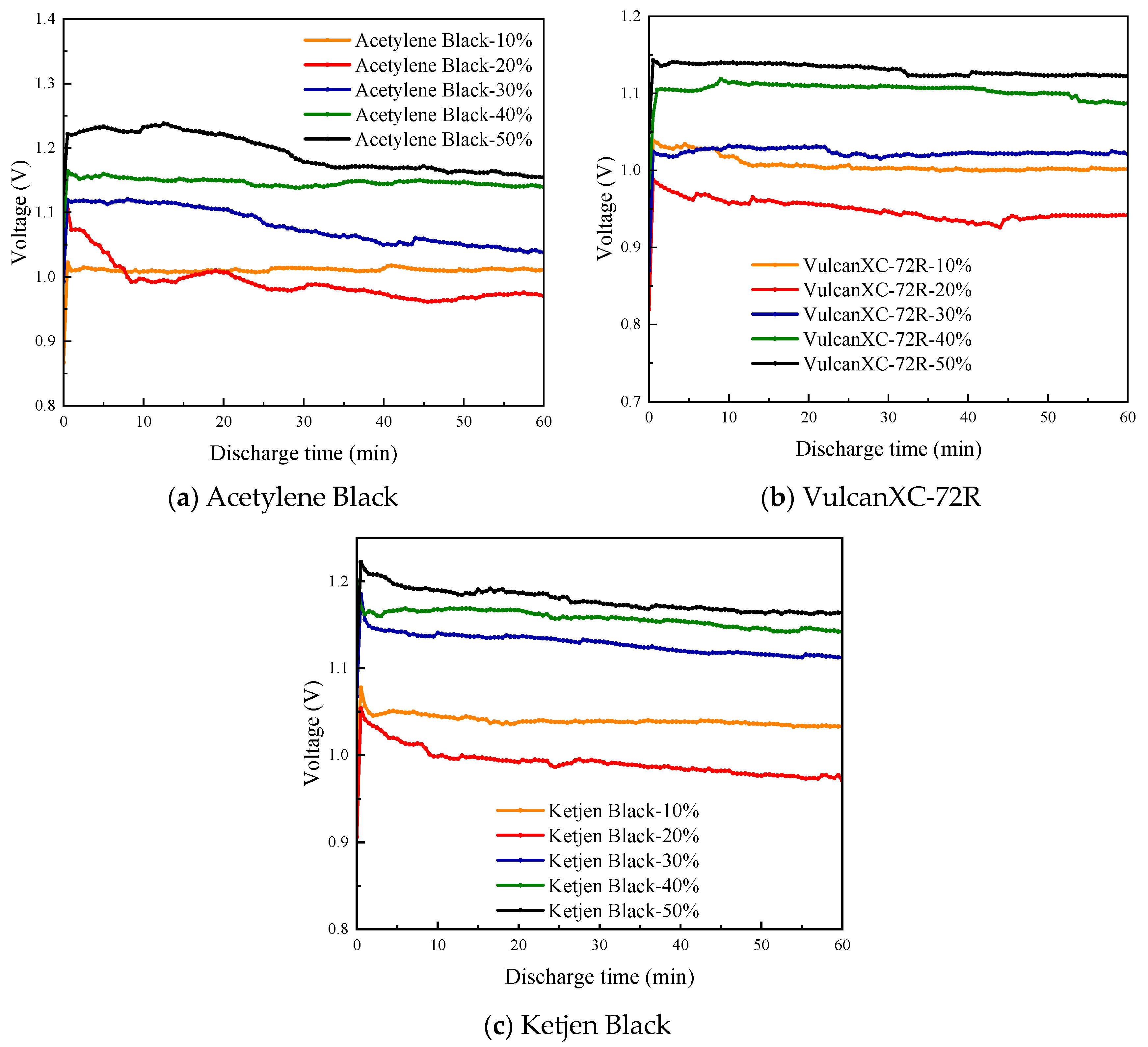
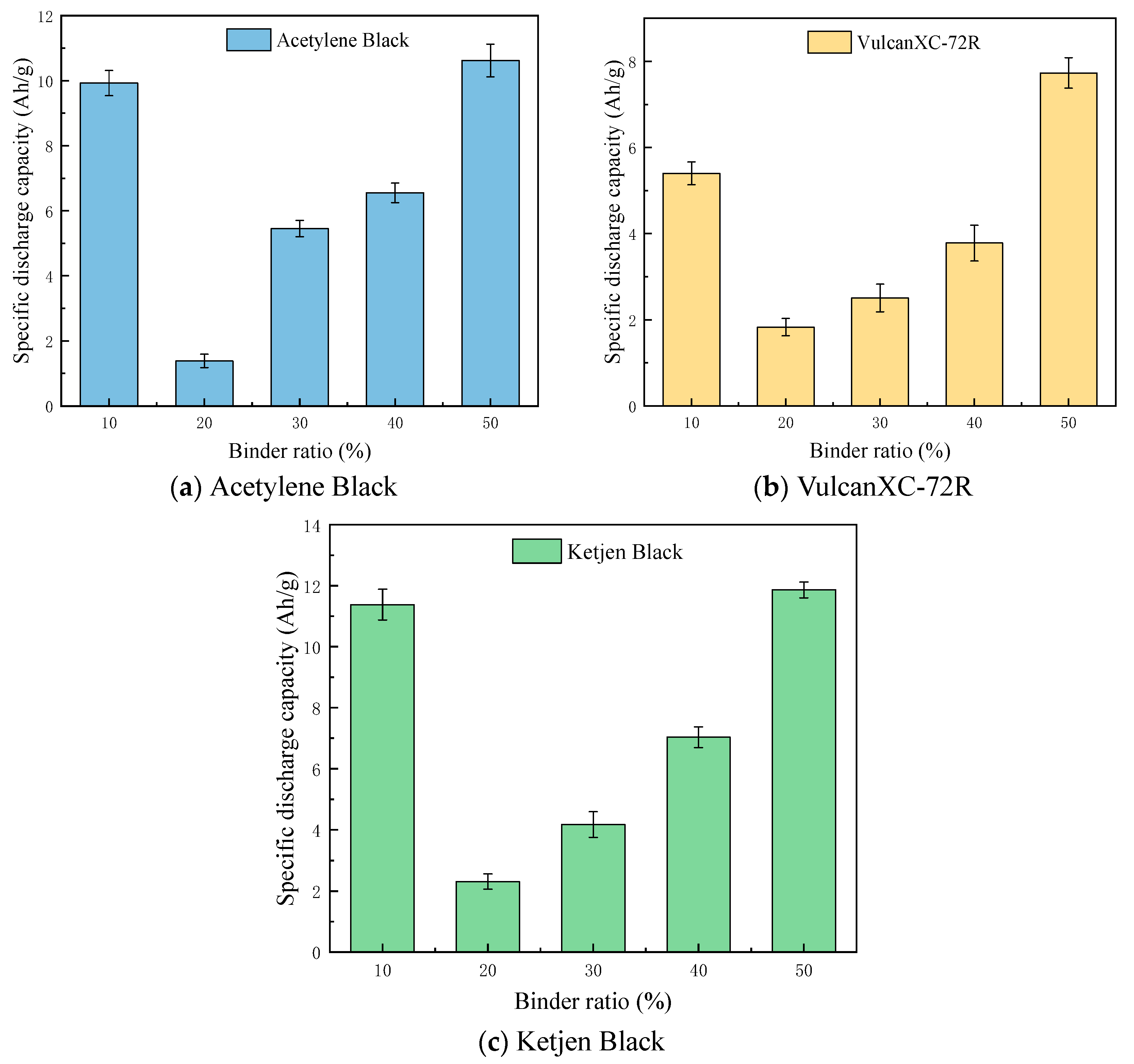
3.2. Discharge Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, T.L.; Huang, J.L.; Li, Z.W.; Liu, Q.; Li, J.; Jiang, K.; Yan, X.; Liu, N. Planning Scheme Design for Multi-time Scale Energy Storage at the City Level. In Proceedings of the 58th IEEE/IAS Industrial and Commercial Power Systems Technical Conference Asia (IEEE I and CPS Asia), Shanghai, China, 8–11 July 2022. [Google Scholar]
- Wang, F.; Kahol, P.K.; Gupta, R.; Li, X. Experimental Studies of Carbon Electrodes With Various Surface Area for Li–O2 Batteries. J. Electrochem. Energy Convers. Storage 2019, 16, 041007. [Google Scholar] [CrossRef]
- Lung, L.-Y.; Chou, T.-Y.; Chang, W.-C.; Kuo, C.-C. Development of Energy Storage Systems for High Penetration of Renewable Energy Grids. Appl. Sci. 2023, 13, 11978. [Google Scholar] [CrossRef]
- Saravanakumar, Y.N.; Sultan, M.T.H.; Shahar, F.S.; Giernacki, W.; Łukaszewicz, A.; Nowakowski, M.; Holovatyy, A.; Stępień, S. Power Sources for Unmanned Aerial Vehicles: A State-of-the Art. Appl. Sci. 2023, 13, 11932. [Google Scholar] [CrossRef]
- Vegge, T.; Garcia-Lastra, J.M.; Siegel, D.J. Lithium-oxygen batteries: At a crossroads? Curr. Opin. Electrochem. 2017, 6, 100–107. [Google Scholar] [CrossRef]
- Chen, Y.H.; Xu, J.J.; He, P.; Qiao, Y.; Guo, S.; Yang, H.; Zhou, H. Metal-air batteries: Progress and perspective. Sci. Bull. 2022, 67, 2449–2486. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P.; Blanco, M.; Bugga, R. Boron-based anion receptors in lithium-ion and metal-air batteries. J. Power Sources 2014, 247, 813–820. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.G.; Xie, Z.J.; Zhou, Z. Recent progress in rechargeable alkali metal-air batteries. Green Energy Environ. 2016, 1, 4–17. [Google Scholar] [CrossRef]
- Wang, F.; Liang, C.S.; Xu, D.L.; Cao, H.-Q.; Sun, H.-Y.; Luo, Z.-K. Research Progress of Lithium-air Battery. J. Inorg. Mater. 2012, 27, 1233–1242. [Google Scholar] [CrossRef]
- Aurbach, D.; Mccloskey, B.D.; Nazar, L.F.; Bruce, P.G. Advances in understanding mechanisms underpinning lithium-air batteries. Nat. Energy 2016, 1, 16128. [Google Scholar] [CrossRef]
- Yu, Z.; Shi, G.; Ju, D.Y. Electrochemical Properties Evaluation of a Novel Mg Alloy Anode on Mg-1 Air Batteries. Int. J. Electrochem. Sci. 2014, 9, 6668–6676. [Google Scholar]
- Rahman, M.A.; Wang, X.J.; Wen, C.E. High Energy Density Metal-Air Batteries: A Review. J. Electrochem. Soc. 2013, 160, A1759–A1771. [Google Scholar] [CrossRef]
- Yu, J.X.; Ma, L.; Duan, T.G.; Xin, Y.; Lv, Y.; Zhang, H. Electrocatalytic oxygen reduction of three-dimensional carbon fiber-based composites for seawater oxygen-dissolved battery. Carbon Lett. 2022, 32, 537–546. [Google Scholar] [CrossRef]
- Wilcock, W.S.D.; Kauffman, P.C. Development of a seawater battery for deep-water applications. J. Power Sources 1997, 66, 71–75. [Google Scholar] [CrossRef]
- Song, J.; Ji, Y.; Li, Y.; Tong, R.; Tian, Q.; Chen, J.; Chen, Z. Porous carbon with carbon nanotube scaffold for embedding Cu2O/Cu nanoparticles towards high lithium storage. Chem. Phys. Lett. 2021, 780, 138934. [Google Scholar] [CrossRef]
- Wu, A.; Li, C.; Han, B.; Hanson, S.; Guan, W.; Singhal, S.C. Effect of air addition to the air electrode on the stability and efficiency of carbon dioxide electrolysis by solid oxide cells. Int. J. Hydrogen Energy 2022, 47, 24268–24278. [Google Scholar] [CrossRef]
- Rao, Y.; Zhong, S.; He, F.; Wang, Z.; Peng, R.; Lu, Y. Cobalt-doped BaZrO3: A single phase air electrode material for reversible solid oxide cells. Int. J. Hydrogen Energy 2012, 37, 12522–12527. [Google Scholar] [CrossRef]
- Kunanusont, N.; Shimoyama, Y. Porous carbon electrode for Li-air battery fabricated from solvent expansion during supercritical drying. J. Supercrit. Fluids 2018, 133, 77–85. [Google Scholar] [CrossRef]
- Ishihara, T.; Yokoe, K.; Miyano, T.; Kusaba, H. Mesoporous MnCo2O4 spinel oxide for a highly active and stable air electrode for Zn-air rechargeable battery. Electrochim. Acta 2019, 300, 455–460. [Google Scholar] [CrossRef]
- Milikić, J.; Nastasić, A.; Martins, M.; Sequeira, C.A.C.; Šljukić, B. Air Cathodes and Bifunctional Oxygen Electrocatalysts for Aqueous Metal–Air Batteries. Batteries 2023, 9, 394. [Google Scholar] [CrossRef]
- Williford, R.E.; Zhang, J.-G. Air electrode design for sustained high power operation of Li/air batteries. J. Power Sources 2009, 194, 1164–1170. [Google Scholar] [CrossRef]
- Lin, C.; Li, X.; Shinde, S.S.; Kim, D.H.; Song, X.; Zhang, H.; Lee, J.H. Long-life rechargeable Zn air battery based on binary metal carbide armored by nitrogen-doped carbon. ACS Appl. Energy Mater. 2019, 2, 1747–1755. [Google Scholar] [CrossRef]
- Tian, W.-W.; Ren, J.-T.; Lv, X.-W.; Yuan, Z.Y. A “gas-breathing” integrated air diffusion electrode design with improved oxygen utilization efficiency for high-performance Zn-air batteries. Chem. Eng. J. 2022, 431, 133210. [Google Scholar] [CrossRef]
- Ding, N.; Chien, S.W.; Hor, T.A.; Lum, R.; Zong, Y.; Liu, Z. Influence of carbon pore size on the discharge capacity of Li–O2 batteries. J. Mater. Chem. A 2014, 2, 12433–12441. [Google Scholar] [CrossRef]
- Read, J. Characterization of the lithium/oxygen organic electrolyte battery. J. Electrochem. Soc. 2002, 149, A1190–A1195. [Google Scholar] [CrossRef]
- Sakai, K.; Iwamura, S.; Mukai, S.R. Influence of the Porous Structure of the Cathode on the Discharge Capacity of Lithium-Air Batteries. J. Electrochem. Soc. 2017, 164, A3075–A3080. [Google Scholar] [CrossRef]
- Ou, Z.H.; Qin, Y.; Xue, C.L.; Lum, R.; Zong, Y.; Liu, Z. Double-activator modulation of ultrahigh surface areas on doped carbon catalysts boosts the primary Zn–Air battery performance. ACS Appl. Energy Mater. 2022, 5, 1701–1709. [Google Scholar] [CrossRef]
- Hayashi, M.; Minowa, H.; Takahashi, M.; Shodai, T. Surface Properties and Electrochemical Performance of Carbon Materials for Air Electrodes of Lithium-Air Batteries. Electrochemistry 2010, 78, 325–328. [Google Scholar] [CrossRef]
- Ferreira, J.; Salgueiro, T.; Marcuzzo, J.; Arruda, E.; Ventura, J.; Oliveira, J. Textile PAN Carbon Fibers Cathode for High-Voltage Seawater Batteries. Batteries 2023, 9, 178. [Google Scholar] [CrossRef]
- Kuboki, T.; Okuyama, T.; Ohsaki, T.; Takami, N. Lithium-air batteries using hydrophobic room temperature ionic liquid electrolyte. J. Power Sources 2005, 146, 766–769. [Google Scholar] [CrossRef]
- Léonard, A.F.; Castro-Muniz, A.; Suarez-Garcia, F.; Job, N.; Paredes, J.I. Understanding the effect of the mesopore volume of ordered mesoporous carbons on their electrochemical behavior as Li-ion battery anodes. Microporous Mesoporous Mater. 2020, 306, 110417. [Google Scholar] [CrossRef]
- Peng, H.; Ma, G.F.; Sun, K.J.; Mu, J.; Lei, Z. One-step preparation of ultrathin nitrogen-doped carbon nanosheets with ultrahigh pore volume for high-performance supercapacitors. J. Mater. Chem. A 2014, 2, 17297–17301. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, J.E.; Kim, S.; Lim, M.S.; Jin, A.; Kim, O.H.; Kim, M.J.; Lee, K.S.; Kim, J.; Kim, S.S.; et al. Biomass-derived air cathode materials: Pore-controlled S, N-Co-doped carbon for fuel cells and metal–air batteries. ACS Catal. 2019, 9, 3389–3398. [Google Scholar] [CrossRef]
- Wang, F.; Li, X. Effects of the Electrode Wettability on the Deep Discharge Capacity of Li–O2 Batteries. ACS Omega 2018, 3, 6006–6012. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhou, X.; Cheng, S.; Xia, R.; Nie, S.; Liang, X.; Sun, Y.; Xiang, H. High-Voltage LiNi0.5Mn1.5O4 Cathode Stability of Fluorinated Ether Based on Enhanced Separator Wettability. J. Electrochem. Soc. 2019, 166, A1456–A1462. [Google Scholar] [CrossRef]
- Schroder, A.; Wippermann, K.; Zehl, G.; Stolten, D. The influence of cathode flow field surface properties on the local and time-dependent performance of direct methanol fuel cells. Electrochem. Commun. 2010, 12, 1318–1321. [Google Scholar] [CrossRef]
- Li, J.; Ye, D.D.; Zhu, X.; Liao, Q.; Ding, Y.D.; Tian, X. Effect of wettability of anode microporous layer on performance and operation duration of passive air-breathing direct methanol fuel cells. J. Appl. Electrochem. 2009, 39, 1771–1778. [Google Scholar] [CrossRef]
- Li, X. A Modeling Study of the Pore Size Evolution in Lithium-Oxygen Battery Electrodes. J. Electrochem. Soc. 2015, 162, A1636–A1645. [Google Scholar] [CrossRef]
- Liu, B.; Dai, Y.K.; Li, L.; Zhang, H.D.; Zhao, L.; Kong, F.R.; Sui, X.L.; Wang, Z.B. Effect of polytetrafluoroethylene (PTFE) in current collecting layer on the performance of zinc-air battery. Prog. Nat. Sci. Mater. Int. 2020, 30, 861–867. [Google Scholar] [CrossRef]
- Liu, S.; Wippermann, K.; Lehnert, W. Mechanism of action of polytetrafluoroethylene binder on the performance and durability of high-temperature polymer electrolyte fuel cells. Int. J. Hydrogen Energy 2021, 46, 14687–14698. [Google Scholar] [CrossRef]

| Carbon Electrode | Average Carbon Content (mg/cm2) |
|---|---|
| Acetylene Black | 3.19 |
| VulcanXC-72R | 3.58 |
| Ketjen Black | 3.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Li, W.; Wang, F.; Hao, X.; Tan, J. Experimental Study of Discharging Magnesium-Dissolved Oxygen Seawater Batteries with Various Binder Ratios. Appl. Sci. 2023, 13, 12996. https://doi.org/10.3390/app132412996
Cao Y, Li W, Wang F, Hao X, Tan J. Experimental Study of Discharging Magnesium-Dissolved Oxygen Seawater Batteries with Various Binder Ratios. Applied Sciences. 2023; 13(24):12996. https://doi.org/10.3390/app132412996
Chicago/Turabian StyleCao, Yimeng, Wanxing Li, Fangzhou Wang, Xiaowen Hao, and Jianyu Tan. 2023. "Experimental Study of Discharging Magnesium-Dissolved Oxygen Seawater Batteries with Various Binder Ratios" Applied Sciences 13, no. 24: 12996. https://doi.org/10.3390/app132412996





