Evaluation of the Effectiveness of Bioaugmentation-Assisted Phytoremediation of Soils Contaminated with Petroleum Hydrocarbons Using Echinacea purpurea
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil, Microbial Consortia, γ-PGA and Plant Description
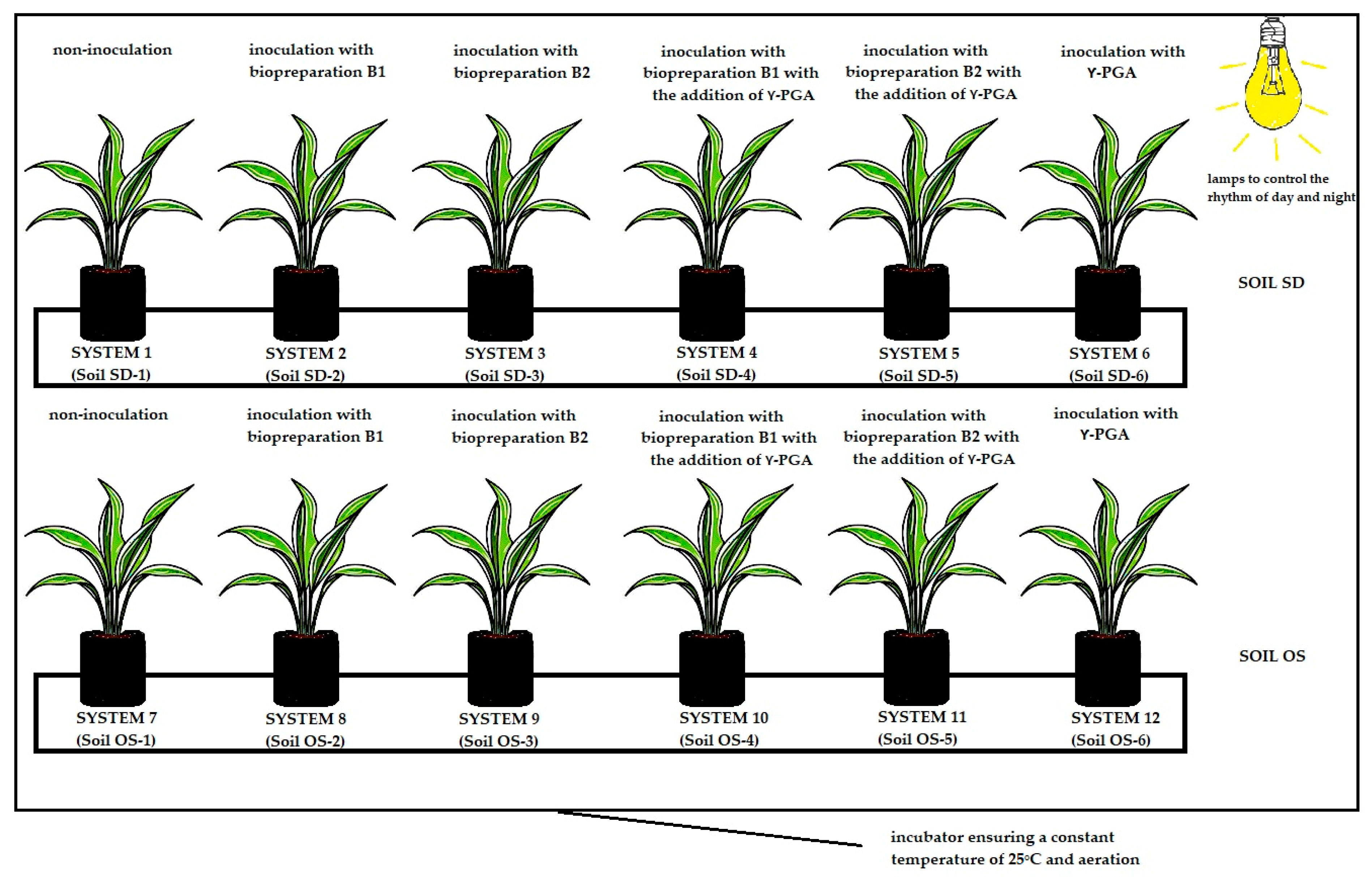
2.2. Course of the Experiment
2.2.1. Analysis of TPH in Soil and Plant Material
2.2.2. Analysis of PAHs in Soil and Plant Material
2.2.3. Soil Toxicity Analysis
3. Results
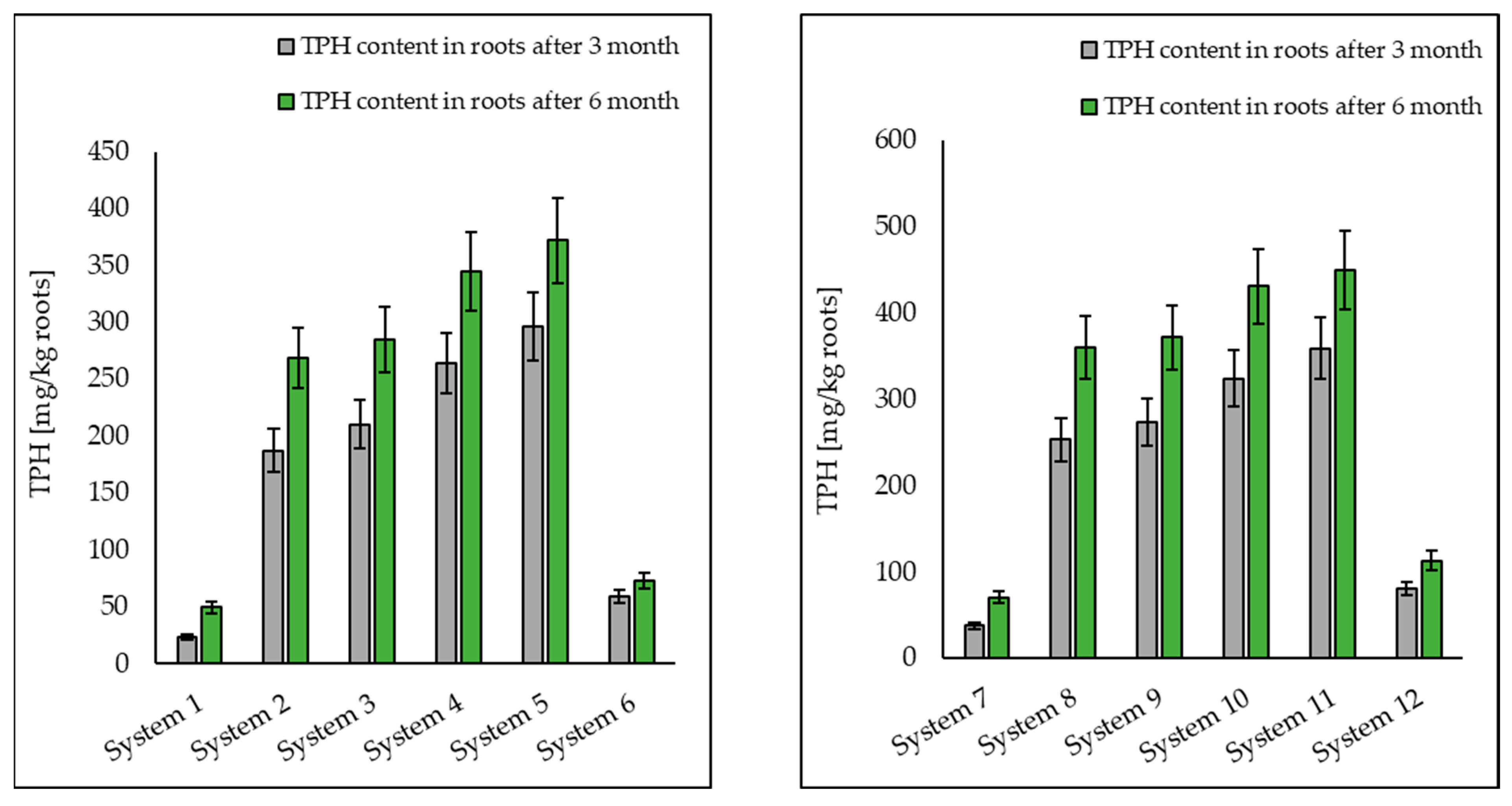
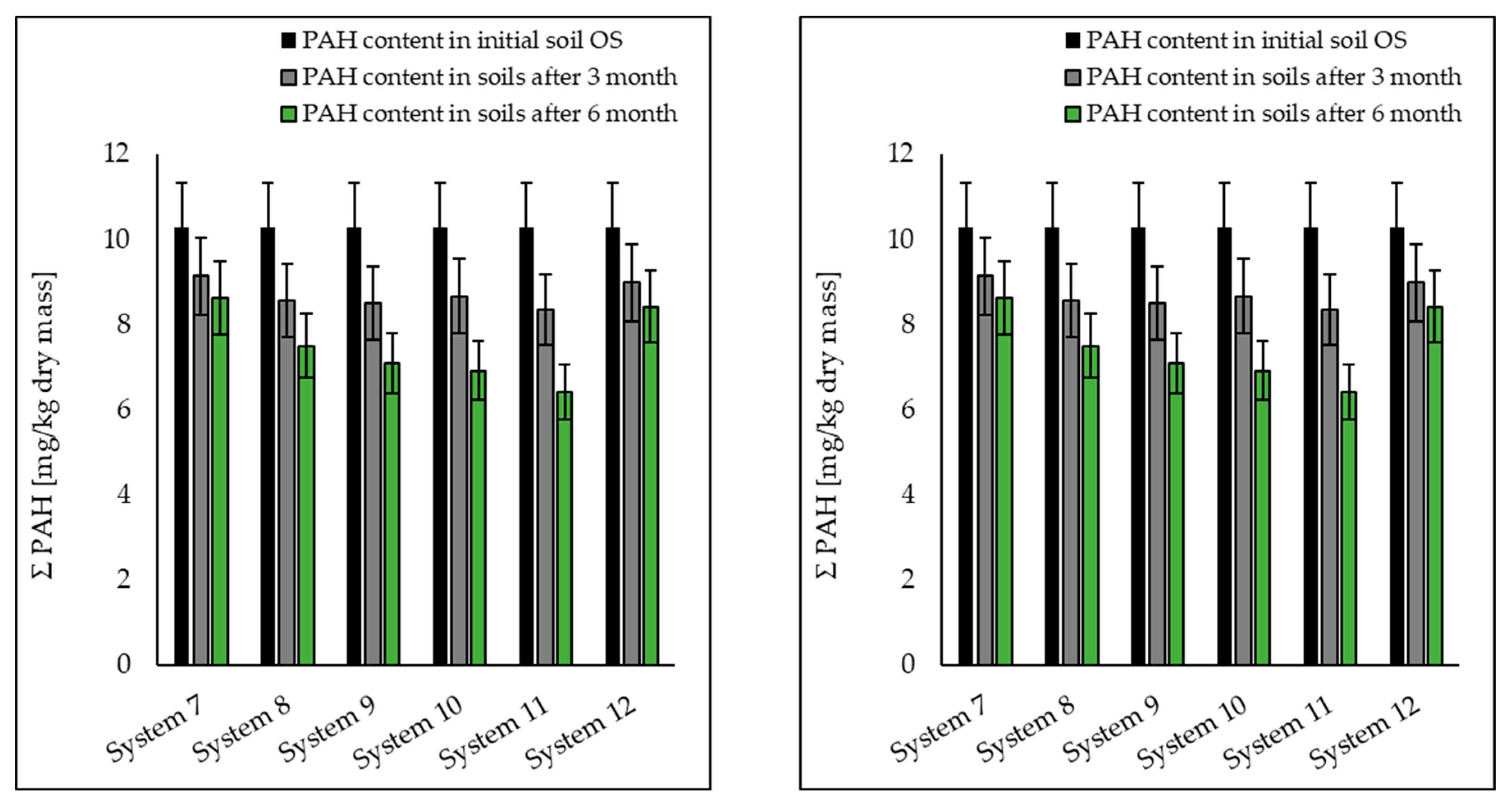
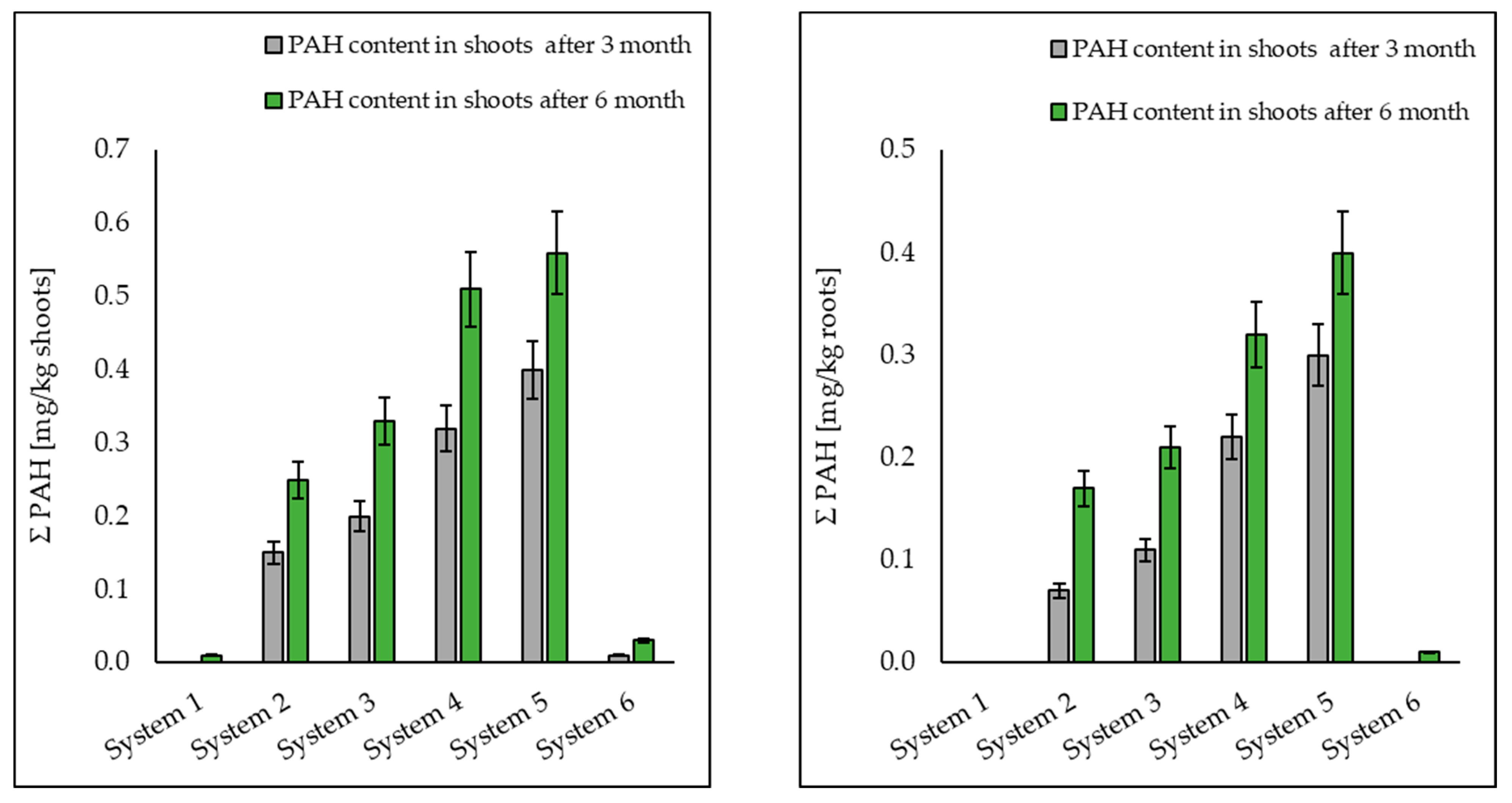
3.1. Analysis of Petroleum Hydrocarbons in Soil, Root and Shoot
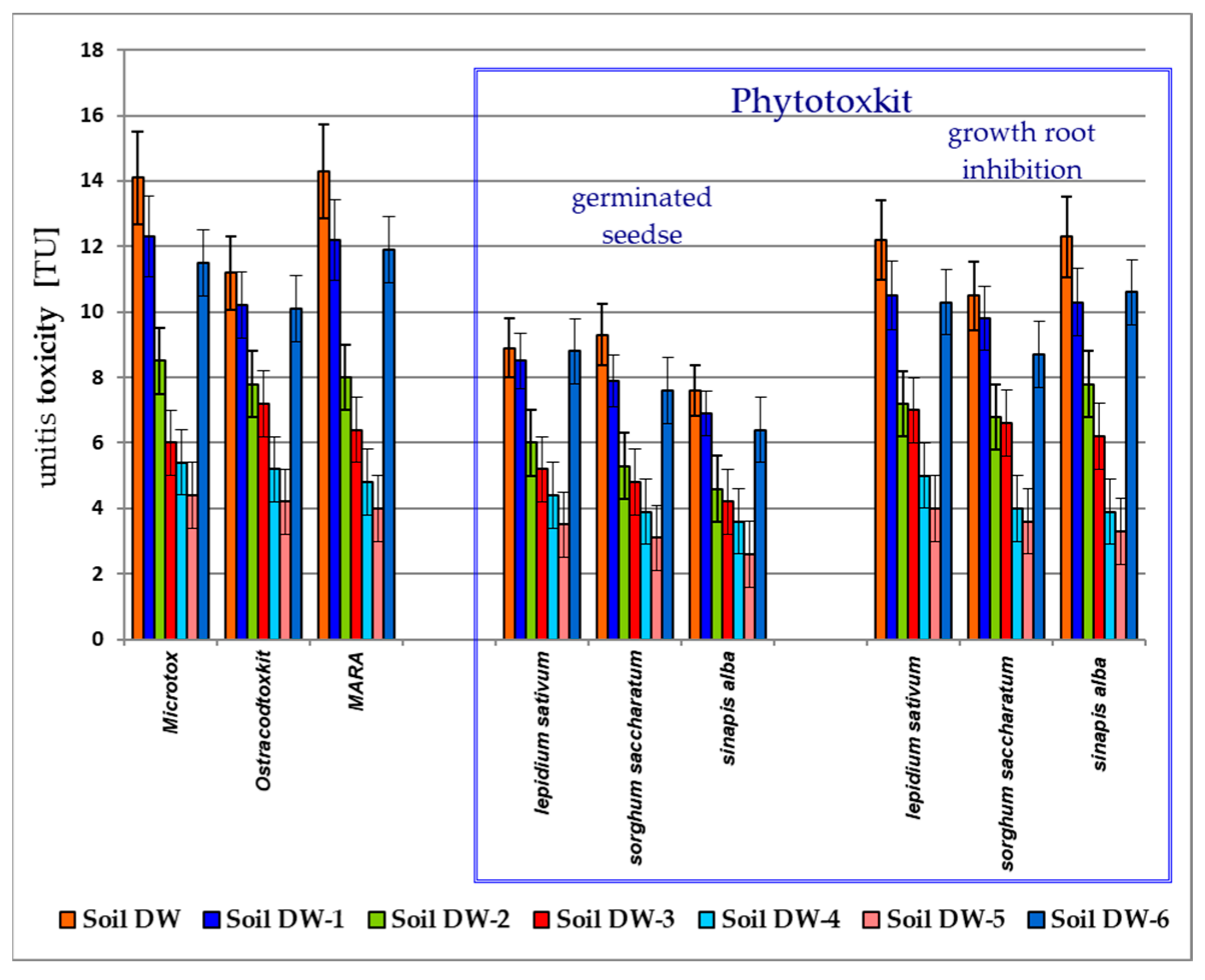
3.2. Toxicological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steliga, T. Bioremediacja Odpadów Wiertniczych Zanieczyszczonych Substancjami Ropopochodnymi ze Starych Dołów Urobkowych; Instytut Nafty i Gazu: Krakow, Poland, 2009. [Google Scholar]
- Kluk, D.; Steliga, T. Efektywna Metoda Identyfikacji Zanieczyszczeń Ropopochodnych (TPH) i Wielopierścieniowych Węglowodorów Aromatycznych (WWA) w Glebach. Nafta-Gaz 2017, 73, 488–495. [Google Scholar] [CrossRef]
- Wojtowicz, K.; Steliga, T.; Skalski, T. Badania Laboratoryjne Wpływu Dodatku γ-PGA Na Efektywność Biodegradacji Węglowodorów Ropopochodnych. Nafta-Gaz 2022, 78, 668–678. [Google Scholar] [CrossRef]
- Tang, K.H.D. Phytoremediation: Where Do We Go from Here? Biocatal. Agric. Biotechnol. 2023, 50, 102721. [Google Scholar] [CrossRef]
- Rai, P.K.; Kim, K.-H.; Lee, S.S.; Lee, J.-H. Molecular Mechanisms in Phytoremediation of Environmental Contaminants and Prospects of Engineered Transgenic Plants/Microbes. Sci. Total Environ. 2020, 705, 135858. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xin, X.; Zhou, Q. Phytoremediation of Contaminated Soils Using Ornamental Plants. Environ. Rev. 2018, 26, 43–54. [Google Scholar] [CrossRef]
- Małachowska-Jutsz, A.; Janosz, W.; Rudek, J. Toksyczność gleby zanieczyszczonej olejem silnikowym poddanej samooczyszczaniu oraz fitoremediacji. Ochr. Sr. 2012, 34, 15–20. [Google Scholar]
- Khan, S.; Afzal, M.; Iqbal, S.; Khan, Q.M. Plant–Bacteria Partnerships for the Remediation of Hydrocarbon Contaminated Soils. Chemosphere 2013, 90, 1317–1332. [Google Scholar] [CrossRef]
- Pawlik, M.; Piotrowska-Seget, Z. Endophytic Bacteria Associated with Hieracium piloselloides: Their Potential for Hydrocarbon-Utilizing and Plant Growth-Promotion. J. Toxicol. Environ. Health Part A 2015, 78, 860–870. [Google Scholar] [CrossRef]
- Saeidnia, S.; Manayi, A.; Vazirian, M. Echinacea purpurea: Pharmacology, Phytochemistry and Analysis Methods. Pharmacogn. Rev. 2015, 9, 63. [Google Scholar] [CrossRef]
- Heidari, S.; Fotouhi Ghazvini, R.; Zavareh, M.; Kafi, M. Physiological Responses and Phytoremediation Ability of Eastern Coneflower (Echinacea purpurea) for Crude Oil Contaminated Soil. CJES 2018, 16, 149–164. [Google Scholar] [CrossRef]
- Kubińska, N. Phytoremediation as an Approach to Clean up Contaminated Soil, Including Petroleum Product Contamination. Nafta-Gaz 2020, 76, 322–330. [Google Scholar] [CrossRef]
- Pretorius, T.R.; Charest, C.; Kimpe, L.E.; Blais, J.M. The Accumulation of Metals, PAHs and Alkyl PAHs in the Roots of Echinacea purpurea. PLoS ONE 2018, 13, e0208325. [Google Scholar] [CrossRef]
- Hou, L.; Liu, R.; Li, N.; Dai, Y.; Yan, J. Study on the Efficiency of Phytoremediation of Soils Heavily Polluted with PAHs in Petroleum-Contaminated Sites by Microorganism. Environ. Sci. Pollut. Res. 2019, 26, 31401–31413. [Google Scholar] [CrossRef]
- Sun, C.; Shen, X.; Zhang, Y.; Song, T.; Xu, L.; Xiao, J. Molecular Defensive Mechanism of Echinacea purpurea (L.) Moench against PAH Contaminations. Int. J. Mol. Sci. 2023, 24, 11020. [Google Scholar] [CrossRef]
- Sun, C.; Xiao, J.; Bai, L.; Bai, J.; Liu, J.; Geng, L.; Zhang, Y. Defined and Natural PAH Contaminations Shift PAH-Degrading Bacterial Community in Rhizosphere of Ornamental Plant Species Echinacea purpurea L. Environ. Technol. Innov. 2023, 31, 103189. [Google Scholar] [CrossRef]
- Wojtowicz, K.; Steliga, T.; Kapusta, P.; Brzeszcz, J. Oil-Contaminated Soil Remediation with Biodegradation by Autochthonous Microorganisms and Phytoremediation by Maize (Zea mays). Molecules 2023, 28, 6104. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Wang, X.; Li, Y.; Ji, H. Remediation of Petroleum-Contaminated Soils with Microbial and Microbial Combined Methods: Advances, Mechanisms, and Challenges. Sustainability 2021, 13, 9267. [Google Scholar] [CrossRef]
- Ptaszek, N.; Pacwa-Płociniczak, M.; Noszczyńska, M.; Płociniczak, T. Comparative Study on Multiway Enhanced Bio- and Phytoremediation of Aged Petroleum-Contaminated Soil. Agronomy 2020, 10, 947. [Google Scholar] [CrossRef]
- Abdullah, S.R.S.; Al-Baldawi, I.A.; Almansoory, A.F.; Purwanti, I.F.; Al-Sbani, N.H.; Sharuddin, S.S.N. Plant-Assisted Remediation of Hydrocarbons in Water and Soil: Application, Mechanisms, Challenges and Opportunities. Chemosphere 2020, 247, 125932. [Google Scholar] [CrossRef]
- Martínez-Rabelo, F.; Gómez-Guzmán, L.A.; García-Segura, D.R.; Villegas-García, E.; Rodriguez-Campos, J.; Velázquez-Fernández, J.B.; Hernández-Castellanos, B.; Barois, I.; Contreras-Ramos, S.M. Hydrocarbon Bioremediation in a Pilot-Scale: A Combination of Bioaugmentation, Phytoremediation, and Vermiremediation. Environ. Technol. Innov. 2023, 31, 103210. [Google Scholar] [CrossRef]
- Cutright, T.J.; Lee, S. Remediation of PAH-Contaminated Soil Using Achromobacter sp. Energy Sources 1994, 16, 279–287. [Google Scholar] [CrossRef]
- Hong, Y.-H.; Ye, C.-C.; Zhou, Q.-Z.; Wu, X.-Y.; Yuan, J.-P.; Peng, J.; Deng, H.; Wang, J.-H. Genome Sequencing Reveals the Potential of Achromobacter sp. HZ01 for Bioremediation. Front. Microbiol. 2017, 8, 1507. [Google Scholar] [CrossRef]
- Cerqueira, V.S.; Hollenbach, E.B.; Maboni, F.; Vainstein, M.H.; Camargo, F.A.O.; Peralba, M.D.C.R.; Bento, F.M. Biodegradation Potential of Oily Sludge by Pure and Mixed Bacterial Cultures. Bioresour. Technol. 2011, 102, 11003–11010. [Google Scholar] [CrossRef]
- Janbandhu, A.; Fulekar, M.H. Biodegradation of Phenanthrene Using Adapted Microbial Consortium Isolated from Petrochemical Contaminated Environment. J. Hazard. Mater. 2011, 187, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Oba, B.T.; Shen, C.; Rong, L.; Zhang, B.; Huang, L.; Feng, L.; Liu, J.; Du, T.; Deng, Y. Effect of the Bacterial Community Assembly Process on the Microbial Remediation of Petroleum Hydrocarbon-Contaminated Soil. Front. Microbiol. 2023, 14, 1196610. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, J.; Lin, J.; Wang, W.; Li, S. Biodegradation of Petroleum Hydrocarbons by Bacillus Subtilis BL-27, a Strain with Weak Hydrophobicity. Molecules 2019, 24, 3021. [Google Scholar] [CrossRef] [PubMed]
- Baburam, C.; Mitema, A.; Tsekoa, T.; Feto, N.A. Bacillus Species and Their Invaluable Roles in Petroleum Hydrocarbon Bioremediation. In Bacilli in Agrobiotechnology; Islam, M.T., Rahman, M., Pandey, P., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 101–126. ISBN 9783030854645. [Google Scholar]
- Rong, L.; Zheng, X.; Oba, B.T.; Shen, C.; Wang, X.; Wang, H.; Luo, Q.; Sun, L. Activating Soil Microbial Community Using Bacillus and Rhamnolipid to Remediate TPH Contaminated Soil. Chemosphere 2021, 275, 130062. [Google Scholar] [CrossRef]
- Wang, X.-B.; Chi, C.-Q.; Nie, Y.; Tang, Y.-Q.; Tan, Y.; Wu, G.; Wu, X.-L. Degradation of Petroleum Hydrocarbons (C6–C40) and Crude Oil by a Novel Dietzia Strain. Bioresour. Technol. 2011, 102, 7755–7761. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, J.; Sun, X.; Min, J.; Hu, X. High Efficiency Degradation of Alkanes and Crude Oil by a Salt-Tolerant Bacterium Dietzia Species CN-3. Int. Biodeterior. Biodegrad. 2017, 118, 110–118. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J.; Liu, Y.; Wu, X. Biological Process of Alkane Degradation by Gordonia sihwaniensis. ACS Omega 2022, 7, 55–63. [Google Scholar] [CrossRef]
- Kim, H.; Dong, K.; Kim, J.; Lee, S. Characteristics of Crude Oil-degrading Bacteria Gordonia iterans Isolated from Marine Coastal in Taean Sediment. MicrobiologyOpen 2019, 8, e00754. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Mou, T.; Wang, J.; Su, J.; Yan, Y.; Zhang, Y.-Q. Characterization of Three Rapidly Growing Novel Mycobacterium Species with Significant Polycyclic Aromatic Hydrocarbon Bioremediation Potential. Front. Microbiol. 2023, 14, 1225746. [Google Scholar] [CrossRef] [PubMed]
- Brzeszcz, J.; Steliga, T.; Kapusta, P.; Turkiewicz, A.; Kaszycki, P. R-Strategist versus K-Strategist for the Application in Bioremediation of Hydrocarbon-Contaminated Soils. Int. Biodeterior. Biodegrad. 2016, 106, 41–52. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Cheng, Z.; Yao, Y.; Chen, J. Biodegradation of Benzene, Toluene, Ethylbenzene, and o-Xylene by the Bacterium Mycobacterium Cosmeticum Byf-4. Chemosphere 2013, 90, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Rehmann, K.; Hertkorn, N.; Kettrup, A.A. Fluoranthene Metabolism in Mycobacterium sp. Strain KR20: Identity of Pathway Intermediates during Degradation and Growth. Microbiology 2001, 147, 2783–2794. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Pettersson, B.M.F.; Behra, P.R.K.; Ramesh, M.; Dasgupta, S.; Bhattacharya, A.; Kirsebom, L.A. Characterization of Three Mycobacterium spp. with Potential Use in Bioremediation by Genome Sequencing and Comparative Genomics. Genome Biol. Evol. 2015, 7, 1871–1886. [Google Scholar] [CrossRef]
- Solano-Serena, F.; Marchal, R.; Casarégola, S.; Vasnier, C.; Lebeault, J.-M.; Vandecasteele, J.-P. A Mycobacterium Strain with Extended Capacities for Degradation of Gasoline Hydrocarbons. Appl. Environ. Microbiol. 2000, 66, 2392–2399. [Google Scholar] [CrossRef]
- Karamalidis, A.K.; Evangelou, A.C.; Karabika, E.; Koukkou, A.I.; Drainas, C.; Voudrias, E.A. Laboratory Scale Bioremediation of Petroleum-Contaminated Soil by Indigenous Microorganisms and Added Pseudomonas aeruginosa Strain Spet. Bioresour. Technol. 2010, 101, 6545–6552. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, Z.; Yang, C.; Ma, C.; Tao, F.; Xu, P. Degradation of N-Alkanes and Polycyclic Aromatic Hydrocarbons in Petroleum by a Newly Isolated Pseudomonas aeruginosa DQ8. Bioresour. Technol. 2011, 102, 4111–4116. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Sarma, P.M.; Krishnan, S.; Mishra, S.; Lal, B. Evaluation of Genetic Diversity among Pseudomonas citronellolis Strains Isolated from Oily Sludge-Contaminated Sites. Appl. Environ. Microbiol. 2003, 69, 1435–1441. [Google Scholar] [CrossRef]
- Ghosh, I.; Jasmine, J.; Mukherji, S. Biodegradation of Pyrene by a Pseudomonas aeruginosa Strain RS1 Isolated from Refinery Sludge. Bioresour. Technol. 2014, 166, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Palleroni, N.J.; Pieper, D.H.; Moore, E.R.B. Microbiology of Hydrocarbon-Degrading Pseudomonas. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1787–1798. ISBN 9783540775843. [Google Scholar]
- Varjani, S.; Upasani, V.N.; Pandey, A. Bioremediation of Oily Sludge Polluted Soil Employing a Novel Strain of Pseudomonas aeruginosa and Phytotoxicity of Petroleum Hydrocarbons for Seed Germination. Sci. Total Environ. 2020, 737, 139766. [Google Scholar] [CrossRef]
- Nazari, M.T.; Simon, V.; Machado, B.S.; Crestani, L.; Marchezi, G.; Concolato, G.; Ferrari, V.; Colla, L.M.; Piccin, J.S. Rhodococcus: A Promising Genus of Actinomycetes for the Bioremediation of Organic and Inorganic Contaminants. J. Environ. Manag. 2022, 323, 116220. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, J.; Wang, Y.; Meng, T.; Li, M. Bioremediation of the Petroleum Contaminated Desert Steppe Soil with Rhodococcus erythropolis KB1 and Its Effect on the Bacterial Communities of the Soils. Geomicrobiol. J. 2021, 38, 842–849. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Chaudhary, D.K.; Jeong, S.-W.; Kim, J. Van Hong Thi Pham; Chaudhary, D.K.; Jeong, S.-W.; Kim, J. Oil-Degrading Properties of a Psychrotolerant Bacterial Strain, Rhodococcus sp. Y2-2, in Liquid and Soil Media. World J. Microbiol. Biotechnol. 2018, 34, 33. [Google Scholar] [CrossRef]
- Pacwa-Płociniczak, M.; Czapla, J.; Płociniczak, T.; Piotrowska-Seget, Z. The Effect of Bioaugmentation of Petroleum-Contaminated Soil with Rhodococcus erythropolis Strains on Removal of Petroleum from Soil. Ecotoxicol. Environ. Saf. 2019, 169, 615–622. [Google Scholar] [CrossRef]
- Chen, X.; Shan, G.; Shen, J.; Zhang, F.; Liu, Y.; Cui, C. In Situ Bioremediation of Petroleum Hydrocarbon–Contaminated Soil: Isolation and Application of a Rhodococcus Strain. Int. Microbiol. 2022, 26, 411–421. [Google Scholar] [CrossRef]
- Płociniczak, T.; Fic, E.; Pacwa-Płociniczak, M.; Pawlik, M.; Piotrowska-Seget, Z. Improvement of Phytoremediation of an Aged Petroleum Hydrocarbon-Contaminated Soil by Rhodococcus erythropolis CD 106 Strain. Int. J. Phytoremediation 2017, 19, 614–620. [Google Scholar] [CrossRef]
- Mukherjee, A. Role of Aspergillus in Bioremediation Process. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 209–214. ISBN 9780444635051. [Google Scholar]
- Peidro-Guzmán, H.; Pérez-Llano, Y.; González-Abradelo, D.; Fernández-López, M.G.; Dávila-Ramos, S.; Aranda, E.; Hernández, D.R.O.; García, A.O.; Lira-Ruan, V.; Pliego, O.R.; et al. Transcriptomic Analysis of Polyaromatic Hydrocarbon Degradation by the Halophilic Fungus Aspergillus sydowii at Hypersaline Conditions. Environ. Microbiol. 2021, 23, 3435–3459. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Song, Q.; Zhang, S.; Xie, L.; Yang, J. Transmembrane Transport Mechanism of N-Hexadecane by Candida tropicalis: Kinetic Study and Proteomic Analysis. Ecotoxicol. Environ. Saf. 2021, 209, 111789. [Google Scholar] [CrossRef]
- Rana, S.; Handa, S.; Aggarwal, Y.; Puri, S.; Chatterjee, M. Role of Candida in the Bioremediation of Pollutants: A Review. Lett. Appl. Microbiol. 2023, 76, ovad103. [Google Scholar] [CrossRef] [PubMed]
- Birolli, W.G.; Santos, D.A.; Alvarenga, N.; Garcia, A.C.F.S.; Romão, L.P.C.; Porto, A.L.M. Biodegradation of Anthracene and Several PAHs by the Marine-Derived Fungus Cladosporium sp. CBMAI 1237. Mar. Pollut. Bull. 2018, 129, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Bakri, M. Assessing Some Cladosporium Species in the Biodegradation of Petroleum Hydrocarbon for Treating Oil Contamination. J. Appl. Microbiol. 2022, 133, 3296–3306. [Google Scholar] [CrossRef] [PubMed]
- Romauld, S.I.; Venkataraghavan, R.; Yuvaraj, D.; Devi, V.I.; Hashika, S. Mycoremediation of Hydrocarbon and Its Products Using Fusarium oxysporum. Res. J. Pharm. Technol. 2019, 12, 4216. [Google Scholar] [CrossRef]
- Wu, Y.-R.; Luo, Z.-H.; Vrijmoed, L.L.P. Biodegradation of Anthracene and Benz[a]Anthracene by Two Fusarium Solani Strains Isolated from Mangrove Sediments. Bioresour. Technol. 2010, 101, 9666–9672. [Google Scholar] [CrossRef] [PubMed]
- Zehra, A.; Dubey, M.K.; Meena, M.; Aamir, M.; Patel, C.B.; Upadhyay, R.S. Role of Penicillium Species in Bioremediation Processes. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 247–260. ISBN 9780444635013. [Google Scholar]
- Vanishree, M.; Thatheyus, A.J.; Ramya, D. Biodegradation of Petrol Using the Fungus Penicillium sp. Sci. Int. 2014, 2, 26–31. [Google Scholar] [CrossRef]
- Wojtowicz, K.; Steliga, T.; Kapusta, P.; Brzeszcz, J.; Skalski, T. Evaluation of the Effectiveness of the Biopreparation in Combination with the Polymer γ-PGA for the Biodegradation of Petroleum Contaminants in Soil. Materials 2022, 15, 400. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Shi, W. Poly-γ-Glutamic Acid Improves Water-Stable Aggregates, Nitrogen and Phosphorus Uptake Efficiency, Water-Fertilizer Productivity, and Economic Benefit in Barren Desertified Soils of Northwest China. Agric. Water Manag. 2021, 245, 106551. [Google Scholar] [CrossRef]
- Zając, E.; Fabiańska, M.J.; Jędrszczyk, E.; Skalski, T. Hydrocarbon Degradation and Microbial Survival Improvement in Response to γ-Polyglutamic Acid Application. Int. J. Environ. Res. Public Health 2022, 19, 15066. [Google Scholar] [CrossRef]
- Steliga, T.; Wojtowicz, K.; Kapusta, P.; Brzeszcz, J. Assessment of Biodegradation Efficiency of Polychlorinated Biphenyls (PCBs) and Petroleum Hydrocarbons (TPH) in Soil Using Three Individual Bacterial Strains and Their Mixed Culture. Molecules 2020, 25, 709. [Google Scholar] [CrossRef]
- Steliga, T.; Jakubowicz, P.; Kapusta, P. Changes in Toxicity during in Situ Bioremediation of Weathered Drill Wastes Contaminated with Petroleum Hydrocarbons. Bioresour. Technol. 2012, 125, 1–10. [Google Scholar] [CrossRef]
- Nwankwegu, A.S.; Orji, M.U.; Onwosi, C.O. Studies on Organic and In-Organic Biostimulants in Bioremediation of Diesel-Contaminated Arable Soil. Chemosphere 2016, 162, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, K. Opracowanie Metodyki Oznaczania WWA w Próbkach Gleb z Wykorzystaniem Chromatografii Cieczowej HPLC. Nafte-Gaz 2022, 78, 141–153. [Google Scholar] [CrossRef]
- Steliga, T.; Jakubowicz, P.; Wojtowicz, K.; Kluk, D. Zastosowanie Testów Toksykologicznych w Przemyśle Naftowym. Nafte-Gaz 2018, 74, 684–689. [Google Scholar] [CrossRef]
- Kamath, R.; Rentz, J.A.; Schnoor, J.L.; Alvarez, P.J.J. Chapter 16 Phytoremediation of Hydrocarbon-Contaminated Soils: Principles and Applications. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2004; Volume 151, pp. 447–478. ISBN 9780444516992. [Google Scholar]
- Wang, D.; Xie, Y.; Jaisi, D.P.; Jin, Y. Effects of Low-Molecular-Weight Organic Acids on the Dissolution of Hydroxyapatite Nanoparticles. Environ. Sci. Nano 2016, 3, 768–779. [Google Scholar] [CrossRef]
- Weyens, N.; Taghavi, S.; Barac, T.; Van Der Lelie, D.; Boulet, J.; Artois, T.; Carleer, R.; Vangronsveld, J. Bacteria Associated with Oak and Ash on a TCE-Contaminated Site: Characterization of Isolates with Potential to Avoid Evapotranspiration of TCE. Environ. Sci. Pollut. Res. 2009, 16, 830–843. [Google Scholar] [CrossRef] [PubMed]
- Weyens, N.; Van Der Lelie, D.; Artois, T.; Smeets, K.; Taghavi, S.; Newman, L.; Carleer, R.; Vangronsveld, J. Bioaugmentation with Engineered Endophytic Bacteria Improves Contaminant Fate in Phytoremediation. Environ. Sci. Technol. 2009, 43, 9413–9418. [Google Scholar] [CrossRef] [PubMed]
- Weyens, N.; Van Der Lelie, D.; Taghavi, S.; Vangronsveld, J. Phytoremediation: Plant–Endophyte Partnerships Take the Challenge. Curr. Opin. Biotechnol. 2009, 20, 248–254. [Google Scholar] [CrossRef]
- Li, D.; Hou, L.; Gao, Y.; Tian, Z.; Fan, B.; Wang, F.; Li, S. Recent Advances in Microbial Synthesis of Poly-γ-Glutamic Acid: A Review. Foods 2022, 11, 739. [Google Scholar] [CrossRef]
- Abbasian, F.; Lockington, R.; Megharaj, M.; Naidu, R. A Review on the Genetics of Aliphatic and Aromatic Hydrocarbon Degradation. Appl. Biochem. Biotechnol. 2016, 178, 224–250. [Google Scholar] [CrossRef]
- Rehman, K.; Imran, A.; Amin, I.; Afzal, M. Inoculation with Bacteria in Floating Treatment Wetlands Positively Modulates the Phytoremediation of Oil Field Wastewater. J. Hazard. Mater. 2018, 349, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Mani, D.; Kumar, C.; Patel, N.K.; Sivakumar, D. Enhanced Clean-up of Lead-Contaminated Alluvial Soil through Chrysanthemum indicum L. Int. J. Environ. Sci. Technol. 2015, 12, 1211–1222. [Google Scholar] [CrossRef]
- Ojuederie, O.; Babalola, O. Microbial and Plant-Assisted Bioremediation of Heavy Metal Polluted Environments: A Review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef]
- Kadam, S.K.; Watharkar, A.D.; Chandanshive, V.V.; Khandare, R.V.; Jeon, B.-H.; Jadhav, J.P.; Govindwar, S.P. Co-Planted Floating Phyto-Bed along with Microbial Fuel Cell for Enhanced Textile Effluent Treatment. J. Clean. Prod. 2018, 203, 788–798. [Google Scholar] [CrossRef]
- Weyens, N.; Van Der Lelie, D.; Taghavi, S.; Newman, L.; Vangronsveld, J. Exploiting Plant–Microbe Partnerships to Improve Biomass Production and Remediation. Trends Biotechnol. 2009, 27, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Yousaf, S.; Reichenauer, T.G.; Sessitsch, A. The Inoculation Method Affects Colonization and Performance of Bacterial Inoculant Strains in the Phytoremediation of Soil Contaminated with Diesel Oil. Int. J. Phytoremediation 2012, 14, 35–47. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Tian, S.; Wang, W.; Qi, Q.; Jiang, P.; Gao, X.; Li, F.; Li, H.; Yu, H. Petroleum Hydrocarbon-Degrading Bacteria for the Remediation of Oil Pollution Under Aerobic Conditions: A Perspective Analysis. Front. Microbiol. 2018, 9, 2885. [Google Scholar] [CrossRef]
- Cherian, S.; Oliveira, M.M. Transgenic Plants in Phytoremediation: Recent Advances and New Possibilities. Environ. Sci. Technol. 2005, 39, 9377–9390. [Google Scholar] [CrossRef]
- Lee, J.H. An Overview of Phytoremediation as a Potentially Promising Technology for Environmental Pollution Control. Biotechnol Bioproc E 2013, 18, 431–439. [Google Scholar] [CrossRef]
- Mani, D.; Kumar, C. Biotechnological Advances in Bioremediation of Heavy Metals Contaminated Ecosystems: An Overview with Special Reference to Phytoremediation. Int. J. Environ. Sci. Technol. 2014, 11, 843–872. [Google Scholar] [CrossRef]
- Kiamarsi, Z.; Kafi, M.; Soleimani, M.; Nezami, A.; Lutts, S. Conjunction of Vetiveria Zizanioides L. and Oil-Degrading Bacteria as a Promising Technique for Remediation of Crude Oil-Contaminated Soils. J. Clean. Prod. 2020, 253, 119719. [Google Scholar] [CrossRef]
- Rohrbacher, F.; St-Arnaud, M. Root Exudation: The Ecological Driver of Hydrocarbon Rhizoremediation. Agronomy 2016, 6, 19. [Google Scholar] [CrossRef]
- Aliku, C.B.; Madu, C.N.; Aliku, O. Accelerating Phytoextraction of Petroleum Hydrocarbon with Organic Stimulant. MethodsX 2021, 8, 101509. [Google Scholar] [CrossRef] [PubMed]
- Fernández Rodríguez, M.D.; García Gómez, M.C.; Alonso Blazquez, N.; Tarazona, J.V. Soil Pollution Remediation. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 344–355. ISBN 9780123864550. [Google Scholar]
- Helmy, Q.; Laksmono, R.; Kardena, E. Bioremediation of Aged Petroleum Oil Contaminated Soil: From Laboratory Scale to Full Scale Application. Procedia Chem. 2015, 14, 326–333. [Google Scholar] [CrossRef]




| Parameter | Soil DW | Soil OS | |
|---|---|---|---|
| pH H2O | 7.06 | 6.99 | |
| Initial water moisture (%) | 21.9 | 23.01 | |
| BTEX (benzene, toluene, p-, m-ksylen) | 0.59 | 0.97 | |
| TPH | 5176.03 | 4025.65 | |
| WWA | 17.71 | 10.30 | |
| Cl− a | 168.5 | 258.6 | |
| S—SO42− a | 781.5 | 826.3 | |
| N—NH4+ a | 70.1 | 79.4 | |
| N—NO3− a | 241.2 | 230.4 | |
| P—PO43− a | 27.26 | 32.1 | |
| Al2O3 b | 688.5 | 426.9 | |
| SiO2 b | 236.5 | 315.6 | |
| Fe2O3 b | 5.3 | 6.2 | |
| MgO b | 3.9 | 3.0 | |
| CaO b | 6.9 | 6.5 | |
| Sand (%) | 54.01 | 75.21 | |
| Silt (%) | 10.37 | 6.92 | |
| Clay (%) | 35.62 | 17.87 | |
| Heavy metal content a | As | 0.9 | 0.7 |
| Ba | 28.9 | 23.5 | |
| Cd | 1.0 | 0.7 | |
| Cr | 11.1 | 9.1 | |
| Co | 3.1 | 3.0 | |
| Cu | 22.2 | 24.5 | |
| Hg | 0.5 | 0.2 | |
| Pb | 22.9 | 15.4 | |
| Mo | 1.8 | 2.1 | |
| Sn | 5.4 | 6.7 | |
| Zn | 20.6 | 17.1 | |
| Ni | 11.8 | 9.3 | |
| Chromatographic Analysis of Aliphatic Hydrocarbons—TPH, nC6–nC44, Pristane, Phytane (Clarus 500 GC Perkin Elmer) Parameter of Chromatography | |||||
|---|---|---|---|---|---|
| Temperature (°C) | Column | Detector | Calibration Standards | ||
| Injector | Carrier Gas | Temperature Program | |||
| 290 °C | He 20 mL min−1 | 30 °C—isothermal run for 2 min 30–105 °C—temp. increase rate 10 °C min−1 105–285 °C—temp. increase rate 5 °C min−1 285 °C—isothermal run for 5 min−1 | RTX-1 30 m × 0.53 mm (Restek, Bellefonte, PA, USA) | FID 300 °C | Reference soil: BAM—K010 (Tusnovic Instruments, Poland) Standard mixture hydrocarbons (nC6–nC44) ASTM® No. D2807 (Supelco, Saint Louis, MO, USA) Fuel Oil Degradation Mix nC17, pristane, nC18, phytane No. A029668 (Restek, Bellefonte, PA, USA) |
| Chromatographic Analysis of Polycyclic Aromatic Hydrocarbons—PAH (Vanquish Core Thermo Scientific) Parameter of Chromatography | |||||
| Eluents | Flow | Gradient | Column | Detector | Calibration Standards |
| A—methanol B—acetonitrile (Chempur, Piekary śląskie, Poland) | 1.5 mL min−1 | 20% B—for 1.5 min 20–50% B—for 1.5 min 50–100% B—for 1 min 100% B—for 1 min 100–0% B—for 3 min 100% A—for 3 min | NUCLEODUR C18 PAH column 125 mm × 4 mm, 3 µm (Marcherey-Nagel, Dueren, Germany) | UV-ViS FLD | Reference soil: PAHs by HPLC No. SQC017-40G (Sigma-Aldrich, Saint Louis, MO, USA) Certified PAH-Mix solution No. 722393 (Marcherey-Nagel, Dueren, Germany) |
| Parameter | Content ± SD (mg/kg Dry Mass Soil) | ||||||
|---|---|---|---|---|---|---|---|
| Initial Soil DW | After 180 Days | ||||||
| Soil DW-1 | Soil DW-2 | Soil DW-3 | Soil DW-4 | Soil DW-5 | Soil DW-6 | ||
| TPH | 5176.03 | 4223.45 | 3335.33 | 2890.1 | 2758.11 | 2381.96 | 4047.68 |
| ∑nC6–nC9 | 26.31 | 20.36 | 7.58 | 3.44 | 2.52 | 1.98 | 18.75 |
| ∑nC10–nC21 | 892.48 | 683.89 | 407.46 | 300.37 | 271.65 | 196.37 | 627.57 |
| ∑nC22–nC30 | 1092.38 | 919.85 | 845.72 | 845.72 | 777.35 | 661.4 | 896.15 |
| ∑nC31–nC36 | 383.94 | 347.88 | 333.86 | 316.11 | 317.99 | 290.3 | 342.39 |
| isoprenoids | 169.93 | 159.37 | 147.65 | 142.73 | 141.63 | 135.23 | 156.52 |
| Unidentified aliphatic hydrocarbons | 2610.98 | 2092.1 | 1593.07 | 1347.24 | 1246.98 | 1096.69 | 2006.31 |
| Parameter | Initial Soil OS | After 180 Days | |||||
| Soil OS-1 | Soil OS-2 | Soil OS-3 | Soil OS-4 | Soil OS-5 | Soil OS-6 | ||
| TPH | 4025.65 | 3145.08 | 2381.17 | 2021.52 | 1965.03 | 1591.26 | 2943.24 |
| ∑nC6–nC9 | 4.09 | 2.99 | 0.8 | 0.46 | 0.39 | 0.28 | 2.75 |
| ∑nC10–nC21 | 1013.77 | 735.64 | 422.35 | 352.87 | 258.55 | 191.87 | 658.24 |
| ∑nC22–nC30 | 358.69 | 290.79 | 263.5 | 263.5 | 231.71 | 193.99 | 285.91 |
| ∑nC31–nC36 | 200.59 | 179.49 | 172.95 | 153.23 | 164.76 | 144.65 | 176.54 |
| isoprenoids | 384.97 | 356.53 | 325.83 | 319.22 | 309.93 | 300.32 | 348.62 |
| Unidentified aliphatic hydrocarbons | 2063.54 | 1579.64 | 1195.74 | 963.57 | 999.69 | 760.15 | 1471.18 |
| Parameter | Content ± SD (mg/kg Dry Mass Soil) | ||||||
|---|---|---|---|---|---|---|---|
| Initial Soil DW | After 180 Days | ||||||
| Soil DW-1 | Soil DW-2 | Soil DW-3 | Soil DW-4 | Soil DW-5 | Soil DW-6 | ||
| ∑PAH | 17.71 | 14.85 | 12.25 | 11.204 | 10.76 | 8.95 | 14.19 |
| ∑two-ring PAHs | 5.985 | 4.667 | 3.309 | 2.795 | 2.646 | 2 | 4.361 |
| ∑ three-ring PAHs | 4.815 | 3.961 | 3.216 | 2.813 | 2.825 | 1.639 | 3.763 |
| ∑ four-ring PAHs | 5.196 | 4.655 | 4.263 | 4.16 | 3.918 | 3.502 | 4.529 |
| ∑ five-ring PAHs | 1.278 | 1.168 | 1.087 | 1.065 | 1.017 | 0.952 | 1.143 |
| ∑ six-ring PAHs | 0.434 | 0.4 | 0.377 | 0.37 | 0.358 | 0.34 | 0.392 |
| Parameter | Initial Soil OS | After 180 Days | |||||
| Soil OS-1 | Soil OS-2 | Soil OS-3 | Soil OS-4 | Soil OS-5 | Soil OS-6 | ||
| ∑PAHs | 10.3 | 8.626 | 7.51 | 7.1 | 6.92 | 6.34 | 8.43 |
| ∑two-ring PAHs | 0.495 | 0.335 | 0.195 | 0.153 | 0.15 | 0.129 | 0.316 |
| ∑ three-ring PAHs | 1.325 | 0.979 | 0.728 | 0.612 | 0.639 | 0.381 | 0.938 |
| ∑ four-ring PAHs | 5.026 | 4.275 | 3.808 | 3.704 | 3.508 | 2.468 | 4.187 |
| ∑ five-ring PAHs | 2.322 | 2.03 | 1.85 | 1.736 | 1.727 | 1.651 | 1.995 |
| ∑ six-ring PAHs | 1.136 | 1.008 | 0.93 | 0.898 | 0.891 | 0.859 | 0.993 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtowicz, K.; Steliga, T.; Kapusta, P. Evaluation of the Effectiveness of Bioaugmentation-Assisted Phytoremediation of Soils Contaminated with Petroleum Hydrocarbons Using Echinacea purpurea. Appl. Sci. 2023, 13, 13077. https://doi.org/10.3390/app132413077
Wojtowicz K, Steliga T, Kapusta P. Evaluation of the Effectiveness of Bioaugmentation-Assisted Phytoremediation of Soils Contaminated with Petroleum Hydrocarbons Using Echinacea purpurea. Applied Sciences. 2023; 13(24):13077. https://doi.org/10.3390/app132413077
Chicago/Turabian StyleWojtowicz, Katarzyna, Teresa Steliga, and Piotr Kapusta. 2023. "Evaluation of the Effectiveness of Bioaugmentation-Assisted Phytoremediation of Soils Contaminated with Petroleum Hydrocarbons Using Echinacea purpurea" Applied Sciences 13, no. 24: 13077. https://doi.org/10.3390/app132413077
APA StyleWojtowicz, K., Steliga, T., & Kapusta, P. (2023). Evaluation of the Effectiveness of Bioaugmentation-Assisted Phytoremediation of Soils Contaminated with Petroleum Hydrocarbons Using Echinacea purpurea. Applied Sciences, 13(24), 13077. https://doi.org/10.3390/app132413077








