Phytomicrobiomes: A Potential Approach for Sustainable Pesticide Biodegradation
Abstract
1. Introduction
2. Pesticides
2.1. Fate of Pesticides
2.2. Pesticide Biodegradation Processes
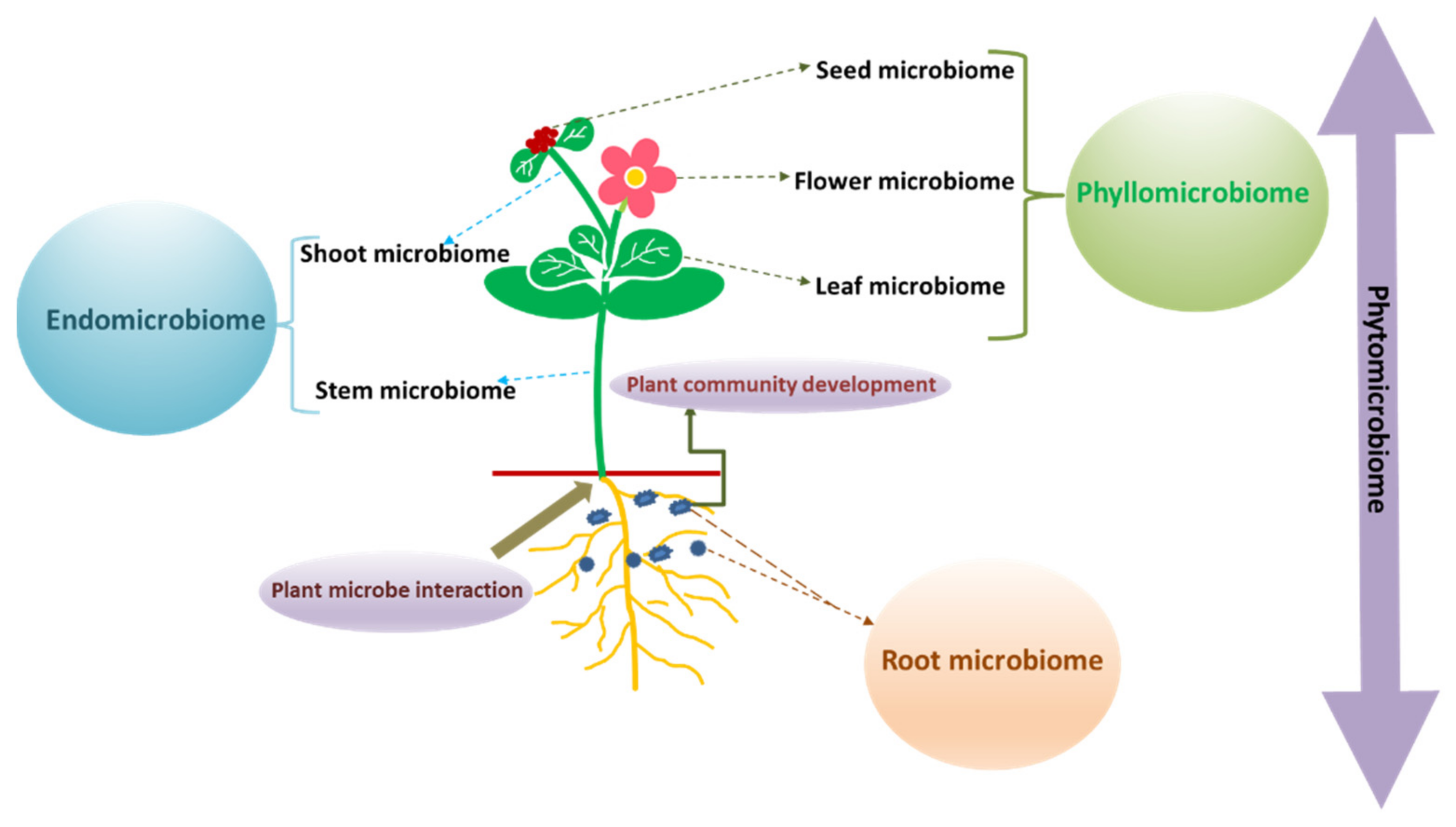
3. Phytomicrobiome and Pesticide Biodegradation
3.1. Shoot Microbiome
3.2. Leaf Microbiome
3.3. Seed Microbiome
3.4. Flower Microbiome
3.5. Root Microbiome
3.6. Translocation and Accumulation
4. Omics Approaches in Pesticide Biodegradation
4.1. Cell Sorting
4.2. Transcriptomics
4.3. Proteomics
4.4. Metabolomics
5. Future Research Scope
6. Concluding Remarks
Funding
Conflicts of Interest
References
- FAO; ITPS; GSBI; SCBD; EC. State of Knowledge of Soil Biodiversity—Status, Challenges and Potentialities; FAO: Rome, Italy, 2020; p. 668. [Google Scholar] [CrossRef]
- Lakshmanan, V.; Selvaraj, G.; Bais, H.P. Functional Soil Microbiome: Belowground Solutions to an Aboveground Problem. Plant Physiol. 2014, 166, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Verma, A.; Verma, S.; Anwar, M.S.; Prasher, P.; Mudila, H.; Chen, S. Understanding Phytomicrobiome: A Potential Reservoir for Better Crop Management. Sustainability 2020, 12, 5446. [Google Scholar] [CrossRef]
- Smith, D.L.; Subramanian, S.; Lamont, J.R.; Bywater-Ekegàrd, M. Signaling in the Phytomicrobiome: Breadth and Potential. Front. Plant Sci. 2015, 6, 709. [Google Scholar] [CrossRef] [PubMed]
- Miliute, I.; Buzaite, O.; Baniulis, D.; Stanys, V. Bacterial Endophytes in Agricultural Crops and Their Role in Stress Tolerance: A Review. Zemdirb. Agric. 2015, 102, 465–478. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant Growth-Promoting Bacterial Endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; Ver Loren van Themaat, E.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing Structure and Assembly Cues for Arabidopsis Root-Inhabiting Bacterial Microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; Del Rio, T.G.; et al. Defining the Core Arabidopsis thaliana Root Microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Batista, B.D.; Bazany, K.E.; Singh, B.K. Plant–Microbiome Interactions under a Changing World: Responses, Consequences and Perspectives. New Phytol. 2022, 234, 1951–1959. [Google Scholar] [CrossRef]
- IPPC Secretariat. Scientific Review of the Impact of Climate Change on Plant Pests—A Global Challenge to Prevent and Mitigate Plant Pest Risks in Agriculture, Forestry and Ecosystems; FAO on behalf of the IPPC Secretariat: Rome, Italy, 2021; p. 88. [Google Scholar] [CrossRef]
- Singh, B.K.; Walker, A. Microbial Degradation of Organophosphorus Compounds. FEMS Microbiol. Rev. 2006, 30, 428–471. [Google Scholar] [CrossRef]
- Walter, G.H.; Chandrasekaran, S.; Collins, P.J.; Jagadeesan, R.; Mohankumar, S.; Alagusundaram, K.; Ebert, P.R.; Daglish, G.J.; Nayak, M.K.; Mohan, S.; et al. The Grand Challenge of Food Security—General Lessons from a Comprehensive Approach to Protecting Stored Grain from Insect Pests in Australia and India. Indian J. Entomol. 2016, 78, 7–16. [Google Scholar] [CrossRef]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide Residues in European Agricultural Soils—A Hidden Reality Unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Riyaz, M.; Shah, R.A.; Sivasankaran, K.; Riyaz, M.; Shah, R.A.; Sivasankaran, K. Pesticide Residues: Impacts on Fauna and the Environment. In Biodegradation Technology of Organic and Inorganic Pollutants; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Salam, M.T.B.; Kataoka, R. Changes in the Endophytic Bacterial Community of Brassica rapa after Application of Systemic Insecticides. Int. J. Mol. Sci. 2023, 24, 15306. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.; Pandey, G.; Hartley, C.J.; Jackson, C.J.; Cheesman, M.J.; Taylor, M.C.; Pandey, R.; Khurana, J.L.; Teese, M.; Coppin, C.W.; et al. The Enzymatic Basis for Pesticide Bioremediation. Indian J. Microbiol. 2008, 48, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Cycoń, M.; Zmijowska, A.; Wójcik, M.; Piotrowska-Seget, Z. Biodegradation and Bioremediation Potential of Diazinon-Degrading Serratia marcescens to Remove Other Organophosphorus Pesticides from Soils. J. Environ. Manage. 2013, 117, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Hernández, M.L.; Sánchez-Salinas, E.; Dantán-González, E.; Castrejón-Godínez, M.L. Pesticide Biodegradation: Mechanisms, Genetics and Strategies to Enhance the Process. In Biodegradation-Life of Science; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Singh, D.K. Biodegradation and Bioremediation of Pesticide in Soil: Concept, Method and Recent Developments. Indian J. Microbiol. 2008, 48, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, C.; Jiwal, S.; Rout, P.; Ramya, M. Biodegradation of Chlorpyrifos by Bacterial Consortium Isolated from Agriculture Soil. World J. Microbiol. Biotechnol. 2012, 28, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Romeh, A.A. Phytoremediation of Cyanophos Insecticide by Plantago major L. in Water. J. Environ. Health Sci. Eng. 2014, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Rousidou, K.; Chanika, E.; Georgiadou, D.; Soueref, E.; Katsarou, D.; Kolovos, P.; Ntougias, S.; Tourna, M.; Tzortzakakis, E.A.; Karpouzas, D.G. Isolation of Oxamyl-Degrading Bacteria and Identification of CehA as a Novel Oxamyl Hydrolase Gene. Front. Microbiol. 2016, 7, 616. [Google Scholar] [CrossRef]
- Salam, J.A.; Hatha, M.A.A.; Das, N. Microbial-Enhanced Lindane Removal by Sugarcane (Saccharum officinarum) in Doped Soil-Applications in Phytoremediation and Bioaugmentation. J. Environ. Manag. 2017, 193, 394–399. [Google Scholar] [CrossRef]
- López, J.L.; Alvarez, F.; Príncipe, A.; Salas, M.E.; Lozano, M.J.; Draghi, W.O.; Jofré, E.; Lagares, A. Isolation, Taxonomic Analysis, and Phenotypic Characterization of Bacterial Endophytes Present in Alfalfa (Medicago sativa) Seeds. J. Biotechnol. 2018, 267, 55–62. [Google Scholar] [CrossRef]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root Exudates: From Plant to Rhizosphere and Beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Batista, B.D.; de Almeida, J.R.; Bezerra, T.E.; de Azevedo, J.L.; Quecine, M.C. Describing the Unexplored Microorganisms Associated with Guarana: A Typical Tropical Plant. In Diversity and Benefits of Microorganisms from the Tropics; Springer: Cham, Switzerland, 2017; pp. 293–312. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Paungfoo-Lonhienne, C.; Dennis, P.G.; Robinson, N.; Ragan, M.A.; Schmidt, S.; Hugenholtz, P. The Core Root Microbiome of Sugarcanes Cultivated under Varying Nitrogen Fertilizer Application. Environ. Microbiol. 2016, 18, 1338–1351. [Google Scholar] [CrossRef]
- Dunlop, E.S.; McLaughlin, R.; Adams, J.V.; Jones, M.; Birceanu, O.; Christie, M.R.; Criger, L.A.; Hinderer, J.L.M.; Hollingworth, R.M.; Johnson, N.S.; et al. Rapid Evolution Meets Invasive Species Control: The Potential for Pesticide Resistance in Sea Lamprey. Can. J. Fish. Aquat. Sci. 2018, 75, 152–168. [Google Scholar] [CrossRef]
- Battaglin, W.A.; Meyer, M.T.; Kuivila, K.M.; Dietze, J.E. Glyphosate and Its Degradation Product AMPA Occur Frequently and Widely in U.S. Soils, Surface Water, Groundwater, and Precipitation. J. Am. Water Resour. Assoc. 2014, 50, 275–290. [Google Scholar] [CrossRef]
- Norris, K.; Terry, A.; Hansford, J.P.; Turvey, S.T. Biodiversity Conservation and the Earth System: Mind the Gap. Trends Ecol. Evol. 2020, 35, 919. [Google Scholar] [CrossRef]
- Onwona-kwakye, M.; Plants-paris, K.; Keita, K.; Lee, J.; den Brink, P.J.V.; Hogarh, J.N.; Darkoh, C. Pesticides Decrease Bacterial Diversity and Abundance of Irrigated Rice Fields. Microorganisms 2020, 8, 318. [Google Scholar] [CrossRef] [PubMed]
- Tosi, S.; Costa, C.; Vesco, U.; Quaglia, G.; Guido, G. A 3-Year Survey of Italian Honey Bee-Collected Pollen Reveals Widespread Contamination by Agricultural Pesticides. Sci. Total Environ. 2018, 615, 208–218. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee Declines Driven by Combined Stress from Parasites, Pesticides, and Lack of Flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Gavrilescu, M. Fate of Pesticides in the Environment and Its Bioremediation. Eng. Life Sci. 2005, 5, 497–526. [Google Scholar] [CrossRef]
- Besse-Hoggan, P.; Alekseeva, T.; Sancelme, M.; Delort, A.M.; Forano, C. Atrazine Biodegradation Modulated by Clays and Clay/Humic Acid Complexes. Environ. Pollut. 2009, 157, 2837–2844. [Google Scholar] [CrossRef]
- Worrall, F.; Fernandez-Perez, M.; Johnson, A.C.; Flores-Cesperedes, F.; Gonzalez-Pradas, E. Limitations on the Role of Incorporated Organic Matter in Reducing Pesticide Leaching. J. Contam. Hydrol. 2001, 49, 241–262. [Google Scholar] [CrossRef]
- Torres-Duarte, C.; Roman, R.; Tinoco, R.; Vazquez-Duhalt, R. Halogenated Pesticide Transformation by a Laccase-Mediator System. Chemosphere 2009, 77, 687–692. [Google Scholar] [CrossRef]
- Ortiz-hernández, M.L.; Sánchez-salinas, E. Biodegradación Del Plaguicida Organofosforado Tetraclorvinfos Por Bacterias Aisladas de Suelos Agrícolas En México. Rev. Int. Contam. Ambient. 2010, 26, 27–38. [Google Scholar]
- Dua, M.; Singh, A.; Sethunathan, N.; Johri, A. Biotechnology and Bioremediation: Successes and Limitations. Appl. Microbiol. Biotechnol. 2002, 59, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, H.; Mitsuoka, M.; Ura, Y.; Watanabe, T.; Furukawa, K. Directed Evolution of Biphenyl Dioxygenase: Emergence of Enhanced Degradation Capacity for Benzene, Toluene, and Alkylbenzenes. J. Bacteriol. 2001, 183, 5441–5444. [Google Scholar] [CrossRef] [PubMed]
- Germaine, K.J.; Liu, X.; Cabellos, G.G.; Hogan, J.P.; Ryan, D.; Dowling, D.N. Bacterial Endophyte-Enhanced Phytoremediation of the Organochlorine Herbicide 2,4-Dichlorophenoxyacetic Acid. FEMS Microbiol. Ecol. 2006, 57, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Wan, Q.; Wang, Y.; Cheng, J.; Yu, X.; Ge, J. An Endophytic Bacterial Strain, Enterobacter cloacae TMX-6, Enhances the Degradation of Thiamethoxam in Rice Plants. Chemosphere 2021, 269, 128751. [Google Scholar] [CrossRef]
- Männistö, M.K.; Tiirola, M.A.; Puhakka, J.A. Degradation of 2,3,4,6-Tetrachlorophenol at Low Temperature and Low Dioxygen Concentrations by Phylogenetically Different Groundwater and Bioreactor Bacteria. Biodegradation 2001, 12, 291–301. [Google Scholar] [CrossRef]
- Pandey, G.; Dorrian, S.J.; Russell, R.J.; Oakeshott, J.G. Biotransformation of the Neonicotinoid Insecticides Imidacloprid and Thiamethoxam by Pseudomonas sp. 1G. Biochem. Biophys. Res. Commun. 2009, 380, 710–714. [Google Scholar] [CrossRef]
- Wang, M.; Yang, G.; Wang, X.; Yao, Y.; Min, H.; Lu, Z. Nicotine Degradation by Two Novel Bacterial Isolates of Acinetobacter sp. TW and Sphingomonas sp. TY and Their Responses in the Presence of Neonicotinoid Insecticides. World J. Microbiol. Biotechnol. 2011, 27, 1633–1640. [Google Scholar] [CrossRef]
- Dai, Y.J.; Ji, W.W.; Chen, T.; Zhang, W.J.; Liu, Z.H.; Ge, F.; Sheng, Y. Metabolism of the Neonicotinoid Insecticides Acetamiprid and Thiacloprid by the Yeast Rhodotorula mucilaginosa Strain IM-2. J. Agric. Food Chem. 2010, 58, 2419–2425. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Dong, W.; Xin, F.; Liu, J.; Zhou, X.; Xu, F.; Lv, Z.; Ma, J.; Zhang, W.; Fang, Y.; et al. Characteristics and Metabolic Pathway of Acetamiprid Biodegradation by Fusarium sp. Strain CS-3 Isolated from Soil. Biodegradation 2018, 29, 593–603. [Google Scholar] [CrossRef]
- Guo, L.; Fang, W.W.; Guo, L.L.; Yao, C.F.; Zhao, Y.X.; Ge, F.; Dai, Y.J. Biodegradation of the Neonicotinoid Insecticide Acetamiprid by Actinomycetes Streptomyces canus CGMCC 13662 and Characterization of the Novel Nitrile Hydratase Involved. J. Agric. Food Chem. 2019, 67, 5922–5931. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Zhou, L.Y.; Wang, Y.; Ma, Y.; Zhai, S.; Liu, Z.H.; Dai, Y.J.; Yuan, S. Hydrolysis of the Neonicotinoid Insecticide Thiacloprid by the N2-Fixing Bacterium Ensifer meliloti CGMCC 7333. Int. Biodeterior. Biodegrad. 2014, 93, 10–17. [Google Scholar] [CrossRef]
- Mori, T.; Wang, J.; Tanaka, Y.; Nagai, K.; Kawagishi, H.; Hirai, H. Bioremediation of the Neonicotinoid Insecticide Clothianidin by the White-Rot Fungus Phanerochaete sordida. J. Hazard. Mater. 2017, 321, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tanaka, Y.; Ohno, H.; Jia, J.; Mori, T.; Xiao, T.; Yan, B.; Kawagishi, H.; Hirai, H. Biotransformation and Detoxification of the Neonicotinoid Insecticides Nitenpyram and Dinotefuran by Phanerochaete sordida YK-624. Environ. Pollut. 2019, 252, 856–862. [Google Scholar] [CrossRef]
- Verma, J.P.; Jaiswal, D.K.; Sagar, R. Pesticide Relevance and Their Microbial Degradation: A-State-of-Art. Rev. Environ. Sci. Biotechnol. 2014, 13, 429–466. [Google Scholar] [CrossRef]
- Upadhyay, L.S.B.; Dutt, A. Microbial Detoxification of Residual Organophosphate Pesticides in Agricultural Practices. Microb. Biotec. 2017, 1, 225–242. [Google Scholar] [CrossRef]
- Hai, F.I.; Modin, O.; Yamamoto, K.; Fukushi, K.; Nakajima, F.; Nghiem, L.D. Pesticide Removal by a Mixed Culture of Bacteria and White-Rot Fungi. Fac. Eng. Pap. 2012, 43, 459. [Google Scholar] [CrossRef]
- Huang, J.; Ai, G.; Liu, N.; Huang, Y. Environmental Adaptability and Organic Pollutant Degradation Capacity of a Novel Rhodococcus Species Derived from Soil in the Uninhabited Area of the Qinghai-Tibet Plateau. Microorganisms 2022, 10, 1935. [Google Scholar] [CrossRef]
- Myresiotis, C.K.; Vryzas, Z.; Papadopoulou-Mourkidou, E. Biodegradation of Soil-Applied Pesticides by Selected Strains of Plant Growth-Promoting Rhizobacteria (PGPR) and Their Effects on Bacterial Growth. Biodegradation 2012, 23, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wei, H.; Zhu, C.; Geng, B. Biodegradation of Atrazine by the Novel Citricoccus sp. Strain TT3. Ecotoxicol. Environ. Saf. 2018, 147, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Abigail, E.A.; Abdul Salam, J.; Das, N. Atrazine Degradation in Liquid Culture and Soil by a Novel Yeast Pichia kudriavzevii Strain Atz-EN-01 and Its Potential Application for Bioremediation. J. Appl. Pharm. Sci. 2013, 3, 35–43. [Google Scholar]
- Swissa, N.; Nitzan, Y.; Langzam, Y.; Cahan, R. Atrazine Biodegradation by a Monoculture of Raoultella planticola Isolated from a Herbicides Wastewater Treatment Facility. Int. Biodeterior. Biodegrad. 2014, 92, 6–11. [Google Scholar] [CrossRef]
- Lin, X.Y.; Yang, Y.Y.; Zhao, Y.H.; Fu, Q.L. Biodegradation of Bensulfuron-Methyl and Its Effect on Bacterial Community in Paddy Soils. Ecotoxicology 2012, 21, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Anwar, S.; Firdous, S.; Da-Chuan, Y.; Iqbal, S. Biodegradation of Bispyribac Sodium by a Novel Bacterial Consortium BDAM: Optimization of Degradation Conditions Using Response Surface Methodology. J. Hazard. Mater. 2018, 349, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Sehnem, N.T.; Souza-Cruz, P.; Peralba, M.D.C.R.; Ayub, M.A.Z. Biodegradation of Tebuconazole by Bacteria Isolated from Contaminated Soils. J. Environ. Sci. Health B 2010, 45, 67–72. [Google Scholar] [CrossRef]
- Egea, T.C.; da Silva, R.; Boscolo, M.; Rigonato, J.; Monteiro, D.A.; Grünig, D.; da Silva, H.; van der Wielen, F.; Helmus, R.; Parsons, J.R.; et al. Diuron Degradation by Bacteria from Soil of Sugarcane Crops. Heliyon 2017, 3, e00471. [Google Scholar] [CrossRef]
- Birolli, W.G.; da Silva, B.F.; Rodrigues-Filho, E. Biodegradation of the Fungicide Pyraclostrobin by Bacteria from Orange Cultivation Plots. Sci. Total Environ. 2020, 746, 140968. [Google Scholar] [CrossRef]
- Lyu, D.; Backer, R.; Subramanian, S.; Smith, D.L. Phytomicrobiome Coordination Signals Hold Potential for Climate Change-Resilient Agriculture. Front. Plant Sci. 2020, 11, 634. [Google Scholar] [CrossRef]
- Pascale, A.; Proietti, S.; Pantelides, I.S.; Stringlis, I.A. Modulation of the Root Microbiome by Plant Molecules: The Basis for Targeted Disease Suppression and Plant Growth Promotion. Front. Plant Sci. 2020, 10, 1741. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Pandey, G.; Pandey, J.; Jain, R.K. Accessing Microbial Diversity for Bioremediation and Environmental Restoration. Trends Biotechnol. 2005, 23, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Rheinwald, J.G.; Chakrabarty, A.M.; Gunsalus, I.C. A Transmissible Plasmid Controlling Camphor Oxidation in Pseudomonas putida. Proc. Natl. Acad. Sci. USA 1973, 70, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Pemberton, J.M.; Fisher, P.R. 2,4-D Plasmids and Persistence. Nature 1977, 268, 732–733. [Google Scholar] [CrossRef] [PubMed]
- Pemberton, J.M.; Schmidt, R. Catabolic Plasmids. eLS 2006. [Google Scholar] [CrossRef]
- Vamsee-Krishna, C.; Phale, P.S. Bacterial Degradation of Phthalate Isomers and Their Esters. Indian J. Microbiol. 2008, 48, 19. [Google Scholar] [CrossRef]
- Malhotra, S.; Sharma, P.; Kumari, H.; Singh, A.; Lal, R. Localization of HCH Catabolic Genes (Lin Genes) in Sphingobium indicum B90A. Indian J. Microbiol. 2007, 47, 271. [Google Scholar] [CrossRef]
- Abraham, W.R.; Nogales, B.; Golyshin, P.N.; Pieper, D.H.; Timmis, K.N. Polychlorinated Biphenyl-Degrading Microbial Communities in Soils and Sediments. Curr. Opin. Microbiol. 2002, 5, 246–253. [Google Scholar] [CrossRef]
- Sun, W.; Chen, Y.; Liu, L.; Tang, J.; Chen, J.; Liu, P. Conidia Immobilization of T-DNA Inserted Trichoderma atroviride Mutant AMT-28 with Dichlorvos Degradation Ability and Exploration of Biodegradation Mechanism. Bioresour. Technol. 2010, 101, 9197–9203. [Google Scholar] [CrossRef]
- Jeon, C.O.; Madsen, E.L. In Situ Microbial Metabolism of Aromatic-Hydrocarbon Environmental Pollutants. Curr. Opin. Biotechnol. 2013, 24, 474–481. [Google Scholar] [CrossRef]
- Brisson, V.L.; Schmidt, J.E.; Northen, T.R.; Vogel, J.P.; Gaudin, A.C.M. Impacts of Maize Domestication and Breeding on Rhizosphere Microbial Community Recruitment from a Nutrient Depleted Agricultural Soil. Sci. Rep. 2019, 9, 15611. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Ryu, C.M. Understanding Plant Social Networking System: Avoiding Deleterious Microbiota but Calling Beneficials. Int. J. Mol. Sci. 2021, 22, 3319. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M. Plant Phenotypic Plasticity in the Phytobiome: A Volatile Issue. Curr. Opin. Plant Biol. 2016, 32, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, B.; Cernava, T.; Müller, H.; Berg, C.; Berg, G. Seeds of Native Alpine Plants Host Unique Microbial Communities Embedded in Cross-Kingdom Networks. Microbiome 2019, 7, 108. [Google Scholar] [CrossRef]
- Agler, M.T.; Ruhe, J.; Kroll, S.; Morhenn, C.; Kim, S.T.; Weigel, D.; Kemen, E.M. Microbial Hub Taxa Link Host and Abiotic Factors to Plant Microbiome Variation. PLoS Biol. 2016, 14, e1002352. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Spinelli, F.; Vandelle, E. The Unseen Effect of Pesticides: The Impact on Phytobiota Structure and Functions. Front. Agron. 2022, 4, 77. [Google Scholar] [CrossRef]
- Tamošiūnė, I.; Stanienė, G.; Haimi, P.; Stanys, V.; Rugienius, R.; Baniulis, D. Endophytic Bacillus and Pseudomonas spp. Modulate Apple Shoot Growth, Cellular Redox Balance, and Protein Expression under in Vitro Conditions. Front. Plant Sci. 2018, 9, 324495. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, A.C.; Thomas, P.; Sekhar, A.C.; Thomas, P. Isolation and Identification of Shoot-Tip Associated Endophytic Bacteria from Banana Cv. Grand Naine and Testing for Antagonistic Activity against Fusarium oxysporum f. sp. Cubense. Am. J. Plant Sci. 2015, 6, 943–954. [Google Scholar] [CrossRef]
- Chialva, M.; Lanfranco, L.; Bonfante, P. The Plant Microbiota: Composition, Functions, and Engineering. Curr. Opin. Biotechnol. 2022, 73, 135–142. [Google Scholar] [CrossRef]
- Fuentes-Ramirez, L.E.; Jimenez-Salgado, T.; Abarca-Ocampo, I.R.; Caballero-Mellado, J. Acetobacter diazotrophicus, an Indoleacetic Acid Producing Bacterium Isolated from Sugarcane Cultivars of México. Plant Soil 1993, 154, 145–150. [Google Scholar] [CrossRef]
- Hurek, T.; Reinhold-Hurek, B.; Van Montagu, M.; Kellenberger, E. Root Colonization and Systemic Spreading of Azoarcus sp. Strain BH72 in Grasses. J. Bacteriol. 1994, 176, 1913. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, H.; Chen, S. Colonization of Maize and Rice Plants by Strain Bacillus megaterium C4. Curr. Microbiol. 2006, 52, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A Review on the Plant Microbiome: Ecology, Functions, and Emerging Trends in Microbial Application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahi, M.; Pourbabaei, A.A.; Etesami, H.; Talebi, K. Diazinon Degradation by Bacterial Endophytes in Rice Plant (Oryzia sativa L.): A Possible Reason for Reducing the Efficiency of Diazinon in the Control of the Rice Stem-Borer. Chemosphere 2020, 246, 125759. [Google Scholar] [CrossRef] [PubMed]
- Luna, M.F.; Galar, M.L.; Aprea, J.; Molinari, M.L.; Boiardi, J.L. Colonization of Sorghum and Wheat by Seed Inoculation with Gluconacetobacter diazotrophicus. Biotechnol. Lett. 2010, 32, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Montiel, L.G.; Chiquito-Contreras, C.J.; Murillo-Amador, B.; Vidal-Hernández, L.; Quiñones-Aguilar, E.E.; Chiquito-Contreras, R.G. Efficiency of Two Inoculation Methods of Pseudomonas putida on Growth and Yield of Tomato Plants. J. Soil Sci. Plant Nutr. 2017, 17, 1003–1012. [Google Scholar] [CrossRef]
- Strigul, N.S.; Kravchenko, L.V. Mathematical Modeling of PGPR Inoculation into the Rhizosphere. Environ. Model. Softw. 2006, 21, 1158–1171. [Google Scholar] [CrossRef]
- Chai, Y.N.; Futrell, S.; Schachtman, D.P. Assessment of Bacterial Inoculant Delivery Methods for Cereal Crops. Front. Microbiol. 2022, 13, 791110. [Google Scholar] [CrossRef]
- Wang, G.; Chen, M.; Jiang, L.; Zhang, Y. Nitenpyram Biodegradation by a Novel Nitenpyram-Degrading Bacterium, Ochrobactrum sp. Strain DF-1, and Its Novel Degradation Pathway. Front. Microbiol. 2023, 14, 1209322. [Google Scholar] [CrossRef]
- Tiwari, S.; Tripathi, P.; Mohan, D.; Singh, R.S. Imidacloprid Biodegradation Using Novel Bacteria Tepidibacillus decaturensis Strain ST1 in Batch and in Situ Microcosm Study. Environ. Sci. Pollut. Res. Int. 2023, 30, 61562–61572. [Google Scholar] [CrossRef]
- Ito, K.; Mahmood, A.; Kataoka, R.; Takagi, K. Dichlorodiphenyltrichloroethane (DDT) Degradation by Streptomyces sp. Isolated from DDT Contaminated Soil. Bioremediat. J. 2021, 25, 148–158. [Google Scholar] [CrossRef]
- Ji, X.; Lu, G.; Gai, Y.; Zheng, C.; Mu, Z. Biological Control against Bacterial Wilt and Colonization of Mulberry by an Endophytic Bacillus subtilis Strain. FEMS Microbiol. Ecol. 2008, 65, 565–573. [Google Scholar] [CrossRef]
- Naveed, M.; Mitter, B.; Sessitsch, A.; Reichenauer, T.G. Endophytic Colonization of Burkholderia phytofirmans Strain psJN Induces Drought-Stress Tolerance in Maize. Acta Hortic. 2012, 1009, 117–126. [Google Scholar] [CrossRef]
- Elbeltagy, A.; Nishioka, K.; Sato, T.; Suzuki, H.; Ye, B.; Hamada, T.; Isawa, T.; Mitsui, H.; Minamisawa, K. Endophytic Colonization and in Planta Nitrogen Fixation by a Herbaspirillum sp. Isolated from Wild Rice Species. Appl. Environ. Microbiol. 2001, 67, 5285–5293. [Google Scholar] [CrossRef] [PubMed]
- Brusamarello-Santos, L.C.; Gilard, F.; Brulé, L.; Quilleré, I.; Gourion, B.; Ratet, P.; De Souza, E.M.; Lea, P.J.; Hirel, B. Metabolic Profiling of Two Maize (Zea Mays L.) Inbred Lines Inoculated with the Nitrogen Fixing Plant-Interacting Bacteria Herbaspirillum seropedicae and Azospirillum brasilense. PLoS ONE 2017, 12, e0174576. [Google Scholar] [CrossRef] [PubMed]
- Doty, S.L.; Freeman, J.L.; Cohu, C.M.; Burken, J.G.; Firrincieli, A.; Simon, A.; Khan, Z.; Isebrands, J.G.; Lukas, J.; Blaylock, M.J. Enhanced Degradation of TCE on a Superfund Site Using Endophyte-Assisted Poplar Tree Phytoremediation. Environ. Sci. Technol. 2017, 51, 10050–10058. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Ye, Y.; Ke, M.; Xu, N.; Zhang, Z.; Zhang, F.; Kang, J.; Yu, Y.; Lu, T.; Qian, H. Effects of Chiral Herbicide Dichlorprop on Arabidopsis thaliana Metabolic Profile and Its Implications for Microbial Communities in the Phyllosphere. Environ. Sci. Pollut. Res. 2022, 29, 28256–28266. [Google Scholar] [CrossRef] [PubMed]
- Perazzolli, M.; Antonielli, L.; Storari, M.; Puopolo, G.; Pancher, M.; Giovannini, O.; Pindo, M.; Pertot, I. Resilience of the Natural Phyllosphere Microbiota of the Grapevine to Chemical and Biological Pesticides. Appl. Environ. Microbiol. 2014, 80, 3585–3596. [Google Scholar] [CrossRef]
- Chesneau, G.; Torres-Cortes, G.; Briand, M.; Darrasse, A.; Preveaux, A.; Marais, C.; Jacques, M.A.; Shade, A.; Barret, M. Temporal Dynamics of Bacterial Communities during Seed Development and Maturation. FEMS Microbiol. Ecol. 2020, 96, fiaa190. [Google Scholar] [CrossRef]
- Rodríguez, C.E.; Antonielli, L.; Mitter, B.; Trognitz, F.; Sessitsch, A. Heritability and Functional Importance of the Setaria viridis Bacterial Seed Microbiome. Phytobiomes J. 2020, 4, 40–52. [Google Scholar] [CrossRef]
- Johnston-Monje, D.; Lundberg, D.S.; Lazarovits, G.; Reis, V.M.; Raizada, M.N. Bacterial Populations in Juvenile Maize Rhizospheres Originate from Both Seed and Soil. Plant Soil 2016, 405, 337–355. [Google Scholar] [CrossRef]
- Pirttilä, A.; Frank, A.C. Endophytes of Forest Trees; Biology and Applications; Springer: Berlin/Heidelberg, Germany, 2018; Volume 86. [Google Scholar] [CrossRef]
- Adam, E.; Bernhart, M.; Müller, H.; Winkler, J.; Berg, G. The Cucurbita pepo Seed Microbiome: Genotype-Specific Composition and Implications for Breeding. Plant Soil 2018, 422, 35–49. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Carvalhais, L.C.; Percy, C.D.; Prakash Verma, J.; Schenk, P.M.; Singh, B.K. Evidence for the Plant Recruitment of Beneficial Microbes to Suppress Soil-Borne Pathogens. New Phytol. 2021, 229, 2873–2885. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, B.; Rybakova, D.; Adam, E.; Zachow, C.; Bernhard, M.; Müller, M.; Mancinelli, R.; Berg, G. Studying Seed Microbiomes. Methods Mol. Biol. 2021, 2232, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Asaf, S.; Lee, I.J. What Is There in Seeds? Vertically Transmitted Endophytic Resources for Sustainable Improvement in Plant Growth. Front. Plant Sci. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Kuźniar, A.; Włodarczyk, K.; Grządziel, J.; Woźniak, M.; Furtak, K.; Gałązka, A.; Dziadczyk, E.; Skórzyńska-Polit, E.; Wolińska, A. New Insight into the Composition of Wheat Seed Microbiota. Int. J. Mol. Sci. 2020, 21, 4634. [Google Scholar] [CrossRef] [PubMed]
- Verma, J.P.; Macdonald, C.; Gupta, V.K.; Podile, A.R. New and Future Developments in Microbial Biotechnology and Bioengineering. In Phytomicrobiome for Sustainable Agriculture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 25–33. [Google Scholar] [CrossRef]
- Jacobsen, C.S. Plant Protection and Rhizosphere Colonization of Barley by Seed Inoculated Herbicide Degrading Burkholderia (Pseudomonas) cepacia DBO1(PRO101) in 2,4-D Contaminated Soil. Plant Soil 1997, 189, 139–144. [Google Scholar] [CrossRef]
- Cubitto, M.A.; Gentili, A.R. Bioremediation of Crude Oil–Contaminated Soil By Immobilized Bacteria on an Agroindustrial Waste—Sunflower Seed Husks. Bioremediation 2015, 19, 277–286. [Google Scholar] [CrossRef]
- Massoni, J.; Bortfeld-Miller, M.; Jardillier, L.; Salazar, G.; Sunagawa, S.; Vorholt, J.A. Consistent Host and Organ Occupancy of Phyllosphere Bacteria in a Community of Wild Herbaceous Plant Species. ISME J. 2019, 14, 245–258. [Google Scholar] [CrossRef]
- Vannette, R.L. The Floral Microbiome: Plant, Pollinator, and Microbial Perspectives. Annu Rev. Ecol. Sys. 2020, 51, 363–386. [Google Scholar] [CrossRef]
- Wagner, M.R.; Lundberg, D.S.; Del Rio, T.G.; Tringe, S.G.; Dangl, J.L.; Mitchell-Olds, T. Host Genotype and Age Shape the Leaf and Root Microbiomes of a Wild Perennial Plant. Nat. Commun. 2016, 7, 12151. [Google Scholar] [CrossRef] [PubMed]
- Steven, B.; Huntley, R.B.; Zeng, Q. The Influence of Flower Anatomy and Apple Cultivar on the Apple Flower Phytobiome. Phytobiomes J. 2018, 2, 171–179. [Google Scholar] [CrossRef]
- Lessl, J.T.; Fessehaie, A.; Walcott, R.R. Colonization of Female Watermelon Blossoms by Acidovorax avenae ssp. Citrulli and the Relationship between Blossom Inoculum Dosage and Seed Infestation. J. Phytopathol. 2007, 155, 114–121. [Google Scholar] [CrossRef]
- Kuiper, I.; Lagendijk, E.L.; Bloemberg, G.V.; Lugtenberg, B.J.J. Rhizoremediation: A Beneficial Plant-Microbe Interaction. Mol. Plant. Microbe. Interact. 2004, 17, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Quiza, L.; St-Arnaud, M.; Yergeau, E. Harnessing Phytomicrobiome Signaling for Rhizosphere Microbiome Engineering. Front. Plant Sci. 2015, 6, 507. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Trivedi, P.; Singh, S.; Macdonald, C.A.; Verma, J.P. Emerging Microbiome Technologies for Sustainable Increase in Farm Productivity and Environmental Security. Microbiol. Aust. 2018, 39, 17–23. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Andreote, F.D.; Reinhold-Hurek, B.; Sessitsch, A.; van Overbeek, L.S.; van Elsas, J.D. Rice Root-Associated Bacteria: Insights into Community Structures across 10 Cultivars. FEMS Microbiol. Ecol. 2011, 77, 154–164. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Toots, M.; Diédhiou, A.G.; Henkel, T.W.; Kjoller, R.; Morris, M.H.; Nara, K.; Nouhra, E.; Peay, K.G.; et al. Towards Global Patterns in the Diversity and Community Structure of Ectomycorrhizal Fungi. Mol. Ecol. 2012, 21, 4160–4170. [Google Scholar] [CrossRef]
- Dean, S.L.; Farrer, E.C.; Lee Taylor, D.; Porras-Alfaro, A.; Suding, K.N.; Sinsabaugh, R.L. Nitrogen Deposition Alters Plant–Fungal Relationships: Linking Belowground Dynamics to Aboveground Vegetation Change. Mol. Ecol. 2014, 23, 1364–1378. [Google Scholar] [CrossRef]
- Dean, S.L.; Farrer, E.C.; Porras-Alfaro, A.; Suding, K.N.; Sinsabaugh, R.L. Assembly of Root-Associated Bacteria Communities: Interactions between Abiotic and Biotic Factors. Environ. Microbiol. Rep. 2015, 7, 102–110. [Google Scholar] [CrossRef]
- Faist, H.; Keller, A.; Hentschel, U.; Deeken, R. Grapevine (Vitis vinifera) Crown Galls Host Distinct Microbiota. Appl. Environ. Microbiol. 2016, 82, 5542. [Google Scholar] [CrossRef] [PubMed]
- Samad, A.; Trognitz, F.; Compant, S.; Antonielli, L.; Sessitsch, A. Shared and Host-Specific Microbiome Diversity and Functioning of Grapevine and Accompanying Weed Plants. Environ. Microbiol. 2017, 19, 1407–1424. [Google Scholar] [CrossRef] [PubMed]
- Correa-Galeote, D.; Bedmar, E.J.; Arone, G.J. Maize Endophytic Bacterial Diversity as Affected by Soil Cultivation History. Front. Microbiol. 2018, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Reiter, B.; Sessitsch, A.; Nowak, J.; Clément, C.; Barka, E.A. Endophytic Colonization of Vitis vinifera L. by Plant Growth-Promoting Bacterium Burkholderia sp. Strain PsJN. Appl. Environ. Microbiol. 2005, 71, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The Rhizosphere Microbiome: Significance of Plant Beneficial, Plant Pathogenic, and Human Pathogenic Microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Kristin, A.; Miranda, H. The Root Microbiota—A Fingerprint in the Soil? Plant Soil. 2013, 370, 671–686. [Google Scholar] [CrossRef]
- Nemergut, D.R.; Schmidt, S.K.; Fukami, T.; O’Neill, S.P.; Bilinski, T.M.; Stanish, L.F.; Knelman, J.E.; Darcy, J.L.; Lynch, R.C.; Wickey, P.; et al. Patterns and Processes of Microbial Community Assembly. Microbiol. Mol. Biol. Rev. 2013, 77, 342–356. [Google Scholar] [CrossRef]
- Chaudhry, Q.; Blom-Zandstra, M.; Gupta, S.; Joner, E.J. Utilising the Synergy between Plants and Rhizosphere Microorganisms to Enhance Breakdown of Organic Pollutants in the Environment. Environ. Sci. Pollut. Res. Int. 2005, 12, 34–48. [Google Scholar] [CrossRef]
- Kahlon, R.S. Biodegradation and Bioremediation of Organic Chemical Pollutants by Pseudomonas. In Pseudomonas: Molecular and Applied Biology; Springer: Cham, Switzerland, 2016; pp. 343–417. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Liu, H.; Wang, Y.; Xia, X. Study on Pseudomonas sp. WBC-3 Capable of Complete Degradation of Methylparathion. Wei Sheng Wu Xue Bao 2002, 42, 490–497. [Google Scholar]
- Dong, W.; Chen, Q.; Hou, Y.; Li, S.; Zhuang, K.; Huang, F.; Zhou, J.; Li, Z.; Wang, J.; Fu, L.; et al. Metabolic Pathway Involved in 2-Methyl-6-Ethylaniline Degradation by Sphingobium sp. Strain MEA3-1 and Cloning of the Novel Flavin-Dependent Monooxygenase System MeaBA. Appl. Environ. Microbiol. 2015, 81, 8254–8264. [Google Scholar] [CrossRef]
- Inui, H.; Wakai, T.; Gion, K.; Kim, Y.S.; Eun, H. Differential Uptake for Dioxin-like Compounds by Zucchini Subspecies. Chemosphere 2008, 73, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.; Fryer, M.; Grosso, A. Plant Uptake of Non Ionic Organic Chemicals. Environ. Sci. Technol. 2006, 40, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yao, F.E.N.G.; Liu, Y.W.; Chang, H.Q.; Li, Z.J.; Xue, J.M. Uptake and Translocation of Organic Pollutants in Plants: A Review. J. Integr. Agric. 2017, 16, 1659–1668. [Google Scholar] [CrossRef]
- Kvesitadze, G.; Khatisashvili, G.; Sadunishvili, T.; Kvesitadze, E. Plants for Remediation: Uptake, Translocation and Transformation of Organic Pollutants. In Plants, Pollutants and Remediation; Springer: Dordrecht, The Netherlands, 2016; pp. 241–308. [Google Scholar] [CrossRef]
- Karas, P.A.; Baguelin, C.; Pertile, G.; Papadopoulou, E.S.; Nikolaki, S.; Storck, V.; Ferrari, F.; Trevisan, M.; Ferrarini, A.; Fornasier, F.; et al. Assessment of the Impact of Three Pesticides on Microbial Dynamics and Functions in a Lab-to-Field Experimental Approach. Sci. Total Environ. 2018, 637–638, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Sun, D.; Lu, Y.; Dai, S.; Chen, L.; An, Y. Effects of Pesticide–Fertilizer Combinations on the Rhizosphere Microbiome of Sugarcane: A Preliminary Study. Sugar Tech 2021, 23, 571–579. [Google Scholar] [CrossRef]
- Chen, X.; Wicaksono, W.A.; Berg, G.; Cernava, T. Bacterial Communities in the Plant Phyllosphere Harbour Distinct Responders to a Broad-Spectrum Pesticide. Sci. Total Environ. 2021, 751, 141799. [Google Scholar] [CrossRef]
- Erlacher, A.; Cardinale, M.; Grube, M.; Berg, G. Biotic Stress Shifted Structure and Abundance of Enterobacteriaceae in the Lettuce Microbiome. PLoS ONE 2015, 10, e0118068. [Google Scholar] [CrossRef]
- Cernava, T.; Erlacher, A.; Soh, J.; Sensen, C.W.; Grube, M.; Berg, G. Enterobacteriaceae Dominate the Core Microbiome and Contribute to the Resistome of Arugula (Eruca sativa Mill.). Microbiome 2019, 7, 13. [Google Scholar] [CrossRef]
- Vryzas, Z. The Plant as Metaorganism and Research on Next-Generation Systemic Pesticides—Prospects and Challenges. Front. Microbiol. 2016, 7, 1968. [Google Scholar] [CrossRef]
- Birnbaum, K.; Shasha, D.E.; Wang, J.Y.; Jung, J.W.; Lambert, G.M.; Galbraith, D.W.; Benfey, P.N. A Gene Expression Map of the Arabidopsis Root. Science 2003, 302, 1956–1960. [Google Scholar] [CrossRef]
- Kleber, R.; Kehr, J. Preparation and Quality Assessment of RNA from Cell-Specific Samples Obtained by Laser Microdissection. Methods Mol. Biol. 2006, 323, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Dvořák, P.; Nikel, P.I.; Damborský, J.; de Lorenzo, V. Bioremediation 3.0: Engineering Pollutant-Removing Bacteria in the Times of Systemic Biology. Biotechnol. Adv. 2017, 35, 845–866. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.W.; Wang, T.; Bedzyk, L.; Croker, K.M. Applications of DNA Microarrays in Microbial Systems. J. Microbiol. Methods 2001, 47, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.J.; Griffiths, M.W. Impact of Hydroxyl- and Superoxide Anion-Based Oxidative Stress on Logarithmic and Stationary Phase Escherichia coli O157:H7 Stress and Virulence Gene Expression. Food Microbiol. 2012, 29, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Dennis, P.; Edwards, E.A.; Liss, S.N.; Fulthorpe, R. Monitoring Gene Expression in Mixed Microbial Communities by Using DNA Microarrays. Appl. Environ. Microbiol. 2003, 69, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; Wang, Z.C.; Huang, H.Y.; Huang, H.D.; Peng, H.L. YjcC, a c-Di-GMP Phosphodiesterase Protein, Regulates the Oxidative Stress Response and Virulence of Klebsiella pneumoniae CG43. PLoS ONE 2013, 8, e66740. [Google Scholar] [CrossRef]
- Cheng, Y.; Zang, H.; Wang, H.; Li, D.; Li, C. Global Transcriptomic Analysis of Rhodococcus erythropolis D310-1 in Responding to Chlorimuron-Ethyl. Ecotoxicol. Environ. Saf. 2018, 157, 111–120. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Dileep, A.; Lebonah, D.E.; Pramoda Kumari, J. A Short Review on Proteomics and Its Applications. Int. Lett. Nat. Sci. 2014, 12, 77–84. [Google Scholar] [CrossRef]
- Seo, J.S.; Keum, Y.S.; Li, Q.X. Metabolomic and Proteomic Insights into Carbaryl Catabolism by Burkholderia sp. C3 and Degradation of Ten N-Methylcarbamates. Biodegradation 2013, 24, 795. [Google Scholar] [CrossRef]
- Schiffmann, C.L.; Jehmlich, N.; Otto, W.; Hansen, R.; Nielsen, P.H.; Adrian, L.; Seifert, J.; von Bergen, M. Proteome Profile and Proteogenomics of the Organohalide-Respiring Bacterium Dehalococcoides mccartyi Strain CBDB1 Grown on Hexachlorobenzene as Electron Acceptor. J. Proteom. 2014, 98, 59–64. [Google Scholar] [CrossRef]
- Tiwari, B.; Verma, E.; Chakraborty, S.; Srivastava, A.K.; Mishra, A.K. Tolerance Strategies in Cyanobacterium Fischerella sp. under Pesticide Stress and Possible Role of a Carbohydrate-Binding Protein in the Metabolism of Methyl Parathion (MP). Int. Biodeterior. Biodegrad. 2018, 127, 217–226. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Chrysayi-Tokousbalides, M. Metabolomics in Pesticide Research and Development: Review and Future Perspectives. Metabolomics 2011, 7, 35–53. [Google Scholar] [CrossRef]
- Wang, P.; Wu, Y.-J. Applications of Metabonomics in Pesticide Toxicology. Curr. Drug Metab. 2015, 16, 191–199. [Google Scholar] [CrossRef]
- Lénárt, J.; Bujna, E.; Kovács, B.; Békefi, E.; Száraz, L.; Dernovics, M. Metabolomic Approach Assisted High Resolution LC-ESI-MS Based Identification of a Xenobiotic Derivative of Fenhexamid Produced by Lactobacillus casei. J. Agric. Food Chem. 2013, 61, 8969–8975. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.V.; Booth, S.C.; Vantomme, E.A.N.; Afroj, S.; Yost, C.K.; Dahms, T.E.S. Oxidative Stress and Metabolic Perturbations in Escherichia coli Exposed to Sublethal Levels of 2,4-Dichlorophenoxyacetic Acid. Chemosphere 2015, 135, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Deng, Y.; Chang, C.; Lee, J.; Cheng, Y.; Cui, Z.; Zhou, J.; He, F.; Hu, M.; Zhang, L.H. Pathway and Kinetics of Cyhalothrin Biodegradation by Bacillus thuringiensis Strain ZS-19. Sci. Rep. 2015, 5, 8784. [Google Scholar] [CrossRef] [PubMed]
- Fulekar, M.H.; Sharma, J. Bioinformatics Applied in Bioremediation. Innov. Rom. Food Biotechnol. 2008, 3, 28–36. [Google Scholar]
- Dejonghe, W.; Berteloot, E.; Goris, J.; Boon, N.; Crul, K.; Maertens, S.; Höfte, M.; De Vos, P.; Verstraete, W.; Top, E.M. Synergistic Degradation of Linuron by a Bacterial Consortium and Isolation of a Single Linuron-Degrading Variovorax Strain. Appl. Environ. Microbiol. 2003, 69, 1532. [Google Scholar] [CrossRef]
- Pawelczyk, S.; Abraham, W.R.; Harms, H.; Müller, S. Community-Based Degradation of 4-Chorosalicylate Tracked on the Single Cell Level. J. Microbiol. Methods 2008, 75, 117–126. [Google Scholar] [CrossRef]
- Singh, V.; Braddick, D.; Dhar, P.K. Exploring the Potential of Genome Editing CRISPR-Cas9 Technology. Gene 2017, 599, 1–18. [Google Scholar] [CrossRef]

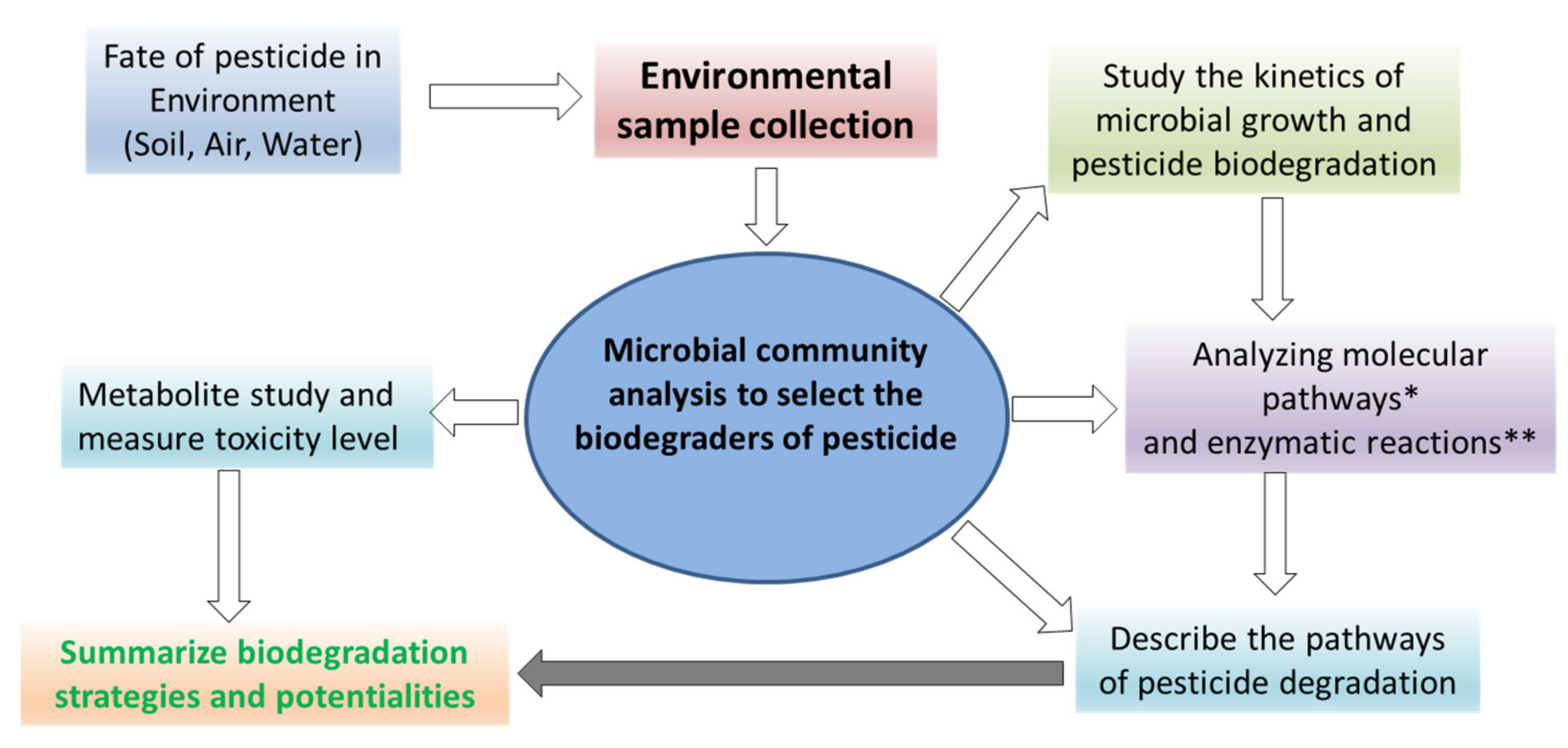

| Name of Pesticide | Isolation Source | Microbe Used | Microbe Type | Reference |
|---|---|---|---|---|
| 2,4-D | Pea (Pisum sativum) | Pseudomonas putida VM1450 | Bacterium | [41] |
| Thiamethoxam | Rice (Oryza sativa) | Enterobacter cloacae TMX-6 | Bacterium | [42] |
| 2,3,4,6-tetrachlorophenol, 2,4,6-Trichloropropane | Contaminated groundwater | Herbaspirillum spp. K1 | Bacterium | [43] |
| Imadcloprid | Coarse textured Rhizosphere soil | Pseudomonas spp. 1G | Bacterium | [44] |
| Acetamiprid and Imidacloprid | Tobacco plant (Nicotiana tabacum) | Acinetobacter spp. TW | Bacterium | [45] |
| Acetamiprid and Imidacloprid | Tobacco plant (Nicotiana tabacum) | Sphingomonas spp. TY | Bacterium | [45] |
| Acetamiprid | Rhizosphere Zone | Rhodotorula mucilaginosa Strain IM-2 | Yeast | [46] |
| Acetamiprid | Pesticide factory Rhizosphere Zone | Fusarium spp. strain CS-3 | Fungus | [47] |
| Acetamiprid | Rhizosphere Zone | Streptomyces canus CGMCC 13662 | Actinomycete | [48] |
| Thiacloprid | Rhizosphere soil | Ensifer meliloti CGMCC7333 | Bacterium | [49] |
| Clothianidin | Rotted wood | Phanerochaete sordida | Fungus | [50] |
| Nitenpyram | Rotted wood | Phanerochaete sordida YK-624 | Fungus | [51] |
| Dinotifuran | Rotted wood | Phanerochaete sordida YK-624 | Fungus | [51] |
| Chloropyrifos | Rhizosphere Zone | Bacillus spp. | Bacterium | [52,53] |
| Endosulfan | Rhizosphere Zone | Pseudomonas spp. | Bacterium | [52] |
| Alachlor | NITE Biological 53 Resource Center (NBRC), Japan | Aspergillus fumigates | Fungus | [54] |
| Diazion | Rhizosphere Zone | Pseudomonas spp. | Bacterium | [52,53] |
| Parathion | Rhizosphere Zone | Bacillus spp. | Bacterium | [52,53] |
| Malathion | Rhizosphere Zone | Rhodococcus spp. | Bacterium | [55] |
| Polychlorinated biphenyls (PCBs), Polycyclic aromatic hydrocarbons (PAHs) | Rhizosphere Zone | Rhodococcus spp. | Bacterium | [55] |
| Acibenzolar-S-methyl | Rhizosphere Zone | B. pumilus SE34 | Bacterium | [56] |
| Atrazine | Rhizosphere Zone | Cryptococcus strain TT3 | Bacterium | [57] |
| Rhizosphere Zone | Pichia kudriavzevii strain Atz-EN-01 | Yeast | [58] | |
| Wastewater | Raoultella planticola | Bacterium | [59] | |
| bensulfuron-methyl | Rhizosphere Zone | Bacillus megaterium L1 and Brevibacterium spp. BH | Bacteria | [60] |
| Bispyribac sodium | Madhana ghas (Dactyloctenium aegyptium) and lamp grass (Paspalum delatatum) | Chromobacter xylosoxidans (BD1), Achromobacter pulmonis (BA2), and Ochrobactrum intermedium (BM2) | Bacterial consortium | [61] |
| Tebuconazole | Rhizosphere Zone | Enterobacter sakazakii and Serratia spp. | Bacteria | [62] |
| Diuron | Rhizosphere Zone of sugarcane crop | Stenotrophomonas acidophila TD4.7, Bacillus cereus TD4.31 | Bacteria | [63] |
| Pyraclostrobin | Rhizosphere Zone of Orange tree | Bacillus spp. CSA-13, Bacillus aryabhattai CBMAI2223 | Bacteria | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salam, M.T.B.; Mahmood, A.; Asghar, W.; Ito, K.; Kataoka, R. Phytomicrobiomes: A Potential Approach for Sustainable Pesticide Biodegradation. Appl. Sci. 2024, 14, 2740. https://doi.org/10.3390/app14072740
Salam MTB, Mahmood A, Asghar W, Ito K, Kataoka R. Phytomicrobiomes: A Potential Approach for Sustainable Pesticide Biodegradation. Applied Sciences. 2024; 14(7):2740. https://doi.org/10.3390/app14072740
Chicago/Turabian StyleSalam, Md. Tareq Bin, Ahmad Mahmood, Waleed Asghar, Koji Ito, and Ryota Kataoka. 2024. "Phytomicrobiomes: A Potential Approach for Sustainable Pesticide Biodegradation" Applied Sciences 14, no. 7: 2740. https://doi.org/10.3390/app14072740
APA StyleSalam, M. T. B., Mahmood, A., Asghar, W., Ito, K., & Kataoka, R. (2024). Phytomicrobiomes: A Potential Approach for Sustainable Pesticide Biodegradation. Applied Sciences, 14(7), 2740. https://doi.org/10.3390/app14072740









