RObotic-Assisted Rehabilitation of Lower Limbs for Orthopedic Patients (ROAR-O): A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Clinical and Technological Assessment
2.3. Procedures
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.Z. Falls in Older People: Epidemiology, Risk Factors and Strategies for Prevention. Age Ageing 2006, 35 (Suppl. S2), ii37–ii41. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.Z.; Solomon, D.H.; Roth, C.P.; Young, R.T.; Shekelle, P.G.; Chang, J.T.; MacLean, C.H.; Kamberg, C.J.; Saliba, D.; Wenger, N.S. Detection and Management of Falls and Instability in Vulnerable Elders by Community Physicians. J. Am. Geriatr. Soc. 2004, 52, 1527–1531. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, S.; Brau, F.; Galluzzo, V.; Santagada, D.A.; Loreti, C.; Biscotti, L.; Laudisio, A.; Zuccalà, G.; Bernabei, R. Falls among Older Adults: Screening, Identification, Rehabilitation, and Management. Appl. Sci. 2022, 12, 7934. [Google Scholar] [CrossRef]
- Giovannini, S.; Iacovelli, C.; Brau, F.; Loreti, C.; Fusco, A.; Caliandro, P.; Biscotti, L.; Padua, L.; Bernabei, R.; Castelli, L. RObotic-Assisted Rehabilitation for Balance and Gait in Stroke Patients (ROAR-S): Study Protocol for a Preliminary Randomized Controlled Trial. Trials 2022, 23, 872. [Google Scholar] [CrossRef] [PubMed]
- Falls. Available online: https://www.who.int/news-room/fact-sheets/detail/falls (accessed on 20 October 2023).
- Dennison, E. Osteoporosis Treatment A Clinical Overview; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Carfì, A.; Liperoti, R.; Fusco, D.; Giovannini, S.; Brandi, V.; Vetrano, D.L.; Meloni, E.; Mascia, D.; Villani, E.R.; Manes Gravina, E.; et al. Bone Mineral Density in Adults with Down Syndrome. Osteoporos. Int. 2017, 28, 2929–2934. [Google Scholar] [CrossRef]
- Quintana, J.M.; Arostegui, I.; Escobar, A.; Azkarate, J.; Goenaga, J.I.; Lafuente, I. Prevalence of Knee and Hip Osteoarthritis and the Appropriateness of Joint Replacement in an Older Population. Arch. Intern. Med. 2008, 168, 1576–1584. [Google Scholar] [CrossRef]
- Laurence, B.D.; Michel, L. The Fall in Older Adults: Physical and Cognitive Problems. Curr. Aging Sci. 2017, 10, 185–200. [Google Scholar] [CrossRef]
- Song, R.; Roberts, B.L.; Lee, E.O.; Lam, P.; Bae, S.C. A Randomized Study of the Effects of t’ai Chi on Muscle Strength, Bone Mineral Density, and Fear of Falling in Women with Osteoarthritis. J. Altern. Complement. Med. 2010, 16, 227–233. [Google Scholar] [CrossRef]
- Giovannini, S.; Coraci, D.; Brau, F.; Galluzzo, V.; Loreti, C.; Caliandro, P.; Padua, L.; Maccauro, G.; Biscotti, L.; Bernabei, R. Neuropathic Pain in the Elderly. Diagnostics 2021, 11, 613. [Google Scholar] [CrossRef]
- Giovannini, S.; Brau, F.; Forino, R.; Berti, A.; D’ignazio, F.; Loreti, C.; Bellieni, A.; D’angelo, E.; Di Caro, F.; Biscotti, L.; et al. Sarcopenia: Diagnosis and Management, State of the Art and Contribution of Ultrasound. J. Clin. Med. 2021, 10, 5552. [Google Scholar] [CrossRef] [PubMed]
- Rouzi, A.A.; Ardawi, M.S.M.; Qari, M.H.; Bahksh, T.M.; Raddadi, R.M.; Ali, A.Y.; Jalal, M.M.; Taha, A.A.; Kary, H.S. Risk Factors for Falls in a Longitudinal Cohort Study of Saudi Postmenopausal Women: The Center of Excellence for Osteoporosis Research Study. Menopause 2015, 22, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- van Schoor, N.M.; Dennison, E.; Castell, M.V.; Cooper, C.; Edwards, M.H.; Maggi, S.; Pedersen, N.L.; van der Pas, S.; Rijnhart, J.J.M.; Lips, P.; et al. Clinical Osteoarthritis of the Hip and Knee and Fall Risk: The Role of Low Physical Functioning and Pain Medication. Semin. Arthritis Rheum. 2020, 50, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Li, X.; Zhang, Y.; Wang, Y.; Ma, Y.; Xu, S.; Shuai, Z.; Peng, X.; Pan, F. Knee Symptom but Not Radiographic Knee Osteoarthritis Increases the Risk of Falls and Fractures: Results from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2022, 30, 436–442. [Google Scholar] [CrossRef]
- Kramer, J.F.; Speechley, M.; Bourne, R.; Rorabeck, C.; Vaz, M. Comparison of Clinic- and Home-Based Rehabilitation Programs after Total Knee Arthroplasty. Clin. Orthop. Relat. Res. 2003, 410, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.D.; Lin, L.F.; Huang, Y.C.; Huang, S.W.; Chou, L.C.; Liou, T.H. Functional Outcomes of Outpatient Balance Training Following Total Knee Replacement in Patients with Knee Osteoarthritis: A Randomized Controlled Trial. Clin. Rehabil. 2014, 29, 855–867. [Google Scholar] [CrossRef]
- Sherrington, C.; Fairhall, N.; Wallbank, G.; Tiedemann, A.; Michaleff, Z.A.; Howard, K.; Clemson, L.; Hopewell, S.; Lamb, S. Exercise for Preventing Falls in Older People Living in the Community: An Abridged Cochrane Systematic Review. Br. J. Sports Med. 2020, 54, 885–891. [Google Scholar] [CrossRef]
- McCrum, C.; Bhatt, T.S.; Gerards, M.H.G.; Karamanidis, K.; Rogers, M.W.; Lord, S.R.; Okubo, Y. Perturbation-Based Balance Training: Principles, Mechanisms and Implementation in Clinical Practice. Front. Sports Act. Living 2022, 4, 1015394. [Google Scholar] [CrossRef]
- Okubo, Y.; Schoene, D.; Lord, S.R. Step Training Improves Reaction Time, Gait and Balance and Reduces Falls in Older People: A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2017, 51, 586–593. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Khan, A.A.; Muzammil, M. Lower Limb Rehabilitation Robotics: The Current Understanding and Technology. Work 2021, 69, 775–793. [Google Scholar] [CrossRef]
- Calafiore, D.; Negrini, F.; Tottoli, N.; Ferraro, F.; Ozyemisci-Taskiran, O.; De Sire, A. Efficacy of Robotic Exoskeleton for Gait Rehabilitation in Patients with Subacute Stroke: A Systematic Review. Eur. J. Phys. Rehabil. Med. 2022, 58, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.; Ali, H.; Hassan, T.; Marie, H. Open Issues for Intelligent Connectivity Wireless Sensor Networks (WSNs) and IoT: State of the Art. Delta Univ. Sci. J. 2022, 5, 352–366. [Google Scholar] [CrossRef]
- Kotani, N.; Morishita, T.; Saita, K.; Kamada, S.; Maeyama, A.; Abe, H.; Yamamoto, T.; Shiota, E.; Inoue, T. Feasibility of Supplemental Robot-Assisted Knee Flexion Exercise Following Total Knee Arthroplasty. J. Back Musculoskelet. Rehabil. 2020, 33, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Mutsuzaki, H.; Sano, A.; Koseki, K.; Fukaya, T.; Mizukami, M.; Yamazaki, M. Training with Hybrid Assistive Limb for Walking Function after Total Knee Arthroplasty. J. Orthop. Surg. Res. 2018, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Setoguchi, D.; Kinoshita, K.; Kamada, S.; Sakamoto, T.; Kise, N.; Kotani, N.; Goto, K.; Shiota, E.; Inoue, T.; Yamamoto, T. Hybrid Assistive Limb Improves Restricted Hip Extension after Total Hip Arthroplasty. Assist. Technol. 2022, 34, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Kubota, S.; Sugaya, H.; Arai, N.; Hyodo, K.; Kanamori, A.; Yamazaki, M. Feasibility and Efficacy of Knee Extension Training Using a Single-Joint Assistive Limb, versus Conventional Rehabilitation during the Early Postoperative after Total Knee Arthroplasty. J. Rural Med. 2021, 16, 22. [Google Scholar] [CrossRef]
- Morone, G.; Paolucci, S.; Cherubini, A.; De Angelis, D.; Venturiero, V.; Coiro, P.; Iosa, M. Robot-Assisted Gait Training for Stroke Patients: Current State of the Art and Perspectives of Robotics. Neuropsychiatr. Dis. Treat. 2017, 13, 1303–1311. [Google Scholar] [CrossRef]
- Caliandro, P.; Molteni, F.; Simbolotti, C.; Guanziroli, E.; Iacovelli, C.; Reale, G.; Giovannini, S.; Padua, L. Exoskeleton-Assisted Gait in Chronic Stroke: An EMG and Functional near-Infrared Spectroscopy Study of Muscle Activation Patterns and Prefrontal Cortex Activity. Clin. Neurophysiol. 2020, 131, 1775–1781. [Google Scholar] [CrossRef]
- Doma, K.; Grant, A.; Morris, J. The Effects of Balance Training on Balance Performance and Functional Outcome Measures Following Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. Sports Med. 2018, 48, 2367–2385. [Google Scholar] [CrossRef]
- Payedimarri, A.B.; Ratti, M.; Rescinito, R.; Vanhaecht, K.; Panella, M. Effectiveness of Platform-Based Robot-Assisted Rehabilitation for Musculoskeletal or Neurologic Injuries: A Systematic Review. Bioengineering 2022, 9, 129. [Google Scholar] [CrossRef]
- Domínguez-Navarro, F.; Igual-Camacho, C.; Silvestre-Muñoz, A.; Roig-Casasús, S.; Blasco, J.M. Effects of Balance and Proprioceptive Training on Total Hip and Knee Replacement Rehabilitation: A Systematic Review and Meta-Analysis. Gait Posture 2018, 62, 68–74. [Google Scholar] [CrossRef]
- Berg, K.; Wood-Dauphinee, S.; Williams, J.I. The Balance Scale: Reliability Assessment with Elderly Residents and Patients with an Acute Stroke. Scand. J. Rehabil. Med. 1995, 27, 27–36. [Google Scholar] [PubMed]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Nilsdotter, A.K.; Lohmander, L.S.; Klässbo, M.; Roos, E.M. Hip Disability and Osteoarthritis Outcome Score (HOOS)—Validity and Responsiveness in Total Hip Replacement. BMC Musculoskelet. Disord. 2003, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Roos, E.M.; Lohmander, L.S. The Knee Injury and Osteoarthritis Outcome Score (KOOS): From Joint Injury to Osteoarthritis. Health Qual. Life Outcomes 2003, 1, 64. [Google Scholar] [CrossRef]
- Volpato, S.; Cavalieri, M.; Sioulis, F.; Guerra, G.; Maraldi, C.; Zuliani, G.; Fellin, R.; Guralnik, J.M. Predictive Value of the Short Physical Performance Battery Following Hospitalization in Older Patients. J. Gerontol. Ser. A 2011, 66, 89–96. [Google Scholar] [CrossRef]
- Cassidy, B.; Arena, S. The Short Physical Performance Battery as a Predictor of Functional Decline. Home Healthc. Now 2022, 40, 168–169. [Google Scholar] [CrossRef]
- Fayazi, M.; Dehkordi, S.N.; Dadgoo, M.; Salehi, M. Test-Retest Reliability of Motricity Index Strength Assessments for Lower Extremity in Post Stroke Hemiparesis. Med. J. Islam. Repub. Iran 2012, 26, 27–30. [Google Scholar]
- Hauser, S.L.; Dawson, D.M.; Lehrich, J.R.; Beal, M.F.; Kevy, S.V.; Propper, R.D.; Mills, J.A.; Weiner, H.L. Intensive Immunosuppression in Progressive Multiple Sclerosis. A Randomized, Three-Arm Study of High-Dose Intravenous Cyclophosphamide, Plasma Exchange, and ACTH. N. Engl. J. Med. 1983, 308, 173–180. [Google Scholar] [CrossRef]
- Perry, J.; Garrett, M.; Gronley, J.K.; Mulroy, S.J. Classification of Walking Handicap in the Stroke Population. Stroke 1995, 26, 982–989. [Google Scholar] [CrossRef]
- Mehrholz, J.; Wagner, K.; Rutte, K.; Meißner, D.; Pohl, M. Predictive Validity and Responsiveness of the Functional Ambulation Category in Hemiparetic Patients after Stroke. Arch. Phys. Med. Rehabil. 2007, 88, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Comfortable and Maximum Walking Speed of Adults Aged 20–79 Years: Reference Values and Determinants. Age Ageing 1997, 26, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.D.; Chiu, V.; Stewart, A.L. Mobility-Related Function in Older Adults: Assessment with a 6-Minute Walk Test. Arch. Phys. Med. Rehabil. 1999, 80, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Balestroni, G.; Bertolotti, G. EuroQol-5D (EQ-5D): An Instrument for Measuring Quality of Life. Monaldi Arch. Chest Dis. Card. Ser. 2012, 78, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Learmonth, Y.C.; Dlugonski, D.; Pilutti, L.A.; Sandroff, B.M.; Klaren, R.; Motl, R.W. Psychometric Properties of the Fatigue Severity Scale and the Modified Fatigue Impact Scale. J. Neurol. Sci. 2013, 331, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Strober, L.B.; Bruce, J.M.; Arnett, P.A.; Alschuler, K.N.; DeLuca, J.; Chiaravalloti, N.; Lebkuecher, A.; Di Benedetto, M.; Cozart, J.; Thelen, J.; et al. Tired of Not Knowing What That Fatigue Score Means? Normative Data of the Modified Fatigue Impact Scale (MFIS). Mult. Scler. Relat. Disord. 2020, 46, 102576. [Google Scholar] [CrossRef] [PubMed]
- Saglia, J.A.; De Luca, A.; Squeri, V.; Ciaccia, L.; Sanfilippo, C.; Ungaro, S.; De Michieli, L. Design and Development of a Novel Core, Balance and Lower Limb Rehabilitation Robot: Hunova®. IEEE Int. Conf. Rehabil. Robot. 2019, 2019, 417–422. [Google Scholar] [CrossRef]
- Julious, S.A. Sample Size of 12 per Group Rule of Thumb for a Pilot Study. Pharm. Stat. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Moutzouri, M.; Gleeson, N.; Billis, E.; Panoutsopoulou, I.; Gliatis, J. What Is the Effect of Sensori-Motor Training on Functional Outcome and Balance Performance of Patients’ Undergoing TKR? A Systematic Review. Physiotherapy 2016, 102, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.B.; Elmallah, R.D.K.; Bhave, A.; Chughtai, M.; Cherian, J.J.; McGinn, T.; Harwin, S.F.; Mont, M.A. Rehabilitative Guidelines after Total Knee Arthroplasty: A Review. J. Knee Surg. 2016, 29, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Castelli, L.; Iacovelli, C.; Loreti, C.; Malizia, A.M.; Barone Ricciardelli, I.; Tomaino, A.; Fusco, A.; Biscotti, L.; Padua, L.; Giovannini, S. Robotic-Assisted Rehabilitation for Balance in Stroke Patients (ROAR-S): Effects of Cognitive, Motor and Functional Outcomes. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 8198–8211. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.I.; Polyak, M.; Coughlan, D.; Nichols, M.; Roche, A. Natural History of Recovery from Brain Injury after Prolonged Disorders of Consciousness: Outcome of Patients Admitted to Inpatient Rehabilitation with 1–4 Year Follow-Up. Prog. Brain Res. 2009, 177, 73–88. [Google Scholar] [CrossRef]
| G-Hun (n = 12) | G-Conv (n = 12) | p Value | ||
|---|---|---|---|---|
| Gender, % | Male vs. Female | 33.33 % vs. 66.67 % | 50.00 % vs. 50.00 % | p = 0.410 |
| Age, years | Mean ± SD | 69.1 ± 24.6 | 65.6 ± 24.3 | p = 0.887 |
| Latency, days | Mean ± SD | 5.0 ± 1.4 | 5.0 ± 1.6 | p = 0.514 |
| Type of prosthesis, % | Knee vs. Hip | 58.33 % vs. 41.67 % | 41.67 % vs. 58.33 % | p = 1.000 |
| Affected size, % | Left vs. Right | 66.67 % vs. 33.33 % | 58.33 % vs. 41.67 % | p = 0.755 |
| G-Hun | G-Con | ||||||
|---|---|---|---|---|---|---|---|
| T0 Median (IQR) | T1 Median (IQR) | p Value | T0 Median (IQR) | T1 Median (IQR) | p Value | p Value G-Hun vs. G-Con | |
| Motor function, balance and gait | |||||||
| MI-LL prosthetic side | 61.5 (48.75–65.5) | 77 (74.5–92) | p = 0.002 | 64 (52.5–66) | 87 (74.5–91.25) | p = 0.013 | p = 0.319 |
| MI-LL non-prosthetic side | 88 (82–100) | 100 (92–100) | p = 0.016 | 88 (76–94) | 95.5 (91–100) | p = 0.017 | p = 0.799 |
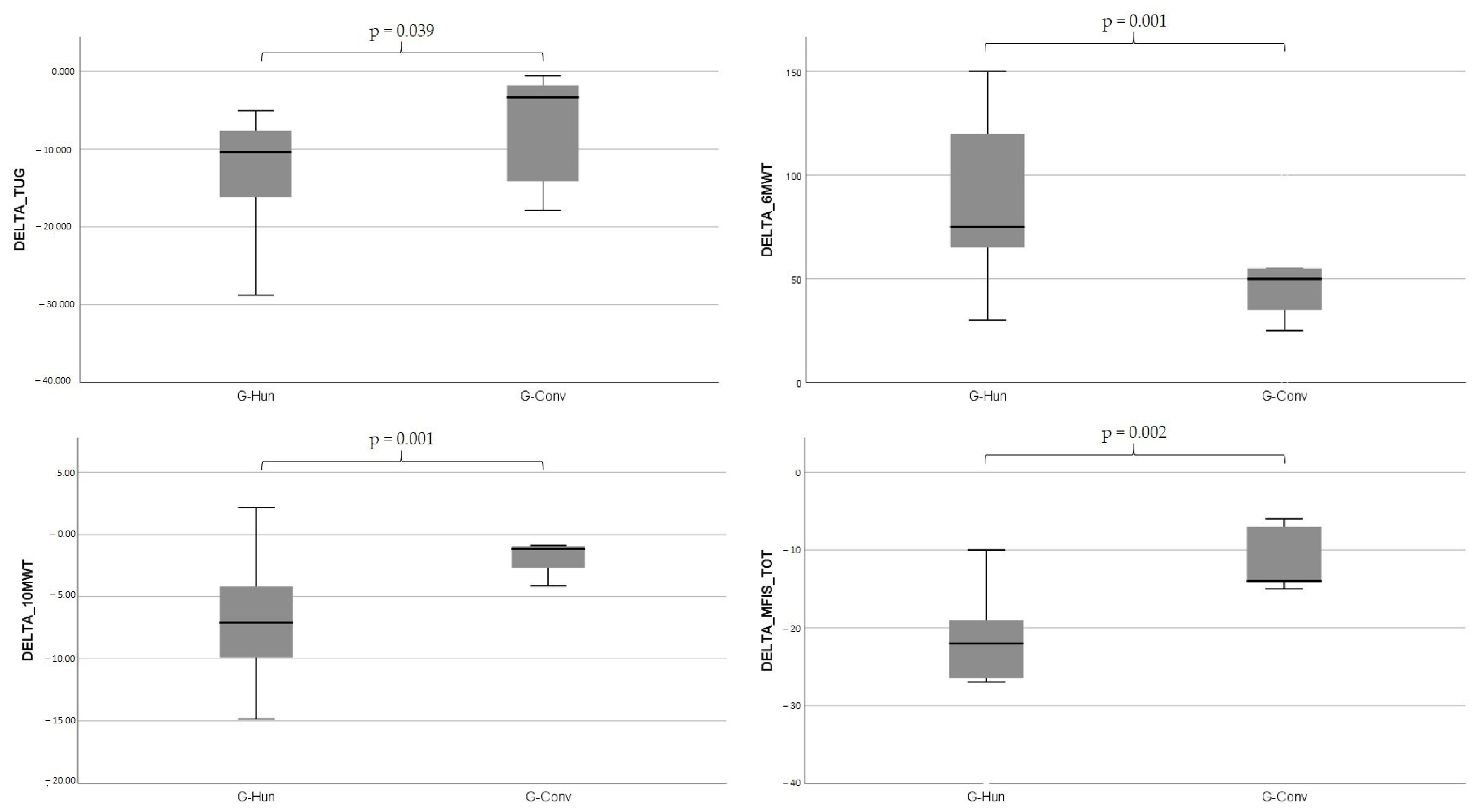
| TUG | 31.60 (27.63–63.5) | 22.93 (19.14–26.86) | p = 0.002 | 23.85 (17.32–27.62) | 12.82 (8.3–24.29) | p = 0.002 | p = 0.039 |
| BBS | 30 (22.75–32.25) | 42 (38.75–48.5) | p = 0.02 | 28 (18–33) | 38.5 (33–54) | p = 0.003 | p = 0.089 |
| SPPB_B | 1 (1–2) | 2 (2–3) | p = 0.002 | 1 (1–1) | 2 (1.75–4) | p = 0.007 | p = 0.713 |
| SPPB_W | 1 (1–1) | 2 (1–2) | p = 0.007 | 1 (1–1) | 1 (1–3) | p = 0.039 | p = 0.843 |
| SPPB_STS | 1 (1–1) | 2 (1.75–2) | p = 0.004 | 1 (1–1) | 1 (1–4) | p = 0.007 | p = 0.843 |
| SPPB_TOT | 3 (2.75–4) | 6 (5–6) | p = 0.002 | 3 (3–3) | 4 (3.75–11) | p = 0.011 | p = 0.514 |
| HAI | 5 (4.75–6) | 3 (2.75–4) | p = 0.002 | 4.5 (2–5.25) | 2 (2–2.5) | p = 0.053 | p = 0.671 |
| FAC | 1.5 (1–2) | 4 (3–4) | p = 0.002 | 2 (2–3) | 3 (2.75–5) | p = 0.002 | p = 0.219 |
| WHS | 2 (2–1) | 3.5 (3–4) | p = 0.001 | 2 (2–2) | 3 (3–5) | p = 0.002 | p = 0.932 |
| 10 MWT | 20.16 (16.86–28.61) | 12.19 (11.29–18.58) | p = 0.001 | 17.4 (14.8–18.24) | 15.69 (13.79–16.38) | p = 0.002 | p = 0.001 |
| 6 MWT | 77.5 (60–106.25) | 175 (133.75–212.5) | p = 0.002 | 100 (85–1656) | 150 (143.75–190) | p = 0.003 | p = 0.001 |
| Fatigue, autonomy, and quality of life | |||||||
| mBI | 50.5 (42.75–58.5) | 91 (84–92.75) | p = 0.002 | 43 (37.75–43) | 64 (56–87) | p = 0.002 | p = 0.219 |
| EQ-5D_VAS | 52.5 (43.75–60) | 85 (80–86.25) | p = 0.003 | 47.5 (45–53.75) | 80 (70–85) | p = 0.003 | p = 0.630 |
| EQ-5D TOT | 11 (10.5–15.25) | 7 (6–7.25) | p = 0.002 | 15 (14–15) | 9 (7–11) | p = 0.002 | p = 0.713 |
| MFIS_PHY | 25 (22.5–28.25) | 14 (10–16.5) | p = 0.002 | 22 (21–22.25) | 19 (16–19) | p = 0.002 | p = 0.001 |
| MFIS_COG | 13 (12–18.25) | 3 (2–3.25) | p = 0.003 | 11 (9–16.75) | 8 (6–12) | p = 0.002 | p = 0.05 |
| MFIS_PSY | 7 (5.5–7.25) | 3 (2–3.25) | p = 0.003 | 5 (4.75–6.25) | 3 (2–3) | p = 0.003 | p = 0.514 |
| MFIS_TOT | 43.5 (42–50.75) | 23.5 (17.75–29.25) | p = 0.002 | 41 (36–44) | 30 (28–30) | p = 0.002 | p = 0.002 |
| G-Hun | G-Conv | |||||
|---|---|---|---|---|---|---|
| T0 Median (IQR) | T1 Median (IQR) | p Value | T0 Median (IQR) | T1 Median (IQR) | p Value | |
| KOOS-I | ||||||
| KOOS_S | 70 (65–75) | 35 (25–45) | p = 0.013 | 91.66 (91.66–100) | 75 (75–75) | p = 0.046 |
| KOOS_P | 69.45 (66.66–75) | 41.67 (41.67–52.77) | p = 0.043 | 80 (80–100) | 33.34 (33.34 + 75) | p = 0.046 |
| KOOS_ADL | 69.12 (67.64–79.42) | 48.53 (45.49–52.95) | p = 0.080 | 70.58 (70.58–92.65) | 51.18 (30.89–72.05) | p = 0.170 |
| KOOS_sport | 100 (100–100) | 50 (52–75) | p = 0.024 | 100 (100–100) | 81.25 (45–81.25) | p = 0.042 |
| KOOS_QoL | 75 (62.5–81.25) | 27.5 (25–31.25) | p = 0.033 | 75 (68.75–75) | 62.25 (56.25–62.25) | p = 0.036 |
| KOOS_TOT | 74.50 (74.18–78.21) | 42.02 (31.45–45.10) | p = 0.013 | 82.04 (82.04–93.57) | 58.43 (45.25–70.34) | p = 0.016 |
| HOOS-I | ||||||
| HOOS_S | 66.67 (63.33–79.17) | 33.33 (25–45.83) | p = 0.018 | 75 (75–75) | 66.67 (66.67–84.3) | p = 0.680 |
| HOOS_p | 69.95 (62.5–83.75) | 52 (32.5–37.5) | p = 0.018 | 57.5 (57.5–57.5) | 52 (32.5–80) | p = 0.892 |
| HOOS_ADL | 70.59 (60.88–75.74) | 43.75 (31.61–52.8) | p = 0.018 | 60.3 (60.3–60.3) | 54.42 (51.18–64.71) | p = 0.684 |
| HOOS_sport | 100 (100–100) | 50 (46.88–62.5) | p = 0.017 | 100 (43.75–81.2) | 100 (100–100) | p = 0.180 |
| HOOS_QoL | 68.42 (65.25–75) | 18.75 (11.13–31.25) | p = 0.018 | 52 (43.75–81.2) | 62.75 (62.75–62.75) | p = 0.684 |
| HOOS_TOT | 78.32 (70.70–80.09) | 36.89 (31.93–47.63) | p = 0.018 | 67.47 (54.70–70.67) | 60.10 (58.43–72.60) | p = 0.684 |
| G-Hun | G-Conv | ||||||
|---|---|---|---|---|---|---|---|
| T0 Mediana (IQR) | T1 Mediana (IQR) | p Value | T0 Mediana (IQR) | T1 Mediana (IQR) | p Value | p Value G-Huv vs. G-Conv | |
| Static Condition | |||||||
| Area-EC [cm2] | 4.96 [4.17–6.50] | 2.67 [1.87–6.08] | p = 0.169 | 7.73 [5.98–9.5] | 7.73 [5.98–9.5] | p = 0.142 | p = 0.186 |
| Area-EO [cm2] | 2.50 [2.05–3.34] | 1.99 [1.18–3.58] | p = 0.959 | 1.17 [0.95–3.1] | 1.35 [1.17–3.1] | p = 0.155 | p = 0.361 |
| Romberg Index | 0.37 [0.36–0.55] | 0.86 [0.37–1.40] | p = 0.471 | 0.23 [0.14–0.33] | 0.23 [0.14–0.47] | p = 0.241 | p = 0.047 |
| COP path-EO [cm] | 49.78 [36.02–70.64] | 42.96 [31.00–61.78] | p = 0.333 | 33.53 [28.25–35.79] | 35.32 [29.3–35.79] | p = 0.177 | p = 0.303 |
| COP path-EC [cm] | 64.38 [49.44–118.26] | 60.68 [49.56–88.22] | p = 0.169 | 62.89 [46.76–115.83] | 62.89 [46.76–115.83] | p = 0.220 | p = 0.119 |
| Trunk movement-EO [deg/s2] | 0.05 [0.04–0.07] | 0.05 [0.05–0.07] | p = 0.735 | 0.04 [0.04–0.06] | 0.04 [0.04–0.08] | p = 1.000 | p = 0.569 |
| Trunk movement-EC [deg/s2] | 0.05 [0.05–0.12] | 0.05 [0.05–0.05] | p = 0.075 | 0.06 [0.04–0.1] | 0.06 [0.04–0.01] | p = 0.237 | p = 0.277 |
| Trunk sway range AP-EO [deg] | 2.80 [2.12–3.22] | 3.03 [2.31–6.97] | p = 0.059 | 2.12 [2.1–2.97] | 2.12 [2.1–2.97] | p = 0.256 | p = 0.186 |
| Trunk sway range AP-EC [deg] | 3.07 [2.42–4.16] | 2.78 [2.24–3.32] | p = 0.575 | 3.21 [2.52–4.09] | 3.21 [2.52–4.09] | p = 0.242 | p = 0.608 |
| Trunk sway range ML-EO [deg] | 1.25 [0.83–1.54] | 1.28 [0.97–2.07] | p = 0.507 | 0.81 [0.43.1.17] | 0.81 [0.45–1.17] | p = 0.158 | p = 0.691 |
| Trunk sway range ML-EC [deg] | 1.41 [1.26–1.90] | 1.31 [0.98–2.03] | p = 0.507 | 1.21 [0.86–1.43] | 1.21 [0.86–1.43] | p = 0.189 | p = 0.331 |
| COP sway range AP-EO [cm] | 1.96 [1.83–2.67] | 1.75 [1.36–3.87] | p = 0.959 | 2.96 [2.22–3.43] | 2.96 [2.22–3.43] | p = 0.164 | p = 0.424 |
| COP sway range AP-EC [cm] | 2.35 [2.21–3.01] | 2.47 [2.14–2.77] | p = 0.878 | 1.77 [1.5–2.23] | 1.78 [1.5–2.23] | p = 0.157 | p = 0.424 |
| COP sway range ML-EO [cm] | 3.63 [2.59–4.41] | 2.83 [2.31–3.48] | p = 0.92 | 3.74 [2.99–5.2] | 3.74 [2.99–5.1] | p = 0.174 | p = 0.026 |
| COP sway range ML-OC [cm] | 1.41 [1.27–2.12] | 1.73 [1.10–2.29] | p = 0.959 | 1.21 [0.65–1.49] | 1.30 [1.03–1.49] | p = 0.144 | p = 0.691 |
| Ratio of axes of the ellipse-EO [%] | 54.43 [48.07–57.68] | 39.93 [35.21–49.86] | p = 0.600 | 62.64 [50.72–71.79] | 71.79 [60.55–71.9] | p = 0.182 | p = 0.055 |
| Ratio of axes of the ellipse -EC [%] | 55.96 [46.60] | 48.89 [46.46–73.37] | p = 0.646 | 60.90 [48.86–81.69] | 60.90 [54.77–81.69] | p = 0.157 | p = 0.119 |
| Mean speed COP AP-EO [cm/s] | 1.38 [1.16–1.82] | 1.29 [0.95–1.84] | p = 0.721 | 0.86 [0.83–0.98] | 0.98 [0.86–1.02] | p = 0.139 | p = 0.361 |
| Mean speed COP AP-EC [cm/s] | 1.90 [1.48–4.06] | 1.68 [1.56–1.96] | p = 0.874 | 1.81 [1.41–4.02] | 1.81 [1.41–4.02] | p = 0.157 | p = 0.186 |
| Mean speed COP ML-EO [cm/s] | 0.77 [0.50–0.97] | 0.66 [0.47–0.97] | p = 0.283 | 0.70 [0.38–0.71] | 0.70 [0.38–0.71] | p = 0.177 | p = 0.018 |
| Mean speed COP ML-EC [cm/s] | 0.93 [0.66–1.54] | 0.87 [0.67–1.87] | p = 0.859 | 0.99 [0.8–1.19] | 1.04 [0.99–1.19] | p = 0.128 | p = 0.569 |
| Dynamic Condition | |||||||
| Area-EO [cm2] | 38.47 [17.95–44.66] | 3.06 [2.44–10.33] | p = 0.006 | 39.50 [27.06–66.38] | 39.50 [22.08–66.38] | p = 0.177 | p = 0.006 |
| COP path-EO [cm] | 85.00 [49.94–115.41] | 28.17 [17.19–30.82] | p = 0.004 | 94.26 [91.26–125.6] | 94.26 [91.26–125.6] | p = 0.240 | p = 0.002 |
| Trunk movement-EO [deg/s2] | 0.08 [0.08–0.16] | 0.06 [0.05–0.07] | p = 0.028 | 0.08 [0.07–0.11] | 0.08 [0.07–0.11] | p = 0.347 | p = 0.035 |
| Trunk sway range AP-EO [deg] | 4.40 [2.70–5.01] | 2.87 [2.37–4.20] | p = 0.248 | 3.96 [3.45–4.09] | 3.96 [3.45–4.09] | p = 0.184 | p = 0.459 |
| Trunk sway range ML-EO [deg] | 4.97 [2.96–5.91] | 1.89 [1.48–2.45] | p = 0.005 | 2.37 [1.92–5] | 2.37 [1.92–5] | p = 0.664 | p = 0.001 |
| COP sway range AP-EO [cm] | 7.16 [5.06–9.21] | 3.23 [2.42–5.04] | p = 0.059 | 7.82 [7.11–8.9] | 7.11 [5.73–8.9] | p = 0.378 | p = 0.134 |
| COP sway range ML-EO [cm] | 6.32 [4.32–7.41] | 2.07 [1.55–3.81] | p = 0.013 | 7.59 [5.6–10.38] | 7.59 [5.6–10.38] | p = 0.157 | p = 0.093 |
| Mean speed COP AP-EO [cm/s] | 2.49 [0.95–2.82] | 0.56 [0.33–0.62] | p = 0.012 | 2.42 [1.41–3.11] | 2.42 [1.41–3.11] | p = 0.124 | p = 0.035 |
| Mean speed COP ML-EO [cm/s] | 1.44 [0.93–2.23] | 0.59 [0.34–0.78] | p = 0.021 | 1.81 [1.36–2.54] | 1.81 [1.36–2.54] | p = 0.237 | p = 0.055 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castelli, L.; Iacovelli, C.; Ciccone, S.; Geracitano, V.; Loreti, C.; Fusco, A.; Biscotti, L.; Padua, L.; Giovannini, S. RObotic-Assisted Rehabilitation of Lower Limbs for Orthopedic Patients (ROAR-O): A Randomized Controlled Trial. Appl. Sci. 2023, 13, 13208. https://doi.org/10.3390/app132413208
Castelli L, Iacovelli C, Ciccone S, Geracitano V, Loreti C, Fusco A, Biscotti L, Padua L, Giovannini S. RObotic-Assisted Rehabilitation of Lower Limbs for Orthopedic Patients (ROAR-O): A Randomized Controlled Trial. Applied Sciences. 2023; 13(24):13208. https://doi.org/10.3390/app132413208
Chicago/Turabian StyleCastelli, Letizia, Chiara Iacovelli, Siria Ciccone, Valerio Geracitano, Claudia Loreti, Augusto Fusco, Lorenzo Biscotti, Luca Padua, and Silvia Giovannini. 2023. "RObotic-Assisted Rehabilitation of Lower Limbs for Orthopedic Patients (ROAR-O): A Randomized Controlled Trial" Applied Sciences 13, no. 24: 13208. https://doi.org/10.3390/app132413208






