Abstract
Leptadenia pyrotechnica is considered a wild herb used to enhance the palatability of food, particularly in the Gulf region. The effect of microwave (5, 8, and 10 min at 900 W) and hot-air heating (60, 120, and 180 min at 100 °C) on the phenolic compounds and antioxidants of L. pyrotechnica stems was investigated. The results showed that microwave heating gave high values of the total phenolic content (TPC), total flavonoid content (TFC), and antioxidant properties, while the control sample was inferior in all these attributes. Moreover, microwave heating, particularly for 8 min, produced the highest TPC, TFC, and DPPH values (significant at p < 0.05) and reduced power in the L. pyrotechnica stem. However, prolonging the heating time caused adverse effects on the bioactive potential of the samples. The HPLC analysis indicates that both processes caused a significant increment in the phenolic compounds of the sample. Tannic acid, vanillin, and acetyl salicylic acid were found to be higher in the microwaved-treated samples than in those heated with hot air. The tannic acid was found to be high after microwave heating for 8 min at 116.06 mg/100 g, while the higher value of acetylsalicylic acid 119.08 mg/100 g was observed after microwave heating for 5 min. The partial least regression (PLS) validation model revealed that microwave heating, particularly at an 8 min application time, offered better results, and owing to its short processing time, which might be adopted for heating the L. pyrotechnica stems in the food industry, and it can be useful for preparing functional foods.
1. Introduction
Leptadenia pyrotechnica (Forssk.) Decne is a wild plant growing in semi-arid deserts, mainly in the Mediterranean region. In the Arabian Peninsula, the common name of the L. pyrotechnica plant is “Markh”, which means flexible and soft plant [1]. In the southwestern region of Saudi Arabia, Leptadenia pyrotechnica grows widely in valleys and coastal areas where sandy and dry conditions exist. Interestingly, L. pyrotechnica (Forssk.) is used in Middle Eastern countries, especially in the Gulf area, to enhance the flavor of lamb meat chunks during cooking. Hence, the fresh or newly harvested stem of L. pyrotechnica is recommended for application as a flavoring agent. Also, this plant is cultivated for its health benefits in farms, forests, and sidewalks in the Middle East [2].
Several reports have attributed the health benefits of L. pyrotechnica to the contents of its phenolic profile, including caffeic acid, vanillin, and ferulic acid [3,4]. These bioactive compounds positively impact human health and reduce the hazard of wide-spectrum diseases such as diabetes, cancer, and cardiovascular disease. However, antioxidants could prevent or delay the oxidation process caused by reactive oxygen species by donating an electron to the free radical or removing the reactive oxygen/nitrogen species initiators [5,6,7,8]. Also, antioxidants occurring naturally and in plants, fruits, and vegetables are highly distinguished and favored for use in food preservation, food coloring, and food flavoring over their synthetic counterparts [9,10].
Preparing food before consuming or while cooking requires many steps (i.e., cutting, portioning, mixing, heating, etc.) to homogenize and improve the food structure, texture, and aroma. The factors that influence the activity of antioxidants play a significant role in limiting or expanding their functional properties, including, but not limited to, food mixture, antioxidant concentration, temperature, pressure, and oxygen concentration [11,12]. In particular, the higher temperatures utilized during the cooking of foods can either increase or decrease the removal of bioactive compounds from plant tissue, which affects the ability and the properties of antioxidants [13,14]. Microwave energy is considered a valuable tool in thermal and drying techniques. It provides multiple advantages (e.g., higher drying rate, short drying time, fast volumetric heating, and enhanced qualities of the product) [15]. Several studies have applied microwaves and hot air to dry herbal plants, fruits, and vegetables. They confirmed the advantage of microwave over hot-air heating regarding color damage, darkness, stickiness, and energy consumption.
Moreover, microwave heating enhanced the antioxidant capability of the plant products [16]. Nevertheless, few accessible data regarding the effect of thermal processing on the phenolic compounds and antioxidant capacity of wild herbs such as Leptadenia pyrotechnica have been stated. Therefore, this study aims to evaluate and compare the differences between microwave and conventional heating processes on the phytochemical compounds and antioxidant activity of the Leptadenia pyrotechnica stems.
2. Materials and Methods
2.1. Chemicals and Materials
Ethanol and methanol of reagent grade were obtained from Merck (Darmstadt, Germany). The Folin–Ciocalteu reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and phenolic compounds (tannic acid, resorcinol, 1,2-dihydroxybenzene, chlorogenic acid, caffeic acid, vanillin, acetylsalicylic acid, 3,5-dinitro salicylic acid, salicylic acid, quercetin, gallic acid, and catechin) were purchased from Sigma Aldrich (St. Louis, MO, USA).
2.2. Leptadenia pyrotechnica Sample Preparation
The branches/stems of L. pyrotechnica were collected at maturity during the harvesting season of October 2022 from the same area in the Tihamah coastal plains of the Jazan region, Saudi Arabia. The samples were cut into small pieces of around 2 cm and then treated at 100 °C in a hot-air oven (Binder D-78532, Tuttlingen, Germany) for 60, 120, and 180 min. The microwave treatments were conducted in a microwave oven (Whirlpool. Model: WMH32517AS; 230 V-50 Hz AC, 2450 MHz) with an output power of 900 W. About 200 g of the cut samples was placed in one layer on a Teflon plate and treated with the microwave for 5, 8, and 10 min, respectively. Each treatment was conducted three times. The heated samples were brought to ambient temperature and ground into a fine powder.
The powder, which passed through a 60-mesh sieve, was stored at room temperature. The untreated sample served as a control sample. The control sample was cut into small pieces, dried under an ambient temperature of 25 °C, and then ground into fine powder.
2.3. Sample Extraction
The phenolic and antioxidant compounds of the sample were extracted in a ratio of 1:10 (w:v). About 2 g of the sample was mixed with 20 mL of ethanol (70%). The mixture was sonicated using an ultrasonic water bath (model 2800 CPX, Branson, Marshall Scientific, Hampton, NH, USA) at 23 °C for 1 h. The mixture was centrifuged at 3000× g for 15 min, filtered, and kept at 4 °C for further analysis.
2.4. Total Phenolic Content (TPC)
The extract (25 µL) was blended with 1500 µL water and 125 µL Folin–Ciocalteu reagent [17]. Subsequently, the mixture was incubated for 1 min, and 375 µL of 20% sodium carbonate was added to reach 2500 µL as the final volume. The absorbance was recorded at 760 nm at room temperature. Gallic acid was used as the standard to express TPC, and the result was calculated as gallic acid equivalent per gram dry weight of the sample (mg GAE/g DW).
2.5. Total Flavonoid Content (TFC)
The extract (250 µL) was diluted with 1000 µL of H2O. Subsequently, NaNO2 and AlCl3 (75 µL) were added to the extract and kept for 5 min at room temperature [17]. After that, 1 M NaOH (500 µL) and nanopore water (600 µL) were added. The absorbance was detected at 510 nm. Catechin was used as the standard to express TFC, and the result was calculated as a catechin equivalent (mg CE/g DW).
2.6. DPPH Scavenging
An aliquot of extract (130 µL) and 0.1 mM DPPH was blended and incubated in the dark for 30 min [18]. The absorbance was detected at 517 nm. Meanwhile, methanol was tested as a blank. The scavenging percentage was calculated according to Equation (1):
2.7. Reducing Power
The extract (120 µL) was mixed with potassium ferricyanide (1000 µL) and incubated for 20 min at 50 °C [19]. Trichloroacetic acid (1000 µL) was added and centrifuged at 3000× g for 10 min. An aliquot (1000 µL) of the supernatant was blended with 1000 µL H2O and 200 µL ferric chloride and then detected at 700 nm. The reducing power was specified as ascorbic acid equivalents (AAEs) per gram of sample.
2.8. HPLC Analysis of Phenolic Compounds
The extracted samples from Section 2.3 were analyzed using HPLC with the method described previously [19]; the quantification of phenolic compounds (tannic acid, resorcinol, 1,2-dihydroxybenzene, chlorogenic acid, caffeic acid, vanillin, acetylsalicylic acid, 3,5-dinitro salicylic acid, salicylic acid, and quercetin) in L. pyrotechnica samples was carried out in the present study with some modifications. HPLC (Agilent Technologies, Santa Clara, CA, USA, 1260 infinity) with a DAD-1260 VL detector, Zorbax SB-C18 (250 × 4.6 mm, 5 µm; Agilent, Santa Clara, CA, USA) column at 30 °C and a mobile phase (A) Milli Q water with 4% acetic acid (HPLC grade) and (B) MeOH (HPLC grade) was used. The gradient program used for the binary solvent was 0–10 min 5–20% B; 10–15 min 20–40% B; 15–20 min 40–60% B; 20–25 min 60% B with 1.0 mL/min flow rate. A 10 µL sample was injected into the HPLC system, and the DAD was set at 280 nm. The phenolic compounds in the L. pyrotechnica samples were detected by comparing the peak retention time and standards and reported as mg/100 g DW (Figure 1).
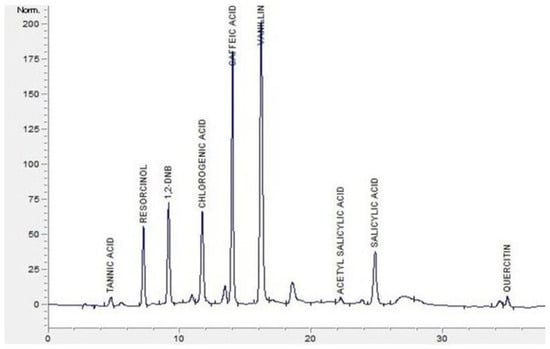
Figure 1.
HPLC chromatogram of phenolic standards.
2.9. Statistical Analysis
The experiments were conducted in three replicates. The ANOVA and Duncan’s multiple range tests were performed using SAS statistical software (Version 9.2) for all the parameters. The principal component analysis (PCA) and the linear partial least squares regression test (PLS) validation were conducted using the XLSTAT software (version 2023.2.1414).
3. Results and Discussion
3.1. Total Phenolic Content of Leptadenia pyrotechnica Stem
The effect of microwave heating and conventional heating on the total phenolic content (TPC) of L. pyrotechnica stem is shown in Figure 2. Generally, the microwave-treated samples exhibited a higher TPC than the oven-treated samples. It was observed that microwave treatment for 8 min showed the highest TPC (10.68 mg GAE/g DW), followed by microwave treatment for 10 min (8.98 mg GAE/g DW) and hot-air heating for 120 min (7.84 mg GAE/g DW). The control sample of L. pyrotechnica showed the lowest TPC value of 4.46 mg/g DW (p < 0.05).
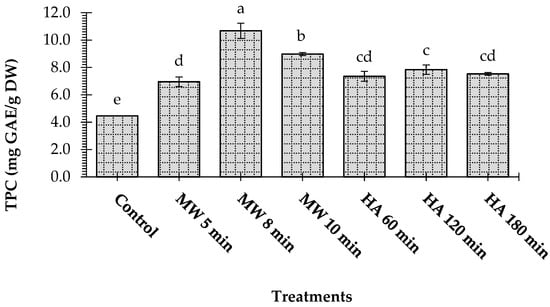
Figure 2.
Effect of microwave heating (MW) and hot-air heating (HA) methods on the total phenolic content (TPC) of Leptadenia pyrotechnica stem samples. The bars for the treatments bearing different letters are significantly different from each other at p < 0.05.
Our results follow those of a previous study of microwaved-dried coriander leaves. The microwave drying (500 W, 430 s) yielded a higher total polyphenol content (48.44 mg GAE/g DW) as compared to that (26.64 mg GAE/g DW) obtained on oven drying (60 °C, 160 min) the coriander leaves [20]. In another study, microwave-dried blueberry leaves showed a higher TPC than oven-dried leaf samples [21]. The highest TPC was recorded in the Strobilanthes crispus tea leaves developed from microwave-dried leaves [22]. On the contrary, the total phenolic content of date fruit flesh decreased after microwave and conventional oven drying compared to the control sample [23]. Microwave, vacuum, and oven drying were applied to celery slices, and it was noticed that the dried samples had 38.51–75.34% less TPC than fresh samples [24]. However, degradation, auto-oxidation, and polymerization can occur during drying, which might explain the decrease in the total phenol contents [25]. Moreover, the heating and drying processes encourage the release of phenolic compounds from the plant matrix, which happens after the breakdown of cellular constituents [26,27].
3.2. Total Flavonoid Content of Leptadenia pyrotechnica Stem
Figure 3 shows the total flavonoid content (TFC) of control and treated L. pyrotechnica samples. Like the total phenolic content, the TFC of the dried samples was higher than that in the control sample. The samples treated for 8 min in the microwave oven exhibited the highest TFC (3.32 mg CE/g DW), while the control sample showed the lowest TFC value (1.38 mg CE/g DW). Statistically, there was no difference (p > 0.05) between the TFC of the sample treated under microwaves for 10 min (3.07 mg CE/g DW) and that of the sample heated conventionally for 120 min (3.04 mg CE/g DW). Although the TFCs were comparable, microwave heating was better in terms of a shorter processing time of only 8 min compared to the 120 min for hot-air oven heating.
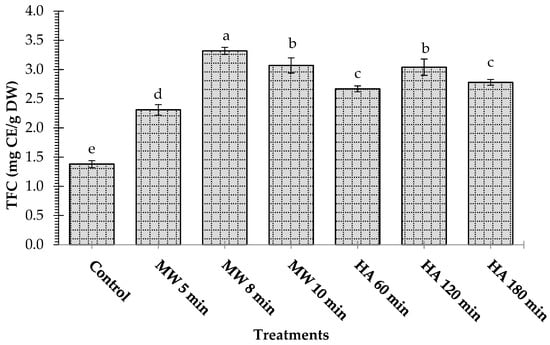
Figure 3.
Effect of microwave heating (MW) and hot-air heating (HA) methods on the total flavonoid content (TFC) of Leptadenia pyrotechnica stem samples. The bars for the treatments bearing different letters are significantly different from each other at p < 0.05.
The microwave drying (500 W, 430 s) yielded a higher total flavonoid content (20.28 mg rutin equivalent (RE)/g) as compared to (19.60 mg RE/g) the oven drying (60 °C, 160 min) of coriander leaves [20]. Previous research stated that the heating process liberated the bound phenolic compounds from citrus peel and pomace powder, increasing the free phenolic compound’s content. In turn, the antioxidant activity was enhanced [28,29]. Also, the drying processes involve lowering the samples’ water content, which might lead to the inactivation of enzymes in the plant matrix and a high level of flavonoids in the extract [30]. The longer drying times for the microwave and hot-air drying resulted in a lower TFC in the samples. For example, the TFC of hot-air dried samples at 60, 120, and 180 min were 2.67, 3.04, and 2.78 mg CE/g DW, respectively. This shows that the phenolic compounds were destroyed under prolonged treatment, and these results follow those of the previous studies [28,29].
3.3. Antioxidant Activity of Leptadenia pyrotechnica Stem
The effect of microwave and conventional heating on the antioxidant activity in terms of DPPH radical scavenging and reducing the power of the L. pyrotechnica stem samples is shown in Figure 4 and Figure 5. Before treatments, the DPPH of the L. pyrotechnica stem was found to be 22.01%. Both microwave and conventional heating processes caused a significant (p < 0.05) increment in the DPPH of the samples. However, it was observed that microwave-heated samples had significantly higher DPPH values than conventionally heated samples (Figure 3). The highest DPPH values were obtained in the samples treated with the microwave for 8 min (76.58%), followed by the samples microwave heated for 10 min (73.43%) and then the samples hot-air oven heated for 120 min (63.03%), respectively.
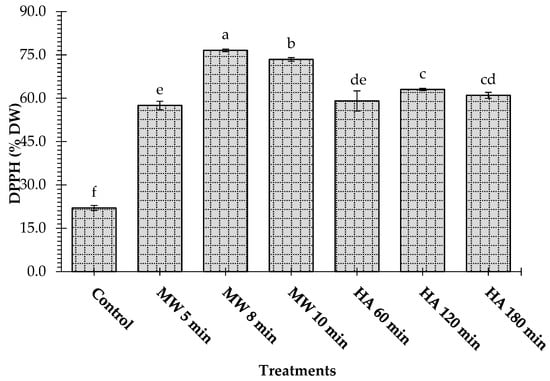
Figure 4.
Effect of microwave heating (MW) and hot-air heating (HA) methods on the DPPH scavenging of Leptadenia pyrotechnica stem samples. The bars for the treatments bearing different letters are significantly different from each other at p < 0.05.
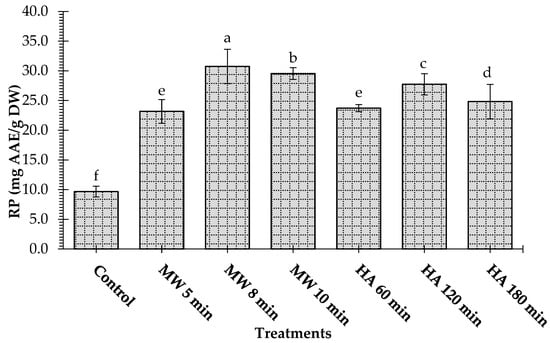
Figure 5.
Effect of microwave heating (MW) and hot-air heating (HA) methods on the reducing power of Leptadenia pyrotechnica stem samples. MD. The bars for the treatments bearing different letters are significantly different from each other at p < 0.05.
A recent study applied various drying methods to dry blueberry fruits. It was found that microwave drying at 300 W for 82 min and 500 W for 64 min was the best way to increase the blueberry fruits’ antioxidant capacity [31]. Coriander leaves dried in microwave ovens showed higher DPPH scavenging than those dried in conventional ovens [21]. Maillard reaction products might be produced during heating, contributing to the sample’s increased antioxidant activity [31]. Although some phenolic compounds are destroyed, some new compounds like hydroxycinnamates and quinic acid are released, which play a role in the antioxidant activity of the material [32].
The effect of heating processes on the reducing power (RP) of L. pyrotechnica stem samples is depicted in Figure 5. The reducing power of the samples is measured in terms of its equivalent to the ascorbic acid. The microwave-treated samples showed a higher reducing power than the hot-air-treated samples. For instance, RPs of 23.18, 30.74, and 29.55 mg AAE/g were noticed for the samples that were microwave heated for 5, 8, and 10 min, respectively. However, the control sample showed a significantly (p < 0.05) lower reducing power value (9.67 mg AAE/g DW) than all the samples. These results followed the findings for the TPC and TFC, which showed that the antioxidant activity of the L. pyrotechnica samples was attributable to the phenolic compounds. Blueberry leaves dried under microwaves exhibited a higher ferric reducing power than oven-dried leaves, and their ferric reducing power was similar to that of freeze-dried blueberry leaves [21]. Microwave, vacuum, and oven-dried celery slices showed more antioxidant activity than the fresh sample. In short, microwave drying provides higher nutrients by increasing the antioxidant capacities [24]. Saifullah [33] stated that the freeze-drying method gave the highest phenolic content and antioxidant activity in lemon myrtle, and no significant differences in phenolic compounds and antioxidant activity were observed among all the other drying methods, i.e., hot-air drying, microwave drying, vacuum drying, shade drying, and sun drying. However, microwave drying was suggested for the industrial-scale drying of lemon myrtle due to its time and/or energy efficiency.
3.4. Phenolic Compound Profile of Leptadenia pyrotechnica Stem
Due to different botanical and regional sources, the phenolic compounds differ between samples. The phenolic substance type and concentration are responsible for the biological activities and rely on the source of the sample [34,35]. The qualitative and quantitative analysis of tannic acid, chlorogenic acid, caffeic acid, vanillin, acetylsalicylic acid, salicylic acid, and quercetin in L. pyrotechnica using HPLC is reported in Table 1. All the samples contained five phenolic compounds. The range of the detected phenolic compounds in the control sample was 2.00–43.95 mg/100 g DW; for the microwave-treated samples, it was 1.52–49.08 mg/100 g DW, and for the hot-air heated samples, it was 1.06–41.98 mg/100 g. Acetylsalicylic acid was the highest (119.08 mg/100 g DW) phenolic compound, while vanillin (1.61 mg/100 g) was the lowest in the 5 min microwave-heated sample. Tannic acid was the highest (116.06 mg/100 g), whereas vanillin (1.65 mg/100 g DW) was the lowest in the 8 min microwave-treated sample. For the 10 min microwave-treated sample, tannic acid was the highest (77.30 mg/100 g DW), while caffeic acid (1.52 mg/100 g DW) was detected at its lowest amount.

Table 1.
Phenolic compounds profile (mg/100g DW) in Leptadenia pyrotechnica stem (ND—not detected).
The results of the hot-air treatment revealed that vanillin was the lowest compound (1.06 mg/100 g DW), while tannic acid was found to be the highest one (28.12 mg/100 g DW) when samples were treated for 60 min. The caffeic acid (3.54 mg/100 g DW) was the lowest, and acetylsalicylic acid (25.82 mg/100 g DW) was the highest in the 120 min hot-air dried sample. In contrast, for the 180 min treatment, vanillin (1.17 mg/100 g DW) was the lowest and tannic acid (41.98 mg/100 g DW) was the highest phenolic compound.
These results reveal that the total phenolic compounds were higher in microwave-heated samples than in the hot-air treated samples. The range of total detected phenolic compounds for the microwave treatment was 95.43–214.66 mg/100 g DW compared to 68.90–82.47 mg/100 g DW for the hot-air treatment. Kasawneh et al. [3] reported the phenolic compounds in L. pyrotechnica with different solvent extractions. The results showed that ethyl acetate extraction had a higher caffeic acid content, 0.78 mg/g, whereas n-butanol extract had the lowest amount of caffeic acid, 0.23 mg/g. The amounts of vanillic acid and gallic acid were found to be 1.2 and 0.42 mg/g, respectively; in water extraction, they were found to be in minute concentrations. Preet and Gupta [2] reported the phenolic compounds in Indian L. pyrotechnica, in which caffeic acid was the most abundant, 3.305%. Munazir et al. [36] reported the total tannin content in the L. pyrotechnica aerial part and root as 154.96 and 62.71 mg/100 g, respectively. The sharp reduction in the acetylsalicylic acid content after microwave treatment for more than 5 min might be due to the increase in the temperature during the treatment. It has been stated that acetylsalicylic acid begins to significantly decrease at high temperatures above 150 °C [37]. Our study showed an improvement in the phenolic compound content due to heating at a particular time, and it was more evident for the microwave-treated sample; this might contribute to the antioxidant activity of L. pyrotechnica. Fu et al. [38] reported a weak correlation between the free radical scavenging antioxidant activity and the total phenolic content. They have suggested that phenolic compounds could not be the main components responsible for free radical scavenging ability.
3.5. Multivariable Analysis
In this work, principal component analysis (PCA) was conducted to describe the mutual relationship between the treatment processes and the quality parameters of the Leptadenia pyrotechnica stem. Based on the PCA, the interrelationship between thermal treatments and the antioxidant capacity of Leptadenia pyrotechnica stem is described in Figure 6. The samples treated with microwaves and the hot air-treated samples were mostly separated. However, a minor intersection between the groups was verified. The biplot axes PC1 and PC2 together were responsible for 72.21% of the variation (Figure 7). PC1 and PC2 described 56.65% and 20.55% of the total deviation, respectively. As reported by Yan and Fregeau-Reid [39], the variable eccentricity and observation that appear at <90° angle were positively correlated, while that at angles > 90° were correlated with a negative relationship, and those with a 90° angle did not show an association in the PCA.
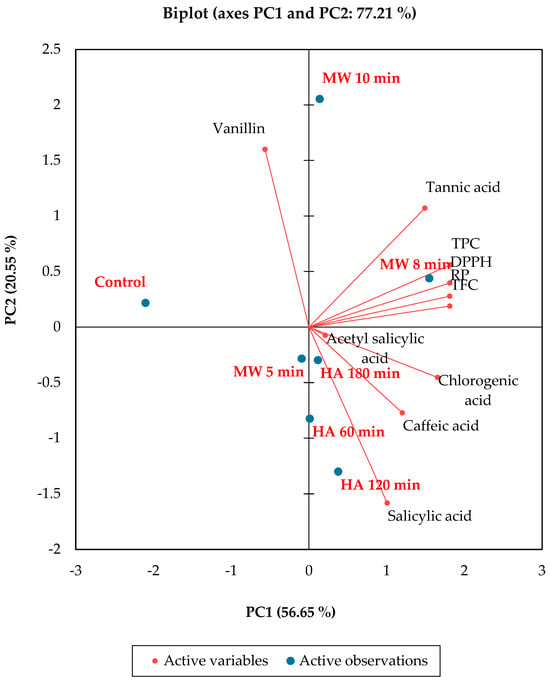
Figure 6.
Principal component analysis (PCA) scores for the experimental variables determined in L. pyrotechnica stem.
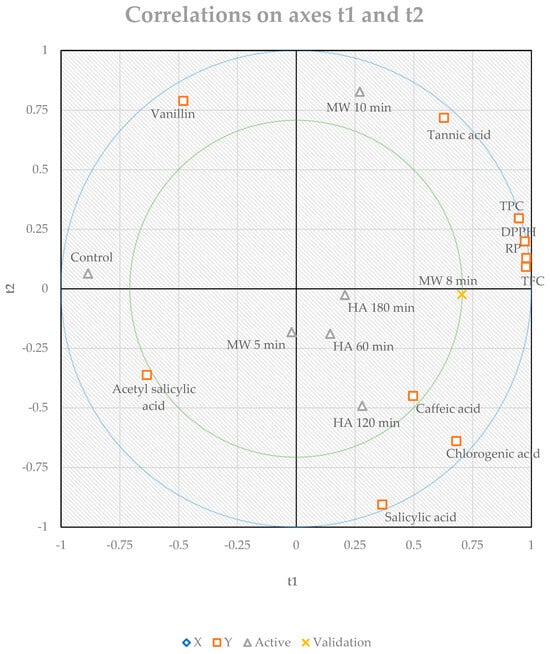
Figure 7.
The partial least squares regression analysis (PLS) for the experimental variables determined in Leptadenia pyrotechnica stem.
Accordingly, the PCA displayed a progressive solid correlation between the tested parameters and the microwave heating process. These explanations show that microwave heating could improve the content of phytochemicals and the antioxidant capacity of Leptadenia pyrotechnica stem.
The interactive effects of conventional heating and microwave heating on the total phenolic content, total flavonoid content, antioxidant activities, and phenolic profile of the Leptadenia pyrotechnica stem were described using the PLS model (Figure 7). The PLS analysis termed the shared effects of thermal treatments (x variables) on the measured parameters (y variables) of Leptadenia pyrotechnica stem (Figure 7). According to this model, conventional and microwave heating showed a positive validation score for most of the studied parameters in the Leptadenia pyrotechnica stem. However, PLS specified that applying microwave heating for 8 min (MW 8 min) was the most valid method, which might be considered for use in the food industry and processing. Generally, improving the bioactive compounds in food enhances its antioxidant, anticarcinogenic, and antimicrobial properties, which positively affects human health. Thus, the bioactive compounds in plants are particularly essential for the manufacture of functional foods and medicinal products, which may have industrial relevance too.
4. Conclusions
This study evaluated the effect of two thermal treatment methods, conventional and microwave heating, on the bioactive properties of Leptadenia pyrotechnica stems. It was found that microwave heating showed better results in terms of TPC, TFC, DPPH scavenging, and reducing power than the hot-air oven drying method. Moreover, even if the two different treatments of both processing methods showed statistically similar results, the microwave power was better in terms of its short processing time. A prolonged drying time negatively impacted the bioactive properties of L. pyrotechnica. The control sample was inferior among all the samples in terms of its bioactive properties tested based on the attributes mentioned above. Based on the results of this study, microwave heating could be a suitable method for enhancing the antioxidant capacity of Leptadenia pyrotechnica stems. However, according to the validation model (PLS), the application of the microwaves for 8 min showed the most positive validation score for most of the studied parameters. Traditionally, Leptadenia pyrotechnica is a rich source of natural antioxidants since the higher intake of foods with functional attributes, including high levels of antioxidants in functional foods, is one strategy that is gaining importance. Hence, additional collaborative research and innovative technologies such as microwave energy could be utilized to enhance the nutritional value of food and uphold traditional health principles by developing new supplementary foods.
Author Contributions
Conceptualization, M.S.A. and K.H.; methodology, M.S.A., K.H. and M.A.A.; data curation, K.H., M.A.A., A.M.S. and A.B.H.; writing review and editing, M.S.A., A.M.S., K.H. and M.A.A.; project administration, M.S.A. and K.H.; funding acquisition, M.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Researchers Supporting Project Number (RSPD2023R917), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors extend thanks to the Researchers Supporting Project number (RSPD2023R917), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ali, M.S.; Kausar, F.; Malik, A. Pyrotechnoic acid: A glycol-oleanolic acid conjugate from Leptadenia pyrotechnica (Asclepiadaceae). J. Chem. Soc. Pak. 2001, 23, 180–181. [Google Scholar]
- Preet, R.; Chand Gupta, R. Simultaneous Determination of Phenolic Compounds in Leptadenia pyrotechnica (Forssk.) Decne. By Using High-Performance Liquid Chromatography (HPLC-DAD-U V). Adv. Pharmacol. Sci. 2018, 2018, 9604972. [Google Scholar] [CrossRef]
- Khasawneh, M.A.; Elwy, H.M.; Hamza, A.A.; Fawzi, N.M.; Hassan, A.H. Antioxidant, Anti-Lipoxygenase and Cytotoxic Activity of Leptadenia pyrotechnica (Forssk.) Decne Polyphenolic Constituents. Molecules 2011, 16, 7510–7521. [Google Scholar] [CrossRef]
- Khalid, H.; Abdalla, W.E.; Abdelgadir, H.; Opatz, T.; Efferth, T. Gems from traditional north-African medicine: Medicinal and aromatic plants from Sudan. Nat. Prod. Bioprospect. 2012, 2, 92–103. [Google Scholar] [CrossRef]
- Rasheed, H.M.F.; Rasheed, F.; Qureshi, A.W.; Jabeen, Q. Immunostimulant Activities of the Aqueous Methanolic Extract of Leptadenia pyrotechnica, a Plant from Cholistan Desert. J. Ethnopharmacol. 2016, 186, 244–250. [Google Scholar] [CrossRef]
- Majidinia, M.; Bishayee, A.; Yousefi, B. Polyphenols: Major regulators of key components of DNA damage response in cancer. DNA Repair 2019, 82, 102679. [Google Scholar] [CrossRef]
- Jain, G.; Jhalani, S.; Agarwal, S.; Jain, K. Hypolipidemic and antiatherosclerotic effect of Leptadenia pyrotechnica extract in cholesterol fed rabbits. Asian J. Exp. Sci. 2007, 21, 115–122. [Google Scholar]
- Krinsky, N.I. Mechanism of Action of Biological Antioxidants. Exp. Biol. Med. 1992, 200, 248–254. [Google Scholar] [CrossRef]
- De Gonzalez, M.N.; Hafley, B.S.; Boleman, R.M.; Miller, R.K.; Rhee, K.S.; Keeton, J.T. Antioxidant properties of plum concentrates and powder in precooked roast beef to reduce lipid oxidation. Meat Sci. 2008, 80, 997–1004. [Google Scholar] [CrossRef]
- Zhang, X.; Li, D.; Meng, Q.; He, C.; Ren, L. Effect of mulberry leaf extracts on color, lipid oxidation, antioxidant enzyme activities and oxidative breakdown products of raw ground beef during refrigerated storage. J. Food Qual. 2016, 39, 159–170. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Anese, M.; Parpinel, M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci. Technol. 1999, 10, 94–100. [Google Scholar] [CrossRef]
- Pokorny, J.; Yanishlieva, N.; Gordon, M. (Eds.) Antioxidants in Food: Practical Applications; CRC Press: Boca Raton, FL, USA, 2001; p. 2. [Google Scholar]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. J. Food Process. Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Ciou, J.Y.; Chen, H.C.; Chen, C.W.; Yang, K.M. Relationship between Antioxidant Components and Oxidative Stability of Peanut Oils as Affected by Roasting Temperatures. Agriculture 2021, 11, 300. [Google Scholar] [CrossRef]
- Sanga, E.; Mujumdar, A.S.; Raghavan, G.S.V. Principles and Applications of Microwave Drying. In Drying Technology in Agriculture and Food Sciences; Mujumdar, A.S., Ed.; Science Publisher: Enfield, NH, USA, 2000; pp. 253–289. [Google Scholar]
- Tulasidas, T.N.; Raghavan, G.S.V.; Mujumdar, A.S. Microwave drying of grapes in a single mode cavity at 2450 MHz—II. Quality and energy aspects. Dry. Technol. 1995, 13, 1973–1992. [Google Scholar] [CrossRef]
- Hayat, K.S.; Abbas, C.; Jia, S.; Xia; Zhang, X. Comparative study on phenolic compounds and antioxidant activity of Feutrell’s early and kinnow peel extracts. J. Food Biochem. 2011, 35, 454–471. [Google Scholar] [CrossRef]
- Noreen, H.; Semmar, N.; Farman, M.; McCullagh, J.S.O. Measurement of total phenolic content and antioxidant activity of aerial parts of medicinal plant Coronopus didymus. Asian Pac. J. Trop. Med. 2011, 10, 792–801. [Google Scholar] [CrossRef]
- He, J.; Yin, T.; Chen, Y.; Cai, L.; Tai, Z.; Li, Z.; Liu, C.; Wang, Y.; Ding, Z. Phenolic compounds and antioxidant activities of edible flowers of Pyrus pashia. J. Funct. Foods 2015, 17, 371–379. [Google Scholar] [CrossRef]
- Hihat, S.; Remini, H.; Madani, K. Effect of oven and microwave drying on phenolic compounds and antioxidant capacity of coriander leaves. Int. Food Res. J. 2017, 24, 503–509. [Google Scholar]
- Routray, W.; Orsat, V. MAE of phenolic compounds from blueberry leaves and comparison with other extraction methods. Ind. Crops Prod. 2014, 58, 36–45. [Google Scholar] [CrossRef]
- Lasano, N.F.; Rahmat, A.; Ramli, N.S.; Abu Bakar, M.F. Effect of oven and microwave drying on polyphenols content and antioxidant capacity of herbal tea from Strobilanthes crispus leaves. Asian J. Pharm. Clin. Res. 2018, 11, 363–368. [Google Scholar] [CrossRef]
- Juhaimi, F.A.; Özcan, M.M.; Uslu, N. The effect of microwave and conventional drying on antioxidant activity, phenolic compounds and mineral profile of date fruit (Phoenix dactylifera L.) flesh. Food Meas. 2017, 11, 58–63. [Google Scholar] [CrossRef]
- Karabacak, A.Ö.; Suna, S.; Tamer, C.E.; Çopur, U. Effects of oven, microwave and vacuum drying on drying characteristics, colour, total phenolic content and antioxidant capacity of celery slices. Qual. Assur. Saf. Crops Foods 2018, 10, 193–205. [Google Scholar] [CrossRef]
- Wongsa, P.; Khampa, N.; Horadee, S.; Chaiwarith, J.; Rattanapanone, N. Quality and bioactive compounds of blends of Arabica and Robusta spray-dried coffee. Food Chem. 2019, 283, 579–587. [Google Scholar] [CrossRef]
- Hayat, K.; Zhang, X.; Chen, H.; Xia, S.; Jia, C.; Zhong, F. Liberation and separation of phenolic compounds from citrus mandarin peels by microwave heating and its effect on antioxidant activity. Sep. Purif. Technol. 2010, 73, 371–376. [Google Scholar] [CrossRef]
- Alkaltham, M.; Özcan, M.; Uslu, N.; Salamatullah, A.M.; Hayat, K. Effect of microwave and oven roasting methods on total phenol, antioxidant activity, phenolic compounds, and fatty acid compositions of coffee beans. J. Food Process. Preserv. 2020, 44, 14874. [Google Scholar] [CrossRef]
- Hayat, K.; Zhang, X.; Farooq, U.; Abbas, S.; Xia, S.; Jia, C.; Zhong, F.; Zhang, J. Effect of microwave treatment on phenolic content and antioxidant activity of citrus mandarin pomace. Food Chem. 2010, 123, 423–429. [Google Scholar] [CrossRef]
- Xu, G.; Ye, X.; Chen, J.; Liu, D. Effect of heat treatment on the phenolic compounds and antioxidant capacity of citrus peel extract. J. Agric. Food Chem. 2007, 55, 330–335. [Google Scholar] [PubMed]
- Hossain, M.B.; Barry-Ryan, C.; Martin-Diana, A.B.; Brunton, N.P. Effect of drying method on the antioxidant capacity of six Lamiaceae herbs. Food Chem. 2010, 123, 85–91. [Google Scholar] [CrossRef]
- Zia, M.P.; Alibas, I. Influence of the drying methods on color, vitamin C, anthocyanin, phenolic compounds, antioxidant activity, and in vitro bioaccessibility of blueberry fruits. Food Biosci. 2021, 42, 101179. [Google Scholar] [CrossRef]
- Pastoriza, S.; Rufián-Henares, J.A. Contribution of melanoidins to the antioxidant capacity of the Spanish diet. Food Chem. 2014, 164, 438–445. [Google Scholar] [CrossRef]
- Wei, F.; Tanokura, M. Coffee in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2015; pp. 83–91. [Google Scholar]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Vuong, Q. Effects of different drying methods on extractable phenolic compounds and antioxidant properties from lemon myrtle dried leaves. Heliyon 2019, 5, e03044. [Google Scholar] [CrossRef]
- Ciulu, M.; Solinas, S.; Floris, I.; Panzanelli, A.; Pilo, M.I.; Piu, P.C.; Spano, N.; Sanna, G. RP-HPLC determination of water-soluble vitamins in honey. Talanta 2011, 83, 924–929. [Google Scholar] [CrossRef]
- Munazir, M.; Qureshi, R.; Munir, M. Preliminary phytochemical screening of roots and aerial parts of Leptadenia pyrotechnica. Pak. J. Bot. 2015, 47, 659–664. [Google Scholar]
- Ferrit, M.; Del Valle, C.; Martínez, F. The influence of the structural characteristics of the substrate and the medium on the stability of triflusal and acetylsalicylic acid in micellar systems. J. Mol. Liq. 2008, 142, 64–71. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Yan, W.; Frégeau-Reid, J. Breeding line selection based on multiple traits. Crop Sci. 2008, 48, 417–423. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).