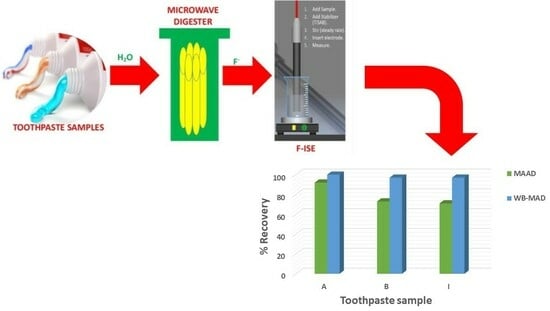
Water-Based Microwave-Assisted Digestion Method for Electrochemical and Chromatographic Determination of Total Fluoride Ions in Toothpaste Samples
Abstract
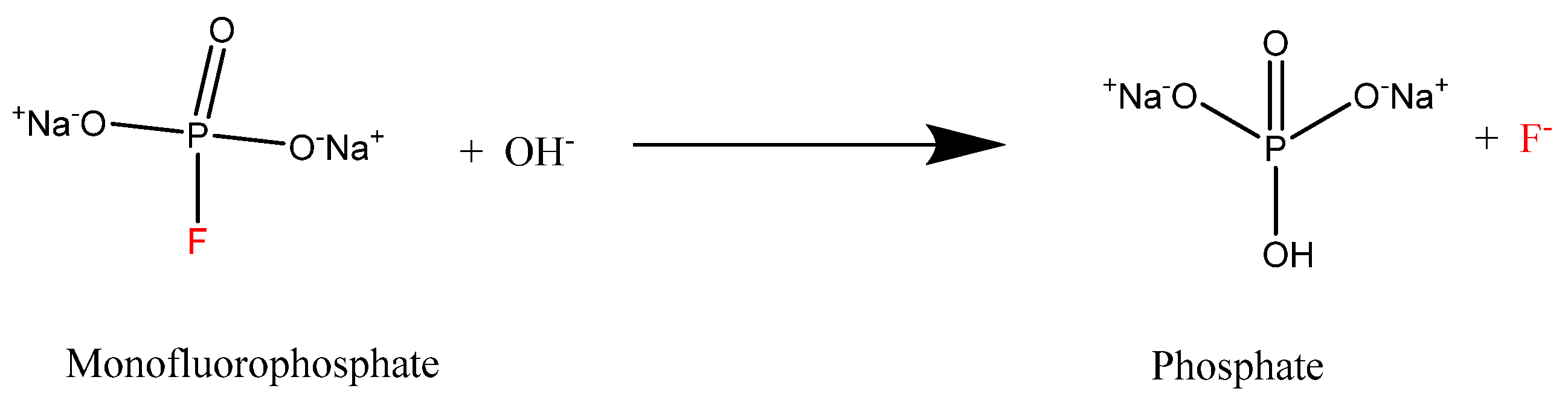
:1. Introduction
2. Experimental Method
2.1. Materials, Reagents and Standards
2.2. Instrumentation
2.3. Water-Based Microwave-Assisted Digestion (WB-MAD) Procedure
3. Results and Discussion
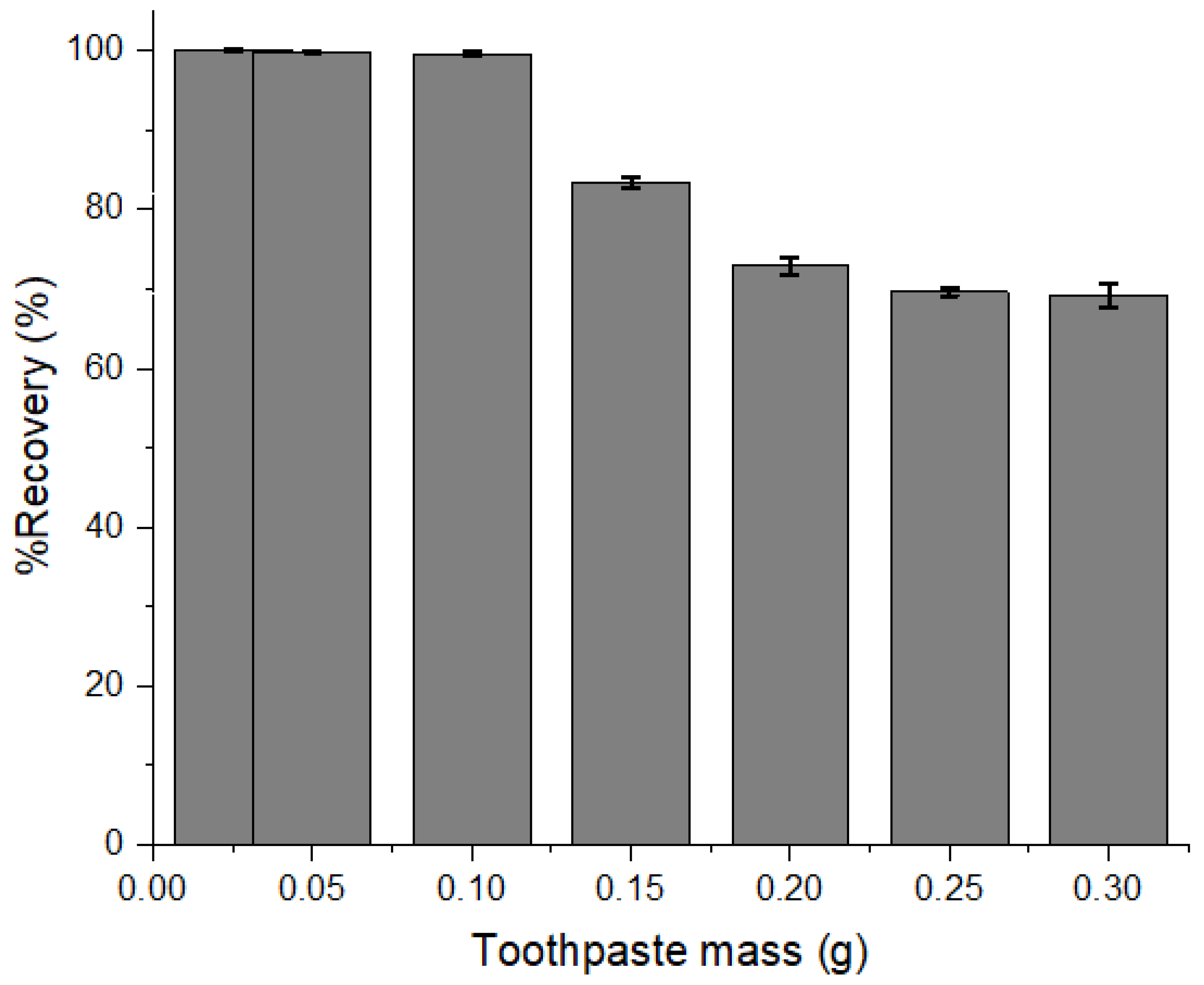
3.1. Optimization of Toothpaste Sample Mass
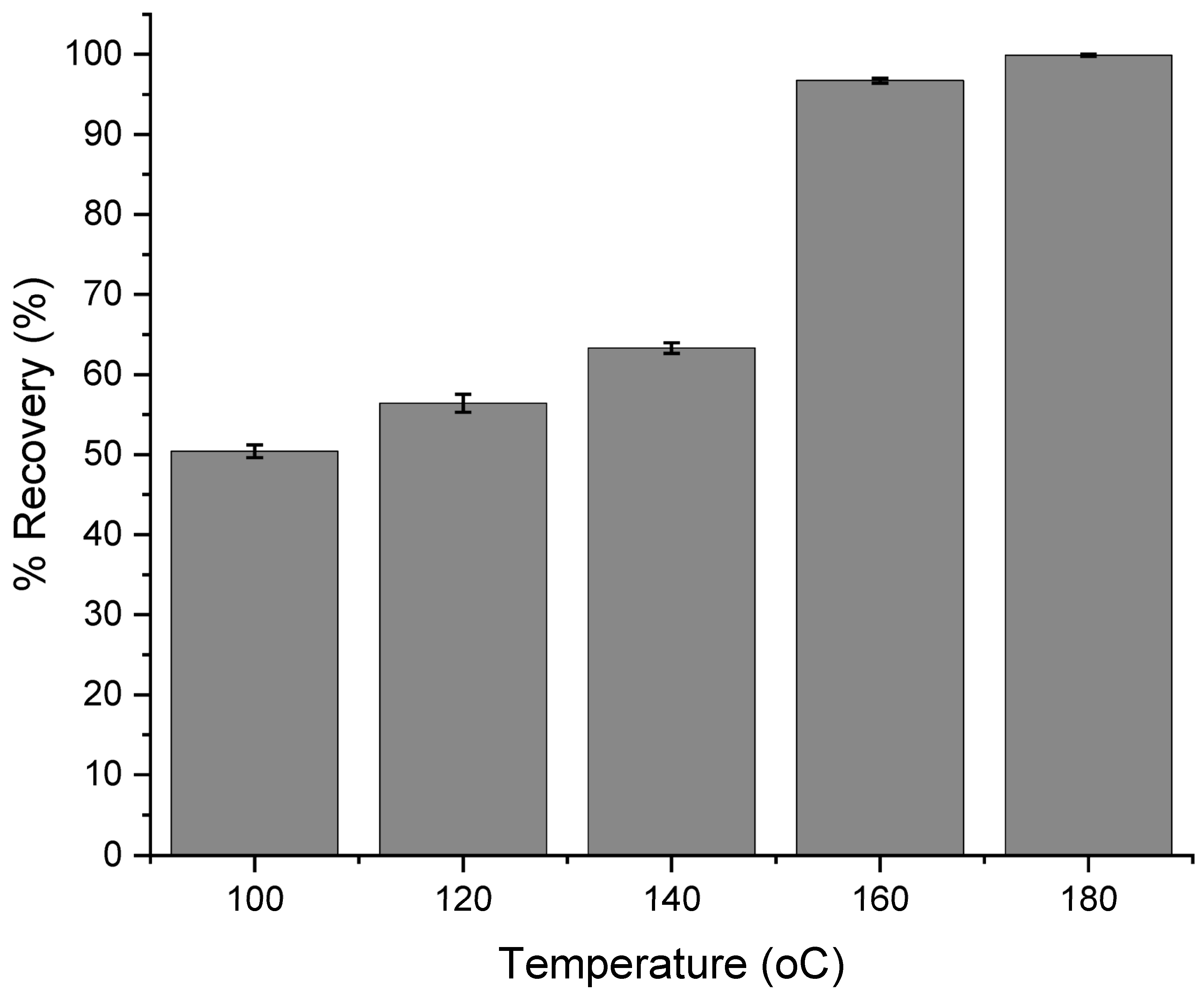
3.2. Optimization of Digestion Temperature
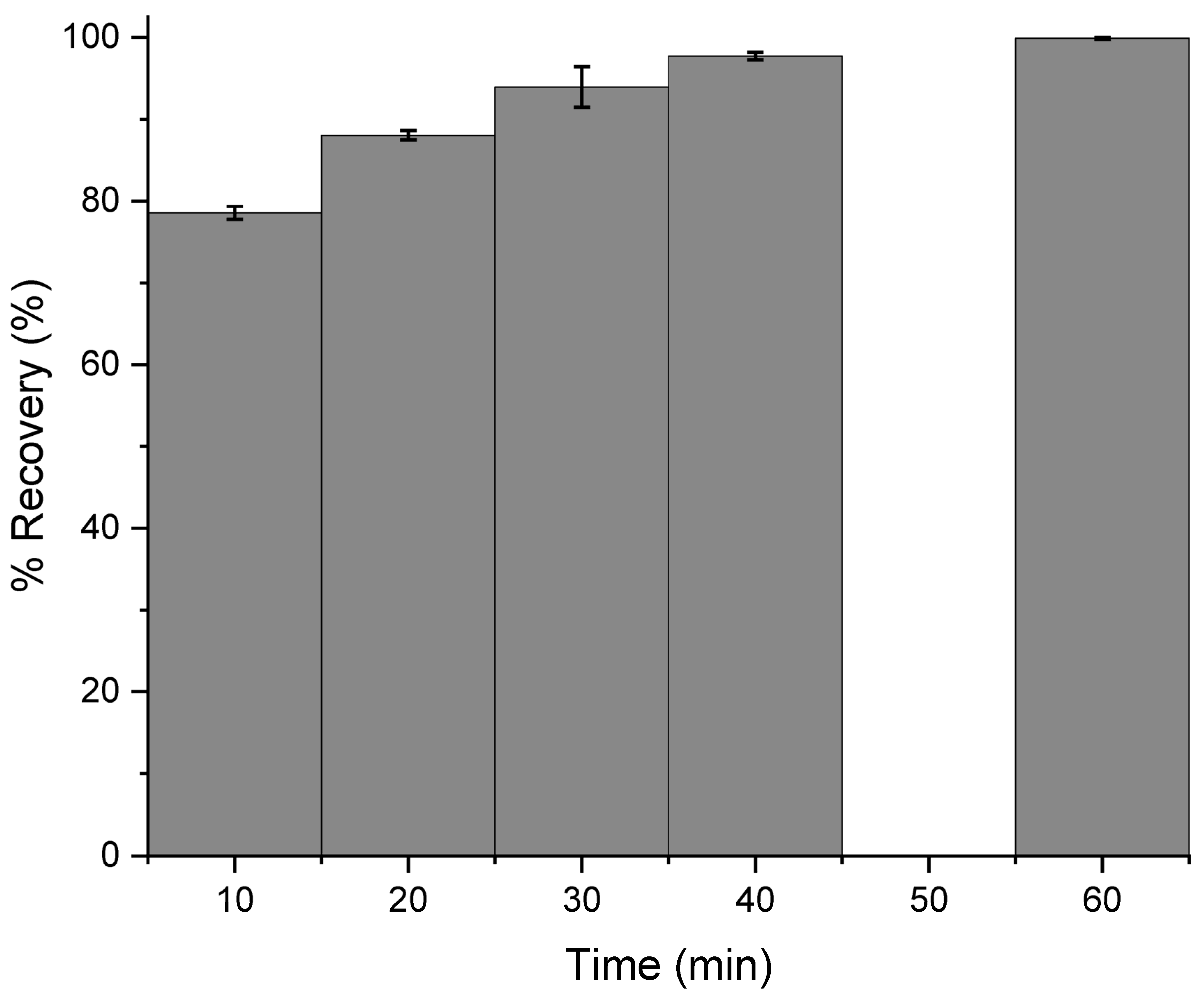
3.3. Optimization of Digestion Time
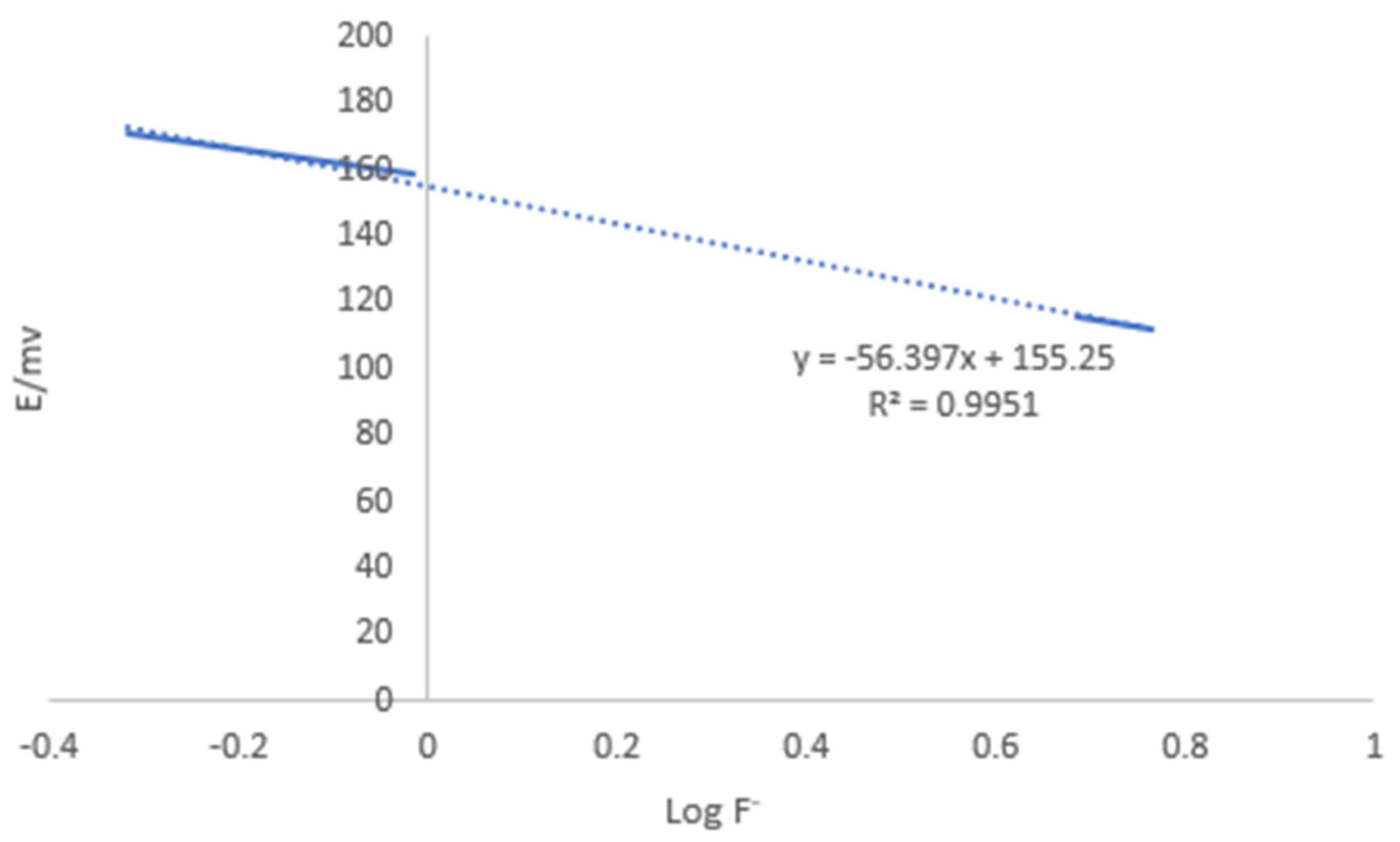
3.4. Analytical Performance of Water-Based Microwave-Assisted Digestion Method
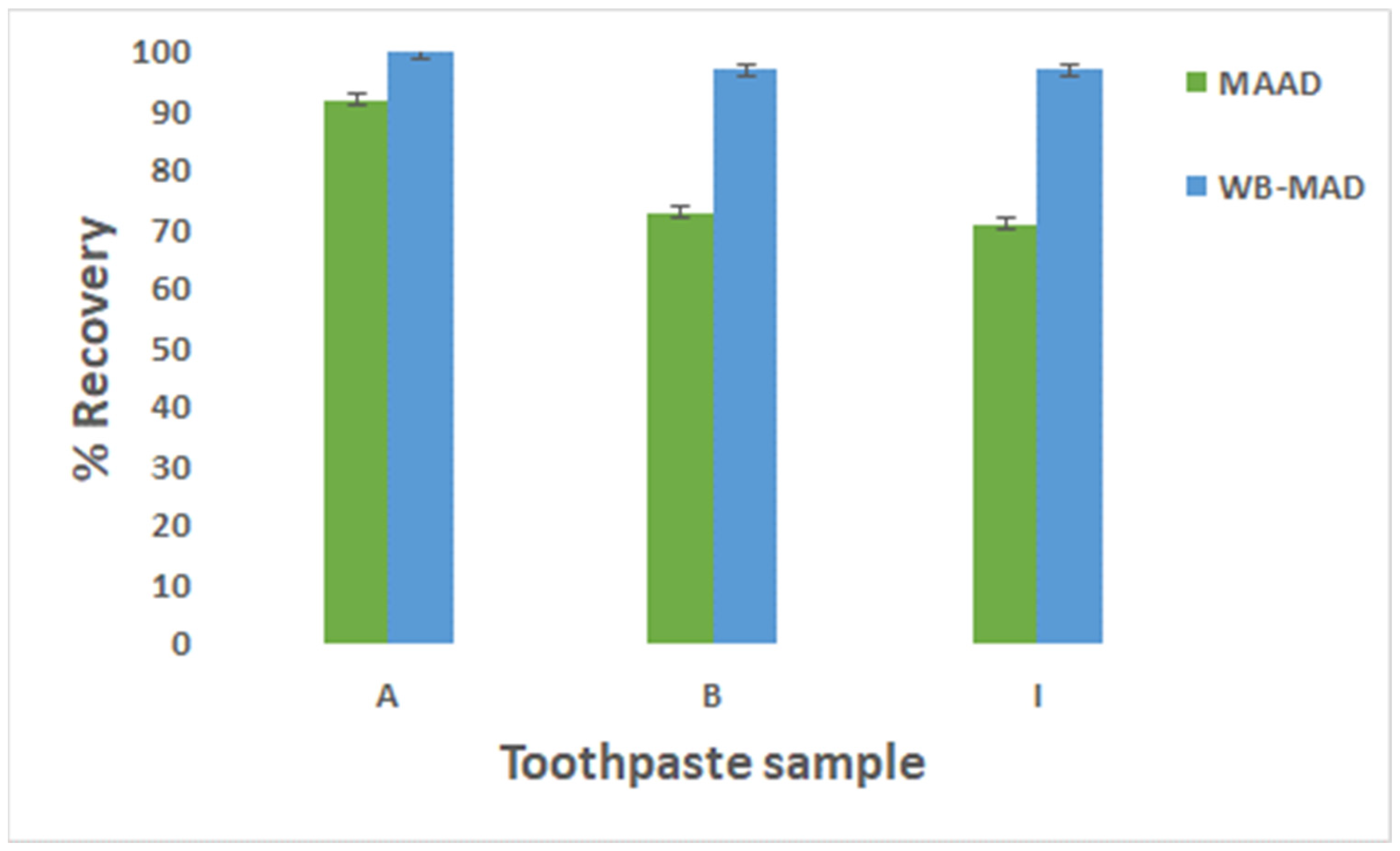
3.5. Validation of Water-Based Microwave-Assisted Digestion Method
3.6. Application of WB-MAD Method for the Determination of Fluoride in Twelve Different Toothpastes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guimarães, I.C.; Rezende, C.C.; da Silva, J.A.F.; de Jesus, D.P. Simultaneous determination of free fluoride and monofluorophosphate in toothpaste by capillary electrophoresis with capacitively coupled contactless conductivity detection. Talanta 2009, 78, 1436–1439. [Google Scholar] [CrossRef]
- Borremans, M.; Van Loco, J.; Van Den Meerssche, P.; Meunier, J.; Vrindts, E.; Goeyens, L. Analysis of fluoride in toothpastes on the Belgian market. Int. J. Cosmet. Sci. 2008, 30, 145–152. [Google Scholar] [CrossRef]
- Iqbal, K.; Asmat, M.; Jawed, S.; Mushtaque, A.; Mohsin, F.; Hanif, S.; Sheikh, N. Role of different ingredients of tooth pastes and mouthwashes in oral health. JPDA 2011, 20, 163–170. [Google Scholar]
- Gleisner, H.; Einax, J.W.; Morés, S.; Welz, B.; Carasek, E. A fast and accurate method for the determination of total and soluble fluorine in toothpaste using high-resolution graphite furnace molecular absorption spectrometry and its comparison with established techniques. J. Pharm. Biomed. Anal. 2011, 54, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Michalski, R.; Mathews, B. Simultaneous determination of fluoride and monofluorophosphate in toothpastes by supressed ion chromatography. Cent. Eur. J. Chem. 2006, 4, 798. [Google Scholar] [CrossRef]
- Michalski, R. Ion chromatography as a reference method for determination of inorganic ions in water and wastewater. Crit. Rev. Anal. Chem. 2006, 36, 107–127. [Google Scholar] [CrossRef]
- Xiang, Q.; Liang, Y.; Chen, L.; Wang, C.; Chen, B.; Chen, X.; Zhou, M. Effect of fluoride in drinking water on children’s intelligence. Floride 2003, 36, 84–94. [Google Scholar]
- Tjabadi, E.; Mketo, N. Recent developments for spectrometric, chromatographic and electroanalytical determination of the total sulfur and halogens in various matrices. TrAC Trends Anal. Chem. 2019, 118, 207–222. [Google Scholar] [CrossRef]
- Tang, Z.; Zhou, R.; Hao, Z.; Zhang, W.; Li, Q.; Zeng, Q.; Li, X.; Zeng, X.; Liu, Y. Determination of fluorine in copper ore using laser-induced breakdown spectroscopy assisted by the SrF molecular emission band. J. Anal. At. Spectrom. 2020, 35, 754–761. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Zhang, Q. Determination of halogen levels in marine geological samples. Spectrosc. Lett. 2016, 49, 151–154. [Google Scholar] [CrossRef]
- Maung, P.; Beauchemin, D. Development of a method for the direct determination of fluorine in solid samples using electrothermal vaporization coupled to inductively coupled plasma optical emission spectrometry. Anal. At. Spectrom. 2020, 35, 1097–1102. [Google Scholar] [CrossRef]
- Cadorim, H.R.; de Gois, J.S.; Borges, A.R.; Vale MG, R.; Welz, B.; Gleisner, H.; Ott, C. Determination of fluorine in copper concentrate via high-resolution graphite furnace molecular absorption spectrometry and direct solid sample analysis—Comparison of three target molecules. Talanta 2018, 176, 178–186. [Google Scholar] [CrossRef]
- Machado, R.C.; Andrade, D.F.; Babos, D.V.; Castro, J.P.; Costa, V.C.; Sperança, M.A.; Garcia, J.A.; Gamela, R.R.; Pereira-Filho, E.R. Solid sampling: Advantages and challenges for chemical element determination-a critical review. J. Anal. At. Spectrom. 2020, 35, 54–77. [Google Scholar] [CrossRef]
- Hoehne, L.; Picoloto, R.S.; Enders, M.S.; Druzian, G.T.; Muller, E.I.; Flores, E.M. Feasibility of pyrohydrolysis as a clean method for further fluorine determination by ISE and IC in high purity nuclear grade alumina. Microchem. J. 2019, 146, 645–649. [Google Scholar] [CrossRef]
- Kaykhaii, M.; Ghalehno, M.H. Rapid and sensitive determination of fluoride in toothpaste and water samples using headspace single drop microextraction-gas chromatography. Anal. Methods 2013, 5, 5622–5626. [Google Scholar] [CrossRef]
- Muhammad, N.; Zhang, Y.; Subhani, Q.; Intisar, A.; Mingli, Y.; Cui, H.; Zhu, Y. Comparative steam distillation based digestion of complex inorganic copper concentrates samples followed by ion chromatographic determination of halogens. Microchem. J. 2020, 158, 105176. [Google Scholar] [CrossRef]
- Li, T.; Min, H.; Li, C.; Yan, C.; Zhang, L.; Liu, S. Simultaneous Determination of Trace Fluorine and Chlorine in Iron Ore by Combustion-Ion Chromatography (C-IC). Anal. Lett. 2021, 54, 2498–2508. [Google Scholar] [CrossRef]
- Pereira, L.S.F.; Pedrotti, M.F.; Dalla Vecchia, P.; Pereira, J.S.F.; Flores, E.M.M. A simple and automated sample preparation system for subsequent halogens determination: Combustion followed by pyrohydrolysis. Anal. Chimi. Acta 2018, 1010, 29–36. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Xie, J.; Hu, Z.; Liu, T.; Zhang, W.; Chen, H.; Liu, Y.; Zong, K.; Li, M. A Rapid Acid Digestion Technique for the Simultaneous Determination of Bromine and Iodine in Fifty-Three Chinese Soils and Sediments by ICP-MS. Geostand. Geoanal. Res. 2018, 42, 309–318. [Google Scholar] [CrossRef]
- Taflik, T.; Duarte, F.A.; Flores, L.M.; Antes, F.G.; Paniz, J.N.G.; Flores, M.M.; Dressler, V.L. Determination of bromine, fluorine and iodine in mineral supplements using pyrohydrolysis for sample preparation. J. Braz. Chem. Soc. 2012, 23, 488–495. [Google Scholar] [CrossRef]
- Sredović Ignjatović, I.D.; Onjia, A.E.; Ignjatović, L.M.; Todorović, Ž.N.; Rajaković, L.V. Experimental Design Optimization of the Determination of Total Halogens in Coal by Combustion–Ion Chromatography. Anal. Lett. 2015, 48, 2597–2612. [Google Scholar] [CrossRef]
- Mesko, M.F.; Balbinot, F.P.; Scaglioni, P.T.; Nascimento, M.S.; Picoloto, R.S.; da Costa, V.C. Determination of halogens and sulfur in honey: A green analytical method using a single analysis. Anal. Bioanal. Chem. 2020, 412, 6475–6484. [Google Scholar] [CrossRef] [PubMed]
- Itota, T.; Carrick, T.E.; Rusby, S.; Al-Naimi, O.T.; Yoshiyama, M.; McCabe, J.F. Determination of fluoride ions released from resin-based dental materials using ion-selective electrode and ion chromatograph. J. Dent. 2004, 32, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Švarc-Gajić, J.; Stojanović, Z.; Vasiljević, I.; Kecojević, I. Determination of fluorides in pharmaceutical products for oral hygiene. J. Food Drug Anal. 2013, 21, 384–389. [Google Scholar] [CrossRef]
- Yildiz, Y.; Patel, S.; Jan, A. Potentiometric determination of % fluoride ion content (w/v) in toothpastes by ion selective electrode. Anal. Chem. Ind. J. 2018, 18, 132. [Google Scholar]
- Gupta, R.; Kumar, A.N.; Bandhu, S.; Gupta, S. Skeletal fluorosis mimicking seronegative arthritis. Scand. J. Rheumatol. 2007, 36, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Bralić, M.; Buljac, M.; Prkić, A.; Buzuk, M.; Brinić, S. Determination Fluoride in Products for Oral Hygiene Using Flow-Injection (FIA) and Continuous Analysis (CA) with HomeMade FISE. Int. J. Electrochem. Sci. 2015, 10, 2253–2264. [Google Scholar] [CrossRef]
- Costa, V.C.; Pereira, R.M.; Mello, J.E.; Brum, J.R.; Picoloto, R.S.; Mesko, M.F. Indirect determination of chlorine and fluorine in eye shadow by ion chromatography after an eco-friendly sample preparation method based on combustion reaction. Microchem. J. 2019, 150, 104125. [Google Scholar] [CrossRef]
- World Health Organization. Preventing Disease through Healthy Environments: Inadequate or Excess Fluoride: A Major Public Health Concern; WHO: Geneva, Switzerland, 2019; Available online: https://apps.who.int/iris/handle/10665/329484 (accessed on 1 June 2023).
- Tokalioğlu, Ş.; Kartal, Ş.; Şahin, U. Determination of fluoride in various samples and some infusions using a fluoride selective electrode. Turk. J. Chem. 2004, 28, 203–212. [Google Scholar]
- Introduction to Ion-Selective Measurement. Available online: https://us.vwr.com/assetsvc/asset/en_US/id/7979405/contents#:~:text=Calibration%20%E2%80%93%202%2Dpoints,2%2Dpoint%20calibration%20is%20sufficient (accessed on 1 June 2023).
- Masanabo, N.M.; Zinyemba, O.; Mketo, N. A greener microwave-assisted extraction method for rapid spectroscopic determination of selected metals in river and freshwater sediment certified reference materials. Int. J. Environ. Anal. Chem. 2019, 99, 33–46. [Google Scholar] [CrossRef]
- Mketo, N.; Nomngongo, P.N.; Ngila, J.C. Development of a novel and green microwave-assisted hydrogen peroxide digestion method for total sulphur quantitative extraction in coal samples prior to inductively coupled plasma-optical emission spectroscopy and ion chromatography determination. Talanta 2015, 15, 567–573. [Google Scholar] [CrossRef]
- Patel, S.; Omid, N.; Zohoori, F.V.; Maguire, A.; Waldron, K.J.; Valentine, R.A. Comparison of total ionic strength adjustment buffers III and IV in the measurement of fluoride concentration of teas. Nutr. Health 2018, 24, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Forsido, T.; Ndibewu, P. Comparison of Different Total Ionic Strength Adjustment Buffer Compositions for Determination of Low Level Fluoride in Environmental Water Samples with Fluoride Ion Selective Electrode. Res. Sq. 2021; preprint. [Google Scholar] [CrossRef]
- Johansson, R. Optimization. In Numerical Python; Apress: Berkeley, CA, USA, 2019. [Google Scholar] [CrossRef]
- Bazaraa, M.S.; Shetty, C.M. Nonlinear Programming: Theory and Algorithms; John Wiley and Sons: Hoboken, NJ, USA, 1979. [Google Scholar]
- Cheng, M.-D. Atmospheric chemistry of hydrogen fluoride. J. Atmos. Chem. 2018, 75, 1–16. [Google Scholar] [CrossRef]
- Rundle, C.C. A Beginners Guide to Ion-Selective Electrode Measurements; Nico2000 Ltd.: London, UK, 2006. [Google Scholar]
- Barzingi, A.N.A. Potentiometric determination of fluoride in various brands of toothpaste. ZANCO J. Pure Appl. Sci. Off. Sci. J. Salahaddin Univ. 2021, 33, 12–20. [Google Scholar] [CrossRef]
- Farcas, F.; Chaussadent, T.; Fiaud, C.; Mabille, I. Determination of the sodium monofluorophosphate in a hardened cement paste by ion chromatography. Anal. Chim. Acta 2002, 472, 37–43. [Google Scholar] [CrossRef]
- Skoog, D.A.; Holler, F.J.; Crouch, S.R. Principles of Instrumental Analysis, 7th ed.; Cengage Learning: New York, NY, USA, 2017; ISBN 978-1-305-57721-3. [Google Scholar]
- WHO. Summary of Principles for Evaluating Health Risks in Children Associated with Exposure to Chemicals; World Health Organization: Geneva, Switzerland, 2011; Available online: https://apps.who.int/iris/handle/10665/44533 (accessed on 1 June 2023).
- WHO. Fluorides. In Air Quality Guidelines for Europe, 2nd ed.; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2000; pp. 143–146. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; Incorporating the First Addendum; World Health Organization: Geneva, Switzerland, 2017; pp. 370–373. Available online: https://apps.who.int/iris/bitstream/handle/10665/254637/9789241549950-eng.pdf (accessed on 1 June 2023).


| Toothpaste Sample | Declared F− Concentration (mg/kg) | Age Group | F− Source |
|---|---|---|---|
| A | 1450 | Adult | Na2FPO3 |
| B | 1000 | Adult | Na2FPO3 |
| C | 1450 | Adult | Na2FPO3 |
| D | 1450 | Adult | Na2FPO3 |
| E | 1000 | Adult | Na2FPO3 |
| F | 1450 | Adult | Na2FPO3 |
| G | 1450 | Adult | Na2FPO3 |
| H | 1450 | Adult | Na2FPO3 |
| I | 500 | Toddler | Na2FPO3 |
| J | 500 | Toddler | Na2FPO3 |
| K | 500 | Toddler | Na2FPO3 |
| L | 500 | Toddler | Na2FPO3 |
| Analytical Features | Specification |
|---|---|
| Correlation coefficient (R2) | 0.995 |
| Standard deviation (SD) in mV | 0.01039. |
| Limit of detection (LOD) in µg/kg | 0.10070 |
| Limit of quantification (LOQ) in µg/kg | 0.33820 |
| Method detection limit (MDL) in µg/kg | 0.00302 |
| Method quantification limit (MDQ) in µg/kg | 0.01007 |
| Precision based on intraday (%) | ≤0.7 |
| Accuracy (%) | ≤96.56 |
| Toothpaste Sample | F− Content Determined Using ISE | F− Content Determined Using IC | Declared F− Concentration | ||
|---|---|---|---|---|---|
| (mg/kg ± SD), n = 3 | (%R) | (mg/kg ± SD), n = 3 | (%R) | (mg/kg) | |
| A | 1448.36 ± 1.74 | 99.9 | 1443.34 ± 0.34 | 99.5 | 1450 |
| B | 970.21 ± 1.90 | 97.0 | 997.31 ± 0.36 | 99.7 | 1000 |
| C | 1452.13 ± 3.99 | 100.1 | 1447.17 ± 1.07 | 99.8 | 1450 |
| D | 1437.72 ± 4.34 | 99.2 | 1460.61 ± 2.17 | 100.7 | 1450 |
| E | 989.41 ± 3.78 | 98.9 | 908.01 ± 0.24 | 90.9 | 1000 |
| F | 1449.17 ± 2.86 | 99.9 | 1454.83 ± 3.53 | 100.3 | 1450 |
| G | 1353.31 ± 4.85 | 93.3 | 1445.76 ± 1.56 | 99.7 | 1450 |
| H | 1456.85 ± 4.76 | 100.5 | 1452.31 ± 0.84 | 100.2 | 1450 |
| I | 482.81 ± 0.103 | 96.6 | 508.22 ± 0.98 | 101.6 | 500 |
| J | 499.33 ± 1.70 | 99.9 | 495.65 ± 1.05 | 99.1 | 500 |
| K | 496.71 ± 1.25 | 99.2 | 518.44 ± 1.11 | 103.7 | 500 |
| L | 500.12 ± 1.05 | 100 | 497.01 ± 0.78 | 99.4 | 500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukendi, M.D.; Mketo, N. Water-Based Microwave-Assisted Digestion Method for Electrochemical and Chromatographic Determination of Total Fluoride Ions in Toothpaste Samples. Appl. Sci. 2023, 13, 13315. https://doi.org/10.3390/app132413315
Mukendi MD, Mketo N. Water-Based Microwave-Assisted Digestion Method for Electrochemical and Chromatographic Determination of Total Fluoride Ions in Toothpaste Samples. Applied Sciences. 2023; 13(24):13315. https://doi.org/10.3390/app132413315
Chicago/Turabian StyleMukendi, Mbuyamba Divin, and Nomvano Mketo. 2023. "Water-Based Microwave-Assisted Digestion Method for Electrochemical and Chromatographic Determination of Total Fluoride Ions in Toothpaste Samples" Applied Sciences 13, no. 24: 13315. https://doi.org/10.3390/app132413315
APA StyleMukendi, M. D., & Mketo, N. (2023). Water-Based Microwave-Assisted Digestion Method for Electrochemical and Chromatographic Determination of Total Fluoride Ions in Toothpaste Samples. Applied Sciences, 13(24), 13315. https://doi.org/10.3390/app132413315