The Influence of Different Cleaning Protocols on the Surface Roughness of Orthodontic Retainers
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Sample Size
2.3. Cleaning Protocol
2.4. Surface Roughness
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Littlewood, S.; Kandasamy, S.; Huang, G. Retention and Relapse in Clinical Practice. Aust. Dent. J. 2017, 62, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Wouters, C.; Lamberts, T.A.; Kuijpers-Jagtman, A.M.; Renkema, A.M. Development of a Clinical Practice Guideline for Orthodontic Retention. Ortho. Craniofac. Res. 2019, 22, 69–80. [Google Scholar] [CrossRef]
- Kiatwarawut, K. The Interesting Types of Plastic of Invisible Retainers. Open Access J. Dent. Oral Surg. 2022, 3, 1040. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.W.; Ha, H.R.; Lim, H.N.; Choi, S. Effects of aging procedures on the molecular, biochemical, morphological, and mechanical properties of vacuum-formed retainers. J. Mech. Behav. Biomed. Mater. 2015, 51, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-H.; Kwon, J.-S.; Jiang, H.B.; Cha, J.-Y.; Kim, K.-M. Effects of Thermoforming on the Physical and Mechanical Properties of Thermoplastic Materials for Transparent Orthodontic Aligners. Korean J. Orthod. 2018, 48, 316. [Google Scholar] [CrossRef]
- Ryokawa, H.; Miyazaki, Y.; Fujishima, A.; Miyazaki, T.; Maki, K. The mechanical properties of dental thermoplastic materials in a simulated intraoral environment. Orthod. Waves 2006, 65, 64–72. [Google Scholar] [CrossRef]
- Kiesow, A.; Sarembe, S.; Pizzey, R.L.; Axe, A.S.; Bradshaw, D.J. Material compatibility and antimicrobial activity of consumer products commonly used to clean dentures. J. Prosthet. Dent. 2016, 115, 189–198.e188. [Google Scholar] [CrossRef]
- Albanna, R.H.; Farawanah, H.M.; Aldrees, A.M. Microbial evaluation of the effectiveness of different methods for cleansing clear orthodontic retainers: A randomized clinical trial. Angle Orthod. 2017, 87, 460–465. [Google Scholar] [CrossRef]
- Batoni, G.; Pardini, M.; Giannotti, A.; Ota, F.; Rita Giuca, M.; Gabriele, M.; Campa, M.; Senesi, S. Effect of removable orthodontic appliances on oral colonisation by mutans streptococci in children. Eur. J. Oral Sci. 2001, 109, 388–392. [Google Scholar] [CrossRef]
- Pathak, A.K.; Sharma, D.S. Biofilm associated microorganisms on removable oral orthodontic appliances in children in the mixed dentition. J. Clin. Pediatr. Dent. 2013, 37, 335–340. [Google Scholar] [CrossRef]
- Akgün, F.A.; Şenışık, N.E.; Çetin, E.S. Evaluation of the Efficacy of Different Cleaning Methods for Orthodontic Thermoplastic Retainers in terms of Bacterial Colonization. Turk. J. Orthod. 2019, 32, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Mueller, H.J.; Greener, E.H. Characterization of some denture cleansers. J. Prosthet. Dent. 1980, 43, 491–496. [Google Scholar] [CrossRef]
- Kaizer, M.R.; De Oliveira-Ogliari, A.; Cenci, M.S.; Opdam, N.J.; Moraes, R.R. Do nanofill or submicron composites show improved smoothness and gloss? A systematic review of in vitro studies. Dent. Mater. 2014, 30, e41–e78. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, N.; Matsumura, H.; Atsuta, M. Wear and surface roughness of current prosthetic composites after tooth-brush/dentifrice abrasion. J. Prosthet. Dent. 2000, 84, 93–97. [Google Scholar] [CrossRef]
- AlAli, M.; Silikas, N.; Satterthwaite, J. The Effects of Toothbrush Wear on the Surface Roughness and Gloss of Resin Composites with Various Types of Matrices. Dent. J. 2021, 9, 8. [Google Scholar] [CrossRef]
- Tsolakis, A.Ι.; Kakali, L.; Prevezanos, P.; Bitsanis, I.; Polyzois, G. Use of Different Cleaning Methods for Removable Orthodontic Appliances: A Questionnaire Study. Oral Health Prev. Dent. 2019, 17, 299–302. [Google Scholar] [PubMed]
- Mai, W.; He, J.A.; Meng, H.; Jiang, Y.; Huang, C.; Li, M.; Yuan, K.; Kang, N. Comparison of vacuum-formed and Hawley retainers: A systematic review. Am. J. Orthod. Dentofac. Orthop. 2014, 145, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Hichens, L.; Rowland, H.; Williams, A.; Hollinghurst, S.; Ewings, P.; Clark, S.; Ireland, A.; Sandy, J. Cost-effectiveness and patient satisfaction: Hawley and vacuum-formed retainers. Eur. J. Orthod. 2007, 29, 372–378. [Google Scholar] [CrossRef]
- Edman Tynelius, G.; Bondemark, L.; Lilja-Karlander, E. Evaluation of orthodontic treatment after 1 year of retention—A randomized controlled trial. Eur. J. Orthod. 2010, 32, 542–547. [Google Scholar] [CrossRef]
- Barth, E.; Myrvik, Q.M.; Wagner, W.; Gristina, A.G. In vitro and in vivo comparative colonization of Staphylococcus aureus and Staphylococcus epidermidis on orthopaedic implant materials. Biomaterials 1989, 10, 325–328. [Google Scholar] [CrossRef]
- Oga, M.; Sugioka, Y.; Hobgood, C.; Gristina, A.; Myrvik, Q. Surgical biomaterials and differential colonization by Staphylococcus epidermidis. Biomaterials 1988, 9, 285–289. [Google Scholar] [CrossRef]
- Hogt, A.; Dankert, J.; De Vries, J.; Feijen, J. Adhesion of coagulase-negative staphylococci to biomaterials. Microbiology 1983, 129, 2959–2968. [Google Scholar] [CrossRef] [PubMed]
- Pringle, J.; Fletcher, M. Influence of substratum hydration and adsorbed macromolecules on bacterial attachment to surfaces. Appl. Environ. Microbiol. 1986, 51, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Locci, R.; Peters, G.; Pulverer, G. Microbial colonization of prosthetic devices. I. Microtopographical characteristics of intravenous catheters as detected by scanning electron microscopy. Zent. Fur Bakteriol. Mikrobiol. Und Hyg. 1. Abt. Orig. B Hyg. 1981, 173, 285–292. [Google Scholar]
- Pilloni, J.A. Human Saliva Biofilm Reduction by Thermoplastic Retainer Cleaners: An In Vitro Study; State University of New York at Stony Brook: New York, NY, USA, 2019. [Google Scholar]
- Agarwal, M.; Wible, E.; Ramir, T.; Altun, S.; Viana, G.; Evans, C.; Lukic, H.; Megremis, S.; Atsawasuwan, P. Long-term effects of seven cleaning methods on light transmittance, surface roughness, and flexural modulus of polyurethane retainer material. Angle Orthod. 2018, 88, 355–362. [Google Scholar] [CrossRef]
- Wible, E.; Agarwal, M.; Altun, S.; Ramir, T.; Viana, G.; Evans, C.; Lukic, H.; Megremis, S.; Atsawasuwan, P. Long-term effects of different cleaning methods on copolyester retainer properties. Angle Orthod. 2019, 89, 221–227. [Google Scholar] [CrossRef]
- Kiatwarawut, K.; Rokaya, D.; Sirisoontorn, I. Antimicrobial Activity of Various Disinfectants to Clean Thermoplastic Polymeric Appliances in Orthodontics. Polymers 2022, 14, 2256. [Google Scholar] [CrossRef]
- Zheng, Y.; Yanful, E.K.; Bassi, A.S. A review of plastic waste biodegradation. Crit. Rev. Biotechnol. 2005, 25, 243–250. [Google Scholar] [CrossRef]
- Allen, A.B.; Hilliard, N.P.; Howard, G.T. Purification and characterization of a solublepolyurethane degrading enzyme from Comamonasacidovorans. Int. Biodeterior. Biodegrad. 1999, 43, 37–41. [Google Scholar] [CrossRef]
- Abou-Zeid, D.-M.; Müller, R.-J.; Deckwer, W.-D. Biodegradation of Aliphatic Homopolyesters and Aliphatic−Aromatic Copolyesters by Anaerobic Microorganisms. Biomacromolecules 2004, 5, 1687–1697. [Google Scholar] [CrossRef]
- Müller, R.-J.; Kleeberg, I.; Deckwer, W.-D. Biodegradation of polyesters containing aromatic constituents. J. Biotechnol. 2001, 86, 87–95. [Google Scholar] [CrossRef]
- Raja, T.A.; Littlewood, S.J.; Munyombwe, T.; Bubb, N.L. Wear resistance of four types of vacuum formed retainer materials: A laboratory study. Angle Orthod. 2014, 84, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Gardner, G.D.; Dunn, W.J.; Taloumis, L. Wear comparison of thermoplastic materials used for orthodontic retainers. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Vidaković, A.; Anić-Milošević, S.; Borić, D.N.; Meštrović, S. Mesiodistal and Buccolingual Dimensions in Croatian Orthodontic Hypodontia Patients’ Teeth. Acta Stomatol. Croat. 2018, 52, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Hwang, C.J.; Yu, H.S.; Lee, S.B.; Cha, J.Y. The effect of thickness and deflection of orthodontic thermoplastic materials on its mechanical properties. Korean J. Orthod. 2010, 40, 16–26. [Google Scholar] [CrossRef]
- ISO 4287:1997; Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Terms, Definitions and Surface Texture Parameters. International Standards Organization: Geneva, Switzerland, 2005. Available online: https://www.iso.org/standard/10132.html (accessed on 16 January 2023).
- Marchado, A.L.; Breeding, L.C.; Vergani, C.E.; Cruz Perez, L.E. Hardness and surface roughness of reline and denture base acrylic resins after repeated disinfection procedures. J. Prosthet. Dent. 2009, 102, 115–122. [Google Scholar] [CrossRef]
- Loxley, E.C.; Liewehr, F.R.; Buxton, T.B.; McPherson, J.C., III. The effect of various intracanal oxidizing agents on the push-out strength of various perforation repair materials. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2003, 95, 490–494. [Google Scholar] [CrossRef]
- Ozylimaz, O.Y.; Akin, C. Effect of cleansers on denture base resins’ structural properties. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019827797. [Google Scholar] [CrossRef]
- Babanouri, N.; Ahmadi, N.; Pakshir, H.R.; Ajami, S.; Habibagahi, R. Influence of a bleaching agent on surface and mechanical properties of orthodontic thermoplastic retainer materials: An in vitro study. J. Orofac. Orthop. 2022, 83, 332–338. [Google Scholar] [CrossRef]
- Pascual, A.L.; Beeman, C.S.; Hicks, E.P.; Bush, H.M.; Mitchell, R.J. The essential work of fracture of thermoplastic orthodontic retainer materials. Angle Orthod. 2010, 80, 554–561. [Google Scholar] [CrossRef]
- Porojan, L.; Vasiliu, R.D.; Porojan, S.D.; Birdeanu, M.I. Surface quality evaluation of removable thermoplastic dental appliances related to staining beverages and cleaning agents. Polymers 2020, 12, 1736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Bai, Y.X.; Ding, X.J. Preparation and characterization of thermoplastic materials for invisible orthodontics. Dent. Mater. J. 2011, 30, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.S.; Al-Awadi, S.; Ready, D.; Noar, J. An assessment of the effectiveness of mechanical and chemical cleaning of Essix orthodontic retainer. J. Orthod. 2014, 41, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Halis, G.; Köroğlu, A.; Sahin, O.; Dede, D.Ö.; Yilmaz, B. Effect of simulated tootbrushing on surface roughness of sealant agent coupled nanohybrid composite resins. J. Esthet. Restor. Dent. 2022, 34, 907–914. [Google Scholar] [CrossRef]
- Köroğlu, A.; Şahin, O.; Küçükekenci, A.S.; Dede, D.O.; Yıldırım, H.; Yilmaz, B. Influences of Toothbrushing and Different Toothpastes on the Surface Roughness and Color Stability of Interim Prosthodontic Materials. Materials 2022, 15, 5831. [Google Scholar] [CrossRef]
- Azmuddin, I.; Mustapha, N.M.N.; Khan, H.B.S.G.; Sinniah Saraswathy, D. Physical effects of cleaning agents on orthodontic thermoplastic retainer polymer: A narrative review. J. Int. Oral Health 2022, 14, 349–356. [Google Scholar]
- Dos Santos, J.H.; Silva, N.L.; Gomes, M.G.; Paschoal, M.A.; Gomes, I.A. Whitening toothpaste effect on nanoparticle resin composite roughness after a brushing challenge: An in vitro study. J. Clin. Exp. Dent. 2019, 11, e334–e339. [Google Scholar] [CrossRef]
- Luo, M.R.; Cui, G.; Rigg, B. The development of the CIE 2000 color difference formula: CIEDE2000. Color Res. Appl. 2001, 26, 340–350. [Google Scholar] [CrossRef]
- Hamza, B.; Tanner, M.; Attin, T.; Wegehaupt, F.J. Dentin Abrasivity and Cleaning Efficacy of Novel/Alternative Toothpastes. Oral Health Prev. Dent. 2020, 18, 713–718. [Google Scholar]
- ISO 11609; Dentistry—Dentifrices—Requirements, Test Methods and Marking. International Organisation for Standardization: Geneva, Switzerland, 2010.
- Philpotts, C.J.; Weader, E.; Joiner, A. The measurement in vitro of enamel and dentine wear by toothpastes of different abrasivity. Int. Dent. J. 2005, 55, 183–187. [Google Scholar] [CrossRef]
- Soares, C.N.; Amaral, F.L.; Mesquita, M.F.; Franca, F.M.; Basting, R.T.; Turssi, C.P. Tooth- pastes containing abrasive and chemical whitening agents: Efficacy in reducing extrinsic dental staining. Gen. Dent. 2015, 63, e24–e28. [Google Scholar]
- Hara, A.T.; Turssi, C.P. Baking soda as an abrasive in toothpastes: Mechanism of action and safety and effectiveness considerations. J. Am. Dent. Assoc. 2017, 148, 27–33. [Google Scholar] [CrossRef]
- Hamza, B.; Attin, T.; Cucuzza, C.; Gubler, A.; Wegehaubt, F.J. RDA and REA values of commercially available toothpastes utilising diamond powder and traditional abrasives. Oral Health Prev. Dent. 2020, 18, 807–814. [Google Scholar] [PubMed]
- Levrini, L.; Paracchini, L.; Bakaj, R.; Diaconu, A.; Cortese, S. Dental bleaching during orthodontic treatment with aligners. Int. J. Esthet. Dent. 2020, 15, 44–54. [Google Scholar] [PubMed]
- Al-Groosh, D.; Bozec, L.; Pratten, J.; Hunt, P.N. The influence of surface roughness and surface dynamics on the attachment of Methicillin-Resistant Staphylococcus aureus onto orthodontic retainer materials. Dent. Mater. J. 2015, 34, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.S.; Billington, R.W.; Pearson, G.J. The in vivo perception of roughness and restorations. Br. Dent. J. 2004, 196, 42–45. [Google Scholar] [CrossRef]
- Sarret, D.C. Polishing systems. ADA Prof. Product Rev. 2010, 5, 1–16. [Google Scholar]
- Jindal, P.; Juneja, M. Mechanical, and geometric properties of thermoformed and 3D printed clear dental aligners. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 694–701. [Google Scholar] [CrossRef]
- Malysa, K.; Krasowska, M.; Krzan, M. Influence of surface active substances on bubble motion and collision with various interfaces. Adv. Colloid Interface Sci. 2005, 114, 205–225. [Google Scholar] [CrossRef]
- Suter, F.; Zinelis, S.; Patcas, R.; Schätzle, M.; Eliades, G.; Eliades, T. Roughness and wettability of aligner materials. J. Orthod. 2020, 47, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.M.; Li, K.K.; Guilleminault, C. Risk factors for sleep bruxism in the general population. Chest 2001, 119, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Cacciafesta, V.; Sfondrini, M.F.; Lena, A.; Scribante, A.; Vallittu, P.K.; Lassila, L.V. Flexural Strengths of Fiber-Reinforced Composites Polymerized with Conventional Light-Curing and Additional Postcuring. Am. J. Orthod. Dentofac. Orthop. 2007, 132, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Pieniak, D.; Walczak, A.; Walczak, M.; Przystupa, K.; Niewczas, A.M. Hardness and Wear Resistance of Dental Biomedical Nanomaterials in a Humid Environment with Non-Stationary Temperatures. Materials 2020, 13, 1255. [Google Scholar] [CrossRef] [PubMed]

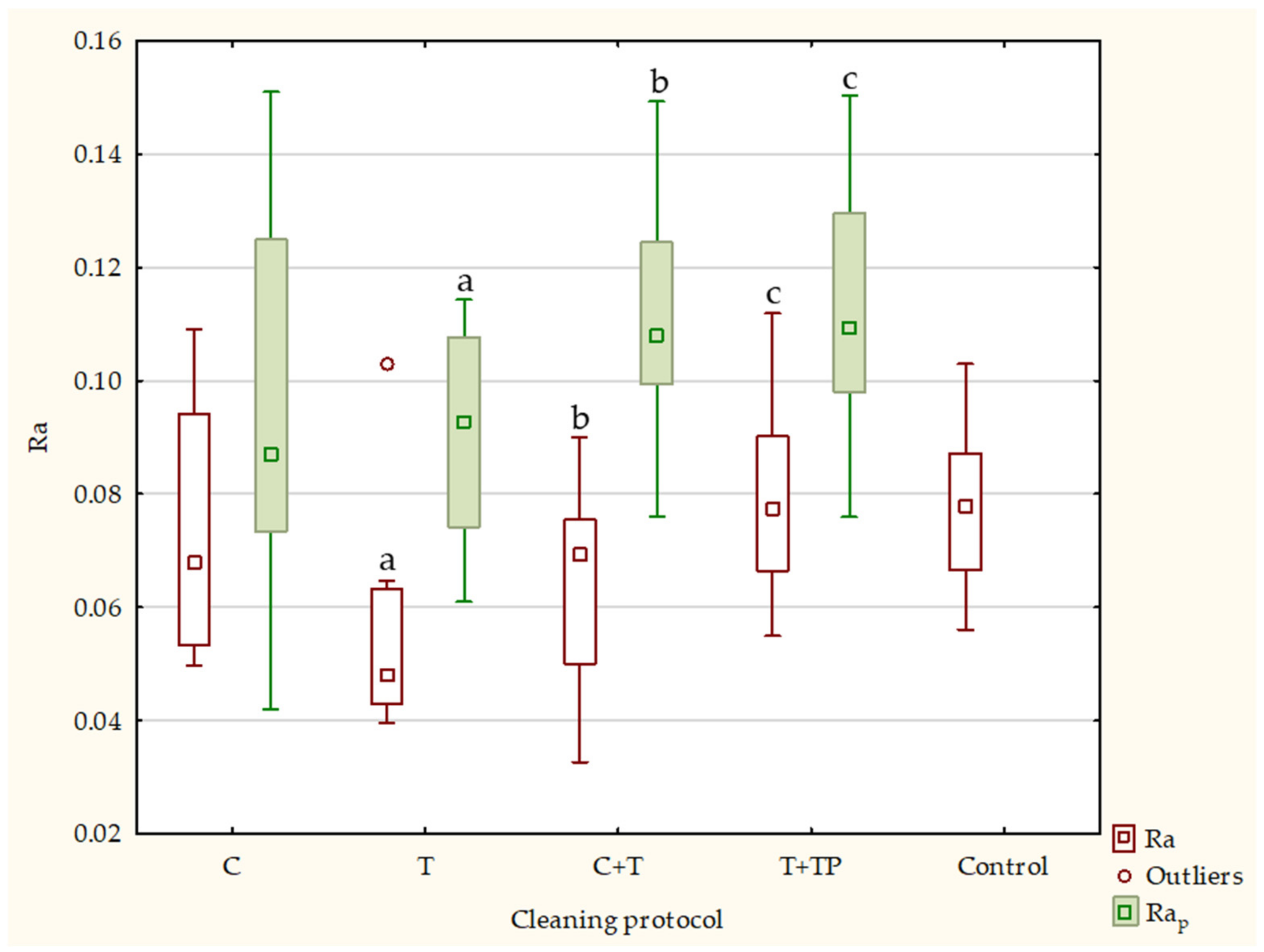
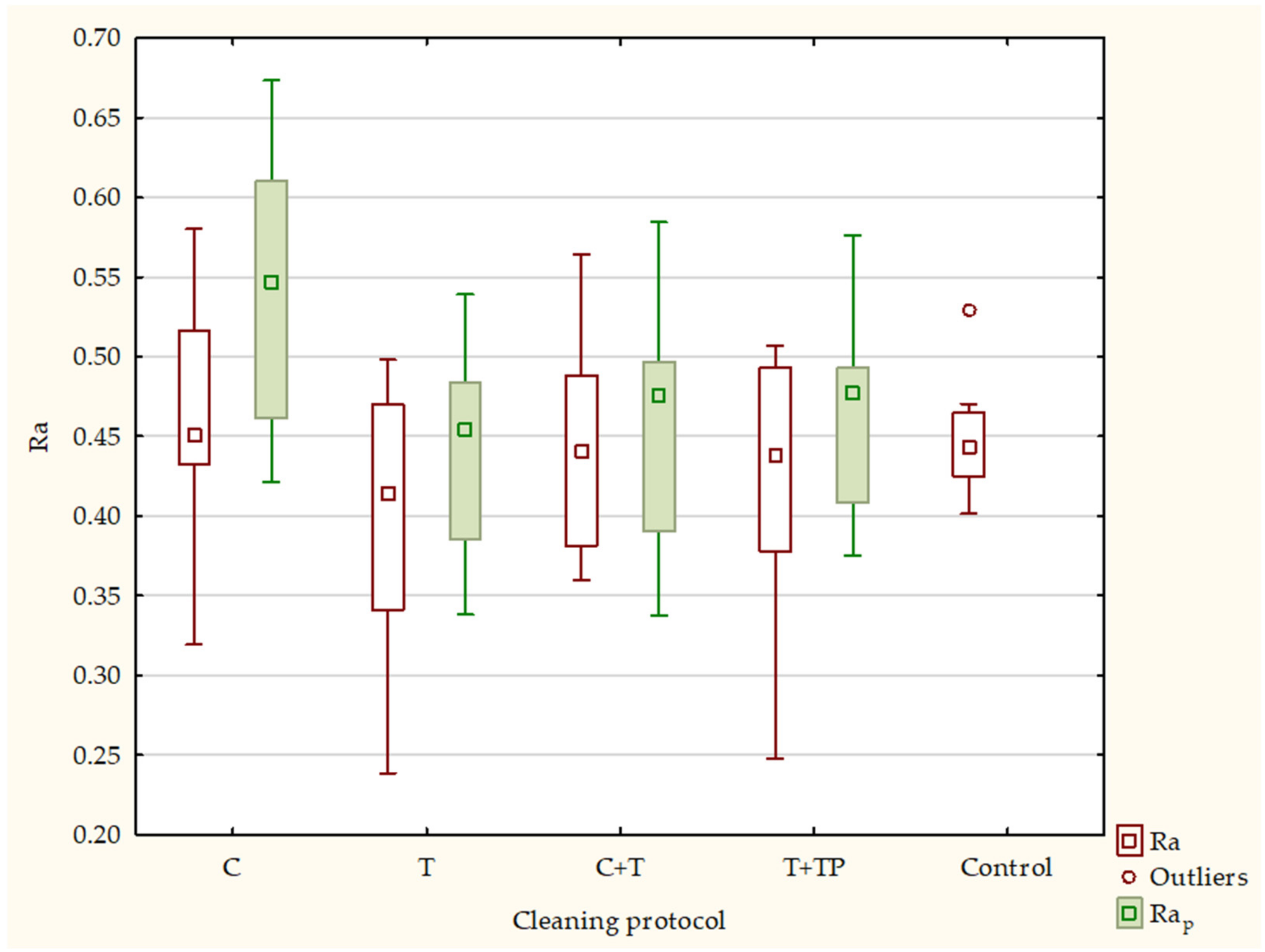
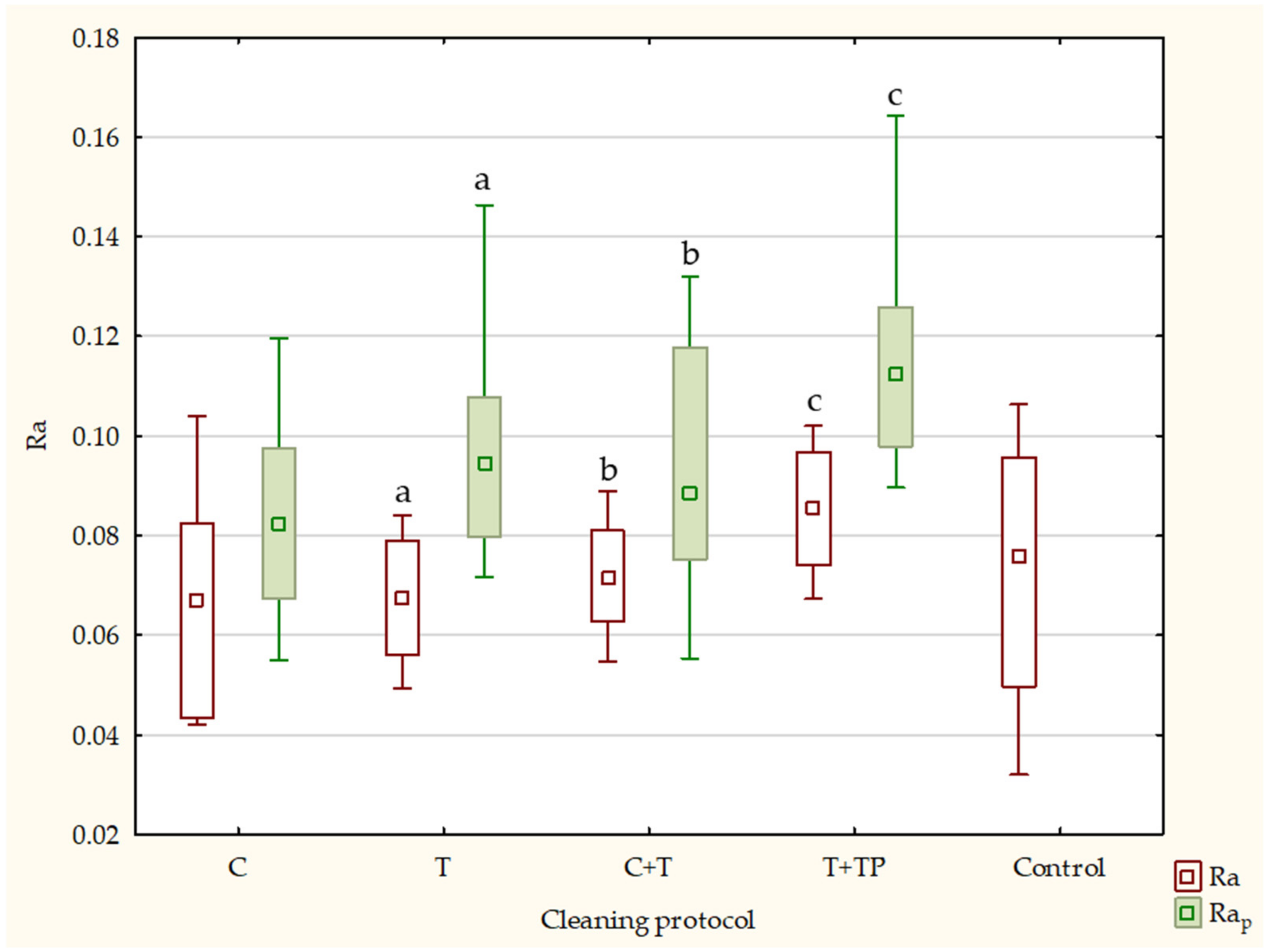
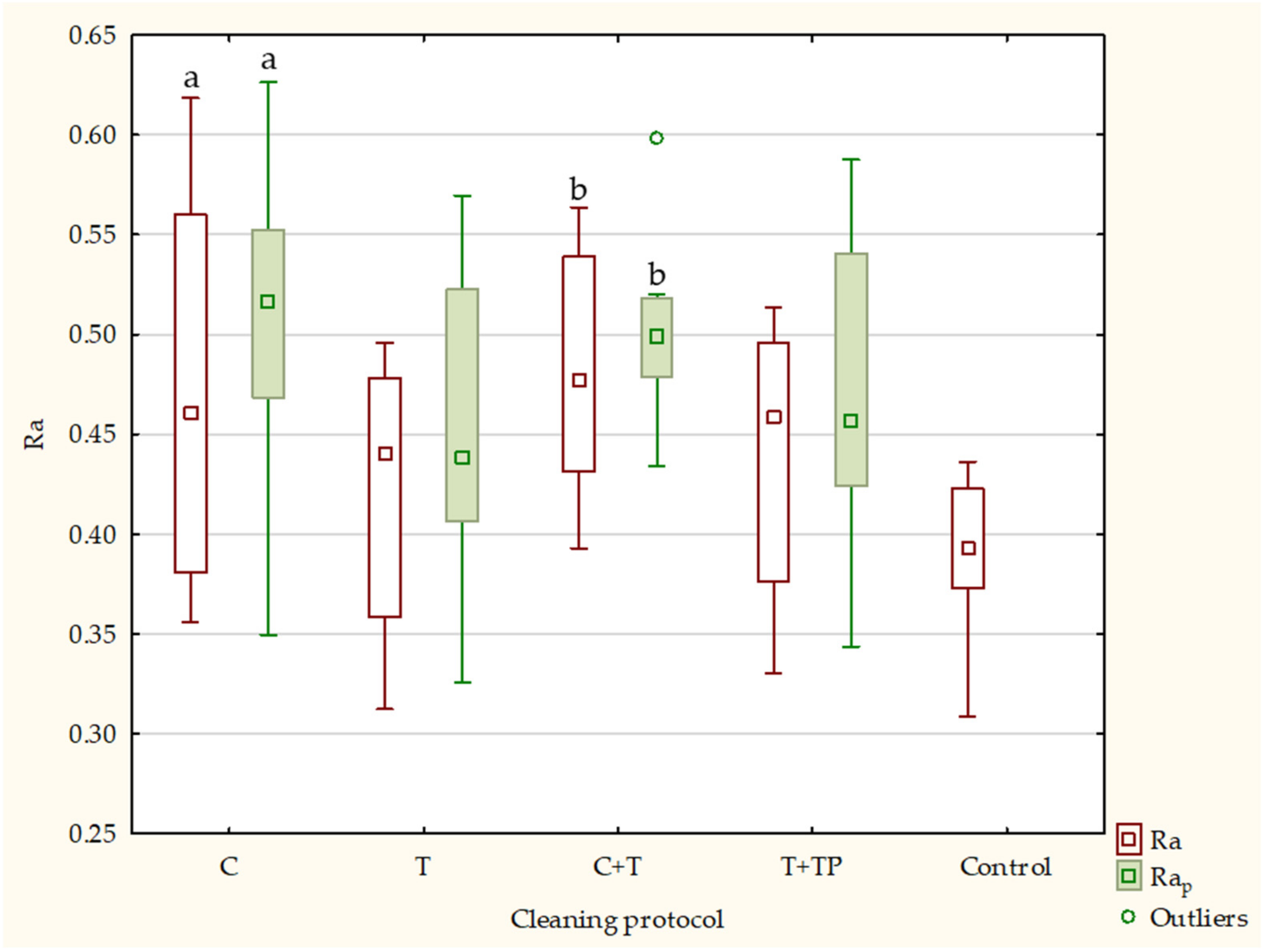
| Ra | Rap | Rq | Rqp | Rz | Rzp | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solution | Side | Median | IQR | Median | IQR | Median | IQR | Median | IQR | Median | IQR | Median | IQR |
| Corega | A-top | 0.068 | 0.053–0.094 | 0.087 | 0.073–0.125 | 0.082 | 0.063–0.11 | 0.108 | 0.092–0.147 | 0.352 c | 0.266–0.416 | 0.492 c | 0.45–0.64 |
| A-bot | 0.451 | 0.432–0.516 | 0.546 | 0.461–0.61 | 0.558 | 0.549–0.622 | 0.695 | 0.562–0.762 | 2.131 c | 2.046–2.349 | 2.623 c | 2.067–2.992 | |
| B-top | 0.067 | 0.043–0.083 | 0.082 | 0.067–0.082 | 0.082 | 0.052–0.098 | 0.107 | 0.095–0.117 | 0.291 c | 0.212–0.315 | 0.497 c | 0.385–0.595 | |
| B-bot | 0.461 | 0.381–0.56 | 0.516 | 0.469–0.553 | 0.557 | 0.478–0.673 | 0.628 | 0.573–0.694 | 1.953 | 1.808–2.378 | 2.165 | 1.994–2.48 | |
| Toothbrush | A-top | 0.048 a | 0.043–0.063 | 0.093 a | 0.074–0.108 | 0.059 b | 0.054–0.076 | 0.111 b | 0.086–0.124 | 0.276 | 0.235–0.385 | 0.391 | 0.317–0.467 |
| A-bot | 0.415 | 0.341–0.47 | 0.454 | 0.386–0.484 | 0.516 | 0.416–0.572 | 0.556 | 0.472–0.584 | 1.896 | 1.526–2.122 | 1.98 | 1.8–2.173 | |
| B-top | 0.068 a | 0.056–0.079 | 0.095 a | 0.08–0.109 | 0.08 b | 0.068–0.095 | 0.115 b | 0.097–0.127 | 0.3 | 0.243–0.337 | 0.422 | 0.376–0.492 | |
| B-bot | 0.441 | 0.358–0.478 | 0.439 | 0.406–0.523 | 0.552 | 0.441–0.582 | 0.531 | 0.498–0.631 | 2.006 | 1.602–2.037 | 1.927 | 1.885–2.146 | |
| Corega + Toothbrush | A-top | 0.069 a | 0.05–0.076 | 0.108 a | 0.099–0.125 | 0.082 b | 0.059–0.092 | 0.122 b | 0.113–0.141 | 0.296 c | 0.229–0.378 | 0.366 c | 0.336–0.473 |
| A-bot | 0.44 | 0.381–0.488 | 0.476 | 0.391–0.497 | 0.541 | 0.469–0.593 | 0.575 | 0.486–0.612 | 1.976 | 1.746–2.115 | 2.044 | 1.8–2.212 | |
| B-top | 0.071 a | 0.063–0.081 | 0.088 a | 0.075–0.118 | 0.086 b | 0.074–0.095 | 0.106 b | 0.091–0.143 | 0.323 c | 0.283–0.36 | 0.403 c | 0.338–0.505 | |
| B-bot | 0.477 | 0.431–0.539 | 0.499 | 0.479–0.518 | 0.566 | 0.537–0.656 | 0.612 | 0.586–0.634 | 2.07 | 1.964–2.324 | 2.223 | 2.096–2.314 | |
| Toothbrush + toothpaste | A-top | 0.078 a | 0.066–0.09 | 0.109 a | 0.098–0.13 | 0.089 b | 0.076–0.103 | 0.125 b | 0.112–0.149 | 0.307 | 0.263–0.393 | 0.427 | 0.353–0.475 |
| A-bot | 0.437 | 0.378–0.493 | 0.477 | 0.409–0.493 | 0.539 | 0.453–0.595 | 0.579 | 0.494–0.592 | 1.918 | 1.563–2.145 | 2.003 | 1.823–2.182 | |
| B-top | 0.086 a | 0.074–0.097 | 0.113 a | 0.098–0.126 | 0.098 b | 0.086–0.113 | 0.133 b | 0.115–0.145 | 0.318 | 0.261–0.355 | 0.44 | 0.394–0.51 | |
| B-bot | 0.459 | 0.376–0.496 | 0.457 | 0.424–0.541 | 0.57 | 0.459–0.6 | 0.55 | 0.516–0.649 | 2.024 | 1.62–2.055 | 1.945 | 1.903–2.164 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šimunović, L.; Blagec, T.; Meštrović, S. The Influence of Different Cleaning Protocols on the Surface Roughness of Orthodontic Retainers. Appl. Sci. 2023, 13, 1319. https://doi.org/10.3390/app13031319
Šimunović L, Blagec T, Meštrović S. The Influence of Different Cleaning Protocols on the Surface Roughness of Orthodontic Retainers. Applied Sciences. 2023; 13(3):1319. https://doi.org/10.3390/app13031319
Chicago/Turabian StyleŠimunović, Luka, Tadeja Blagec, and Senka Meštrović. 2023. "The Influence of Different Cleaning Protocols on the Surface Roughness of Orthodontic Retainers" Applied Sciences 13, no. 3: 1319. https://doi.org/10.3390/app13031319
APA StyleŠimunović, L., Blagec, T., & Meštrović, S. (2023). The Influence of Different Cleaning Protocols on the Surface Roughness of Orthodontic Retainers. Applied Sciences, 13(3), 1319. https://doi.org/10.3390/app13031319








