Spontaneous Epileptic Recordings from hiPSC-Derived Cortical Neurons Cultured with a Human Epileptic Brain Biopsy on a Multi Electrode Array
Abstract
:1. Introduction
2. Materials and Methods
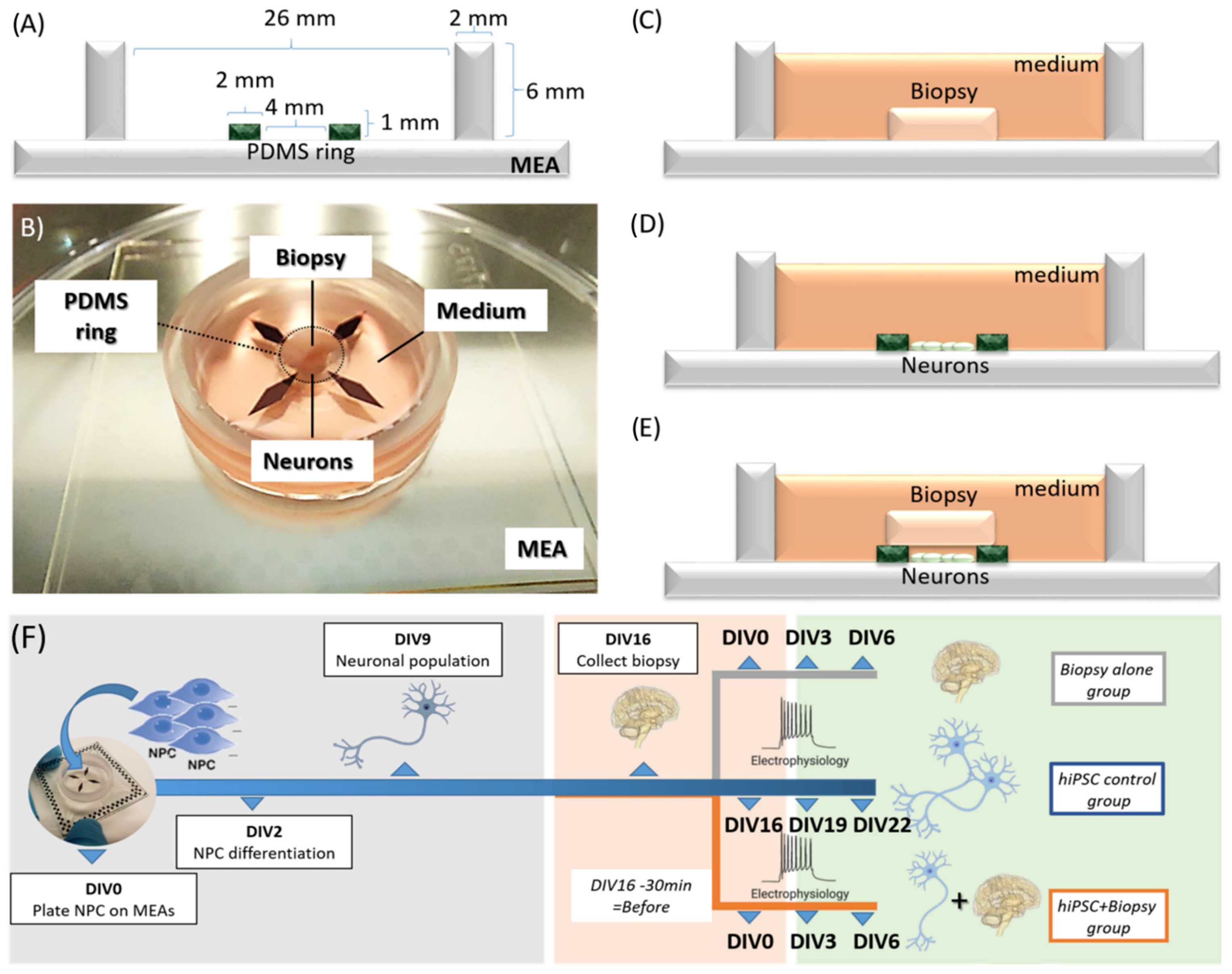
2.1. Concept of the Model
2.2. Preparation of Human Induced Pluripotent Stem Cell (hiPSC)-Derived Neuron Cultures
2.3. Biopsy Collection and Assembly of Hybrid Model
2.4. Data Collection by Multi-Electrode Array (MEA) Recording
2.5. Immunohistochemistry
3. Results
3.1. Immunohistochemical Characterization of hiPSC-Derived Neurons
3.2. Electrophysiological Phenotype of the Hybrid Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhtar, A. The Flaws and Human Harms of Animal Experimentation. Camb. Q. Healthc. Ethics 2015, 24, 407–419. [Google Scholar] [CrossRef] [Green Version]
- Sayed, N.; Liu, C.; Wu, J.C. Translation of Human-Induced Pluripotent Stem Cells from Clinical Trial in a Dish to Precision Medicine. J. Am. Coll. Cardiol. 2016, 67, 2161–2176. [Google Scholar] [CrossRef]
- Hubrecht, R.C.; Carter, E. The 3Rs and Humane Experimental Technique: Implementing Change. Animals 2019, 9, 754. [Google Scholar] [CrossRef] [Green Version]
- Hampshire, V.A.; Gilbert, S.H. Refinement, Reduction, and Replacement (3R) Strategies in Preclinical Testing of Medical Devices. Toxicol. Pathol. 2019, 47, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Beghi, E.; Giussani, G.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; Abraha, H.N.; Adib, M.G.; Agrawal, S.; Alahdab, F.; Awasthi, A.; et al. Global, Regional, and National Burden of Epilepsy, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 357–375. [Google Scholar] [CrossRef] [Green Version]
- Dalic, L.; Cook, M.J. Managing Drug-Resistant Epilepsy: Challenges and Solutions. Neuropsychiatr. Dis. Treat. 2016, 12, 2605–2616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humpel, C. Neuroscience Forefront Review Organotypic Brain Slice Cultures: A Review. Neuroscience 2015, 305, 86–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eugène, E.; Cluzeaud, F.; Cifuentes-Diaz, C.; Fricker, D.; Le Duigou, C.; Clemenceau, S.; Baulac, M.; Poncer, J.C.; Miles, R. An Organotypic Brain Slice Preparation from Adult Patients with Temporal Lobe Epilepsy. J. Neurosci. Methods 2014, 235, 234–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, R.S.G.; da Silva, A.B.; Whittaker, R.G.; Woodhall, G.L.; Cunningham, M.O. Human Brain Slices for Epilepsy Research: Pitfalls, Solutions and Future Challenges. J. Neurosci. Methods 2016, 260, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Valente, C.A.; Meda, F.J.; Carvalho, M.; Sebastião, A.M. A Model of Epileptogenesis in Rhinal Cortex-Hippocampus Organotypic Slice Cultures. J. Vis. Exp. 2021, 169, e61330. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Yu, P.N.; Song, D.; Liu, C.Y.; Heck, C.N.; Millett, D.; Berger, T.W. An in VitroSeizure Model from Human Hippocampal Slices Using Multi-Electrode Arrays. J. Neurosci. Methods 2015, 244, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Dossi, E.; Blauwblomme, T.; Nabbout, R.; Huberfeld, G.; Rouach, N. Multi-electrode Array Recordings of Human Epileptic Postoperative Cortical Tissue. J. Vis. Exp. 2014, 92, e51870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Duigou, C.; Savary, E.; Morin-Brureau, M.; Gomez-Dominguez, D.; Sobczyk, A.; Chali, F.; Milior, G.; Kraus, L.; Meier, J.C.; Kullmann, D.M.; et al. Imaging pathological activities of human brain tissue in organotypic culture. J. Neur. Meth. 2018, 298, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, N.; Hedrich, U.B.S.; Schwarz, H.; Harshad, P.A.; Dammeier, N.; Auffenberg, E.; Bedogni, F.; Honegger, J.B.; Lerche, H.; Wuttke, T.V.; et al. Human Cerebrospinal fluid promotes long-term neuronal viability and network function in human neocortical organotypic brain slice cultures. Sci. Rep. 2017, 7, 12249. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [Green Version]
- Logan, S.; Arzua, T.; Canfield, S.G.; Seminary, E.R.; Sison, S.L.; Ebert, A.D.; Bai, X. Studying Human Neurological Disorders Using Induced Pluripotent Stem Cells: From 2D Monolayer to 3D Organoid and Blood Brain Barrier Models. Compr. Physiol. 2019, 9, 565–611. [Google Scholar] [CrossRef]
- Mateos-Aparicio, P.; Bello, S.A.; Rodríguez-Moreno, A. Challenges in Physiological Phenotyping of HiPSC-Derived Neurons: From 2D Cultures to 3D Brain Organoids. Front. Cell Dev. Biol. 2020, 8, 797. [Google Scholar] [CrossRef]
- Jalink, P.; Caiazzo, M. Brain Organoids: Filling the Need for a Human Model of Neurological Disorder. Biology 2021, 10, 740. [Google Scholar] [CrossRef]
- Farkhondeh, A.; Li, R.; Gorshkov, K.; Chen, K.G.; Might, M.; Rodems, S.; Lo, D.C.; Zheng, W. Induced Pluripotent Stem Cells for Neural Drug Discovery. Drug Discov. Today 2019, 24, 992–999. [Google Scholar] [CrossRef]
- Nikolakopoulou, P.; Rauti, R.; Voulgaris, D.; Shlomy, I.; Maoz, B.M.; Herland, A. Recent Progress in Translational Engineered in Vitro Models of the Central Nervous System. Brain 2021, 143, 3181–3213. [Google Scholar] [CrossRef]
- Tóth, E.; Fabó, D.; Entz, L.; Ulbert, I.; Eross, L. Intracranial Neuronal Ensemble Recordings and Analysis in Epilepsy. J. Neurosci. Methods 2016, 260, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Parent, J.M. Using Patient-Derived Induced Pluripotent Stem Cells to Model and Treat Epilepsies. Curr. Neurol. Neurosci. Rep. 2015, 15, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dulla, C.G.; Janigro, D.; Jiruska, P.; Raimondo, J.V.; Ikeda, A.; Lin, C.C.K.; Goodkin, H.P.; Galanopoulou, A.S.; Bernard, C.; de Curtis, M. How Do We Use in Vitro Models to Understand Epileptiform and Ictal Activity? A Report of the TASK1-WG4 Group of the ILAE/AES Joint Translational Task Force. Epilepsia Open 2018, 3, 460–473. [Google Scholar] [CrossRef] [Green Version]
- Smeets, J.S.J.; Horstman, A.M.H.; Schijns, O.E.M.G.; Dings, J.T.A.; Hoogland, G.; Gijsen, A.P.; Goessens, J.P.B.; Bouwman, F.G.; Wodzig, W.K.W.H.; Mariman, E.C.; et al. Brain Tissue Plasticity: Protein Synthesis Rates of the Human Brain. Brain 2018, 141, 1122–1129. [Google Scholar] [CrossRef]
- Hu, H.; Frega, M.; Tolner, E.A.; van den Maagdenberg, A.M.J.M.; Frimat, J.P.; le Feber, J. MEA-ToolBox: An Open Source Toolbox for Standardized Analysis of Multi-Electrode Array Data. Neuroinformatics 2022, 20, 1077–1092. [Google Scholar] [CrossRef]
- Wagenaar, D.; Demarse, T.B.; Potter, S.M. MeaBench: A Toolset for Multi-Electrode Data Acquisition and on-Line Analysis. In Proceedings of the 2nd International IEEE EMBS Conference on Neural Engineering, Arlington, VA, USA, 16–19 March 2005; pp. 518–521. [Google Scholar] [CrossRef] [Green Version]
- Cotterill, E.; Charlesworth, P.; Thomas, C.W.; Paulsen, O.; Eglen, S.J. A Comparison of Computational Methods for Detecting Bursts in Neuronal Spike Trains and Their Application to Human Stem Cell-Derived Neuronal Networks. J. Neurophysiol. 2016, 116, 306–321. [Google Scholar] [CrossRef] [Green Version]
- Mendis, G.D.C.; Morrisroe, E.; Petrou, S.; Halgamuge, S.K. Use of Adaptive Network Burst Detection Methods for Multielectrode Array Data and the Generation of Artificial Spike Patterns for Method Evaluation. J. Neural Eng. 2016, 13, 026009. [Google Scholar] [CrossRef]
- Mossink, B.; Verboven, A.H.A.; van Hugte, E.J.H.; Klein Gunnewiek, T.M.; Parodi, G.; Linda, K.; Schoenmaker, C.; Kleefstra, T.; Kozicz, T.; van Bokhoven, H.; et al. Human Neuronal Networks on Micro-Electrode Arrays Are a Highly Robust Tool to Study Disease-Specific Genotype-Phenotype Correlations in Vitro. Stem Cell Rep. 2021, 16, 2182–2196. [Google Scholar] [CrossRef]
- Bradley, J.A.; Luithardt, H.H.; Metea, M.R.; Strock, C.J. In Vitro Screening for Seizure Liability Using Microelectrode Array Technology. Toxicol. Sci. 2018, 163, 240–253. [Google Scholar] [CrossRef]
- Ishii, M.N.; Yamamoto, K.; Shoji, M.; Asami, A.; Kawamata, Y. Human Induced Pluripotent Stem Cell (HiPSC)-Derived Neurons Respond to Convulsant Drugs When Co-Cultured with HiPSC-Derived Astrocytes. Toxicology 2017, 389, 130–138. [Google Scholar] [CrossRef]
- Tukker, A.M.; Wijnolts, F.M.J.; de Groot, A.; Westerink, R.H.S. Human IPSC-Derived Neuronal Models for in Vitro Neurotoxicity Assessment. Neurotoxicology 2018, 67, 215–225. [Google Scholar] [CrossRef]
- Odawara, A.; Saitoh, Y.; Alhebshi, A.H.; Gotoh, M.; Suzuki, I. Long-Term Electrophysiological Activity and Pharmacological Response of a Human Induced Pluripotent Stem Cell-Derived Neuron and Astrocyte. Biochem. Biophys. Res. Commun. 2014, 443, 1176–1181. [Google Scholar] [CrossRef] [Green Version]
- Odawara, A.; Katoh, H.; Matsuda, N.; Suzuki, I. Physiological Maturation and Drug Responses of Human Induced Pluripotent Stem Cell-Derived Cortical Neuronal Networks in Long-Term Culture. Sci. Rep. 2016, 6, 26181. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.T.; Kim, J.; López-Valdés, H.E.; Brennan, K.C.; Ju, Y.S. Development and Characterization of a Microfluidic Chamber Incorporating Fluid Ports with Active Suction for Localized Chemical Stimulation of Brain Slices. Lab Chip 2011, 11, 2247–2254. [Google Scholar] [CrossRef] [Green Version]
- Yalcin, Y.D.; Bastiaens, A.J.; Frimat, J.P.; Luttge, R. Long-term brain-on-chip: Multielectrode array recordings in 3D neural cell cultures. J. Vac. Sci. Technol. B 2021, 39, 064004. [Google Scholar] [CrossRef]
- Osaki, T.; Uzel, S.G.M.; Kamm, R.D. On-Chip 3D Neuromuscular Model for Drug Screening and Precision Medicine in Neuromuscular Disease. Nat. Protoc. 2020, 15, 421–449. [Google Scholar] [CrossRef]
- Kubaczkova, V.; Sedlarikova, L.; Bollova, B.; Sandecka, V.; Stork, M.; Pour, L.; Sevcikova, S. Liquid Biopsies—The Clinics and the Molecules. Klin. Onkol. 2017, 30, 2S13–2S20. [Google Scholar] [CrossRef]
- Buskila, Y.; Breen, P.P.; Tapson, J.; Van Schaik, A.; Barton, M.; Morley, J.W. Extending the Viability of Acute Brain Slices. Sci. Rep. 2014, 4, 5309. [Google Scholar] [CrossRef] [Green Version]
- Didier, C.; Kundu, A.; Rajaraman, S. Capabilities and Limitations of 3D Printed Microserpentines and Integrated 3D Electrodes for Stretchable and Conformable Biosensor Applications. Microsyst. Nanoeng. 2020, 6, 15. [Google Scholar] [CrossRef]
- Liu, M.G.; Chen, X.F.; He, T.; Li, Z.; Chen, J. Use of Multi-Electrode Array Recordings in Studies of Network Synaptic Plasticity in Both Time and Space. Neurosci. Bull. 2012, 28, 409–422. [Google Scholar] [CrossRef]




| High pass Butterworth filter | Cut off Frequency | 200 Hz |
| Filter order | 2 | |
| Baseline noise detection | 0.05 s | |
| Pure noise window | 2 s | |
| Spike detection | Spike detection threshold | 7 RMS |
| Interval time | 0 | |
| Min. spike amplitude | 30 µV | |
| Min. fire frequency | 0.1 Hz | |
| Burst detection | Start interval | 0.05 s |
| (Max Interval method) | Spike number | 4 |
| Inter-burst interval | 0.1 s | |
| Intra-burst interval | 0.1 s | |
| Min. burst duration | 0.03 s | |
| Network Burst detection | Synchronized time windows | 0.1 s |
| Min. Synchronized burst number | 2 | |
| Min. channel participation | 50% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, M.H.Y.; Frimat, J.-P.; Rijkers, K.; Schijns, O.E.M.G.; van den Maagdenberg, A.M.J.M.; Dings, J.T.A.; Luttge, R.; Hoogland, G. Spontaneous Epileptic Recordings from hiPSC-Derived Cortical Neurons Cultured with a Human Epileptic Brain Biopsy on a Multi Electrode Array. Appl. Sci. 2023, 13, 1432. https://doi.org/10.3390/app13031432
Hu MHY, Frimat J-P, Rijkers K, Schijns OEMG, van den Maagdenberg AMJM, Dings JTA, Luttge R, Hoogland G. Spontaneous Epileptic Recordings from hiPSC-Derived Cortical Neurons Cultured with a Human Epileptic Brain Biopsy on a Multi Electrode Array. Applied Sciences. 2023; 13(3):1432. https://doi.org/10.3390/app13031432
Chicago/Turabian StyleHu, Michel H. Y., Jean-Philippe Frimat, Kim Rijkers, Olaf E. M. G. Schijns, Arn M. J. M. van den Maagdenberg, Jim T. A. Dings, Regina Luttge, and Govert Hoogland. 2023. "Spontaneous Epileptic Recordings from hiPSC-Derived Cortical Neurons Cultured with a Human Epileptic Brain Biopsy on a Multi Electrode Array" Applied Sciences 13, no. 3: 1432. https://doi.org/10.3390/app13031432
APA StyleHu, M. H. Y., Frimat, J. -P., Rijkers, K., Schijns, O. E. M. G., van den Maagdenberg, A. M. J. M., Dings, J. T. A., Luttge, R., & Hoogland, G. (2023). Spontaneous Epileptic Recordings from hiPSC-Derived Cortical Neurons Cultured with a Human Epileptic Brain Biopsy on a Multi Electrode Array. Applied Sciences, 13(3), 1432. https://doi.org/10.3390/app13031432







