Reclaimed Salt-Affected Soils Can Effectively Contribute to Carbon Sequestration and Food Grain Production: Evidence from Pakistan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Treatments and Experimental Setup
2.3. Crop Husbandry
2.4. Plant and Soil Analysis
2.5. Biological Parameters
2.5.1. Microbial Biomass Carbon
2.5.2. Soil Respiration
2.5.3. Dehydrogenase Activity
2.6. Source of Experimental Materials
2.7. Simulation of Potential Carbon Sequestration in Soils
2.7.1. Initialization of Soil Organic Matter Pools and Plant Inputs in the Control
2.7.2. Plant Inputs in the Treatments
2.7.3. Organic Manure Inputs in the Treatments
2.7.4. Model Evaluation
2.7.5. Simulations of Long-Term Changes in Soil Organic Carbon
2.8. Economic Analysis
2.9. Statistical Analysis
3. Results
3.1. Physical, Chemical and Biological Properties
3.1.1. Pre-Analysis of Soils
3.1.2. Temporal Changes in the Soil’s Physical, Chemical and Biological Properties (Fallow Period)
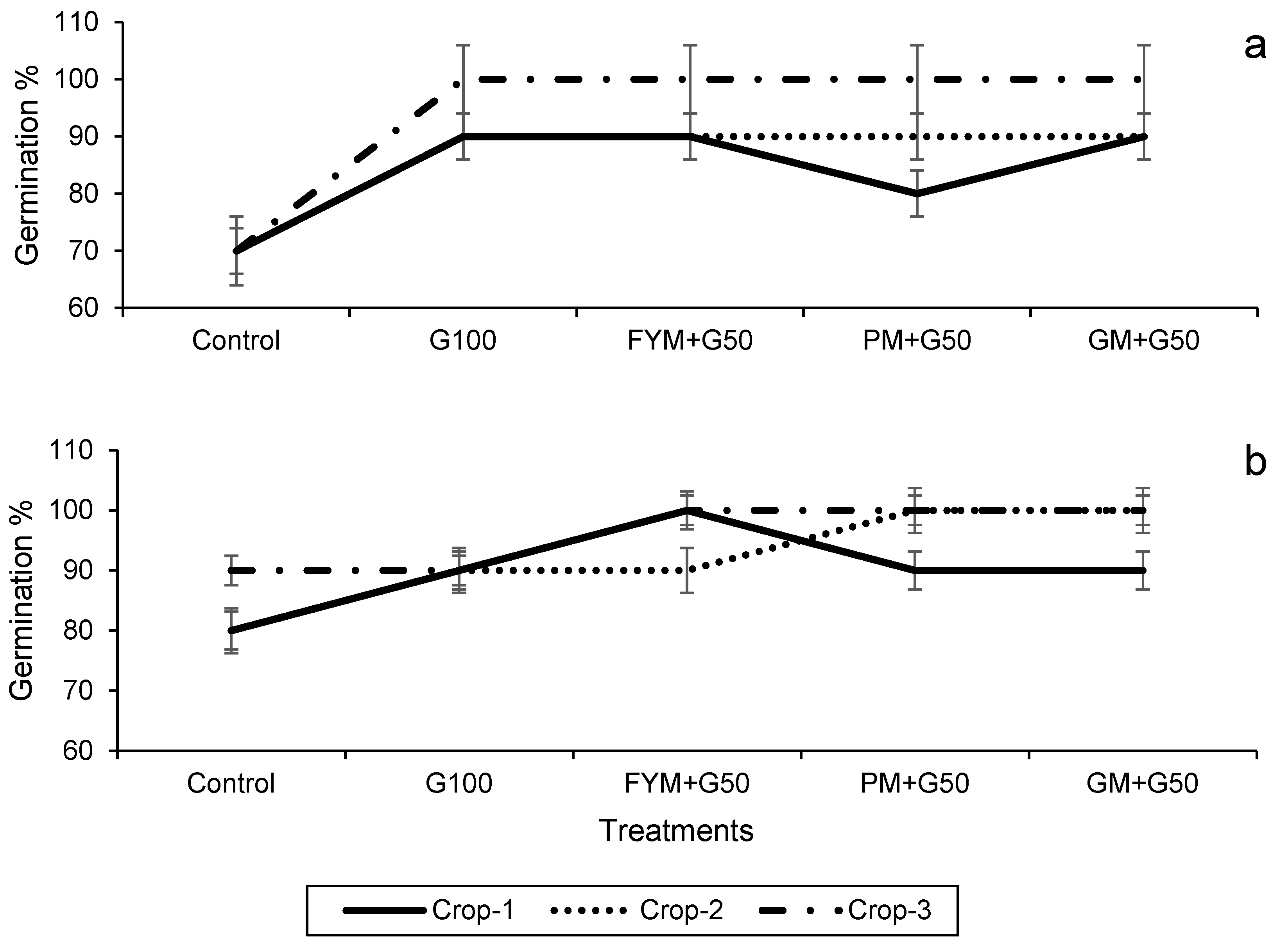
3.2. The Seed Germination, Physiology and Productivity of the Wheat–Maize Cropping System
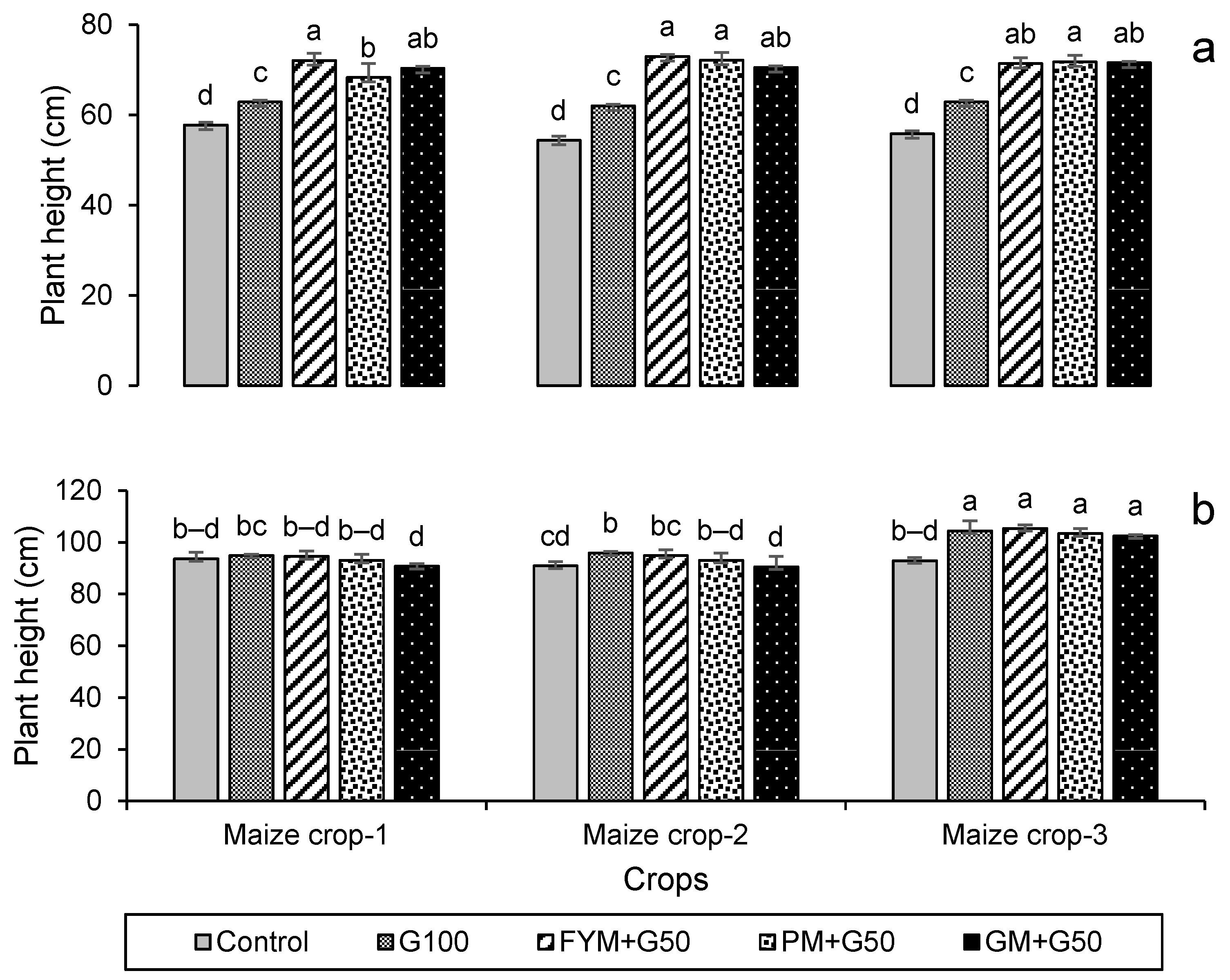
3.2.1. Seed Germination and Plant Height
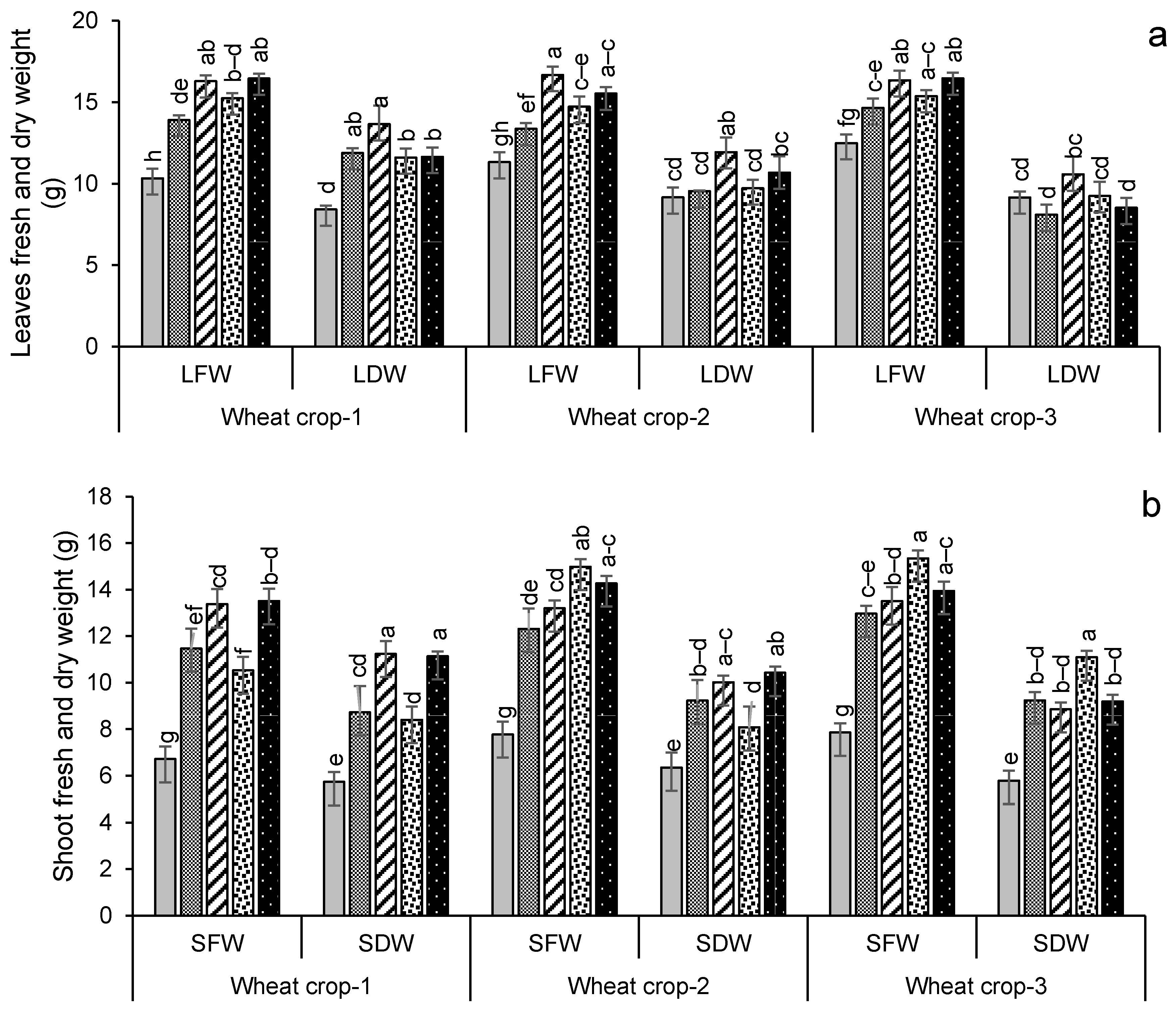
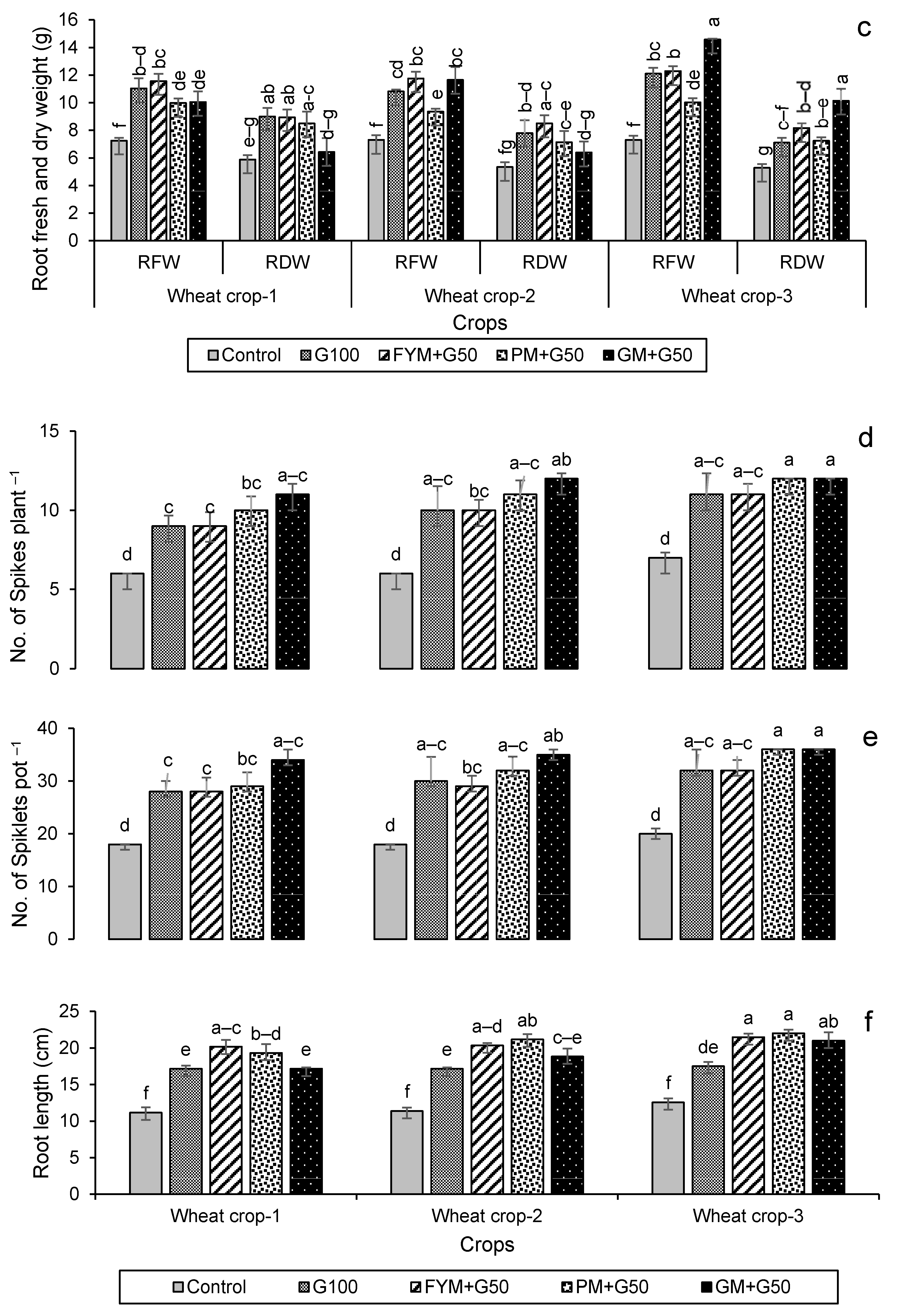
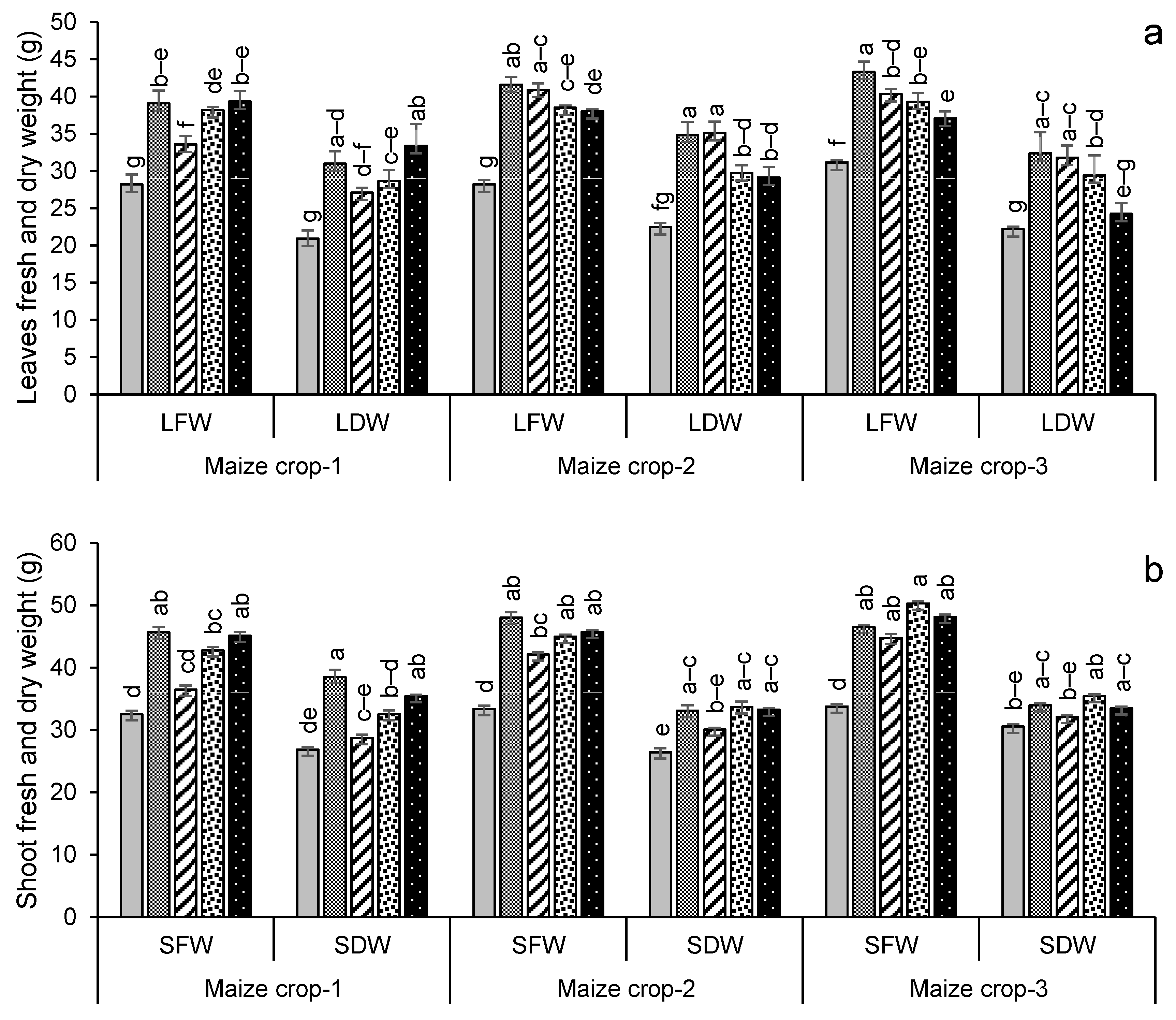
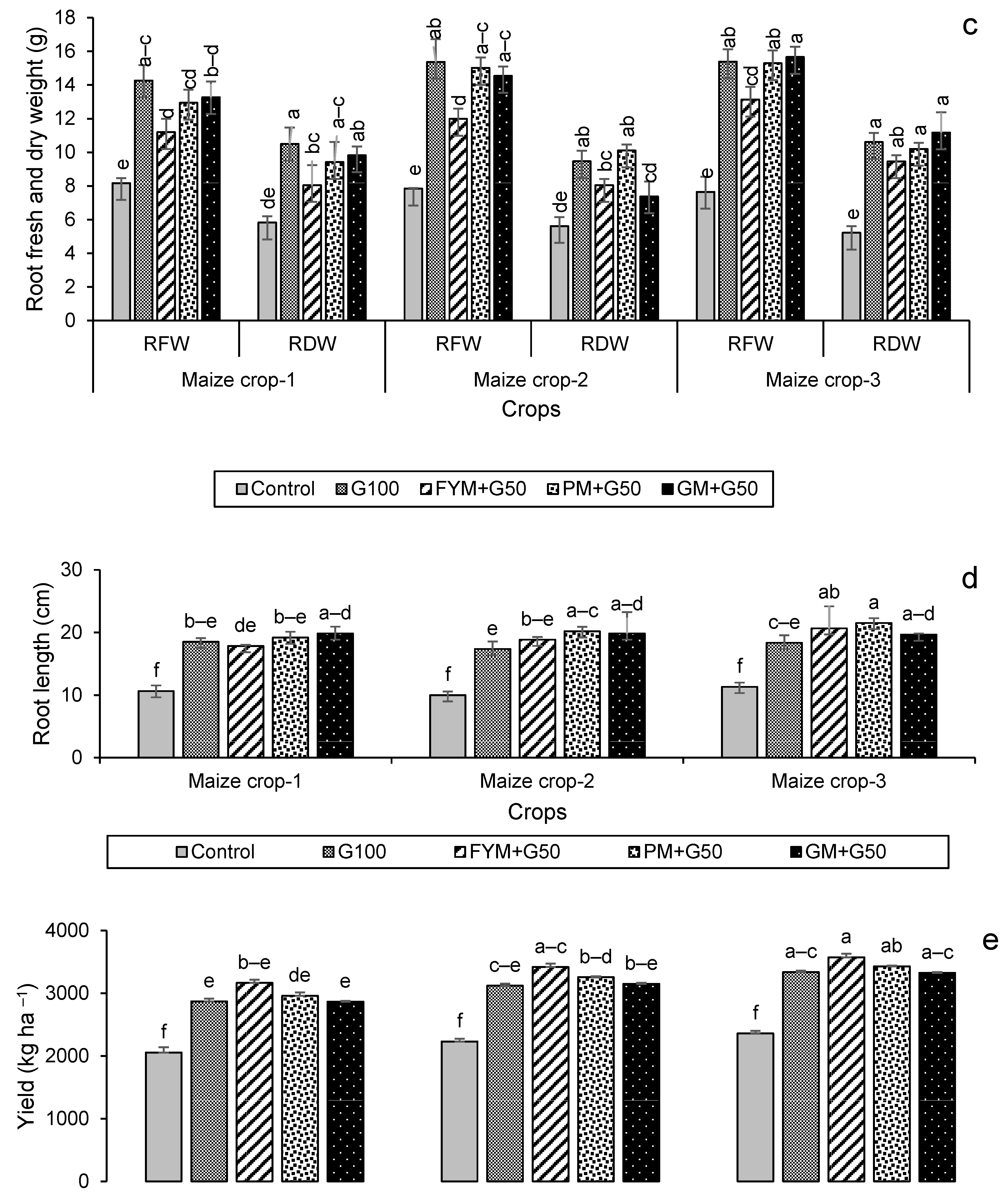
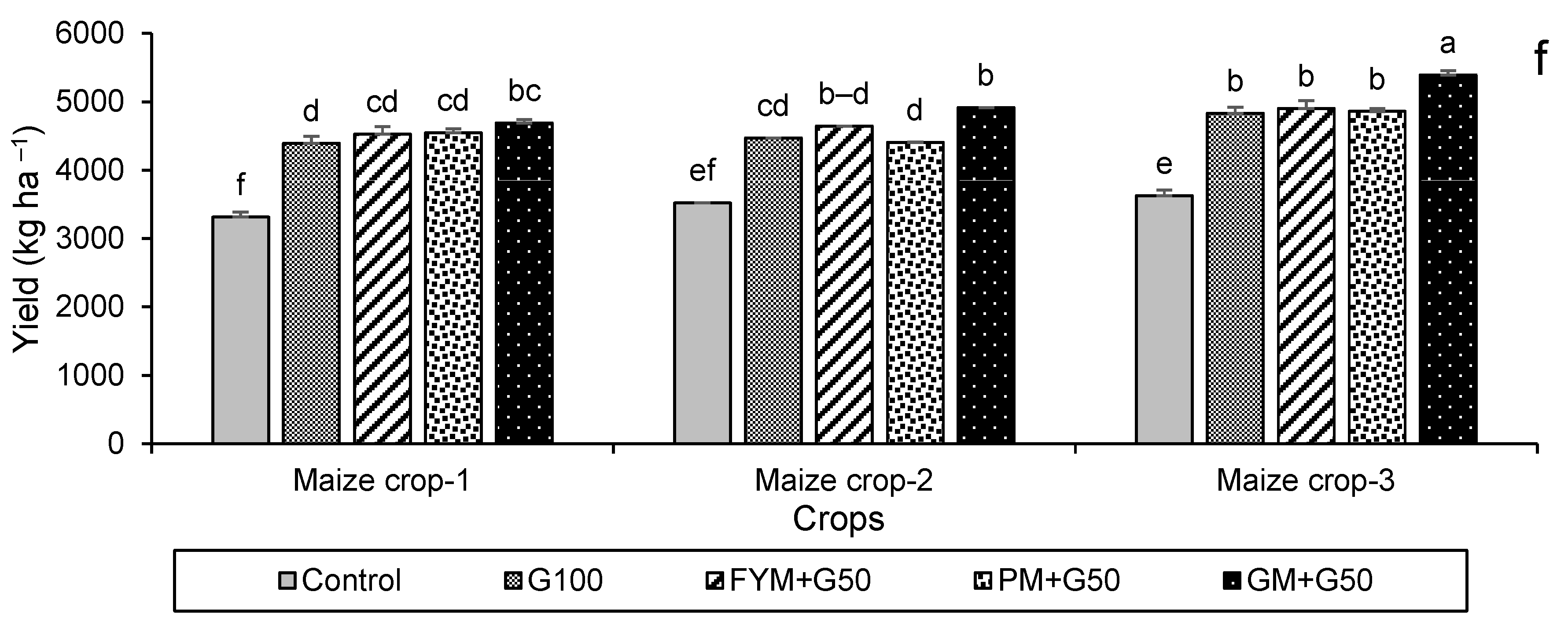
3.2.2. Crop Growth and Yield Attributes
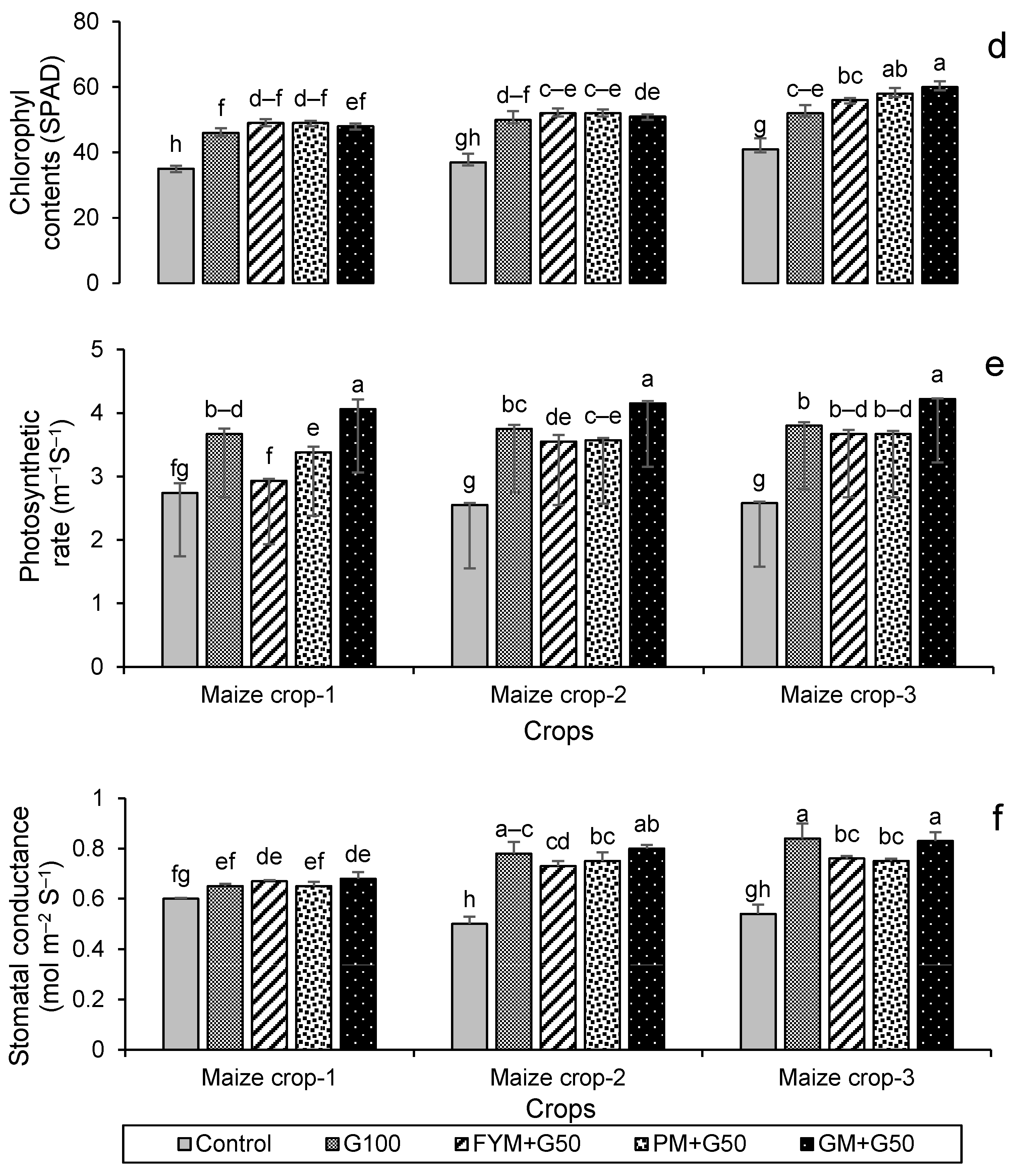
3.2.3. Crop Physiological Attributes
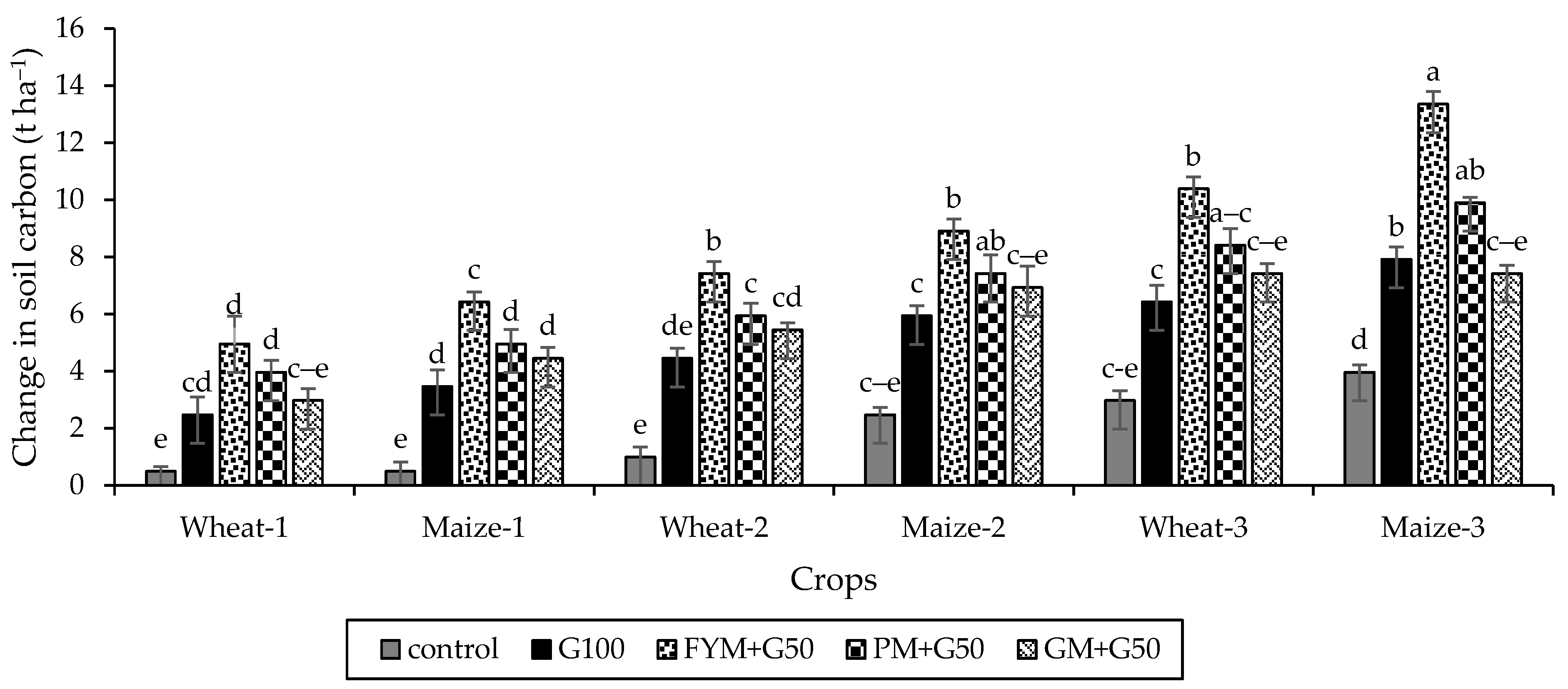
3.3. Soil Organic Carbon
3.4. Post-Harvest Soil Analysis
3.5. Economic Analysis of the Crop Production
3.6. Simulation of Short-Term Changes in Soil Carbon
3.6.1. Derivation of Organic Waste Parameters Using Soils from Pot Experiments at Dijkot and Uchkera
3.6.2. Evaluation of Organic Waste Parameters Using Pot and Field Measurements at an Independent Site, Jhang
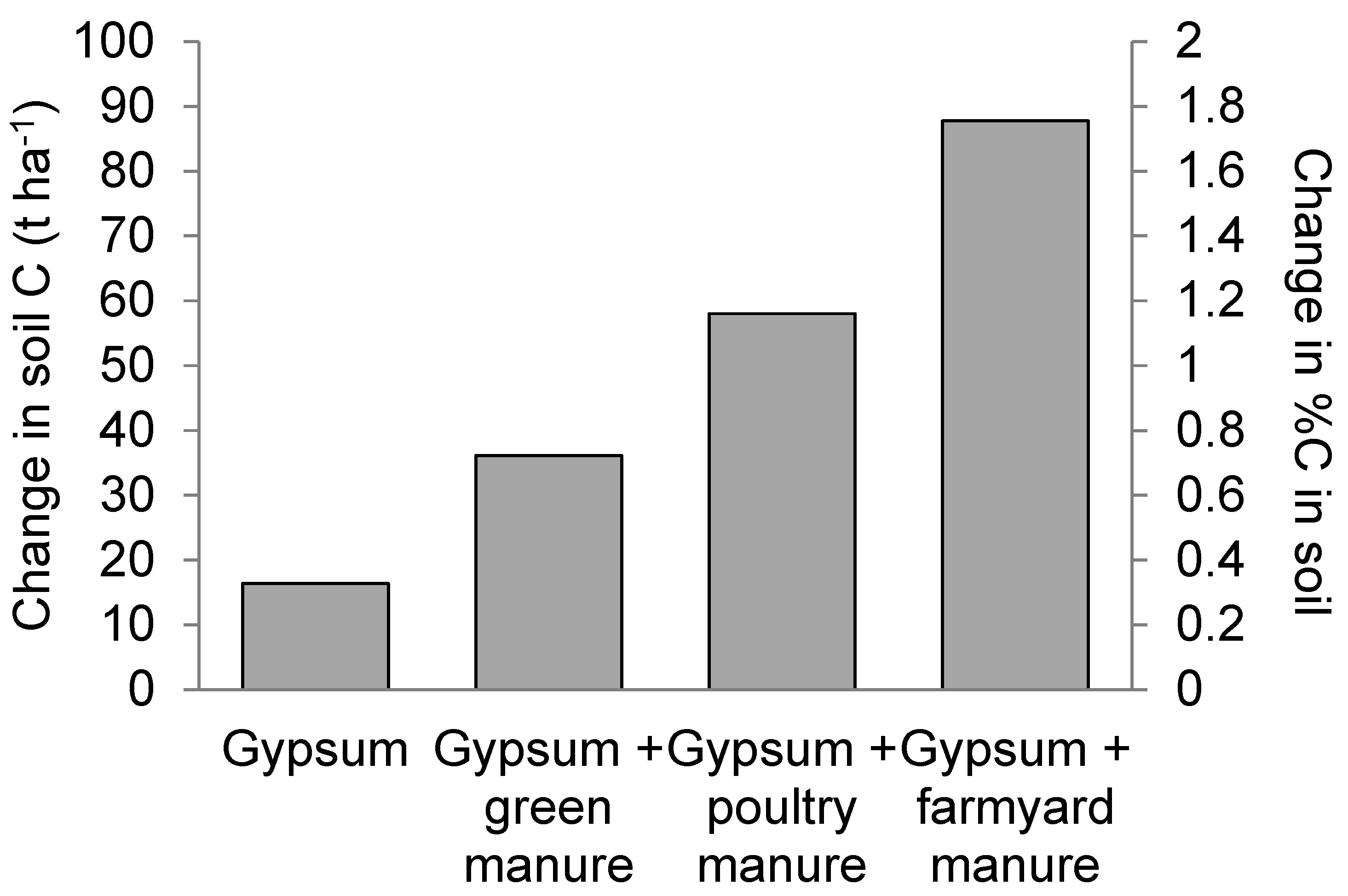
3.6.3. Simulation of Long-Term Carbon Sequestration
4. Discussion
4.1. Impacts of Different Treatments on Soil Properties
4.2. Impacts of Soil Restoration on Plant Growth, Physiology and Yield
4.3. The Impacts of Organic Amendments and Crop Rotation on Soil Carbon Storage
4.4. Simulations of Soil Carbon Sequestration in Freshly Reclaimed Marginally Salt-Affected Soils under Wheat–Maize Cropping System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil salinity under climate change: Challenges for sustainable agriculture and food security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Berlin/Heidelberg, Germany, 2018; pp. 43–53. [Google Scholar]
- Hossain, M.S. Present scenario of global salt affected soils, its management and importance of salinity research. Int. Res. J. Biol. Sci. 2019, 1, 1–3. [Google Scholar]
- Smyth, S.J.; Phillips, P.W.; Kerr, W.A. EU failing FAO challenge to improve global food security. Trends Biotechnol. 2016, 34, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Singh, A.K.; Singh, Y.P. Bioremediation of salt affected soils: An Indian perspective; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Haile, M.G.; Wossen, T.; Tesfaye, K.; von Braun, J. Impact of climate change, weather extremes, and price risk on global food supply. Econ. Disasters Clim. Chang. 2017, 1, 55–75. [Google Scholar] [CrossRef]
- Abate, S.; Belayneh, M.; Ahmed, F. Reclamation and amelioration of saline-sodic soil using gypsum and halophytic grasses: Case of Golina-Addisalem irrigation scheme, Raya Kobo Valley, Ethiopia. Cogent Food Agric. 2021, 7, 1859847. [Google Scholar] [CrossRef]
- Haneklaus, N.; Barbossa, S.; Basallote, M.D.; Bertau, M.; Bilal, E.; Chajduk, E.; Chernysh, Y.; Chubur, V.; Cruz, J.; Dziarczykowski, K.; et al. Closing the upcoming EU gypsum gap with phosphogypsum. Resour. Conserv. Recycl. 2022, 182, 106328. [Google Scholar] [CrossRef]
- Khan, A.; Faisal, S.; Shafique, M.; Khan, S.; Bacha, A.S. ASTER-based remote sensing investigation of gypsum in the Kohat Plateau, north Pakistan. Carbonates Evaporites 2020, 35, 3. [Google Scholar] [CrossRef]
- Amini, S.; Ghadiri, H.; Chen, C.; Marschner, P. Salt-affected soils, reclamation, carbon dynamics, and biochar: A review. J. Soils Sediments 2016, 16, 939–953. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, N.; Wu, L.; Xu, M.; Bingham, I.J.; Li, Z. Effects of enhancing soil organic carbon sequestration in the topsoil by fertilization on crop productivity and stability: Evidence from long-term experiments with wheat-maize cropping systems in China. Sci. Total Environ. 2016, 562, 247–259. [Google Scholar] [CrossRef] [Green Version]
- Bright, M.B.; Diedhiou, I.; Bayala, R.; Assigbetse, K.; Chapuis-Lardy, L.; Ndour, Y.; Dick, R.P. Long-term Piliostigma reticulatum intercropping in the Sahel: Crop productivity, carbon sequestration, nutrient cycling, and soil quality. Agric. Ecosyst. Environ. 2017, 242, 9–22. [Google Scholar] [CrossRef]
- Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar]
- Rashid, M.F.; Aziz, T.; Maqsood, M.A.; Farooq, M. Soil phosphorus fractions and their transformation in normal and salt affected soils as affected by organic amendments. Pak. J. Agric. Sci. 2019, 56, 301–312. [Google Scholar]
- Oldfield, E.E.; Wood, S.A.; Bradford, M.A. Direct effects of soil organic matter on productivity mirror those observed with organic amendments. Plant Soil 2018, 423, 363–373. [Google Scholar] [CrossRef]
- Wood, S.A.; Tirfessa, D.; Baudron, F. Soil organic matter underlies crop nutritional quality and productivity in smallholder agriculture. Agric. Ecosyst. Environ. 2018, 266, 100–108. [Google Scholar] [CrossRef]
- Leifeld, J.; Menichetti, L. The underappreciated potential of peatlands in global climate change mitigation strategies. Nat. Commun. 2018, 9, 1071. [Google Scholar] [CrossRef] [Green Version]
- Farooqi, Z.U.R.; Sabir, M.; Zia-Ur-Rehman, M.; Hussain, M.M. Mitigation of climate change through carbon sequestration in agricultural soils. In Climate Change and Agroforestry Systems; Apple Academic Press: Burlington, ON, USA, 2020; pp. 87–118. [Google Scholar]
- del Mar Montiel-Rozas, M.; Panettieri, M.; Madejón, P.; Madejón, E. Carbon sequestration in restored soils by applying organic amendments. Land Degrad. Dev. 2016, 27, 620–629. [Google Scholar] [CrossRef]
- Haque, M.M.; Biswas, J.; Maniruzaman, M.; Akhter, S.; Kabir, M. Carbon sequestration in paddy soil as influenced by organic and inorganic amendments. Carbon Manag. 2020, 11, 231–239. [Google Scholar] [CrossRef]
- Qureshi, A.S.; McCornick, P.G.; Qadir, M.; Aslam, Z. Managing salinity and waterlogging in the Indus Basin of Pakistan. Agric. Water Manag. 2008, 95, 1–10. [Google Scholar] [CrossRef]
- Wicke, B.; Smeets, E.M.; Akanda, R.; Stille, L.; Singh, R.K.; Awan, A.R.; Mahmood, K.; Faaij, A.P. Biomass production in agroforestry and forestry systems on salt-affected soils in South Asia: Exploration of the GHG balance and economic performance of three case studies. J. Environ. Manag. 2013, 127, 324–334. [Google Scholar] [CrossRef]
- Qadir, M.; Schubert, S.; Oster, J.D.; Sposito, G.; Minhas, P.S.; Cheraghi, S.A.; Murtaza, G.; Mirzabaev, A.; Saqib, M. High-magnesium waters and soils: Emerging environmental and food security constraints. Sci. Total Environ. 2018, 642, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, G.; Murtaza, B.; Kahlon, U.Z.; Yaseen, M. A Comparative study of different amendments on amelioration of saline-sodic soils irrigated with water having different EC: SAR ratios. Commun. Soil Sci. Plant Anal. 2017, 48, 2630–2641. [Google Scholar] [CrossRef]
- Ghafoor, A.; Murtaza, G.; Rehman, M.; Sabir, M. Reclamation and salt leaching efficiency for tile drained saline-sodic soil using marginal quality water for irrigating rice and wheat crops. Land Degrad. Dev. 2012, 23, 1–9. [Google Scholar] [CrossRef]
- Murtaza, G.; Ghafoor, A.; Owens, G.; Qadir, M.; Kahlon, U. Environmental and economic benefits of saline-sodic soil reclamation using low-quality water and soil amendments in conjunction with a rice–wheat cropping system. J. Agron. Crop Sci. 2009, 195, 124–136. [Google Scholar] [CrossRef]
- Ghafoor, A.; Murtaza, G.; Ahmad, B.; Boers, T.M. Evaluation of amelioration treatments and economic aspects of using saline–sodic water for rice and wheat production on salt-affected soils under arid land conditions. Irrig. Drain. 2008, 57, 424–434. [Google Scholar] [CrossRef]
- Zaman, G.; Murtaza, B.; Imran, M.; Shahid, M.; Shah, G.M.; Amjad, M.; Naeem, M.A.; Mubeen, M.; Murtaza, G. Utilization of bio-municipal solid waste improves saline-sodic soils and crop productivity in rice-wheat. Compost. Sci. Util. 2020, 28, 16–27. [Google Scholar] [CrossRef]
- Murtaza, G.; Murtaza, B.; Usman, H.M.; Ghafoor, A. Amelioration of Saline-sodic Soil using Gypsum and Low Quality Water in Following Sorghum-berseem Crop Rotation. Int. J. Agric. Biol. 2013, 15, 640–648. [Google Scholar]
- Garcia-Franco, N.; Wiesmeier, M.; Hurtarte, L.C.C.; Fella, F.; Martínez-Mena, M.; Almagro, M.; Martínez, E.G.; Kögel-Knabner, I. Pruning residues incorporation and reduced tillage improve soil organic matter stabilization and structure of salt-affected soils in a semi-arid Citrus tree orchard. Soil Tillage Res. 2021, 213, 105129. [Google Scholar] [CrossRef]
- Basak, N.; Sheoran, P.; Sharma, R.; Yadav, R.K.; Singh, R.K.; Kumar, S.; Krishnamurthy, T.; Sharma, P.C. Gypsum and pressmud amelioration improve soil organic carbon storage and stability in sodic agroecosystems. Land Degrad. Dev. 2021, 32, 4430–4444. [Google Scholar] [CrossRef]
- Soni, P.G.; Rai, A.K.; Basak, N.; Kumar, P.; Sundha, P. Productivity and profitability of sorghum-wheat cropping system in saline soils as influenced by conservation agriculture practices. Range Manag. Agrofor. 2021, 42, 277–285. [Google Scholar]
- Saqib, Z.A.; Akhtar, J.; Haq, M.; Ahmad, I. Salt induced changes in leaf phenology of wheat plants are regulated by accumulation and distribution pattern of Na ion. Pak. J. Agric. Sci 2012, 49, 141–148. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total nitrogen analysis of soil and plant tissues. J. Assoc. Off. Anal. Chem. 1980, 63, 770–778. [Google Scholar] [CrossRef]
- Terry, R.E.; Nelson, S.D.; Carr, J.; Parnell, J.; Hardin, P.J.; Jackson, M.W.; Houston, S.D. Quantitative phosphorus measurement: A field test procedure for archaeological site analysis at Piedras Negras, Guatemala. Geoarchaeology 2000, 15, 151–166. [Google Scholar] [CrossRef]
- Norman, A. Methods of Soil Analysis: Part 2-Chemical and Microbiological Properties; ASA SSSA: Madison, WI, USA, 1965. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Estefan, G. Methods of Soil, Plant, and Water Analysis: A Manual for the West Asia and North Africa Region; International Center for Agricultural Research in the Dry Areas: Beirut, Lebanon, 2013. [Google Scholar]
- Vance, E.; Brookes, P.; Jenkinson, D. An Extraction Method for Measuring Microbial Biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Anderson, J.P. Soil respiration. Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1983; Volume 9, pp. 831–871. [Google Scholar]
- Casida, L., Jr.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Powlson, D.S.; Smith, P.; Smith, J.U. Evaluation of Soil Organic Matter Models: Using Existing Long-Term Datasets; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 38. [Google Scholar]
- Setia, R.; Smith, P.; Marschner, P.; Baldock, J.; Chittleborough, D.; Smith, J. Introducing a decomposition rate modifier in the Rothamsted carbon model to predict soil organic carbon stocks in saline soils. Environ. Sci. Technol. 2011, 45, 6396–6403. [Google Scholar] [CrossRef]
- Smith, J.; Smith, P.; Wattenbach, M.; Zaehle, S.; Hiederer, R.; Jones, R.J.; Montanarella, L.; Rounsevell, M.D.; Reginster, I.; Ewert, F. Projected changes in mineral soil carbon of European croplands and grasslands, 1990–2080. Glob. Chang. Biol. 2005, 11, 2141–2152. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.H. Using primary productivity as an index of coastal eutrophication: The units of measurement matter. J. Plankton Res. 2007, 29, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.; Abegaz, A.; Matthews, R.B.; Subedi, M.; Orskov, E.R.; Tumwesige, V.; Smith, P. What is the potential for biogas digesters to improve soil carbon sequestration in Sub-Saharan Africa? Comparison with other uses of organic residues. Biomass Bioenergy 2014, 70, 73–86. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.; Smith, P.; Addiscott, T. Quantitative methods to evaluate and compare soil organic matter (SOM) models. In Evaluation of Soil Organic Matter Models; Springer: Berlin/Heidelberg, Germany, 1996; pp. 181–199. [Google Scholar]
- Program, C.E.; Maize, I.; Center, W.I. From Agronomic Data to Farmer Recommendations: An Economics Training Manual; CIMMYT: Texcoco, Mexico, 1988. [Google Scholar]
- Urra, J.; Alkorta, I.; Garbisu, C. Potential benefits and risks for soil health derived from the use of organic amendments in agriculture. Agronomy 2019, 9, 542. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Xiong, X.; Wang, L.; Hou, D.; Bolan, N.S.; Ok, Y.S.; Rinklebe, J.; Tsang, D.C. A critical review on performance indicators for evaluating soil biota and soil health of biochar-amended soils. J. Hazard. Mater. 2021, 414, 125378. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Singh, K.; Pankaj, U.; Verma, S.K.; Verma, R.K.; Patra, D. Effect of organic amendments and microbial application on sodic soil properties and growth of an aromatic crop. Ecol. Eng. 2017, 102, 127–136. [Google Scholar] [CrossRef]
- Yang, L.; Bian, X.; Yang, R.; Zhou, C.; Tang, B. Assessment of organic amendments for improving coastal saline soil. Land Degrad. Dev. 2018, 29, 3204–3211. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; Liang, C.; Xu, Q.; Li, Y.; Qin, H.; Fuhrmann, J.J. Response of microbial community structure and function to short-term biochar amendment in an intensively managed bamboo (Phyllostachys praecox) plantation soil: Effect of particle size and addition rate. Sci. Total Environ. 2017, 574, 24–33. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, Y.; Yang, C.; Shen, Q.; Zhou, J.; Yang, L. Soil enzymatic activity and growth of rice and barley as influenced by organic manure in an anthropogenic soil. Geoderma 2003, 115, 149–160. [Google Scholar] [CrossRef]
- Liang, Y.; Si, J.; Nikolic, M.; Peng, Y.; Chen, W.; Jiang, Y. Organic manure stimulates biological activity and barley growth in soil subject to secondary salinization. Soil Biol. Biochem. 2005, 37, 1185–1195. [Google Scholar] [CrossRef]
- Vance, W.; Tisdall, J.; McKenzie, B. Residual effects of surface applications of organic matter and calcium salts on the subsoil of a red-brown earth. Aust. J. Exp. Agric. 1998, 38, 595–600. [Google Scholar] [CrossRef]
- Abdul Qadir, A.; Murtaza, G.; Zia-ur-Rehman, M.; Waraich, E.A. Application of Gypsum or Sulfuric Acid Improves Physiological Traits and Nutritional Status of Rice in Calcareous Saline-Sodic Soils. J. Soil Sci. Plant Nutr. 2022, 22, 1846–1858. [Google Scholar] [CrossRef]
- David, R.; Dimitrios, P. Diffusion and cation exchange during the reclamation of saline-structured soils. Geoderma 2002, 107, 271–279. [Google Scholar] [CrossRef]
- Qadir, A.A.; Murtaza, G.; Zia-ur-Rehman, M.; Waraich, E.A. Effect of chemical reclamation on the physiological and chemical response of rice grown in varying salinity and sodicity conditions. Int. J. Agric. Biol. 2021, 26, 97–104. [Google Scholar] [CrossRef]
- Farooqi, Z.U.R.; Murtaza, G.; Bibi, S.; Sabir, M.; Owens, G.; Saifullah; Ahmad, I.; Zeeshan, N. Immobilization of cadmium in soil-plant system through soil and foliar applied silicon. Int. J. Phytoremediation 2022, 24, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Yang, Y.; Peng, H.; Zhang, L.; Zhou, Y.; Zhang, J.; Du, C.; Liu, J.; Lin, X.; Wang, N.; et al. Silicon fertilizers, humic acid and their impact on physicochemical properties, availability and distribution of heavy metals in soil and soil aggregates. Sci. Total Environ. 2022, 822, 153483. [Google Scholar] [CrossRef] [PubMed]
- Hijbeek, R.; Ten Berge, H.; Whitmore, A.; Barkusky, D.; Schröder, J.; Van Ittersum, M. Nitrogen fertiliser replacement values for organic amendments appear to increase with N application rates. Nutr. Cycl. Agroecosystems 2018, 110, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Gao, W.; Ren, S. Long-term effects of combined application of chemical nitrogen with organic materials on crop yields, soil organic carbon and total nitrogen in fluvo-aquic soil. Soil Tillage Res. 2015, 151, 67–74. [Google Scholar] [CrossRef]
- Marzi, M.; Shahbazi, K.; Kharazi, N.; Rezaei, M. The influence of organic amendment source on carbon and nitrogen mineralization in different soils. J. Soil Sci. Plant Nutr. 2020, 20, 177–191. [Google Scholar] [CrossRef]
- Leogrande, R.; Vitti, C. Use of organic amendments to reclaim saline and sodic soils: A review. Arid. Land Res. Manag. 2019, 33, 1–21. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Olaniran, A.F.; Adenusi, T.T.; Aremu, C.; Ejue, W.S.; Iranloye, Y.M.; Gbadamosi, A.; Olayanju, A. Effects of cow dung and wood biochars and green manure on soil fertility and tiger nut (Cyperus esculentus L.) performance on a savanna Alfisol. Sci. Rep. 2020, 10, 21021. [Google Scholar] [CrossRef]
- Li, J.; Pu, L.; Zhu, M.; Zhang, J.; Li, P.; Dai, X.; Xu, Y.; Liu, L. Evolution of soil properties following reclamation in coastal areas: A review. Geoderma 2014, 226, 130–139. [Google Scholar] [CrossRef]
- Pereg, L.; Morugán-Coronado, A.; McMillan, M.; García-Orenes, F. Restoration of nitrogen cycling community in grapevine soil by a decade of organic fertilization. Soil Tillage Res. 2018, 179, 11–19. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, Y.; Han, I.; Wang, P.; Mei, Q.; Huang, Y. Effects of different straw biochars on soil organic carbon, nitrogen, available phosphorus, and enzyme activity in paddy soil. Sci. Rep. 2020, 10, 8837. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gao, J.; Bai, L.; Wang, Y.; Huang, J.; Kumar, M.; Zeng, X. Influence of green manure and rice straw management on soil organic carbon, enzyme activities, and rice yield in red paddy soil. Soil Tillage Res. 2019, 195, 104428. [Google Scholar] [CrossRef]
- Deng, L.-N.; Feng, G.-N.; Gao, Y.; Shen, Y.-X.; Li, H.-S.; Gu, Y.; Luan, H.-Y. Phytochemical constituents and antioxidant enzyme activity profiles of different barley (Hordeum vulgare L.) cultivars at different developmental stages. Agronomy 2019, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Rao, D.; Pathak, H. Ameliorative influence of organic matter on biological activity of salt-affected soils. Arid. Land Res. Manag. 1996, 10, 311–319. [Google Scholar] [CrossRef]
- Singh, P.; Choudhary, O.; Mavi, M. Irrigation-induced salinization effects on soil chemical and biological properties under cotton-wheat rotation on loamy sand soil in northwest India. J. Indian Soc. Soil Sci. 2018, 66, 386–391. [Google Scholar] [CrossRef]
- García-Gil, J.C.; Plaza, C.; Soler-Rovira, P.; Polo, A. Long-term effects of municipal solid waste compost application on soil enzyme activities and microbial biomass. Soil Biol. Biochem. 2000, 32, 1907–1913. [Google Scholar] [CrossRef]
- Meier, I.C.; Finzi, A.C.; Phillips, R.P. Root exudates increase N availability by stimulating microbial turnover of fast-cycling N pools. Soil Biol. Biochem. 2017, 106, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Frankenberger, W., Jr.; Bingham, F. Influence of salinity on soil enzyme activities. Soil Sci. Soc. Am. J. 1982, 46, 1173–1177. [Google Scholar] [CrossRef]
- Filipović, L.; Romić, M.; Sikora, S.; Huić Babić, K.; Filipović, V.; Gerke, H.H.; Romić, D. Response of soil dehydrogenase activity to salinity and cadmium species. J. Soil Sci. Plant Nutr. 2020, 20, 530–536. [Google Scholar] [CrossRef]
- Edrisi, S.A.; Tripathi, V.; Chaturvedi, R.K.; Dubey, D.K.; Patel, G.; Abhilash, P.C. Saline Soil Reclamation Index as an efficient tool for assessing restoration progress of saline land. Land Degrad. Dev. 2021, 32, 123–138. [Google Scholar] [CrossRef]
- Badar, R.; Batool, B.; Ansari, A.; Mustafa, S.; Ajmal, A.; Perveen, S. Amelioration of salt affected soils for cowpea growth by application of organic amendments. J. Pharmacogn. Phytochem. 2015, 3, 87–90. [Google Scholar]
- Rop, K.; Karuku, G.N.; Mbui, D.; Njomo, N.; Michira, I. Evaluating the effects of formulated nano-NPK slow release fertilizer composite on the performance and yield of maize, kale and capsicum. Ann. Agric. Sci. 2019, 64, 9–19. [Google Scholar] [CrossRef]
- Bah, H.; Zhou, M.; Kizito, S.; Xiao, R.; Raza, S.T.; Dong, Z.; Zhu, B. Carbon balance under organic amendments in the wheat-maize cropping systems of sloppy upland soil. Sustainability 2020, 12, 2747. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, G.; Ansar, M.; Kaleem, S.; Nabi, G.; Hussain, M. Performance of early maturing oats (Avena sativa L.) cultivars for yield and quality. J. Agric. Res. 2008, 46, 341–346. [Google Scholar]
- Ahmad, R.; Shahzad, S.M.; Khalid, A.; Arshad, M.; Mahmood, M.H. Growth and yield response of wheat (Triticum aestivum L.) and maize (Zea mays L.) to nitrogen and L-tryptophan enriched compost. Pak. J. Bot. 2007, 39, 541. [Google Scholar]
- Gómez, C.; Green, D.R. Small unmanned airborne systems to support oil and gas pipeline monitoring and mapping. Arab. J. Geosci. 2017, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Gharbi, M.; Soualmia, A.; Dartus, D.; Masbernat, L. Comparison of 1D and 2D hydraulic models for floods simulation on the Medjerda Riverin Tunisia. J. Mater. Environ. Sci 2016, 7, 3017–3026. [Google Scholar]
- Alcívar, M.; Zurita-Silva, A.; Sandoval, M.; Muñoz, C.; Schoebitz, M. Reclamation of saline–sodic soils with combined amendments: Impact on quinoa performance and biological soil quality. Sustainability 2018, 10, 3083. [Google Scholar] [CrossRef] [Green Version]
- Crusciol, C.A.; Marques, R.R.; Carmeis Filho, A.C.; Soratto, R.P.; Costa, C.H.; Ferrari Neto, J.; Castro, G.S.; Pariz, C.M.; Castilhos, A.M.; Franzluebbers, A.J. Lime and gypsum combination improves crop and forage yields and estimated meat production and revenue in a variable charge tropical soil. Nutr. Cycl. Agroecosyst. 2019, 115, 347–372. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Pu, L.; Shen, H.; Wang, X.; Zhu, M.; Ge, Y.; Sun, L. Effects of soil reclamation on the oat cultivation in the newly reclaimed coastal land, eastern China. Ecol. Eng. 2019, 129, 115–122. [Google Scholar] [CrossRef]
- Abid, M.; Batool, T.; Siddique, G.; Ali, S.; Binyamin, R.; Shahid, M.J.; Rizwan, M.; Alsahli, A.A.; Alyemeni, M.N. Integrated nutrient management enhances soil quality and crop productivity in maize-based cropping system. Sustainability 2020, 12, 10214. [Google Scholar] [CrossRef]
- Racero, F.J.; Bueno, S.; Gallego, M.D. Predicting students’ behavioral intention to use open source software: A combined view of the technology acceptance model and self-determination theory. Appl. Sci. 2020, 10, 2711. [Google Scholar] [CrossRef] [Green Version]
- Azhar, M.; ur Rehman, M.Z.; Ali, S.; Qayyum, M.F.; Naeem, A.; Ayub, M.A.; ul Haq, M.A.; Iqbal, A.; Rizwan, M. Comparative effectiveness of different biochars and conventional organic materials on growth, photosynthesis and cadmium accumulation in cereals. Chemosphere 2019, 227, 72–81. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Papadakis, I.; Papaioannou, A.; Chatzissavvidis, C.; Giannakoula, A. Comparative study effects between manure application and a controlled-release fertilizer on the growth, nutrient uptake, photosystem II activity and photosynthetic rate of Olea europaea L. (cv.‘Koroneiki’). Sci. Hortic. 2020, 264, 109176. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Mohanty, M.; Sinha, N.K.; Somasundaram, J.; McDermid, S.S.; Patra, A.K.; Singh, M.; Dwivedi, A.; Reddy, K.S.; Rao, C.S.; Prabhakar, M. Soil carbon sequestration potential in a Vertisol in central India-results from a 43-year long-term experiment and APSIM modeling. Agric. Syst. 2020, 184, 102906. [Google Scholar] [CrossRef]
- Jarecki, M.K.; Lal, R.; James, R. Crop management effects on soil carbon sequestration on selected farmers’ fields in northeastern Ohio. Soil Tillage Res. 2005, 81, 265–276. [Google Scholar] [CrossRef]
- Li, Z.; Schneider, R.L.; Morreale, S.J.; Xie, Y.; Li, C.; Li, J. Woody organic amendments for retaining soil water, improving soil properties and enhancing plant growth in desertified soils of Ningxia, China. Geoderma 2018, 310, 143–152. [Google Scholar] [CrossRef]
- Yupeng, W.; Yufei, L.; Zhang, Y.; Yanmeng, B.; Zhenjun, S. Responses of saline soil properties and cotton growth to different organic amendments. Pedosphere 2018, 28, 521–529. [Google Scholar]
- Chatterjee, S.; Bandyopadhyay, K.; Pradhan, S.; Singh, R.; Datta, S. Effects of irrigation, crop residue mulch and nitrogen management in maize (Zea mays L.) on soil carbon pools in a sandy loam soil of Indo-gangetic plain region. Catena 2018, 165, 207–216. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, D.; Liu, N.; Yao, P.; Zhao, N.; Li, Y.; Zhang, S.; Zhai, B.; Huang, D.; Wang, Z. Dynamics and sequestration potential of soil organic carbon and total nitrogen stocks of leguminous green manure-based cropping systems on the Loess Plateau of China. Soil Tillage Res. 2019, 191, 108–116. [Google Scholar] [CrossRef]
- Banger, K.; Kukal, S.; Toor, G.; Sudhir, K.; Hanumanthraju, T. Impact of long-term additions of chemical fertilizers and farm yard manure on carbon and nitrogen sequestration under rice-cowpea cropping system in semi-arid tropics. Plant Soil 2009, 318, 27–35. [Google Scholar] [CrossRef]
- Kukal, S.; Benbi, D. Soil organic carbon sequestration in relation to organic and inorganic fertilization in rice–wheat and maize–wheat systems. Soil Tillage Res. 2009, 102, 87–92. [Google Scholar] [CrossRef]
- Ghosh, S.; Wilson, B.; Ghoshal, S.; Senapati, N.; Mandal, B. Organic amendments influence soil quality and carbon sequestration in the Indo-Gangetic plains of India. Agric. Ecosyst. Environ. 2012, 156, 134–141. [Google Scholar] [CrossRef]
- Maltas, A.; Kebli, H.; Oberholzer, H.R.; Weisskopf, P.; Sinaj, S. The effects of organic and mineral fertilizers on carbon sequestration, soil properties, and crop yields from a long-term field experiment under a Swiss conventional farming system. Land Degrad. Dev. 2018, 29, 926–938. [Google Scholar] [CrossRef]
- Alam, M.; Rahman, M.; Biswas, J.C.; Akhter, S.; Maniruzzaman, M.; Choudhury, A.K.; Jahan, M.; Miah, M.; Uddin, M.; Sen, R. Nitrogen transformation and carbon sequestration in wetland paddy field of Bangladesh. Paddy Water Environ. 2019, 17, 677–688. [Google Scholar] [CrossRef]
- Zhang, D.; Yao, P.; Zhao, N.; Cao, W.; Zhang, S.; Li, Y.; Huang, D.; Zhai, B.; Wang, Z.; Gao, Y. Building up the soil carbon pool via the cultivation of green manure crops in the Loess Plateau of China. Geoderma 2019, 337, 425–433. [Google Scholar] [CrossRef]
- Walia, M.K.; Dick, W.A. Selected soil physical properties and aggregate-associated carbon and nitrogen as influenced by gypsum, crop residue, and glucose. Geoderma 2018, 320, 67–73. [Google Scholar] [CrossRef]



| Parameters | Units | Values ± SE |
|---|---|---|
| pH | - | 9.5 ± 0.05 |
| EC | dS m−1 | 5.4 ± 0.14 |
| Texture | - | Sandy clay loam |
| SAR | (mmol L−1)1/2 | 42.5 ± 2.03 |
| Organic Matter | % | 0.3 ± 0.03 |
| Percent carbon | % | 0.2 ± 0.03 |
| CEC | cmolc kg−1 soil | 7.4 ± 3.05 |
| Bulk Density | g cm−3 | 1.6 ± 0.02 |
| Total Nitrogen | mg kg−1 | 0.03 ± 0.01 |
| MBC | mg kg−1 | 57.1 ± 0.04 |
| SR | mmol m−2 s−1 | 16.2 ± 0.05 |
| DHA | μg TPF g−1 h−1 | 161 ± 0.07 |
| Treatments | Parameters | Units | Responses |
|---|---|---|---|
| Control | pH | - | 9.3 ± 0.01 b–d |
| EC | dS m−1 | 5.3 ± 0.01 c | |
| SAR | (mmol L−1)1/2 | 35.8 ± 0.03 d | |
| CEC | cmolc kg−1 | 7.5 ± 0.02 gh | |
| Total Nitrogen | g kg−1 | 0.03 ± 0.00 g | |
| SOM | % | 0.3 ± 0.00 g | |
| MBC | mg kg−1 | 6.1 ± 0.9 g | |
| SR | mmol m−2 s−1 | 7.1 ± 0.3 g | |
| DHA | μg TPF g−1 h−1 | 255 ± 9.9 f | |
| G100 | pH | - | 8.9 ± 0.02 g |
| EC | dS m−1 | 4.8 ± 0.11 f | |
| SAR | (mmol L−1)1/2 | 16.8 ± 0.6 d | |
| CEC | cmolc kg−1 | 7.6 ± 0.04 c | |
| Total Nitrogen | g kg−1 | 0.05 ± 0.05 e | |
| SOM | % | 0.7 ± 0.02 b | |
| MBC | mg kg−1 | 11.1 ± 0.3 a–c | |
| SR | mmol m−2 s−1 | 11.3 ± 0.3 cd | |
| DHA | μg TPF g−1 h−1 | 342 ± 19.3 e | |
| FYM + G50 | pH | - | 8.7 ± 0.02 h |
| EC | dS m−1 | 4.8 ± 0.02 fg | |
| SAR | (mmol L−1)1/2 | 16 ± 1.4 g | |
| CEC | cmolc kg−1 | 7.9 ± 0.02 c | |
| Total Nitrogen | g kg−1 | 0.07 ± 0.01 c | |
| SOM | % | 0.7 ± 0.02 b | |
| MBC | mg kg−1 | 12.3 ± 0.3 a | |
| SR | mmol m−2 s−1 | 11 ± 1 b–d | |
| DHA | μg TPF g−1 h−1 | 419 ± 3.66 bc | |
| PM + G50 | pH | - | 8.7 ± 0.04 h |
| EC | dS m−1 | 4.8 ± 0.03 f | |
| SAR | (mmol L−1)1/2 | 17.8 ± 0.6 g | |
| CEC | cmolc kg−1 | 8.2 ± 0.03 a | |
| Total Nitrogen | g kg−1 | 0.08 ± 0.01 a | |
| SOM | % | 0.7 ± 0.01 a | |
| MBC | mg kg−1 | 12.3 ± 0.2 ab | |
| SR | mmol m−2 s−1 | 121.1 ± 0.3 ab | |
| DHA | μg TPF g−1 h−1 | 436 ± 20 b | |
| GM + G50 | pH | - | 8.7 ± 0.01 h |
| EC | dS m−1 | 4.7 ± 0.01 g | |
| SAR | (mmol L−1)1/2 | 16.2 ± 0.7 g | |
| CEC | cmolc kg−1 | 8.1 ± 0.03 b | |
| Total Nitrogen | g kg−1 | 0.06 ± 0.02 bc | |
| SOM | % | 0.7 ± 0.01 ab | |
| MBC | mg kg−1 | 13.1 ± 0.3 a | |
| SR | mmol m−2 s−1 | 13.3 ± 0.7 a | |
| DHA | μg TPF g−1 h−1 | 507 ± 10 a |
| Parameters | Units | Control | G100 | FYM + G50 | PM + G50 | GM + G50 |
|---|---|---|---|---|---|---|
| pH | - | 9.1 ± 0.1 a | 8.5 ± 0.2 b | 8.2 ± 0.3 ab | 8.3 ± 0.3 ab | 8.4 ± 0.5 ab |
| EC | dS m−1 | 5 ± 0.4 a | 4 ± 0.5 b | 4 ± 0.9 b | 4 ± 0.6 b | 4 ± 0.6 b |
| SAR | - | 18 ± 4 a | 12 ± 2 a | 12 ± 1 a | 12 ± 2 a | 12 ± 2 a |
| OM | % | 0.4 ± 0.1 c | 0.7 ± 0.3 b | 1 ± 0.1 a | 1 ± 0.2 a | 1 ± 0.2 a |
| CEC | cmolc kg−1 soil | 7.7 ± 0.5 c | 8.3 ± 0.1 b | 8.5 ± 0.5 a | 8.7 ± 0.6 a | 8.7 ± 4 a |
| TN | g kg−1 | 0.04 ± 0.1 c | 0.09 ± 0.04 ab | 0.1 ± 0.5 b | 0.2 ± 0.5 a | 0.1 ± 0.07 b |
| MBC | mg kg−1 | 65.1 ± 2 c | 90.2 ± 4 b | 217.3 ± 3 a | 206.1 ± 4 a | 208.3 ± 5 a |
| SR | mmol m−2 s−1 | 21.2 ± 4 c | 27.1 ± 4 b | 25.1 ± 3 ab | 37.1 ± 2 a | 27.4 ± 4 b |
| DHA | μg TPF g−1 h−1 | 330 ± 11 d | 400 ± 21 cd | 571 ± 20 c | 689 ± 6 a | 620 ± 10 b |
| Wheat | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | G100 | FYM + G50 | PM + G50 | GM + G50 | ||||||||||||||||
| Exp. 1 | Earn. 2 | Profit 3 | BCR 4 | Exp. | Earn. | Profit | BCR | Exp. | Earn. | Profit | BCR | Exp. | Earn. | Profit | BCR | Exp. | Earn. | Profit | BCR | |
| Crop-1 | 219.02 | 347.24 | 128.21 | 1.59 | 352.73 | 485.31 | 132.57 | 1.38 | 301.91 | 535.50 | 233.59 | 1.77 | 315.36 | 497.85 | 182.50 | 1.58 | 296.56 | 485.30 | 188.74 | 1.64 |
| Crop-2 | 225.90 | 455.80 | 229.90 | 2.02 | 362.51 | 638.11 | 275.61 | 1.76 | 319.56 | 688.76 | 369.20 | 2.16 | 320.53 | 663.44 | 342.91 | 2.07 | 303.55 | 643.18 | 339.63 | 2.12 |
| Crop-3 | 230.49 | 502.04 | 271.54 | 2.18 | 371.79 | 713.42 | 341.63 | 1.92 | 327.89 | 760.98 | 433.10 | 2.32 | 333.23 | 729.28 | 396.05 | 2.19 | 317.23 | 708.13 | 390.91 | 2.23 |
| Maize | ||||||||||||||||||||
| Control | G100 | FYM + G50 | PM + G50 | GM + G50 | ||||||||||||||||
| Exp. | Earn. | Profit | BCR | Exp. | Earn. | Profit | BCR | Exp. | Earn. | Profit | BCR | Exp. | Earn. | Profit | BCR | Exp. | Earn. | Profit | BCR | |
| Crop-1 | 230.43 | 324.58 | 94.15 | 1.41 | 364.14 | 430.49 | 66.35 | 1.18 | 313.32 | 443.24 | 129.91 | 1.41 | 326.76 | 436.37 | 109.61 | 1.34 | 307.93 | 458.93 | 150.99 | 1.49 |
| Crop-2 | 232.26 | 376.55 | 144.30 | 1.62 | 371.54 | 478.18 | 106.64 | 1.29 | 328.59 | 496.37 | 167.78 | 1.51 | 328.67 | 470.69 | 142.03 | 1.43 | 312.52 | 525.25 | 212.73 | 1.68 |
| Crop-3 | 242.26 | 403.39 | 161.12 | 1.67 | 383.47 | 538.22 | 154.75 | 1.40 | 339.57 | 546.02 | 206.45 | 1.61 | 344.94 | 540.45 | 195.51 | 1.57 | 328.87 | 600.62 | 271.76 | 1.83 |
| Parameter | Farmyard Manure | Poultry Manure | Green Manure |
|---|---|---|---|
| Percent carbon | 11% | 23% | 10% |
| Percent dry matter | 100% | 100% | 100% |
| DPM:HUM ratio | 1.00 | 31.45 | 31.45 |
| Site and Treatment | Uncertainty RMSE (%) | Correlation (R2) | Comment |
|---|---|---|---|
| Dijkot | |||
| FYM + G50 | 3.4% | 0.9989 | Good fit using DPM:HUM ratio = 1 [47]. Suggests FYM is stabilized in the gut of the cow. |
| PM + G50 | 6.3% | 0.9983 | Good fit using DPM:HUM ratio = 31.45 [47]. Suggests PM is less stabilized than FYM. |
| GM + G50 | 6.8% | 0.997 | Good fit using DPM:HUM ratio = 31.45 [47]. Suggests GM is less stabilized than FYM (similar to PM). |
| Uchkera | |||
| FYM + G50 | 7.2% | 0.9972 | Similar result to Dijkot. |
| PM + G50 | 10.7% | 0.9963 | Similar result to Dijkot. |
| GM + G50 | 5.5% | 0.9985 | Similar result to Dijkot. |
| Conditions and Treatment | Uncertainty RMSE (%) | Correlation (R2) |
|---|---|---|
| Pot | ||
| G100 | 3.8% | 0.9989 |
| FYM + G50 | 8.3% | 0.9993 |
| PM + G50 | 9.3% | 0.9994 |
| GM + G50 | 6.0% | 0.9965 |
| Field | ||
| G100 | 4.8% | 0.9998 |
| FYM + G50 | 5.8% | 0.9994 |
| PM + G50 | 4.6% | 0.9995 |
| GM + G50 | 6.2% | 0.9967 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooqi, Z.U.R.; Sabir, M.; Ahmad, H.R.; Shahbaz, M.; Smith, J. Reclaimed Salt-Affected Soils Can Effectively Contribute to Carbon Sequestration and Food Grain Production: Evidence from Pakistan. Appl. Sci. 2023, 13, 1436. https://doi.org/10.3390/app13031436
Farooqi ZUR, Sabir M, Ahmad HR, Shahbaz M, Smith J. Reclaimed Salt-Affected Soils Can Effectively Contribute to Carbon Sequestration and Food Grain Production: Evidence from Pakistan. Applied Sciences. 2023; 13(3):1436. https://doi.org/10.3390/app13031436
Chicago/Turabian StyleFarooqi, Zia Ur Rahman, Muhammad Sabir, Hamaad Raza Ahmad, Muhammad Shahbaz, and Jo Smith. 2023. "Reclaimed Salt-Affected Soils Can Effectively Contribute to Carbon Sequestration and Food Grain Production: Evidence from Pakistan" Applied Sciences 13, no. 3: 1436. https://doi.org/10.3390/app13031436
APA StyleFarooqi, Z. U. R., Sabir, M., Ahmad, H. R., Shahbaz, M., & Smith, J. (2023). Reclaimed Salt-Affected Soils Can Effectively Contribute to Carbon Sequestration and Food Grain Production: Evidence from Pakistan. Applied Sciences, 13(3), 1436. https://doi.org/10.3390/app13031436







