Assessment of Phenolic Content, Antioxidant and Anti-Aging Activities of Honey from Pittosporum undulatum Vent. Naturalized in the Azores Archipelago (Portugal)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sampling
2.3. Sample Preparation
2.4. Pollen Analysis
2.5. Determination of HMF of Honey by High-Performance Liquid Chromatography—Ultraviolet Detector (HPLC-UV)
2.6. Phenolic Content of Honey
2.7. Antioxidant Capacity of Honey
2.8. Anti-Aging Capacity of Honey
2.9. Statistical Analysis
3. Results and Discussion
3.1. Pollen Analysis
3.2. HMF Content
3.3. Phenolic Content of Honey
3.4. Antioxidant Capacity of Honey
3.5. Anti-Aging Capacity of Honey
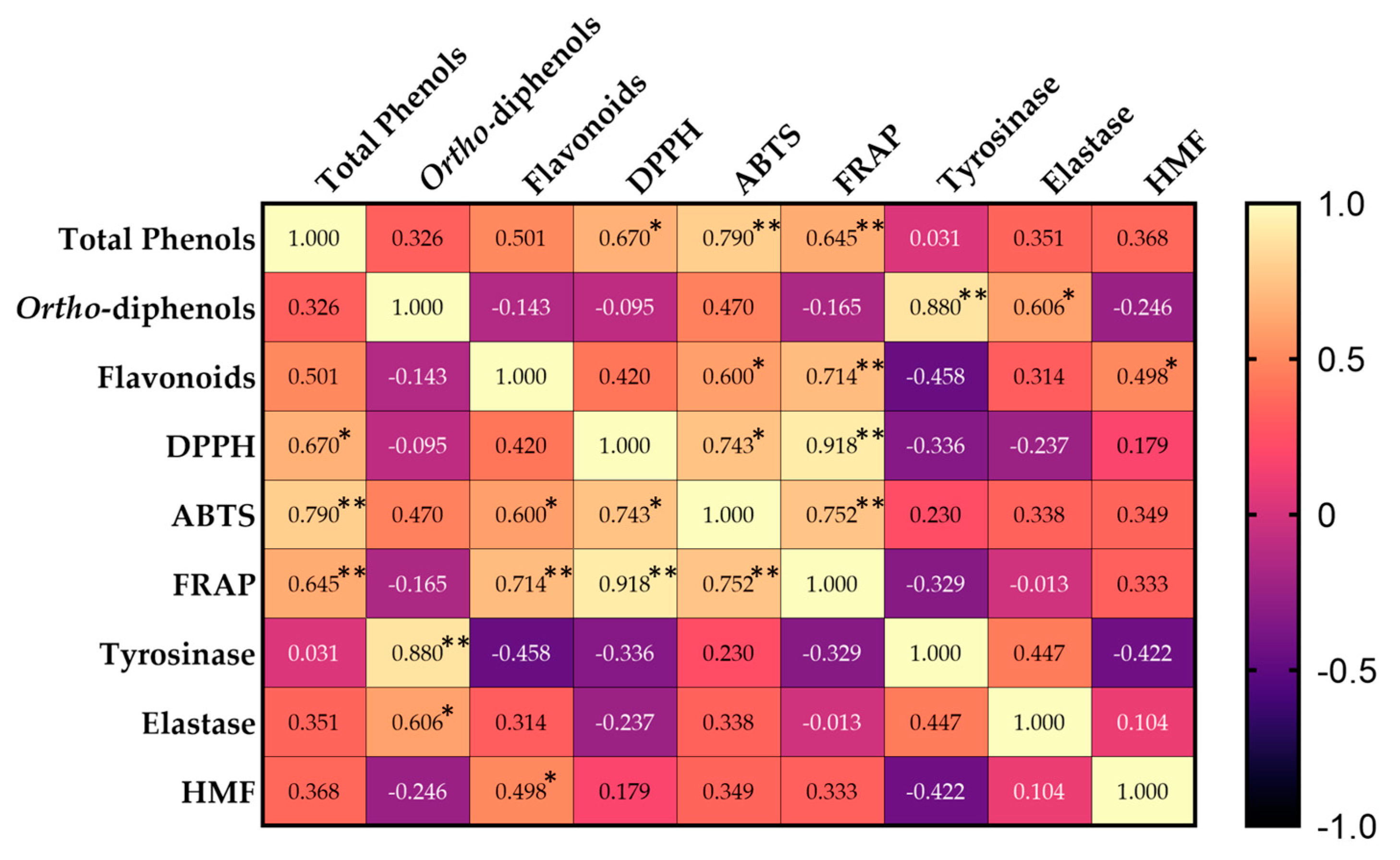
3.6. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pittosporum Undulatum | Flora-On. Available online: https://flora-on.pt/?q=Pittosporum+undulatum (accessed on 23 November 2022).
- Medeiros, J.R.; Campos, L.B.; Mendonça, S.C.; Davin, L.B.; Lewis, N.G. Composition and Antimicrobial Activity of the Essential Oils from Invasive Species of the Azores, Hedychium Gardnerianum and Pittosporum Undulatum. Phytochemistry 2003, 64, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, P.; Medeiros, V.; Gil, A.; Silva, L. Distribution, Habitat and Biomass of Pittosporum Undulatum, the Most Important Woody Plant Invader in the Azores Archipelago. For. Ecol. Manag. 2011, 262, 178–187. [Google Scholar] [CrossRef]
- Ferreira, N.; de Sousa, I.G.M.; Luis, T.C.; Currais, A.J.M.; Figueiredo, A.C.; Costa, M.M.; Lima, A.S.B.; Santos, P.A.G.; Barroso, J.G.; Pedro, L.G.; et al. Pittosporum Undulatum Vent. Grown in Portugal: Secretory Structures, Seasonal Variation and Enantiomeric Composition of Its Essential Oil. Flavour Fragr. J. 2007, 22, 1–9. [Google Scholar] [CrossRef]
- Mendes, M.D.; Lima, A.S.; Trindade, H.; Correia, A.I.D.; Barroso, J.G.; Pedro, L.G.; Figueiredo, A.C. ISSR Molecular Characterization and Leaf Volatiles Analysis of Pittosporum Undulatum Vent. Naturalized in the Azores Archipelago (Portugal). Ind. Crops Prod. 2011, 33, 710–719. [Google Scholar] [CrossRef]
- SIARAM:: Incenso. Available online: http://siaram.azores.gov.pt/flora/infestantes/incenso/2.html (accessed on 25 January 2023).
- De Medeiros, J.M.R.; Macedo, M.; Contancia, J.P.; Nguyen, C.; Cunningham, G.; Miles, D.H. Antithrombin Activity of Medicinal Plants of the Azores. J. Ethnopharmacol. 2000, 72, 157–165. [Google Scholar] [CrossRef]
- MEL DOS AÇORES-DOP-MEL DOS AÇORES-DOP-Instituto de Alimentação e Mercados Agrícolas-Portal. Available online: https://portal.azores.gov.pt/web/iama/-/mel-dos-acores-dop (accessed on 15 December 2022).
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for Nutrition and Health: A Review. J. Apic. Res. 2013, 27, 677–689. [Google Scholar] [CrossRef]
- Boussaid, A.; Chouaibi, M.; Rezig, L.; Hellal, R.; Donsì, F.; Ferrari, G.; Hamdi, S. Physicochemical and Bioactive Properties of Six Honey Samples from Various Floral Origins from Tunisia. Arab. J. Chem. 2018, 11, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Erban, T.; Shcherbachenko, E.; Talacko, P.; Harant, K. The Unique Protein Composition of Honey Revealed by Comprehensive Proteomic Analysis: Allergens, Venom-like Proteins, Antibacterial Properties, Royal Jelly Proteins, Serine Proteases, and Their Inhibitors. J. Nat. Prod. 2019, 82, 1217–1226. [Google Scholar] [CrossRef]
- Sakač, M.B.; Jovanov, P.T.; Marić, A.Z.; Pezo, L.L.; Kevrešan, Ž.S.; Novaković, A.R.; Nedeljković, N.M. Physicochemical Properties and Mineral Content of Honey Samples from Vojvodina (Republic of Serbia). Food Chem. 2019, 276, 15–21. [Google Scholar] [CrossRef]
- Bobis, O.; Moise, A.R.; Ballesteros, I.; Reyes, E.S.; Durán, S.S.; Sánchez-Sánchez, J.; Cruz-Quintana, S.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Eucalyptus Honey: Quality Parameters, Chemical Composition and Health-Promoting Properties. Food Chem. 2020, 325, 126870. [Google Scholar] [CrossRef]
- Pauliuc, D.; Ciursă, P.; Ropciuc, S.; Dranca, F.; Oroian, M. Physicochemical Parameters Prediction and Authentication of Different Monofloral Honeys Based on FTIR Spectra. J. Food Compos. Anal. 2021, 102, 104021. [Google Scholar] [CrossRef]
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic Acids and Flavonoids Profiles of Commercial Honey from Different Floral Sources and Geographic Sources. Int. J. Food Prop. 2019, 22, 290–308. [Google Scholar] [CrossRef] [Green Version]
- Gouvinhas, I.; Queiroz, M.; Rodrigues, M.; Barros, A.I.R.N.A. Evaluation of the Phytochemistry and Biological Activity of Grape (Vitis Vinifera L.) Stems: Toward a Sustainable Winery Industry. In Polyphenols in Plants; Academic Press: Cambridge, MA, USA, 2019; pp. 381–394. [Google Scholar]
- Machado, N.F.L.; Domínguez-Perles, R. Addressing Facts and Gaps in the Phenolics Chemistry of Winery By-Products. molecules 2017, 48, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [Green Version]
- Leal, C.; Gouvinhas, I.; Santos, R.A.; Rosa, E.; Silva, A.M.; José, M.; Barros, A.I.R.N.A. Industrial Crops & Products Potential Application of Grape (Vitis Vinifera L.) Stem Extracts in the Cosmetic and Pharmaceutical Industries: Valorization of a by-Product. Ind. Crop. Prod. 2020, 154, 112675. [Google Scholar] [CrossRef]
- Apraj, V.D.; Pandita, N.S. Evaluation of Skin Anti-Aging Potential of Citrus Reticulata Blanco Peel. Pharmacogn. Res. 2016, 8, 160. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, F.; Cádiz-Gurrea, M.D.L.L.; Nunes, M.A.; Pinto, D.; Vinha, A.F.; Linares, I.B.; Oliveira, M.B.P.P.; Carretero, A.S. Cosmetics. In Polyphenols: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 393–427. ISBN 9780128135723. [Google Scholar]
- Habib, H.M.; Kheadr, E.; Ibrahim, W.H. Inhibitory Effects of Honey from Arid Land on Some Enzymes and Protein Damage. Food Chem. 2021, 364, 130415. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Santos-Buelga, C.; Era, B.; Maria González-Paramás, A.; Tuberoso, C.I.G.; Medda, R.; Pintus, F.; Fais, A. Sardinian Honeys as Sources of Xanthine Oxidase and Tyrosinase Inhibitors. Food Sci. Biotechnol. 2017, 27, 139–146. [Google Scholar] [CrossRef]
- Karaçelik, A.A.; Sahin, H. Determination of Enzyme Inhibition and Antioxidant Activity in Some Chestnut Honeys. Foods Raw Mater. 2018, 6, 210–218. [Google Scholar] [CrossRef]
- Ersoy, E.; Eroglu, E.; Boga, M.; Abdullah, M. Industrial Crops & Products Anti-Aging Potential and Anti-Tyrosinase Activity of Three Hypericum Species with Focus on Phytochemical Composition by LC–MS/MS. Ind. Crops Prod. 2019, 141, 111735. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Liceaga, A.M. Potential Role of Natural Bioactive Peptides for Development of Cosmeceutical Skin Products. Peptides 2019, 122, 170170. [Google Scholar] [CrossRef]
- Aumeeruddy, M.Z.; Aumeeruddy-Elalfi, Z.; Neetoo, H.; Zengin, G.; Blom van Staden, A.; Fibrich, B.; Lambrechts, I.A.; Rademan, S.; Szuman, K.M.; Lall, N.; et al. Pharmacological Activities, Chemical Profile, and Physicochemical Properties of Raw and Commercial Honey. Biocatal. Agric. Biotechnol. 2019, 18, 101005. [Google Scholar] [CrossRef]
- Alimentarius, C. Revised Codex Standard for Honey. Codex Stan 2001, 12, 1982. [Google Scholar]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical Composition, Stability and Authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.; Barros, A.I.R.N.A.; Nunes, F.M. A Novel, Direct, Reagent-Free Method for the Detection of Beeswax Adulteration by Single-Reflection Attenuated Total Reflectance Mid-Infrared Spectroscopy. Talanta 2013, 107, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Küçük, M.; Kolayli, S.; Karaoǧlu, Ş.; Ulusoy, E.; Baltaci, C.; Candan, F. Biological Activities and Chemical Composition of Three Honeys of Different Types from Anatolia. Food Chem. 2007, 100, 526–534. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Maia, M.; Karabournioti, S.; Gatzias, I.; Karabagias, V.K.; Badeka, A.V. Palynological, Physicochemical, Biochemical and Aroma Fingerprints of Two Rare Honey Types. Eur. Food Res. Technol. 2020, 246, 1725–1739. [Google Scholar] [CrossRef]
- Yu, M.; Gouvinhas, I. Variation of the Polyphenolic Composition and Antioxidant Capacity of Freshly Prepared Pomegranate Leaf Infusions over One-Day Storage. Antioxidants 2021, 10. [Google Scholar] [CrossRef]
- Breda, C.; Barros, A.I.; Gouvinhas, I. Characterization of Bioactive Compounds and Antioxidant Capacity of Portuguese Craft Beers. Int. J. Gastron. Food Sci. 2022, 27, 100473. [Google Scholar] [CrossRef]
- Shim, K.B.; Yoon, N.Y. Inhibitory Effect of Fucofuroeckol-A from Eisenia Bicyclis on Tyrosinase Activity and Melanin Biosynthesis in Murine Melanoma B16F10 Cells. Fish. Aquat. Sci. 2018, 21, 35. [Google Scholar] [CrossRef]
- Feás, X.; Pires, J.; Iglesias, A.; Estevinho, M.L. Characterization of Artisanal Honey Produced on the Northwest of Portugal by Melissopalynological and Physico-Chemical Data. Food Chem. Toxicol. 2010, 48, 3462–3470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Önür, İ.; Misra, N.N.; Barba, F.J.; Putnik, P.; Lorenzo, J.M.; Gökmen, V.; Alpas, H. Effects of Ultrasound and High Pressure on Physicochemical Properties and HMF Formation in Turkish Honey Types. J. Food Eng. 2018, 219, 129–136. [Google Scholar] [CrossRef]
- Lee, C.H.; Chen, K.T.; Lin, J.A.; Chen, Y.T.; Chen, Y.A.; Wu, J.T.; Hsieh, C.W. Recent Advances in Processing Technology to Reduce 5-Hydroxymethylfurfural in Foods. Trends Food Sci. Technol. 2019, 93, 271–280. [Google Scholar] [CrossRef]
- Castiglioni, S.; Stefano, M.; Astolfi, P.; Carloni, P. Chemometric Approach to the Analysis of Antioxidant Properties and Colour of Typical Italian Monofloral Honeys. Int. J. Food Sci. Technol. 2017, 52, 1138–1146. [Google Scholar] [CrossRef]
- Machado De-Melo, A.A.; de Almeida-Muradian, L.B.; Sancho, M.T.; Pascual-Maté, A. Composition and Properties of Apis Mellifera Honey: A Review. J. Apic. Res. 2017, 57, 5–37. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Schulz, M.; Brugnerotto, P.; Silva, B.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Quality, Composition and Health-Protective Properties of Citrus Honey: A Review. Food Res. Int. 2021, 143, 110268. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant Activity of Portuguese Honey Samples: Different Contributions of the Entire Honey and Phenolic Extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Alves, A.; Ramos, A.; Gonçalves, M.M.; Bernardo, M.; Mendes, B. Antioxidant Activity, Quality Parameters and Mineral Content of Portuguese Monofloral Honeys. J. Food Compos. Anal. 2013, 30, 130–138. [Google Scholar] [CrossRef]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant Activity, Total Phenolic Content, Individual Phenolics and Physicochemical Parameters Suitability for Romanian Honey Authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- María Muñoz Jáuregui, A.; Alvarado-Ortíz Ureta, C.; Blanco Blasco, T.; Castañeda Castañeda, B.; Ruiz Quiroz, J.; Alvarado Yarasca, Á.; Alvarado-Ortiz Ureta, C. Determinación de Compuestos Fenólicos, Flavonoides Totales y Capacidad Antioxidante En Mieles Peruanas de Diferentes Fuentes Florales. Rev. la Soc. Química del Perú 2014, 80, 287–297. [Google Scholar] [CrossRef]
- Ghorab, A.; Rodríguez-Flores, M.S.; Nakib, R.; Escuredo, O.; Haderbache, L.; Bekdouche, F.; Seijo, M.C. Sensorial, Melissopalynological and Physico-Chemical Characteristics of Honey from Babors Kabylia’s Region (Algeria). Foods 2021, 10, 225. [Google Scholar] [CrossRef]
- Kivima, E.; Tanilas, K.; Martverk, K.; Rosenvald, S.; Timberg, L.; Laos, K. The Composition, Physicochemical Properties, Antioxidant Activity, and Sensory Properties of Estonian Honeys. Foods 2021, 10, 511. [Google Scholar] [CrossRef]
- Gonçalves, J.; Ribeiro, I.; Marçalo, J.; Rijo, P.; Faustino, C.; Pinheiro, L. Physicochemical, Antioxidant and Antimicrobial Properties of Selected Portuguese Commercial Monofloral Honeys. J. Food Nutr. Res. 2018, 6, 645–654. [Google Scholar] [CrossRef] [Green Version]
- Jerković, I.; Radonić, A.; Kranjac, M.; Zekić, M.; Marijanović, Z.; Gudić, S.; Kliškić, M. Red Clover (Trifolium Pratense L.) Honey: Volatiles Chemical-Profiling and Unlocking Antioxidant and Anticorrosion Capacity. Chem. Pap. 2016, 70, i. [Google Scholar] [CrossRef]
- Osés, S.M.; Cantero, L.; Puertas, G.; Fernández-Muiño, M.Á.; Sancho, M.T. Antioxidant, Antimicrobial and Anti-Inflammatory Activities of Ling-Heather Honey Powder Obtained by Different Methods with Several Carriers. LWT 2022, 159, 113235. [Google Scholar] [CrossRef]
- Sousa, C.; Gouvinhas, I.; Barreira, D.; Carvalho, M.T.; Vilela, A.; Lopes, J.; Martins-Lopes, P.; Barros, A.I. ‘Cobrançosa’ Olive Oil and Drupe: Chemical Composition at Two Ripening Stages. J. Am. Oil Chem. Soc. 2014, 91, 599–611. [Google Scholar] [CrossRef] [Green Version]
- Jantakee, K.; Tragoolpua, Y. Activities of Different Types of Thai Honey on Pathogenic Bacteria Causing Skin Diseases, Tyrosinase Enzyme and Generating Free Radicals. Biol. Res. 2015, 48, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.M.; Garcia, J.; Dall’Acqua, S.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Eco-Friendly Insights on Kiwiberry Leaves Valorization through in-Vitro and in-Vivo Studies. Ind. Crops Prod. 2022, 184, 115090. [Google Scholar] [CrossRef]
- Bahadır Acıkara, Ö.; Ilhan, M.; Kurtul, E.; Šmejkal, K.; Küpeli Akkol, E. Inhibitory Activity of Podospermum Canum and Its Active Components on Collagenase, Elastase and Hyaluronidase Enzymes. Bioorg. Chem. 2019, 93, 103330. [Google Scholar] [CrossRef]
- Oulebsir, C.; Mefti-Korteby, H.; Djazouli, Z.-E.; Zebib, B.; Merah, O. Essential Oil of Citrus Aurantium L. Leaves: Composition, Antioxidant Activity, Elastase and Collagenase Inhibition. Agronomy 2022, 12, 1466. [Google Scholar] [CrossRef]
- Olivares-Tenorio, M.L.; Verkerk, R.; van Boekel, M.A.J.S.; Dekker, M. Thermal Stability of Phytochemicals, HMF and Antioxidant Activity in Cape Gooseberry (Physalis Peruviana L.). J. Funct. Foods 2017, 32, 46–57. [Google Scholar] [CrossRef]

| Samples | Predominant Pollen (>30%) | Secondary Pollen (16–30%) | Minority Pollen (3–15%) | |||
|---|---|---|---|---|---|---|
| Species | Percentage (%) | Species | Percentage (%) | Species | Percentage (%) | |
| 1 | Pittosporum undulatum Vent. | 51 | - | - | Eucalyptus spp. | 12 |
| Trifolium spp. | 8 | |||||
| Castanea sativa Mill. | 7 | |||||
| Acacia spp. | 5 | |||||
| Scrophularia spp. | 5 | |||||
| Lathyrus spp. | 3 | |||||
| 2 | Pittosporum undulatum Vent. | 51 | Castanea sativa Mill. | 19 | Acacia spp. | 9 |
| Eucalyptus globulus Labill. | 7 | |||||
| Ranunculus spp. | 3 | |||||
| 3 | Pittosporum undulatum Vent. | 48 | Castanea sativa Mill. | 21 | Acacia spp. | 14 |
| Eucalyptus globulus Labill. | 9 | |||||
| Ranunculus spp. | 3 | |||||
| 4 | Pittosporum undulatum Vent. | 65 | Eucalyptus spp. | 17 | Raphanus raphanistrum L. | 5 |
| Acacia spp. | 3 | |||||
| 5 | Pittosporum undulatum Vent. | 50 | Castanea sativa Mill. | 24 | Trifolium spp. | 8 |
| Acacia spp. | 7 | |||||
| Eucalyptus globulus Labill. | 5 | |||||
| 6 | Pittosporum undulatum Vent. | 69 | - | - | Acacia spp. | 11 |
| Eucalyptus globulus Labill. | 10 | |||||
| Castanea sativa Mill. | 3 | |||||
| Samples | 1 | 2 | 3 | 4 | 5 | 6 | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Quality parameter | HMF (mg/kg) | 13.94 ± 0.11 e | 10.67 ± 0.04 c | 9.78 ± 0.02 b | 15.37 ± 0.02 f | 12.72 ± 0.04 d | 5.20 ± 0.041 | *** |
| Phenolic content | Total phenols (mg GA/100 g) | 30.31 ± 0.42 c | 26.01 ± 0.87 bc | 23.28 ± 1.37 ab | 21.37 ± 1.35 a | 25.87 ± 0.00 b | 20.83 ± 1.42 a | *** |
| Ortho-diphenols (mg GA/100 g) | 28.47 ± 0.59 e | 22.82 ± 0.24 b | 24.91 ± 0.35 c | 21.25 ± 0.12 a | 26.96 ± 0.00 d | 27.29 ± 0.47 d | *** | |
| Flavonoids (mg CAT/100 g) | 7.67 ± 0.84 d | 6.78 ± 0.00 bc | 7.67 ± 0.84 bc | 7.23 ± 0.42 bc | 5.88 ± 0.35 ab | 4.41 ± 0.35 a | ** |
| Samples | 1 | 2 | 3 | 4 | 5 | 6 | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Antioxidant capacity | DPPH (mmol T/100 g) | 0.362 ± 0.007 b | 0.447 ± 0.022 c | 0.039 ± 0.007 a | 0.045 ± 0.004 a | 0.050 ± 0.010 a | 0.098 ± 0.003 a | *** |
| ABTS (mmol T/100 g) | 0.346 ± 0.020 c | 0.203 ± 0.016 b | 0.092 ± 0.001 a | 0.079 ± 0.002 a | 0.102 ± 0.002 a | 0.129 ± 0.005 a | *** | |
| FRAP (mmol T/100 g) | 0.566 ± 0.010 d | 0.612 ± 0.010 c | 0.335 ± 0.013 b | 0.321 ± 0.003 b | 0.184 ± 0.005 a | 0.178 ± 0.003 a | *** | |
| Anti-aging capacity | Tyrosinase inhibition (%) | 9.37 ± 0.20 bc | 4.36 ± 0.28 a | 6.34 ± 0.59 a | 4.65 ± 0.95 a | 6.89 ± 0.06 ab | 9.87 ± 0.70 b | *** |
| Elastase inhibition (%) | 45.88 ± 0.65 b | 37.59 ± 2.16 a | 41.28 ± 0.33 ab | 38.70 ± 2.85 ab | 42.72 ± 2.84 ab | 39.85 ± 1.20 ab | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, S.; Maia, M.; Barros, A.; Gouvinhas, I. Assessment of Phenolic Content, Antioxidant and Anti-Aging Activities of Honey from Pittosporum undulatum Vent. Naturalized in the Azores Archipelago (Portugal). Appl. Sci. 2023, 13, 1788. https://doi.org/10.3390/app13031788
Santos S, Maia M, Barros A, Gouvinhas I. Assessment of Phenolic Content, Antioxidant and Anti-Aging Activities of Honey from Pittosporum undulatum Vent. Naturalized in the Azores Archipelago (Portugal). Applied Sciences. 2023; 13(3):1788. https://doi.org/10.3390/app13031788
Chicago/Turabian StyleSantos, Soraia, Miguel Maia, Ana Barros, and Irene Gouvinhas. 2023. "Assessment of Phenolic Content, Antioxidant and Anti-Aging Activities of Honey from Pittosporum undulatum Vent. Naturalized in the Azores Archipelago (Portugal)" Applied Sciences 13, no. 3: 1788. https://doi.org/10.3390/app13031788








