Featured Application
The results obtained in this work may be of scientific and practical significance for the development of new biocatalytic approaches to the treatment of wastewater of complex composition, contaminated by perfluorocarbons and organophosphorus compounds, with simultaneous production of biomass of phototrophic microorganisms as renewable raw materials with a wide range of possibilities for conversion into valuable products. The results can also be taken into account for predicting changes in aquatic ecosystems when the mentioned pollutants enter them.
Abstract
The effects of the presence of perfluorocarbons (PFC) with a gas transport function in media with different phototrophic microorganisms on their growth rates and the accumulation of their biomass when using free and immobilized cells as inoculums were investigated. The significant increase in the average rate of biomass accumulation as well as levels of biomass accumulation in the presence of various PFCs were established for Chlorella vulgaris cells. When 1 g/L glycerol was introduced into the growth medium with PFCs and C. vulgaris cells, the increase in the rate of biomass accumulation was 9–32%. The maximum intracellular ATP concentrations corresponded to the combination of microalgae (Chlorella vulgaris) with bacterial cells (Pseudomonas esterophilus and Rhodoccus ruber) obtained with a mass ratio of 25:1. It provided for the formation of a consortium, which was able to accumulate the maximum amount of microalgae biomass for 3 days in the medium with PFCs and organophosphorus pesticide. The obtained data allow, on the one hand, predicting the growth of microalgae under environmental conditions in media with PFC pollution and, on the other hand, developing approaches to regulation of phototrophic microorganisms’ growth in order to obtain and use their high biomass yields for various purposes.
1. Introduction
The biomass of phototrophic microorganisms continues to be actively considered as a renewable resource that can be effectively used to produce various commercially significant products [1,2,3]. Unlike lignocellulose-containing waste, microalgae and cyanobacteria biomass does not require delignification before its deep conversion into biofuels [3,4,5,6], organic acids [7], compounds useful for the chemical synthesis of biodegradable polymers, commercially significant polysaccharides [8,9], etc. Commercial production of microalgae biomass is often limited by its relatively low cell productivity per unit volume in comparison with the growth rates of bacterial cells used in biotechnological processes [10].
To increase the economic profitability of the processes of obtaining and processing biomass of phototrophic microorganisms, they can be cultivated using wastewaters with different contents. The cell biomass cultivation can be combined with wastewater treatment and purification [3,11,12,13]. The prospects of using microalgae for the treatment of wastewater of different compositions are due to the flexibility of their metabolism, which allows these cells to use inorganic and organic carbon sources, as well as their mixture. This allows the use of microalgae in the treatment of municipal, agricultural and industrial wastewater in various contexts, including tannery, textiles, paper, pharmaceuticals, food (coming from the production of tofu, dairy products, confectionery and alcoholic beverages, etc.), pig farms, etc. [14,15,16,17].
Among the wastewaters to be treated, those that contain organophosphorus compounds (OPCs) are often found in agriculture and chemical industries related to the production of pesticides. Usually, undecomposed residues of OPCs enter water systems and drains [18]. Perfluorocarbons (PFCs) are also actively used in industry, for example, in the production of cookware with non-stick coating, in water-repellent coatings in clothing and packaging, in fire-fighting equipment as well as in lubricants with a wide range of applications [19]. Due to their chemical and biological inertia, PFC enter the environment with waste and wastewater, where they can stay for a long time and gradually accumulate [20,21]. In contrast to OPCs, the presence of PFCs in river ecosystems is a relatively new problem for the environment [17], as it has been established that only some mycelial fungi and bacteria (mainly of the genus Pseudomonas) are able to decompose PFCs [19,22]. To date, the simultaneous presence of OPCs and PFCs has been determined in municipal wastewater [23,24,25], while their cumulative toxic effect on the human body has been noted [26].
Microalgae are common participants in natural consortia in various aquatic environments [27]. Therefore, when studying the possible influence of xenobiotics on living systems, they represent promising model objects. Today, the metabolic activity of microalgae and cyanobacteria in the presence of xenobiotics is being actively investigated due to the fact that toxic pollutants can be used by these microorganisms as sources of biogenic elements. Such widespread phototrophs as cyanobacteria of the genera Nostoc and Arthrospira (Spirulina), as well as microalgae of the genus Chlorella, can use OPCs as a source of phosphorus [28,29], released under the action of enzymes present in bacterial cells capable of degrading OPCs and, as a rule, coexisting under natural conditions with microalgae and cyanobacterial cells. To estimate the possibilities of implementing such coexistence, artificially created microbial consortia can be investigated.
Some PFCs and their derivatives belong to substances with a gas transport function, for which it is known that they actively carriage oxygen [30]. It is already known that the presence of chemically synthesized substances with a gas transport function in liquid media contributes to the accumulation of increased concentrations of microbial cell biomass [31,32]. In turn, the accumulation of biomass of phototrophic microorganisms is associated with their active consumption of carbon dioxide in aquatic environments. Because the solubility of carbon dioxide in water and in perfluorohexane [33] is significantly higher than the solubility of oxygen, it suggests that PFCs can have a significant intensifying effect on the process of cell growth of phototrophic microorganisms both under environmental conditions and during the implementation of appropriate biotechnological processes aimed at the quick accumulation of microalgae biomass.
Because bacteria among the various microorganisms have the most diverse set of metabolic processes, their combination with the biochemical potential of phototrophic microorganisms makes it possible to solve the problems of treating wastewaters of various compositions and origins as efficiently as possible. The detection of positive trends in the manifestation of interaction between bacterial and microalgae cells for removing xenobiotics is of great scientific and practical significance [34,35,36,37,38,39,40]. However, one of the important drawbacks of the biotechnological process is the possible presence of residual concentrations of toxic components of the treated wastewater (xenobiotics, heavy metals, etc.) in the accumulating microbial biomass [41]. This may limit its further use and therefore requires constant monitoring of its ecotoxicity and the intensification of wastewater treatment due to active cell growth. New knowledge in this direction can be used both to solve current environmental problems and to increase the efficiency of processes of accumulating microalgae biomass in wastewater and for its further transformation into commercial products.
The aim of this work was to study the effect of the presence of different perfluorocarbon compounds with gas transport functions in environments with phototrophic microorganisms (taken in the form of individual cultures of microalgae or cyanobacteria, as well as in the form of participants in synthetically composed consortia with bacteria degrading OPCs) on the growth parameters and viability of microalgae and cyanobacteria cells.
2. Materials and Methods
2.1. Chemicals
Methyl parathion was acquired from Sigma-Aldrich (St. Louis, MO, USA). All other reagents for experiments were purchased from Chimmed (Moscow, Russia). The following perfluorocarbon compounds (PFC) were used in the studies: CF3(CF2)4CF3 (1,1,1,2,2,3,3,4,4,5,5,6,6,6-tetradecafluorohexane, perfluorohexane) from HaloPolymer, Moscow, Russia; and C10F18 (1,1,2,2,3,3,4,4a,5,5,6,6,7,7,8,8,8a-octadecafluorodecalin, perfluorodecalin) from Acros Organics, Geel, Belgium. In addition, C3F7OCF(CF3)CF2OC2F5 (3,6-dioxaperfluoro-5-methylnonane, polyether I) and [C3F3O(C2F4CF2O)4C2F4]2 (4,7,10,13,16,19,22,25-octaoxaperfluoro-5,8,11,14,17,18,21,24-octamethyloctacosane, polyether II) were synthesized as described previously [32].
2.2. Microorganisms and Cultivation Condition
Cells of the phototrophic microorganisms Chlorella vulgaris C-1, Arthrospira (Spirulina) platensis (Nordst.) Geitl. rsemsu 1/02 and Nostoc sp. rsemsu Nss-14/11 were obtained from the IBCP RAS collection and the IPPAS collection of microalgae and cyanobacteria (Moscow, Russia). Bacterial cells of Rhodococcus erythropolis AC-1514D and Pseudomonas esterophilus V-1436D were obtained from the All-Russian Collection of Microorganisms (VKM, Moscow, Russia). The bacterial culture Photobacterium phosphoreum B-1717 was obtained from the Russian National Collection of Industrial Microorganisms (Moscow, Russia).
The preparation of the immobilized inoculums of C. vulgaris cells with and without combination into a bacterial consortium was carried out according to the methods described earlier [13,42]. Briefly, cells separated from the medium were suspended in a solution of poly(vinyl alcohol) (type16/1, 84 kDa, Sinopec Corp., Beijing, China). Then, this suspension was maintained at −70 °C using a DS 78 compact freezer (Dairei Asia Sdn. Bhd, Kuala Lumpur, Malaysia) for 3 days. Then, it was slowly defrosted via the following two stages: the first one, at −20 °C using a GN 3613 freezer (Liebherr, Biberach, Germany) for 3 h, and the second one, at 8 ± 2 °C in a 2201 Combicoldrac II refrigerator (LKB Instruments Haglund, Saffle, Sweden). The obtained samples of cells immobilized by inclusion into a polymer cryogel formed at subzero temperature were used in the further experiments.
Standard media (Tamiya medium, BG-11 medium, Zarrouk medium) [4] were used correspondently for biomass accumulation and cultivation of suspended microalgae (Chlorella vulgaris) and cyanobacteria cells (Nostoc sp. and Arthrospira (spirulina) platensis) when the cell suspensions were used as inoculums. Among investigated variants of media, one of them was used with the addition of 1 g/L glycerol.
Horticultural water from a gardening facility (Sovkhoz dekorativnogo sadovodstva, Moscow, Russia) was used for cultivation of immobilized inoculums of C. vulgaris cells and bacterial consortia. Wastewater with known characteristics [13] was applied in the experiments. It contained 0.99 g COD/L, where COD (chemical oxygen demands) was determined by the standard dichromate method [43]. The main chemical content of the wastewater was as follows: lipids—0.17 g/L; proteins—0.13 g/L; and carbohydrates 0.27 g/L, at a pH of 6.5.
The PFC additives in the growth medium were 0.5 or 1% (v/v). The initial concentration of methyl parathion was 0.15 mM in experiments with artificial consortia.
Cultivation of all microorganisms was carried out in an experimental laboratory-scale closed-type photobioreactor under the conditions described previously [44]. To implement cultivation in a circulating regime for 3 days, cells of all phototrophic microorganisms were loaded into a photobioreactor with a 20 L working volume, into which a medium was pumped at a flowrate of 20 L/day through a system of glass tubes with an inner diameter of 35 mm and an outer diameter of 38 mm by means of a centrifugal pump. Carbon dioxide (99.8% v/v) was bubbled through the tubes around the clock through a silicone membrane at a rate of 1–2 mL/min. Also in this system, constant illumination of 5000 lux was provided by light sources with wavelengths in the intervals of 450–480 nm and 640–700 nm simultaneously. The photobioreactor was equipped with a fully automated system for monitoring the pH (7.2 ± 0.2) of the medium and controlling the cultivation temperature (22 ± 1 °C).
2.3. Biomass Separation and Mechanical Disintegration
Suspended biomass of C. vulgaris cells was separated from the culture medium by centrifugation (8000 rpm, 10 min, Avanti J25, Beckman, Brea, CA, USA). The disintegration of the separated biomass was carried out according to the method detailed in [45].
Mechanical disintegration of C. vulgaris microalgae biomass for the control of toxicity presence was carried out in a Mini-BeadBeater-24 ball mill (BioSpec Products, Bartlesville, OK, USA) (glass bead size 0.5 mm; rotor rotation speed 3000 rpm; in 0.5 mL cells, biomass loading was 80 mg DCW) for 3 min.
2.4. Analytical Methods and Calculated Parameters
Algae growth was controlled by cell counting using an improved Neubauer hemocytometer (Rohem Instruments, Nashik, Maharashtra, India) through an optical microscope (Biomed, Russia with a Biomed Lum 206070112209 nozzle and a Myscope 500 M digital camera for the microscope). Concurrently, the OD540 of the cell suspensions was controlled with an Agilent UV-8453 spectroscopy system (Agilent Technology, Waldbronn, Germany) to investigate the kinetic curve of growth. The calibration graphs reflected correlation between the OD540 and DCW of each culture of studied microorganisms used. The dry cell weight (DCW) of biomass was determined by a standard gravimetrical method using a sample drying at 105 °C to a constant weight.
The initial concentrations of suspended and immobilized cells of phototrophic microorganisms were 1.2 g dry cell weight/L.
For procedures of determination of the concentration of intracellular adenosine triphosphate (ATP) in microbial cells, methyl parathion and p-nitrophenol were used as published previously [42]. The pH value was determined as described previously [13].
The average rate of biomass accumulation (Vbiomass) during the culture period was calculated from the equation: Vbiomass (g DCW/L/d) = ΔC/Δt, where ΔC is the variation of biomass concentration (g DCW/L) within a cultivation time of Δt (days).
To control the presence of residual amounts of PFCs in culture media, the residual bioluminescence of immobilized photobacteria was determined when they were exposed to selected media samples. For this purpose, calibration dependences of the residual bioluminescence of photobacteria on certain concentrations of PFCs in the studied media were plotted and used. For this purpose the Photobacterium phosphoreum B-1717 cells with natural bioluminescence were cultured in a standard Fargaly medium. The accumulated biomass was immobilized by a known method [46] through its inclusion into a cryogel of poly(vinyl alcohol) so that its final concentration in the polymer matrix was 0.1%. The formed samples of immobilized photobacteria were used as sensitive elements for biosensor examination of the toxicity of the studied media. The estimations of toxicity of various media were carried out in a similar way as described earlier [46].
The data are shown as means of at least three independent experiments ± standard deviations (±SD). The statistical analysis was realized using SigmaPlot 12.5 (ver. 12.5, Systat Software Inc., San Jose, CA, USA). The significant (p ≤ 0.05) differences between the obtained results were estimated by a one-way analysis of variance (ANOVA).
3. Results
3.1. Effect of Perfluorocarbon Compounds on the Growth and ATP Concentration of Phototrophic Microorganisms
The biomass accumulation and ATP concentration of the cells growing in media with PFCs were studied. The microalgae C. vulgaris C-1 and cyanobacteria (A. (Spirulina) platensis (Nordst.) Geitl. rsemsu 1/02 and Nostoc sp. rsemsu Nss-14/11) cells were investigated for 3 days under standard conditions for each microorganism when 0.5% or 1% (v/v) of a certain PFC (perfluorohexane, polyether I, perfluorodecalin or polyether II) was introduced into the cell growth medium (Table 1, Figure 1).

Table 1.
Concentration of ATP (nmol/g DCW) * after 3 days of their cultivation in the culture media with various concentrations of different PFCs.
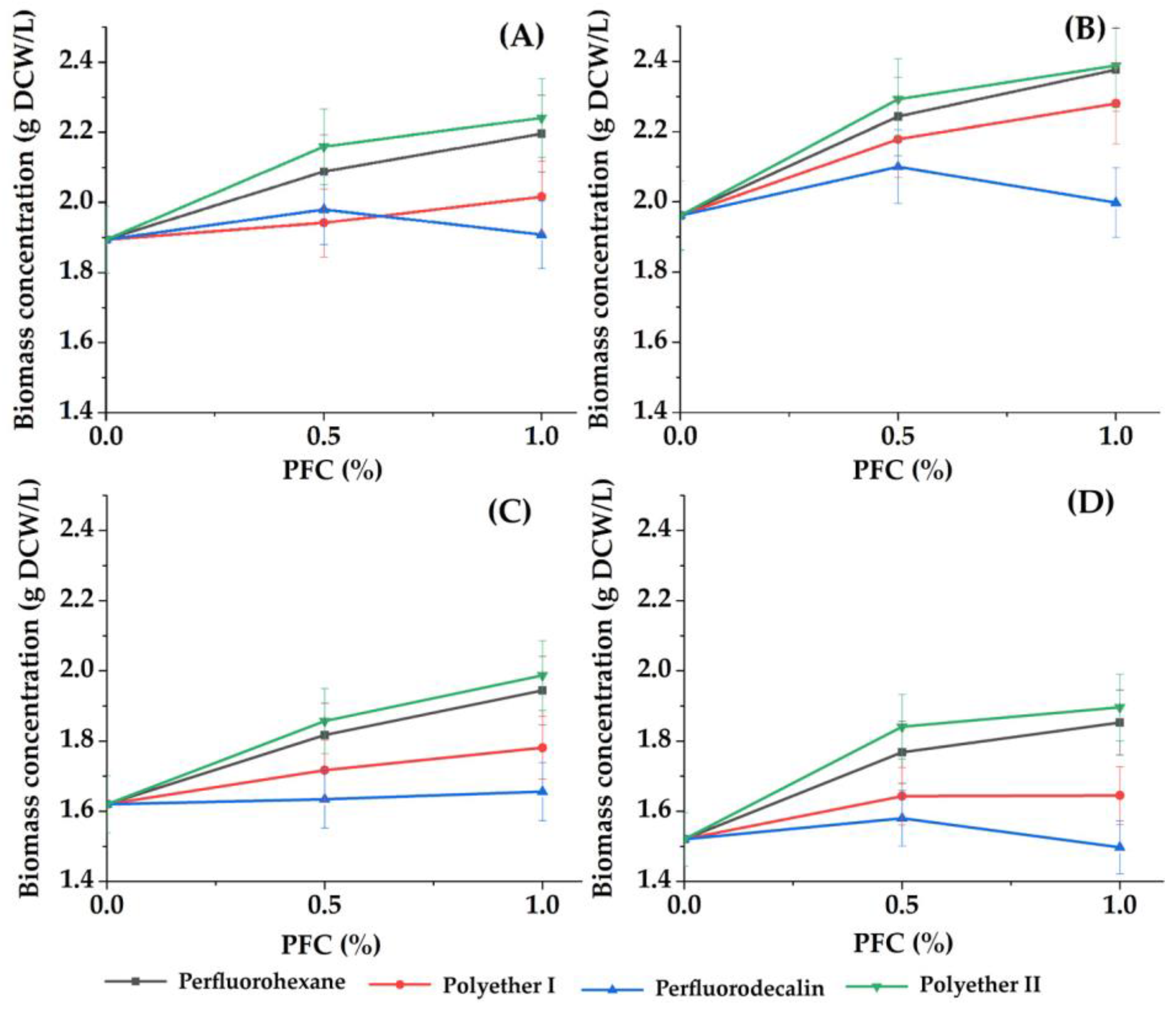
Figure 1.
Dependence of the concentration of accumulated biomass of phototrophic microorganisms ((A) C. vulgaris cells in Tamiya medium, (B) C. vulgaris cells in Tamiya medium with 1 g/L glycerol, (C) A. platensis cells in Zarrouk medium, (D) Nostoc sp. cells in the medium BG-11) on the concentrations of different PFCs. Control: the concentration of accumulated cell biomass of phototrophic microorganisms when concentration of PFC was 0.0%.
The concentration of the suspended cell inoculum was the same in all variants of cells (1.2 g DCW/L), and it was high enough compared to most of the known studies [30,47,48]. The choice of such a cell concentration was based on the data previously obtained by the authors [13], when it was revealed that a high rate of cell growth of phototrophic cultures can be achieved in a short period of time with a high density of the cell population in the inoculum. High cell population density and growth rates of phototrophs, among other things, stimulated the process of “blooming” of the reservoirs. Because the rate of biomass accumulation began to decrease after 3 days, experiments were carried out for no more than 3 days in order to analyze the process according to the initial growth rates.
For all the studied cultures, a positive effect was noted from the presence of perfluorohexane, polyether I or polyether II in the growth medium, manifested in an increased average rate of biomass accumulation in comparison to the parameter value in the control (without PFC) (Figure 1 and Figure 2).
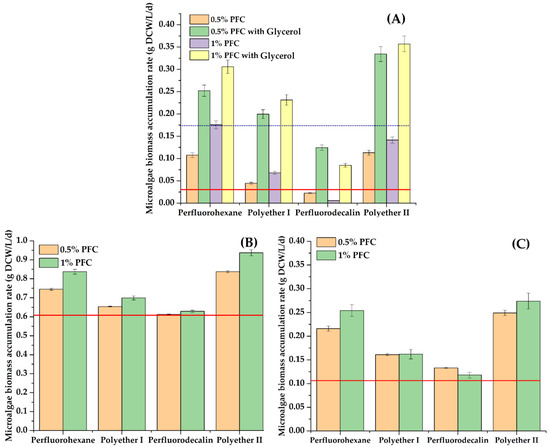
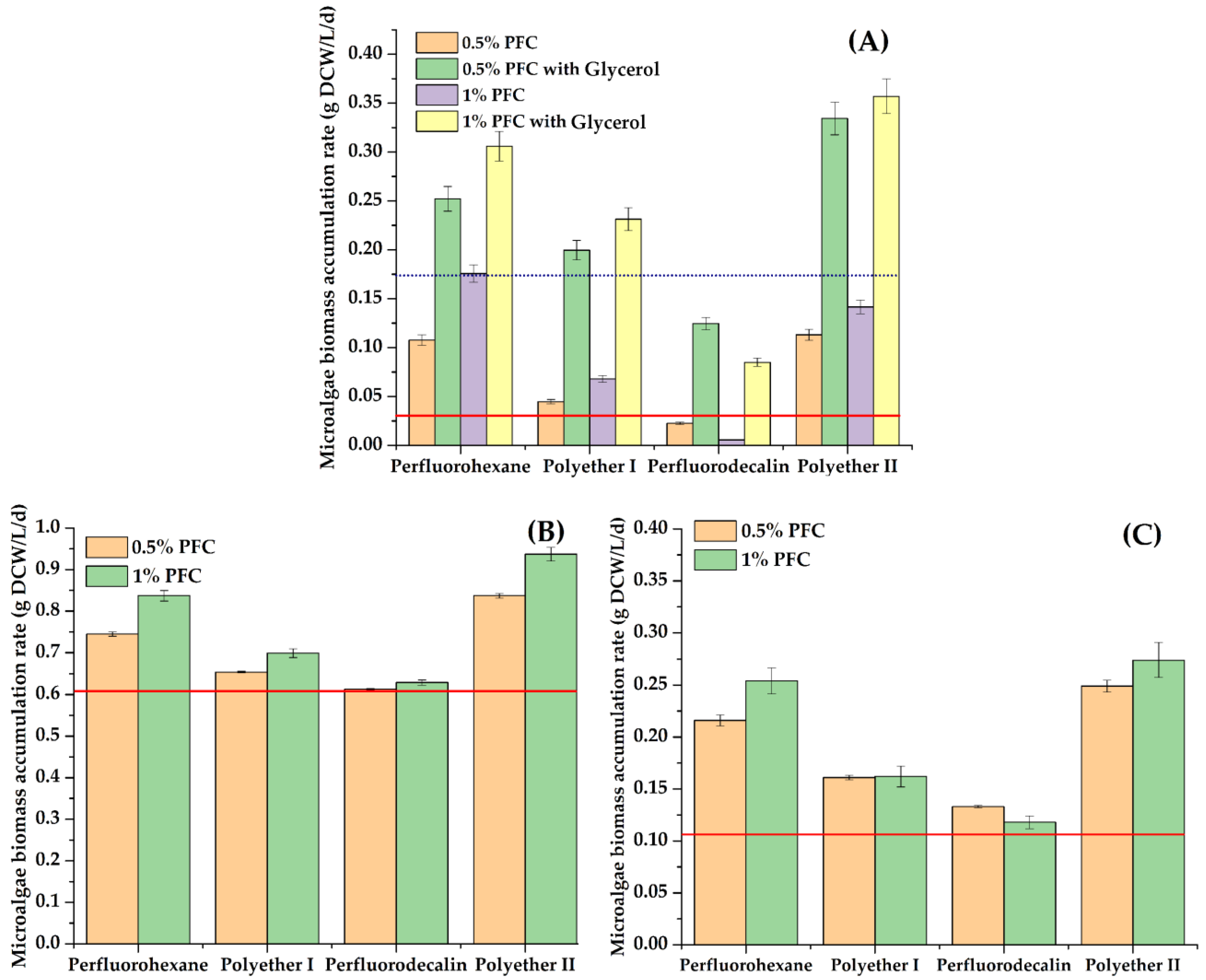
Figure 2.
The average rate of biomass accumulation (g DCW/L/d) for 3 days: C. vulgaris cells in Tamiya medium (A), A. platensis cells in Zarrouk medium (B), Nostoc sp. cells in BG-11 medium (C). Controls: results of cultivation of cells without PFC and with 1 g/L glycerol are marked by a blue dotted line (A), and results of cultivation without both PFCs and glycerol are marked by a red line (A–C).
The most pronounced effect was observed when perfluorohexane or polyether II was introduced into the growth medium. These results were generally consistent with the data obtained earlier regarding the accumulation of E. coli cell biomass in the presence of PFCs [31].
The maximum and lowest values of average rate of biomass accumulation were found for A. platensis and Nostoc sp. cells, respectively (Figure 2). In a standard growth medium without additional additives, an increase in the concentration of perfluorohexane, polyether I or polyether II by 2 times from 0.5% to 1% (v/v) was accompanied by a slight increase (up to 8%) in the concentration of the accumulated biomass of each of the studied phototrophs.
It was noted that by 72 h of cultivation in the presence of a PFC, the level of intracellular ATP concentration in phototrophic cells decreased slightly under experimental conditions (Table 1).
It was found that when 1 g/L glycerol is introduced into the growth medium with C. vulgaris cells as an additional component for mixotrophic nutrition, an increase in the rate of biomass accumulation by 9–32% is possible. This effect was most clearly expressed when glycerol was combined with polyether I (Figure 1). This fact suggests that the replacement of the Tamiya medium with wastewater containing an organic food source will contribute to an increase in the rate of biomass accumulation of C. vulgaris microalgae cells.
Analysis of Figure 2A suggests that in the case of simultaneous ingress into water bodies of sources of heterotrophic nutrition (organic substances) and substances with a gas transport function, a particularly intensive growth of phototrophic microorganisms should be expected, which is important for forecasting the development of ecosystems.
C. vulgaris microalgae cells were selected for further studies, as they are most often used as participants in biosystems for wastewater treatment, and they can also be present in fresh and salty reservoirs and in soil; they can also be part of aerobic active sludge [49].
Commercial perfluorohexane, which is already being produced in industry and is actively used in various fields as a refrigerant, fire-extinguishing agent, component of dielectric media, solvent and foam-blowing agent [50], and polyether II were used as PFCs from among the studied ones, as in the presence of this PFC, the maximum rates of accumulation of phototrophic biomass were noted.
3.2. Effect of PFC on the Characteristics of Chlorella Biomass Accumulation in Presence of Immobilized Inoculum under Conditions of Periodic Cultivation in Wastewater
The toxicity analysis of the studied PFCs, carried out using bioluminescent bacterial cells, confirmed the presence of toxicity in all media with a content of 1% (v/v) PFC. The level of bioluminescence in cells exposed in the analyzed media samples decreased by 20–97% for 0.5 h depending on the type of PFC. Perfluorodecalin turned out to be the most toxic for these cells. Despite the fact that PFCs and their derivatives are actively used in medicine, the presence of cytotoxicity with PFCs is generally consistent with the literature data [51,52,53]. The unique properties of the C-F bond and F-F interactions in PFC structures require increased attention to such compounds in the direction of minimizing the possibility of their ingress into the environment [53]. However, such a negative effect can manifest itself in relation to certain microorganisms, according to the data obtained with phototrophs (Figure 1 and Figure 2), and bioluminescent cells were used for the current control of changes in the toxicity of the media implemented in the work because they are sensitive to PFC presence at a certain concentration (Figure S1).
To study the process of accumulation of suspended biomass of C. vulgaris microalgae, an immobilized inoculum was used as a concentrated form of stabilized cells and as a model of a self-stabilizing ecosystem that can be formed naturally by the phototrophs. This inoculum was introduced into horticultural water from a gardening facility used as medium for cell cultivation. Previously, it was established that this is a good enough medium for the cultivation of C. vulgaris cells [13].
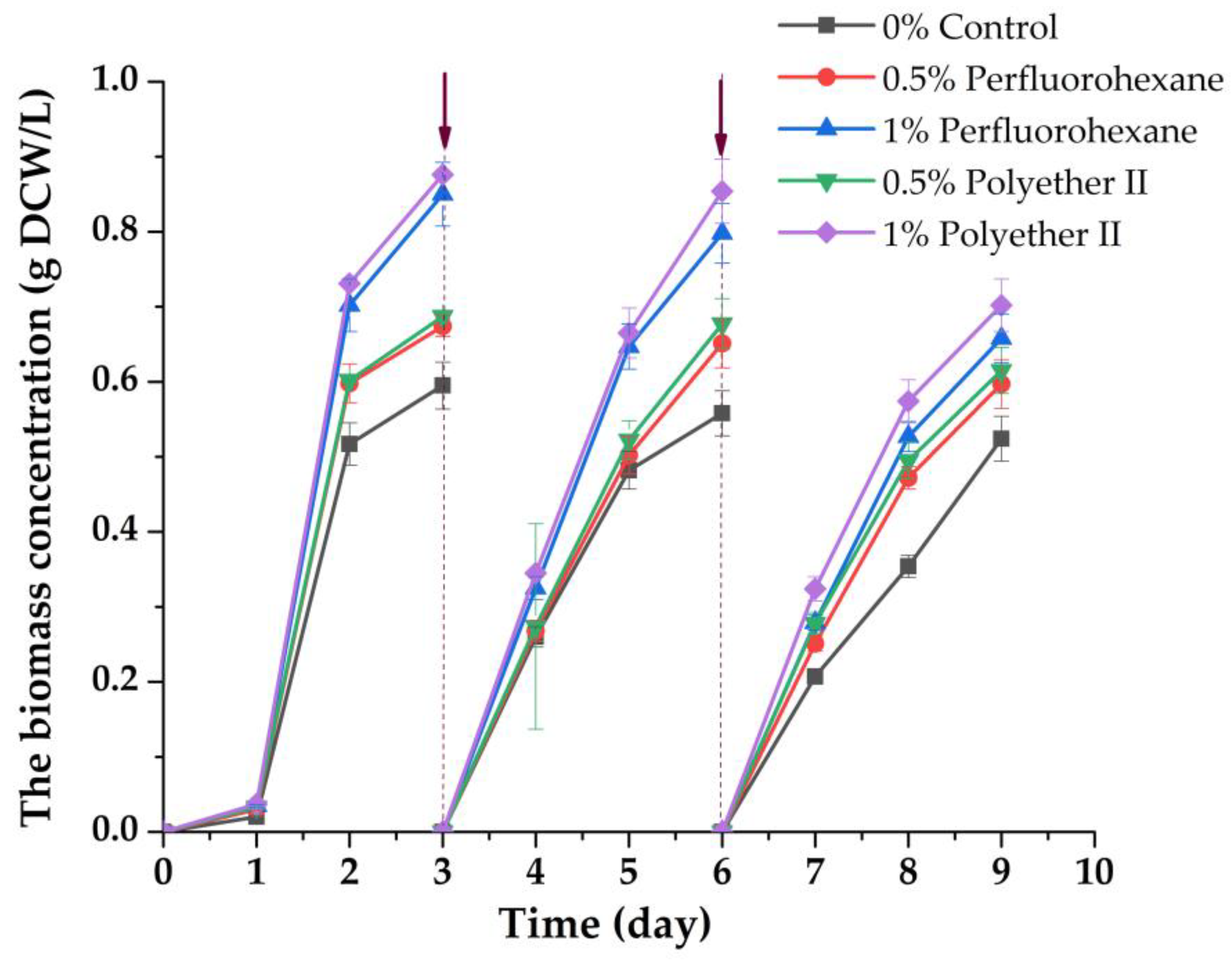
The immobilized inoculum has undoubted advantages when used for wastewater treatment, as it is easily separated from the culture fluid, and cells in this form show resistance to variations in the chemical composition of wastewater and its physical properties [13,54]. The liquid medium was drained with the accumulated free cell biomass from the photobioreactor every 3 days. The accumulated biomass of suspended cells was separated from the medium and analyzed, and the medium with the addition of glycerol was loaded back into the photobioreactor (Figure 3).
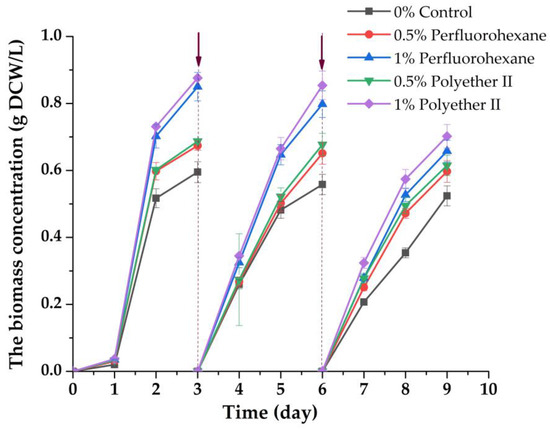
Figure 3.
Changes in the concentration of C. vulgaris suspended biomass during its accumulation in wastewater with or without addition of PFCs, using an immobilized inoculum. The arrows indicate the time of the following procedure: draining of the liquid medium with suspended biomass; separating biomass of free microalgae cells; adding 1 g/L glycerol to the waste medium; returning of the culture liquid medium to the photobioreactor with the immobilized inoculum.
For all the studied variants, in the presence of PFCs at the end of each cycle, the biomass increased the level of the same parameter revealed for cells in the control, where PFCs were absent. The maximum excess of the level of biomass accumulation as compared to the control was about ~25% (Figure 1). The 10.5–12% decrease in the rate of free cell biomass accumulation in the control was comparable with a decrease in the rate of microalgae cell biomass accumulation in the medium with 0.5% (v/v) PFC for three cycles of using an immobilized inoculum. The decrease in the rate of biomass accumulation was 20–23% for three working cycles when 1% (v/v) PFC was introduced into the media.
Thus, it was found for the first time that a significant increase in the levels of biomass accumulation of free microalgae cells should be expected when they enter by case or are directly loaded into wastewater containing different types of PFCs. At the same time, the state of the inoculum of C. vulgaris cells (in the form of a suspension or a sample of cells immobilized in porous gel structures), as it turned out, does not change the overall growth trend, which exceeds the control if a PFC is present in the cell culture medium.
At the end of the process (at the ninth day of immobilized cell cultivation), the toxicity of the culture medium was assessed, and it was found that it remained at the initial level. The suspension biomass of C. vulgaris cells was separated from the liquid and disintegrated. Furthermore, the toxicities of both the spent broth and disintegrated microalgae biomass were separately evaluated with bioluminescent bacteria. It was found that the biomass is absolutely non-toxic, while the liquid medium used for cell cultivation maintained the same initial level of toxicity due to the initial introduction of PFCs to it. In this regard, it seems appropriate in the case to implement similar conditions for the biotechnological process of biomass accumulation of phototrophic microorganisms: the use of a closed-type photobioreactor with the returning of spent culture liquid medium containing PFCs to the production cycle with the addition of a fresh portion of a carbon source for heterotrophic nutrition of cells at a new stage of biomass cultivation with an increased level of accumulation, but without intoxication. This can be an important point for the practical use of such biomass in obtaining commercial products. Actually, glycerol-containing wastes coming from the treatment of the same microalgae biomass in some commercial products can be used as such carbon additives to the photobioreactor. It is known that this is possible [55,56].
3.3. Effect of Joint Presence of PFCs and OPCs on Consortia of Bacterial and Microalgae Cells
As already noted, PFCs and OPCs are present in urban wastewater, as well as in the wastewaters of enterprises using them [23,24,25]. Among other things, these substances are also contained in dust, which can enter water bodies together with precipitation.
Microalgae, especially of the genus Chlorella, are actively used for wastewater treatment, including as parts of consortia. Wastewater is also offered as a medium for the accumulation of phototrophic biomass, where other microorganisms can be cultivated in parallel. It is known that N-acyl homoserine lactones (AHL) produced by Gram-negative bacterial cells stimulate the growth of microalgae, in particular those of the genus Chlorella [57,58]. On the one hand, this information sets a new direction in the search for ways to increase the productivity of the processes of directed accumulation of phototrophic biomass. On the other hand, it causes concern from the point of view of uncontrolled development of bacterial–algal consortia in environmental objects.
In this regard, it is necessary to investigate the process of accumulation of biomass of microalgae of the genus Chlorella in association with bacterial cells when using wastewater containing PFCs and OPCs simultaneously as a nutrient medium. Such a study simulates a situation that may take place in real ecosystems, as xenobiotics can enter water bodies, which helps accelerate the accumulation of phototrophic biomass. The presence of other microorganisms can lead to even more intensive cell growth.
To study changes in the rate of accumulation of Chlorella microalgae biomass in the presence of bacteria capable of producing AHLs, three synthetic consortia were formed. At the same time, they were obtained by joint immobilization of microalgae biomass and a bacterial consortium at different ratios (Table 2). A previously developed immobilized artificial consortium was based on microorganisms belonging to Gram-positive (R. ruber) and Gram-negative (P. esterophilus) bacterial cell types and was capable of jointly performing rapid and efficient degradation of OPCs [42].

Table 2.
Values of study parameters established after 72 h of cultivation of an immobilized consortium of P. esterophilus bacteria with R. ruber and C. vulgaris microalgae in wastewater with 0.15 mM methyl parathion.
Such a combination of components, in our opinion, should ensure the presence of bacterial AHLs in close proximity to C. vulgaris cells, as well as facilitate gas exchange (O2 and CO2) between microorganisms. Among other things, such an immobilized consortium is a kind of nature-like model, as bacterial cells and phototrophic microorganisms coexist in water bodies of the environment, forming self-immobilizing biosystems (biofilms, floccules, mats), as well as being based on natural solid organic–mineral particles present in ecosystems.
The study of changes in the rate of accumulation of microalgae biomass in the presence of bacteria was carried out in wastewater with the simultaneous presence of a PFC (perfluorohexane or polyether II) at different initial concentrations and an OPC (methyl parathion). It was found that in all the studied variations (Table 2), after 20 h, the degree of degradation of methyl parathion in the medium was 100%. Previously, for a bacterial consortium based on P. esterophilus V-1436D and R. ruber AC-1513D cells with a mass ratio of 2:1 [42], a similar result with the same concentration of methyl parathion was achieved in 24 h [42]. In this work, the joint immobilization of bacterial cells and microalgae cells probably provided constant oxygen access to bacteria and the removal of carbon dioxide from them by microalgae cells, which provided intensification of the degradation process of methyl parathion.
From the data obtained (Table 2), it followed that the ratio of the biomasses of different microorganisms in the composition of the pellet affects the level of concentration of intracellular ATP of immobilized and suspended cells. The maximum ATP values corresponded to the combination of “microalgae:bacteria” at a ratio of 25:1, which, apparently, provides for the formation of a consortium with intercellular interaction of cultures. The combination of microalgae with a bacterial consortium was expected to increase the rate of accumulation of phototrophic biomass. It was possible to accumulate the maximum amount of microalgae biomass in 3 days in the medium when the ratio between participants of the microbial combination “microalgae:bacteria” was 25:1.
The presence of a PFC in the medium, as before, had a positive effect on the rate of accumulation of microalgae biomass. It was noted that, unlike individual cultures, when microalgae were combined with bacteria, there was no decrease in ATP concentration in the presence of PFCs as compared to the control (Table 1 and Table 2). Perhaps this effect was due to the formation of mutualistic relations between the participants in microbial societies, manifested in bacterial and microalgal synergy for removing xenobiotics and environmental toxicity. Probably, AHLs synthesized by bacteria contributed to the enhancement of the photoautotrophic growth of C. vulgaris cells, which is generally consistent with the literature data [57].
Evaluation of the intensity of photobacteria bioluminescence after their exposure in culture media containing 0.15 mM methyl parathion and different concentrations of PFCs (Table 3) showed that changes in the residual bioluminescence of cells during the first 24 h already indicated a tendency toward decreasing toxicity in the media.

Table 3.
Residual bioluminescence * (%) of photobacteria in media after 24 h of cultivation of a consortium of P. esterophilus, R. ruber bacteria and C. vulgaris microalgae in wastewater with 0.15 mM methyl parathion and different PFCs.
After 72 h of cultivation, the residual bioluminescence of photobacteria was almost 100%. This is explained by the fact of complete decomposition of methyl parathion and the utilization by cells of its biodegradation products. As for PFCs, they can be partially utilized by P. esterophilus bacterial cells because it is known that individual bacterial strains of the genus Pseudomonas are able to use PFCs as a nutritional source (degradation of 1 g/L PFC can reach 75%) [59]. Based on the above, for the treatment of wastewater containing PFCs, it is possible to recommend the use of artificial consortia of microorganisms containing C. vulgaris microalgae and Pseudomonas bacteria, which are effective destructors of a number of xenobiotics.
4. Discussion
As one of the approaches to the intensification of the growth of phototrophic microorganisms, their co-cultivation with bacteria can be considered. For instance, the co-cultivation was effective in the treatment of wastewater when the following microbial partners were joined in the same biosystem: Chlorella vulgaris and Bacillus subtilis [60]; Pseudomonas and Cyanobacteria cells [17]; Chlorella vulgaris, Ettlia sp. and Chlamydomonas reinhardtii [61]; Chlorella sorokiniana and wastewater bacteria; Auxenochlorella protothecoides and wastewater bacteria; C. vulgaris and Pseudomonas putida; C. vulgaris and Bacillus licheniformis; Desmodesmus sp. and nitrifying bacteria; C. sorokiniana and denitrifying bacteria; C. vulgaris, Ettlia sp., C. reinhartii and hydrogen consumer denitrifiers; and Chlorella, Klebsiella and Acinetobacter cells [39]. Additionally, co-cultivation biosystems are used for commercialization of the microalgae biomass accumulation process [35]. Bacteria synthesizing AHLs are able to regulate the quorum sensing (QS) of microalgae and cyanobacteria. Consortia of bacteria and phototrophic microorganisms are widespread. In nature, they are formed as a result of the symbiotic interaction of microorganisms: microalgae and cyanobacteria cells produce oxygen, which is used by aerobic bacterial cultures that emit carbon dioxide, which phototrophs need. The presence of compounds with a gas transport function in the environment, which appear in the environment as pollutants, in this case can have a positive effect on the structure of mutually beneficial relationships between microorganisms. Studies on this topic are not yet known, but the matter is relevant today. It is possible to obtain new information in this direction by studying synthetic consortia; however, today algal–bacterial synergy is already used in the development of synthetic consortia for the destruction of various xenobiotics [62].
Recently, studies on the influence of the QS mechanism, manifested in the ability of bacterial cells to interact with each other through signaling molecules and to influence algae–bacteria interactions, have been of particular interest [63]. To date, it has been revealed that there are a number of AHL molecules, as well as other lactone-containing QS signaling molecules with different chemical structures and products of their destruction, which can have different effects on microalgae cells [64]. Under certain conditions, the presence of a keto group in the structure of an AHL molecule can lead to its enzyme-free rearrangement and the formation of tetramic acids, which can have a significant algicidal effect on the growth and photosynthesis of individual microalgae [65]. However, it is known that AHLs secreted by bacteria can bind to microalgae cells and contribute to their self-aggregation and stabilization [66]. Previously, it was found that in the combined systems of algae–bacteria, an excess of AHLs can restrain the initial growth of Chlorophyta sp., thereby having a negative effect on the characteristics of microalgae [67]. The results obtained in this work clearly indicate that the variants of combinations of bacteria and Chlorella that were experimentally created in this study turned out to be successful, and this should be taken into account by researchers who work in this field. The concentration of intracellular ATP is a marker of the metabolic activity of cells and the level of their viability [13]. The analysis of this biochemical characteristic of cells by bioluminescent ATP-metry makes it possible to quickly assess with high selectivity and sensitivity the effects of various conditions of cultivation of various cells on their functional activity [68]. In this work, data on the concentration of intracellular ATP were used to monitor the state of cells, both in the presence of xenobiotics and in the formation of consortia of Chlorella cells with bacteria. ATP-metry proved to be indispensable, especially when assessing the state of immobilized cells in granules (Table 2), as it was impossible to isolate them without damage. It was found that an increase in the level of ATP in the analyzed cell samples clearly corresponds to an increase in the level of accumulated biomass in them. Such an analysis of cellular systems by two parameters (ATP and biomass accumulation) allowed us to discuss the positive relationships that developed between cells of different cultures as a part of formed artificial consortia when the cells were combined at certain ratios.
The analysis of the results obtained in this work suggests that if PFCs enter various water bodies, we should expect an intensification of the growth of some microorganisms, in particular a number of microalgae and primarily C. vulgaris microalgae cells. The question remains open about the possibility of using PFCs in biotechnological processes to intensify the growth of microalgae. In our opinion, special use of chemically and biologically inert substances should be carried out in closed systems to avoid their accumulation in the environment.
Interestingly, the ability of some phototrophic microorganisms to utilize OPCs was recently explained as a possible result of the plasmid transfer of necessary genes from bacteria due to the co-existence of microalgae and bacterial cells in native consortia [69,70,71,72]. Thus, the results obtained in this work have practical significance for researchers in the field of developing new biocatalytic approaches to the treatment of wastewaters of complex composition containing OPCs and PFCs while simultaneously obtaining valuable renewable raw materials—biomass of phototrophic microorganisms.
Additionally the results of this work indicate that with a combination of factors such as the presence of various nutrition sources and the presence of PFCs in the medium of microalgae and bacteria capable of regulating the QS of phototrophs, an active increase in microalgae biomass can be registered in environmental objects, which can change the equilibrium in such ecosystems. A recent study of the interaction of PFCs and microorganisms present in the aquatic environment showed that green microalgae Desmodesmus subspicatus cells are not capable of growing in presence of perfluorohexane, perfluorooctane or perfluorodecane phosphonic acids [73]. For these microorganisms, a decrease in the intensity of green coloration in the presence of PFCs was noted, which indirectly indicates a negative effect of PFCs on microalgae chlorophyll, resulting in cell fluorescence being lost. The fact that Chlorella cells were completely resistant to the possible negative effects of PFCs in this study is significant for predicting the viability of these microalgae in vivo under similar conditions.
5. Conclusions
The conditions associated with an increase in the rate of accumulation of biomass of various phototrophic microorganisms by several times compared with their control variants were investigated. It was shown for the first time that the presence of a number of PFCs in the medium significantly stimulates the growth of phototrophs present in the studied media in the form of individual cultures or in combination with a bacterial consortium of soil bacteria of the genera Pseudomonas and Rhodococcus. It turned out that due to cell synergy in wastewater with PFCs and OPCs under the action of a synthetic immobilized consortium based on C. vulgaris cells with P. esterophilus and R. ruber, it is possible to carry out 100% degradation of methyl parathion and obtain intensive accumulation of biomass of free microalgae cells. The study of the vital activity of phototrophic microorganisms and their consortia that are characteristic of water bodies, with the simultaneous presence of OPCs and PFCs in them, has not been carried out by anyone before. The results of the presented experiments make it possible to predict the likely changes in such a system and, in general, indicate the need to assess the possible ecotoxicity of such environments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13031868/s1, Figure S1. Residual intensity of bioluminescence of photobacterial cells dependent on the concentrations of different perfluorocarbon compounds in the medium for bacteria exposition.
Author Contributions
Conceptualization, E.E.; methodology, O.S., O.M., A.A. and E.E.; validation, O.S.; investigation, O.S., A.A. and O.M.; data curation, O.S. and E.E.; writing—original draft preparation, O.S., O.M., A.A. and E.E.; writing—review and editing, O.S., O.M. and E.E.; visualization, O.S. and O.M.; supervision, E.E. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by State Task of Lomonosov Moscow State University (121041500039-8).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research was performed according to the Development Program of the Interdisciplinary Scientific and Educational School of Lomonosov Moscow State University, “The future of the planet and global environmental change”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Magdalena, J.A.; Gonzalez-Fernandez, S. Microalgae biomass as a potential raw material for a carboxylate platform. Molecules 2019, 24, 4404. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hu, K.S.; Chu, K.V.; Selvaraju, A.; Chen, V.H.; Chang, J.S.; Shaw, P.L. Microalgae: A future source of biohydrogen and biogas. Front. Energy Res. 2021, 9, 660399. [Google Scholar] [CrossRef]
- Ansari, F.A.; Singh, P.; Guldhe, A.; Bux, F. Microalgal cultivation using aquaculture wastewater: Integrated biomass generation and nutrient remediation. Algal Res. 2017, 21, 169–177. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Nikolskaya, A.B.; Lyagin, I.V.; Senko, O.V.; Makhlis, T.A.; Stepanov, N.A.; Maslova, O.V.; Mamedova, F.; Varfolomeev, S.D. Production of biofuels from pretreated microalgae biomass by anaerobic fermentation with immobilized Clostridium acetobutylicum cells. Bioresour. Technol. 2012, 114, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Limongi, A.R.; Viviano, E.; De Luca, M.; Radice, R.P.; Bianco, G.; Martelli, G. Biohydrogen from microalgae: Production and application. Appl. Sci. 2021, 11, 1616. [Google Scholar] [CrossRef]
- Schiano di Visconte, G.; Spicer, A.; Chuck, K.J.; Allen, M.J. Bio-purification of microalgae: A look at the current state and future possibilities using genetic modification. Appl. Sci. 2019, 9, 4793. [Google Scholar] [CrossRef]
- Maslova, O.; Stepanov, N.; Senko, O.; Efremenko, E. Preparation of various organic acids from various renewable sources by immobilized cells in the modes of separate hydrolysis and fermentation (microwave) and simultaneous saccharification and fermentation (SFF). Bioresour. Technol. 2019, 272, 1–9. [Google Scholar] [CrossRef]
- Efremenko, E.; Senko, O.; Maslova, O.; Stepanov, N.; Aslanli, A.; Lyagin, I. Biocatalysts in the synthesis of microbial polysaccharides: Properties and development trends. Catalysts 2022, 12, 1377. [Google Scholar] [CrossRef]
- Stepanov, N.; Efremenko, E. “Deceived” concentrated immobilized cells as a biocatalyst for intensive production of bacterial cellulose from various sources. Catalysts 2018, 8, 33. [Google Scholar] [CrossRef]
- Martin, N.; Bernat, T.; Dinasquet, J.; Stofko, A.; Damon, A.; Deheyn, D.D.; Azam, F.; Smith, J.E.; Davey, M.P.; Smith, A.G.; et al. Synthetic consortia of algae and bacteria for the economical cultivation of microalgae in a simple hydrogel system. J. Appl. Physiol. 2021, 33, 2805–2815. [Google Scholar] [CrossRef]
- Senko, O.; Stepanov, N.; Maslova, O.; Efremenko, E. Transformation of enzymatic hydrolysates of Chlorella–fungus mixed biomass into poly(hydroxyalkanoates). Catalysts 2023, 13, 118. [Google Scholar] [CrossRef]
- Maslova, O.; Senko, O.; Stepanov, N.; Gladchenko, M.; Gaydamaka, S.; Akopyan, A.; Yeseva, E.; Anisimov, A.; Efremenko, E. Sulfur-containing mixed waste during anaerobic processing by new immobilized synthetic consortia. Bioresour. Technol. 2022, 362, 127794. [Google Scholar] [CrossRef] [PubMed]
- Senko, O.; Stepanov, N.; Maslova, O.; Efremenko, E. “Nature-like” cryoimmobilization of phototrophic microorganisms: New opportunities for their long-term storage and sustainable use. Sustainability 2022, 14, 661. [Google Scholar] [CrossRef]
- Johnson, D.B.; Schideman, L.C.; Canam, T.; Hudson, R.J. Pilot-scale demonstration of efficient ammonia removal from a high-strength municipal wastewater treatment sidestream by algal-bacterial biofilms affixed to rotating contactors. Algal Res. 2018, 34, 143–153. [Google Scholar] [CrossRef]
- Wollmann, F.; Dietze, S.; Ackermann, J.U.; Bley, T.; Walther, T.; Steingroewer, J.; Krujatz, F. Microalgae wastewater treatment: Biological and technological approaches. Eng. Life Sci. 2019, 19, 860–871. [Google Scholar] [CrossRef]
- Chan, S.S.; Khoo, K.S.; Chew, K.W.; Ling, T.C.; Show, P.L. Recent advances biodegradation and biosorption of organic compounds from wastewater: Microalgae-bacteria consortium—A review. Bioresour. Technol. 2022, 344, 126159. [Google Scholar] [CrossRef]
- Wang, S.; Ji, B.; Zhang, M.; Ma, Y.; Gu, J.; Liu, Y. Defensive responses of microalgal-bacterial granules to tetracycline in municipal wastewater treatment. Bioresour. Technol. 2020, 312, 123605. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Alimohamadi, M.; Khataee, A.; Dragoi, E. A global systematic review on the concentration of organophosphate esters in water resources: Meta-analysis, and probabilistic risk assessment. Sci. Total Environ. 2021, 807 Pt 2, 150876. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Kristanti, R.A. Bioremediation of perfluorochemicals: Current state and the way forward. Bioprocess Biosyst. Eng. 2022, 45, 1–17. [Google Scholar] [CrossRef]
- Eriksson, U.; Haglund, P.; Kärrman, A. Contribution of precursor compounds to the release of per-and polyfluoroalkyl substances (PFASs) from waste water treatment plants (WWTPs). J. Environ. Sci. 2017, 61, 80–90. [Google Scholar] [CrossRef]
- Wu, J.Y.; Hua, Z.L.; Gu, L. Planktonic microbial responses to perfluorinated compound (PFC) pollution: Integrating PFC distributions with community coalescence and metabolism. Sci. Total Environ. 2021, 788, 147743. [Google Scholar] [CrossRef] [PubMed]
- Presentato, A.; Lampis, S.; Vantini, A.; Manea, F.; Daprà, F.; Zuccoli, S.; Vallini, G. On the ability of perfluorohexane sulfonate (PFHxS) bioaccumulation by two Pseudomonas sp. strains isolated from PFAS-contaminated environmental matrices. Microorganisms 2020, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Ofrydopoulou, A.; Nannou, C.; Evgenidou, E.; Christodoulou, A.; Lambropoulou, D. Assessment of a wide array of organic micropollutants of emerging concern in wastewater treatment plants in Greece: Occurrence, removals, mass loading and potential risks. Sci. Total Environ. 2022, 802, 149860. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, K.; Kim, D.; Moon, H.B.; Jeon, J. Ny-Ålesund-oriented organic pollutants in sewage effluent and receiving seawater in the Arctic region of Kongsfjorden. Environ. Pollut. 2020, 258, 113792. [Google Scholar] [CrossRef]
- Lourthuraj, A.A.; Hatshan, M.R.; Hussein, D.S. Biocatalytic degradation of organophosphate pesticide from the wastewater and hydrolytic enzyme properties of consortium isolated from the pesticide contaminated water. Environ. Res. 2022, 205, 112553. [Google Scholar] [CrossRef] [PubMed]
- Petromelidou, S.; Margaritis, D.; Nannou, C.; Keramydas, C.; Lambropoulou, D.A. HRMS screening of organophosphate flame retardants and poly-perfluorinated substances in dust from cars and trucks: Occurrence and human exposure implications. Sci. Total Environ. 2022, 848, 157696. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, D.; Lee, D.J.; Varjani, S.; Lam, S.S.; Allakhverdiev, S.I.; Chang, J.S. Microalgae-based wastewater treatment–microalgae-bacteria consortia, multi-omics approaches and algal stress response. Sci. Total Environ. 2022, 845, 157110. [Google Scholar] [CrossRef] [PubMed]
- Touliabah, H.E.S.; El-Sheekh, M.M.; Ismail, M.M.; El-Kassas, H. A review of microalgae-and cyanobacteria-based biodegradation of organic pollutants. Molecules 2022, 27, 1141. [Google Scholar] [CrossRef]
- Abdel-Razek, M.A.; Abozeid, A.M.; Eltholth, M.M.; Abouelenien, F.A.; El-Midany, S.A.; Moustafa, N.Y.; Mohamed, R.A. Bioremediation of a pesticide and selected heavy metals in wastewater from various sources using a consortium of microalgae and cyanobacteria. Slov. Vet. 2019, 56, 61–73. [Google Scholar] [CrossRef]
- Jägers, J.; Wrobeln, A.; Ferenz, K.B. Perfluorocarbon-based oxygen carriers: From physics to physiology. Pflüg. Arch. Eur. J. Phy. 2021, 473, 139–150. [Google Scholar] [CrossRef]
- Senko, O.; Stepanov, N.; Tyutyunov, A.; Sterlin, S.; Grinberg, V.; Makhlis, T.; Efremenko, E. Intensification of organophosphorus hydrolase synthesis by using substances with gas-transport function. Appl.Sci. 2017, 7, 1305. [Google Scholar] [CrossRef]
- Vandermies, M.; Fickers, P. Bioreactor-scale strategies for the production of recombinant protein in the yeast Yarrowia lipolytica. Microorganisms 2019, 7, 40. [Google Scholar] [CrossRef]
- Costa Gomes, M.F.; Pádua, A.A.H. Interactions of carbon dioxide with liquid fluorocarbons. J. Phys. Chem. B. 2003, 107, 14020–14024. [Google Scholar] [CrossRef]
- Rajapitamahuni, S.; Bachani, P.; Sardar, R.K.; Mishra, S. Co-cultivation of siderophore-producing bacteria Idiomarina loihiensis RS14 with Chlorella variabilis ATCC 12198, evaluation of micro-algal growth, lipid, and protein content under iron starvation. J. Appl. Phycol. 2019, 31, 29–39. [Google Scholar] [CrossRef]
- Han, P.; Lu, Q.; Fan, L.; Zhou, W. A review on the use of microalgae for sustainable aquaculture. Appl. Sci. 2019, 9, 2377. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; Wang, H.; Cheng, Y.; Liu, H.; Jiang, Z.; Li, P.; Wang, Y. Effects of different dissolved organic matter on microbial communities and arsenic mobilization in aquifers. J. Hazard. Mater. 2021, 411, 125146. [Google Scholar] [CrossRef]
- Philippot, L.; Griffiths, B.S.; Langenheder, S. Microbial community resilience across ecosystems and multiple disturbances. Microbiol. Molecul. Biol. Rev. 2021, 85, e00026-20. [Google Scholar] [CrossRef]
- Tawfik, A.; Niaz, H.; Qadeer, K.; Qyyum, M.A.; Liu, J.J.; Lee, M. Valorization of algal cells for biomass and bioenergy production from wastewater: Sustainable strategies, challenges, and techno-economic limitations. Renew. Sust. Energ. Rev. 2022, 157, 112024. [Google Scholar] [CrossRef]
- Fallahi, A.; Rezvani, F.; Asgharnejad, H.; Nazloo, E.K.; Hajinajaf, N.; Higgins, B. Interactions of microalgae-bacteria consortia for nutrient removal from wastewater: A review. Chemosphere 2021, 272, 129878. [Google Scholar] [CrossRef]
- Chai, W.S.; Tan, W.G.; Munawaroh, H.S.H.; Gupta, V.K.; Ho, S.H.; Show, P.L. Multifaceted roles of microalgae in the application of wastewater biotreatment: A review. Environ. Pollut. 2021, 269, 116236. [Google Scholar] [CrossRef]
- Razaviarani, V.; Arab, G.; Lerdwanawattana, N.; Gadia, Y. Algal biomass dual roles in phycoremediation of wastewater and production of bioenergy and value-added products. Int. J. Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Efremenko, E.; Stepanov, N.; Maslova, O.; Senko, O.; Aslanli, A.; Lyagin, I. “Unity and struggle of opposites” as a basis for the functioning of synthetic bacterial immobilized consortium that continuously degrades organophosphorus pesticides. Microorganisms 2022, 10, 1394. [Google Scholar] [CrossRef] [PubMed]
- Dubber, D.; Gray, N.F. Replacement of chemical oxygen demand (COD) with total organic carbon (TOC) for monitoring wastewater treatment performance to minimize disposal of toxic analytical waste. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2010, 45, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.N.; Nikolskaya, A.B.; Mamedova, F.T.; Senko, O.V.; Trusov, L.I. Semicontinuous and continuous process of accumulation of microalgae biomass of Chlorella vulgaris cells. ISJAEE 2013, 2, 44–49. [Google Scholar]
- Bharte, S.; Krutika, D. Techniques for harvesting, cell disruption and lipid extraction of microalgae for biofuel production. Biofuels 2021, 12, 285–305. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Maslova, O.V.; Kholstov, A.V.; Senko, O.V.; Ismailov, A.D. Biosensitive element in the form of immobilized luminescent photobacteria for detecting ecotoxicants in aqueous flow-through systems. Luminescence 2016, 31, 283–1289. [Google Scholar] [CrossRef]
- Yu, H.; Jia, S.; Dai, Y. Growth characteristics of the cyanobacterium Nostoc flagelliforme in photoautotrophic, mixotrophic and heterotrophic cultivation. J. Appl. Phycol. 2009, 21, 127–133. [Google Scholar] [CrossRef]
- Xie, Y.; Jin, Y.; Zeng, X.; Chen, J.; Lu, Y.; Jing, K. Fed-batch strategy for enhancing cell growth and C-phycocyanin production of Arthrospira (Spirulina) platensis under phototrophic cultivation. Bioresour. Technol. 2015, 180, 281–287. [Google Scholar] [CrossRef]
- Coronado-Reyes, J.A.; Salazar-Torres, J.A.; Juarez-Campos, B.; Gonzalez-Hernandez, J.C. Chlorella vulgaris, a microalgae important to be used in Biotechnology: A review. Food Sci. Technol. 2022, 42, e37320. [Google Scholar] [CrossRef]
- Gao, K.; Köster, A.; Thol, M.; Wu, J.; Lemmon, E.W. Equations of state for the thermodynamic properties of n-Perfluorobutane, n-Perfluoropentane, and n-Perfluorohexane. Ind. Eng. Chem. Res. 2021, 60, 17207–17227. [Google Scholar] [CrossRef]
- Menz, D.H.; Feltgen, N.; Menz, H.; Müller, B.K.; Lechner, T.; Dresp, J.; Hoerauf, H. How to ward off retinal toxicity of perfluorooctane and other perfluorocarbon liquids? Invest. Ophthalmol. Vis. Sci. 2018, 59, 4841–4846. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ma, Q.; Cao, J.; Shi, Y.; Wang, J.; Ma, H.; Song, Y. Bifunctional alginate/chitosan stabilized perfluorohexane nanodroplets as smart vehicles for ultrasound and pH responsive delivery of anticancer agents. Int. J. Biol. Macromol. 2021, 191, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yan, K.; Fu, C.; Peng, H.; Hawker, C.J.; Whittaker, A.K. Biological utility of fluorinated compounds: From materials design to molecular imaging, therapeutics and environmental remediation. Chem. Rev. 2021, 122, 167–208. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.N. (Ed.) Immobilized Cells: Biocatalysts and Processes; RIOR: Moscow, Russia, 2018; p. 524. ISBN 978-5-369-02004-3. [Google Scholar] [CrossRef]
- Rana, M.S.; Prajapati, S.K. Stimulating effects of glycerol on the growth, phycoremediation and biofuel potential of Chlorella pyrenoidosa cultivated in wastewater. Environ. Technol. Innov. 2021, 24, 102082. [Google Scholar] [CrossRef]
- Rattanapoltee, P.; Dujjanutat, P.; Muanruksa, P.; Kaewkannetra, P. Biocircular platform for third generation biodiesel production: Batch/fed batch mixotrophic cultivations of microalgae using glycerol waste as a carbon source. Biochem. Eng. J. 2021, 175, 108128. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, W.; Su, Y.; Ren, Q.; Ji, Z.; Zhang, A. Utilization of N-Acyl homoserine lactone-secreting bacteria in algal environment to increase biomass accumulation of Chlorella. BioEnergy Res. 2022, 15, 2111–2121. [Google Scholar] [CrossRef]
- Qixin, L.; Xuan, F.; Zhiya, S.; Wenxin, S.; Shuo, W.; Ji, L. Enhanced wastewater treatment performance by understanding the interaction between algae and bacteria based on quorum sensing. Bioresour. Technol. 2022, 354, 127161. [Google Scholar] [CrossRef]
- Chetverikov, S.P.; Sharipov, D.A.; Korshunova, T.Y.; Loginov, O. Degradation of perfluorooctanyl sulfonate by strain Pseudomonas plecoglossicida 2.4-D. Appl. Biochem. Microbiol. 2017, 53, 533–538. [Google Scholar] [CrossRef]
- Del Rio-Chanona, E.A.; Cong, X.; Bradford, E.; Zhang, D.; Jing, K. Review of advanced physical and data-driven models for dynamic bioprocess simulation: Case study of algae–bacteria consortium wastewater treatment. Biotechnol. Bioeng. 2019, 116, 342–353. [Google Scholar] [CrossRef]
- Rezvani, F.; Sarrafzadeh, M.H.; Oh, H.M. Hydrogen producer microalgae in interaction with hydrogen consumer denitrifiers as a novel strategy for nitrate removal from groundwater and biomass production. Algal Res. 2020, 45, 101747. [Google Scholar] [CrossRef]
- Subashchandrabose, S.R.; Venkateswarlu, K.; Venkidusamy, K.; Palanisami, T.; Naidu, R.; Megharaj, M. Bioremediation of soil long-term contaminated with PAHs by algal–bacterial synergy of Chlorella sp. MM3 and Rhodococcus wratislaviensis strain 9 in slurry phase. Sci. Total Environ. 2019, 659, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Dow, L. How do quorum-sensing signals mediate algae–bacteria interactions? Microorganisms 2021, 9, 1391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lens, P.N.L.; Shi, W.; Zhang, R.; Zhang, Z.; Guo, Y.; Bao, X.; Cui, F. The attachment potential and N-acyl-homoserine lactone-based quorum sensing in aerobic granular sludge and algal-bacterial granular sludge. Appl. Microbiol.Biotechnol. 2018, 102, 5343–5353. [Google Scholar] [CrossRef]
- Stock, F.; Bilcke, G.; De Decker, S.; Osuna-Cruz, C.M.; Van den Berge, K.; Vancaester, E.; De Veylder, L.; Vandepoele, K.; Mangelinckx, S.; Vyverman, W. Distinctive growth and transcriptional changes of the diatom seminavis robusta in response to quorum sensing related compounds. Front. Microbiol. 2020, 11, 1240. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, C.; Fu, L.; Xu, L.; Gui, X.; Li, Q.; Crittenden, J.C. Responses of the microalga Chlorophyta sp. to bacterial quorum sensing molecules (N-acylhomoserine lactones): Aromatic protein-induced self-aggregation. Environ. Sci. Technol. 2017, 51, 3490–3498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, W.; Guo, Y.; Zhang, Z.; Shi, W.; Gui, F.; Lens, P.N.L.; Tay, J.H. Microalgal-bacterial consortia: From interspecies interactions to biotechnological applications. Renew. Sustain. Energy Rev. 2020, 118, 109563. [Google Scholar] [CrossRef]
- Efremenko, E.; Senko, O.; Stepanov, N.; Maslova, O.; Lomakina, G.Y.; Ugarova, N. Luminescent analysis of ATP: Modern objects and processes for sensing. Chemosensors 2022, 10, 493. [Google Scholar] [CrossRef]
- Berman, M.C.; O’Farrell, I.; Huber, P.; Marino, D.; Zagarese, H. A large-scale geographical coverage survey reveals a pervasive impact of agricultural practices on plankton primary producers. Agric. Ecosyst. Environ. 2022, 325, 107740. [Google Scholar] [CrossRef]
- Verasoundarapandian, G.; Lim, Z.S.; Radziff, S.B.M.; Taufik, S.H.; Puasa, N.A.; Shaharuddin, N.A.; Merican, F.; Wong, C.-Y.; Lalung, J.; Ahmad, S.A. Remediation of pesticides by microalgae as feasible approach in agriculture: Bibliometric strategies. Agronomy 2022, 12, 117. [Google Scholar] [CrossRef]
- Goh, P.S.; Lau, W.J.; Ismail, A.F.; Samawati, Z.; Liang, Y.Y.; Kanakaraju, D. Microalgae-enabled wastewater treatment: A sustainable strategy for bioremediation of pesticides. Water 2023, 15, 70. [Google Scholar] [CrossRef]
- Nicodemus, T.J.; DiRusso, C.C.; Wilson, M.; Black, P.N. Reactive oxygen species (ROS) mediated degradation of organophosphate pesticides by the green microalgae Coccomyxa subellipsoidea. Bioresour. Technol. Rep. 2020, 11, 100461. [Google Scholar] [CrossRef]
- Llorca, M.; Farré, M.; Sànchez-Melsió, A.; Villagrasa, M.; Knepper, T.P.; Barceló, D. Perfluoroalkyl phosphonic acids adsorption behaviour and removal by wastewater organisms. Sci. Total Environ. 2018, 636, 273–281. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).