Abstract
Previously, we reported the synthesis of carbosilane and carbosilane-siloxane dendrons of various generations based limonene, a natural terpene. Limonene that contains two double bonds, namely cyclohexene and isoprenyl ones, was shown to undergo regioselective hydrosilylation exclusively at its isoprenyl double bond. This finding was used to prepare carbosilane dendrons (CDs) with a limonene moiety at the focal point. In this study, we present variants for the functionalization of the cyclohexene double bond by an epoxidation reaction in order to use the resulting dendrons for the preparation of various macromolecular objects, including Janus dendrimers (JDs), dendronized polymers, and macroinitiators. Moreover, it was shown that dendrons with peripheral azide functions could be obtained. These methods offer both the possibilities of the further growth of branches and the addition of polymers with a different nature by the azide–alkyne cycloaddition reaction.
1. Introduction
Dendrimers are widely used currently due to their unique structure. In terms of structure, they can be considered as more ordered analogues of branched polymers with a similar chemical structure of the polymer chain. However, due to their mathematically precise molecular geometry, dendrimers differ significantly from their polymer analogs. Since the structure and the number of generations determine the molecular weight, dendrimers exhibit no statistical distribution of molecular weights, in contrast to the polymer analogs. Due to the growth of the dendrimer density from the center to the periphery, such molecules at higher generations become impenetrable spheres and behave as colloidal particles. Nevertheless, these molecules contain cavities for which the size and geometry can be controlled by the chemical structure of the branches and the branching center. Owing to this, dendrimer molecules are used in various fields, for example, in the delivery of drugs and genetic materials [1,2], to transport catalysts and produce nanoparticles [3,4,5,6,7,8], and in nonlinear optics devices [9,10,11,12,13]. Conjugated star-shaped molecules with donor–acceptor links are used as donor components in organic photovoltaic cells [14,15,16]. Functional dendrimers are now widely used in materials science, nanotechnologies, and to modify the physical chemistry of a surface; in particular, in chromatography as a stationary phase [17], for selective sorption [18,19] and for the preparation of organic–inorganic nanocomposites [20], inorganic nanomaterials [21], mesoporous coatings. and nanoparticles [22,23].
Carbosilane dendrimers (CDs) are one of the interesting and promising classes of dendritic molecules. Due to their chemical structure, primarily the high Si-C bond energy, these molecules possess high biological inertness and biological compatibility, high thermal and thermal-oxidative stability, and high hydrophobicity [24,25]. Due to these properties, this type of macromolecules are employed in many areas of today’s science; however, they make the largest contribution to catalysis [26,27,28] and drug delivery [29,30,31,32,33,34].
For the synthesis of these compounds, approaches to the preparation of CDs have been developed by teams guided by Muzafarov [35,36,37] and Frey [38,39]. However, although proven techniques exist, new approaches to the synthesis of carbosilane, carbosilane-siloxane, and other hybrid dendrimers are currently under development. This activity is primarily driven by the need to simplify the synthesis, obtain new functional macromolecules, and obtain hybrid dendrimers [40,41].
We have shown in our previous studies that natural terpenes can be used to obtain block copolymers [42,43] and dendrimers [44]. This approach is based on the phenomenon of regioselective hydrosilylation of the isoprenyl double bond, while the cyclohexene double bond can be involved either in subsequent hydrothiolation or in further functionalization. In this way, based on limonene, carbosilane, and carbosilane-siloxane dendrons of various generations in which limonene is the branching point were obtained. The presence of a cyclohexene double bond in such dendrons allows further functionalizations to be implemented.
The purpose of this study is to show that functional groups can be incorporated at the cyclohexene double bond and that limonene-based dendron chains can be functionalized by simple synthetic methods to obtain macromolecules with various structures, including hybrid dendrimers, Janus dendrimers, and macroinitiators.
2. Experimental
2.1. Material and Methods
All reactions were conducted under an inert atmosphere, and solvents were purified from the appropriate drying agents. D-Limonene, propiolic acid, n-butyllithium (solution 2.5 M in hexane) were purchased from Sigma-Aldrich; allylchloride, dichloromethylsilane, sodium azide, meta-chloroperoxybenzoic acid, lithium aluminum hydride, and Karstedt’s Pt catalyst (2% solution in o-xylene) Pt2(DVDS)3 were obtained from ABCR commercial sources.
Gel permeation chromatography (GPC) analysis was performed on a Shimadzu LC-10A series chromatograph (Tokyo, Japan) equipped with an RID-10A refractometer and SPD-M10A diode matrix detectors. For analytical separation, Phenomenex column (Torrance, USA) with a size of 7.8 mm × 300 mm filled with the Phenogel sorbent with a pour size of 500 Å was used.
The GC analysis was performed on a “Chromatech Analytic 5000” chromatograph (Yoshkar-Ola, Russia) with a katharometer as detector, helium as carrier gas, with 2 m × 3 mm column, and stationary phase SE-30 (5%) was applied to Chromaton-H-AW. Registration and data collection was performed with the help of the program “Chromatech Analyst” (Yoshkar-Ola, Russia).
1H, 13C, 29Si nuclear magnetic resonance (NMR) spectra and their nuclear correlations were recorded using a Bruker Avance II 300 spectrometer (Billerica, USA) at 300, 75 and 60 MHz, respectively.
2.2. Material and Methods
These compounds were synthesized according to [44]. Yield of the product (ϕ) in all the cases is given.
- Diallyl(2-(4-methylcyclohex-3-en-1-yl)propyl)methylsilane (Lim-G0All2)
ϕ = 82%. For C17H30Si: C 77.71%; H 11.43%; Si 10.67%: C 77.39%; H 11.25%; Si 10.31%.
- Triallyl(2-(4-methylcyclohex-3-en-1-yl)propyl)silane (Lim-G0All3)
ϕ = 85%. For C19H32Si: C 79.02%; H 11.09%; Si 9.70%: C 75.57%; H 10.61%; Si 9.23%.
- Dendrimer of the first generation (Lim-G1All4)
ϕ = 7.5 g (66%). For C31H58Si3: C 72.23%; H 11.26%; Si 16.31%: C 72.17%; H 11.16%; Si 16.24%.
- 1,1,1,3,5,5,5-heptamethyl-3-(2-(4-methylcyclohex-3-en-1-yl)propyl)trisiloxane (Lim-G0.5TMS2)
ϕ = 89%. For C17H38O2Si3: C 56.87%; H 10.59%; O 8.92%; Si 23.42%: C 54.36%; H 10.01%; Si 21.00%.
- Bis(heptamethylsilylpropyl)methylsilyllimonene (Lim-G1.5TMS4)
ϕ = 99%. For C31H74O4Si7: C 52.58%; H 10.46%; O 9.05%; Si 27.70%: C 50.02%; H 9.84%; Si 26.56%.
- Tris(heptamethylsilylpropyl)silyllimonene (Lim-G1.5TMS6)
ϕ = 99%. For C40H98O6Si10: C 50.21%; H 10.25%; O 10.04%; Si 29.29%: C 48.96%; H 10.14%; Si 28.44%
- Dendrimer of the 2.5 generation (Lim-G2.5TMS8)
ϕ = 99%. For C59H146O8Si15: C 50.39%; H 10.39%; O 9.11%; Si 29.89%: C 48.91%; H 9.95%; Si 28.27%.
- CD of the first generation (Lim-G1Cl4)
To the stirred solution of Lim-G0All2 (5.0 g, 0.019 mol) and 50 μL of Pt2(DVDS)3 in anhydrous toluene (100 mL), methyldichlorosilane (6.57 g, 0.057 mol) was added. RM was stirred at room temperature for 24 h. The completeness of the process was controlled by 1H NMR spectroscopy from disappearance of proton signals at double bonds in allyl groups. The reaction mixture was evaporated to remove excess dimethylchlorosilane.
- CD of the first generation (Lim-G1Bu4)
To a stirred mixture of 37 mL (2.5 M) of n-BuLi in anhydrous hexane (50 mL), a solution of 9.38 g (0.019 mol) Lim-G1Cl4 in anhydrous hexane (50 mL) at −70 °C was added. When addition of the mixture was finished, THF (70 mL) was added to the solution. RM was stirred at −70 °C, and then at room temperature for 24 h. Excess n-BuLi was deactivated with ethanol. The precipitate of LiCl was filtered off and washed with n-hexane on the filter. The filtrate was evaporated to remove the solvents, and the residue was dissolved in hexane and passed through silica. Solvent was removed by evaporation under reduced pressure. ϕ = 78%. For C35H74Si3: C 72.51%; H 12.78%; Si 14.50%: C 72.52%; H 12.64%; Si 14.30%.
- 3-Chloropropyldimethylsilane
This compound was synthesized according to the method published in [45]. Yield of the product was 72%.
1H NMR (300 MHz, Chloroform-d) δ: 3.87 (m, 1H, SiH), 3.52 (t, J = 6.9 Hz, 2H, CH2), 1.89–1.73 (m, 2H, CH2), 0.77–0.63 (m, 2H, CH2), 0.09 (d, J = 3.5 Hz, 6H, SiCH3).
- Bis(3-chloropropyldimethylsilylpropyl)methylsilyllimonene (Lim-G0(PrCl)2)
To a stirred RM of 1.14 g (0.0084 mol) of 3-chloropropyldimethylsilane and 1.0 g (0.0038 mol) of Lim-G0All2, purged with argon, 11 mL dry toluene and 11 μL of Pt2(DVDS)3 were added. RM was stirred at 60 °C for 10 h. After reaction completed, substance was evaporated to remove the solvents, and the residue was evacuated (0.5 mbar) at 70 °C. ϕ = 99%.
- Tris(3-chloropropyldimethylsilylpropyl)silyllimonene (Lim-G1(PrCl)3)
This compound was obtained similarly to Lim-G1.5Cl2 from 1.0 g (0.0035 mol) Lim-G0All3, 1.56 g (0.0114 mol) 3-chloropropyldimethylsilane, 13 μL of Pt2(DVDS)3, and 13 mL anhydrous toluene. ϕ = 99%.
- Dendrimer of the 2.5 generation (Lim-G2(PrCl)4)
This compound was obtained similarly to Lim-G1.5Cl2 from 1.0 g (0.0019 mol) Lim-G1All4, 1.17 g (0.0085 mol) 3-chloropropyldimethylsilane, 12 μL of Pt2(DVDS)3, and 12 mL anhydrous toluene. Yield of the product was 99%.
- Bis(3-azidopropyldimethylsilylpropyl)methylsilyllimonene (Lim-G0(PrN3)2)
To a stirred RM of 1.5 g (0.0028 mol) of Lim-G1.5Cl2 and 0.44 g (0.0067 mol) of sodium azide, 7.5 mL dry DMF was added. RM was stirred at 70 °C for 6 h. After reaction finished, the product was extracted with toluene and washed with distilled water three times. Organic layer was dried over Na2SO4. Solvent was removed by evaporation under reduced pressure. ϕ = 92%.
- Tris(3-azidopropyldimethylsilylpropyl)silyllimonene (Lim-G1(PrN3)3)
This compound was obtained similarly to Lim-G1.5(N3)2 from 1.5 g (0.0021 mol) Lim-G1.5Cl3, 0.50 g (0.0077 mol) sodium azide, and 7.5 mL anhydrous DMF. ϕ = 89%.
- CD of the 2.5 generation (Lim-G2(PrN3)4)
This compound was obtained similarly to Lim-G1.5(N3)2 from 1.5 g (0.0014 mol) Lim-G2.5Cl4, 0.44 g (0.0068 mol) sodium azide, and 7.5 mL anhydrous DMF. ϕ = 90%.
- 1,1,1,3,5,5,5-Heptamethyl-3-(2-(3,4-epoxy-4-methylcyclohex-1-yl)propyl)trisiloxane (LimOx-G0.5TMS2)
To a stirred, ice-cold solution of Lim-G0.5TMS2 (2.0 g, 0.0056 mol) in DCM (100 mL), NaHCO3 (0.94 g, 0.0112 mol) and m-CPBA (1.44 g, 0.0084 mol) were added sequentially. The mixture was stirred at 0 °C for 30 min. Then, the process was stopped by addition of 0.3 M aqueous solution of Na2S2O3 (200 mL) and extracted with dichloromethane (3 × 50 mL). The combined organic phases were washed sequentially with a saturated aqueous NaHCO3 solution (3 × 100 mL) and brine (100 mL), dried over Na2SO4, and concentrated under reduced pressure. ϕ = 82%.
- Bis(heptamethylsilylpropyl)methylsilyl-1,2-epoxy-limonene (LimOx-G1.5TMS4)
This compound was synthesized similarly to Lim-G0.5TMS2O from 2.0 g (0.0028 mol) Lim-G1.5TMS4, 0.73 g (0.0042 mol) m-CPBA, 0.47 g (0.0057 mol) NaHCO3, and 85 mL DCM. ϕ = 85%.
- Tris(heptamethylsilylpropyl)silyl-1,2-epoxy-limonene (LimOx-G1.5TMS6)
This substance was obtained similarly to Lim-G0.5TMS2O from 2.0 g (0.0021 mol) Lim-G1.5TMS6, 0.54 g (0.0031 mol) m-CPBA, 0.35 g (0.0042 mol) NaHCO3, and 80 mL DCM. ϕ = 83%.
- Epoxidized dendrimer of the 2.5 generation (LimOx-G2.5TMS8)
Synthesized similarly to Lim-G0.5TMS2O from 2.0 g (0.0014 mol) Lim-G2.5TMS8, 0.37 g (0.0021 mol) m-CPBA, 0.24 g (0.0028 mol) NaHCO3, and 76 mL DCM. ϕ = 80%.
- Epoxidized dendrimer of the first generation (LimOx-G1Bu4)
Synthesized similarly to Lim-G0.5TMS2O from 2.0 g (0.0035 mol) Lim-G1Bu4, 0.89 g (0.0052 mol) m-CPBA, 0.58 g (0.0069 mol) NaHCO3, and 90 mL DCM. ϕ = 82%.
- Azide-functionalized dendrimer of the first generation (LimN3-G1Bu4)
To a stirred mixture of 0.50 g (0.84 mmol) of Lim-G1Bu4O, 0.045 g (0.84 mmol) of NH4Cl and 0.11 g (0.0017 mol) of sodium azide, 3.5 mL dry methanol was added. RM was refluxed for 5 h. After that, the product was washed under standard conditions. Organic layer was dried over Na2SO4. Solvent was removed by evaporation under reduced pressure. ϕ = 88%.
- Hydroxy-functionalized dendrimer of the first generation (LimOH-G1Bu4)
First, 0.038 g (0.0010 mol) of LiAlH4 was added to a 5% solution of Lim-G1Bu4O (0.5 g, 0.84 mmol) in anhydrous THF, and the mixture was refluxed for 5 h under argon. Then, CH3COOH was added dropwise and the product was extracted with diethyl ether and washed with distilled water three times. Organic layer was dried over Na2SO4. Solvent was evaporated. ϕ = 86%.
- Propargylate-functionalized dendrimer of the first generation (LimC≡C-G1Bu4)
First, 0.5 g (0.84 mmol) of Lim-G1Bu4O and 0.071 g (0.0010 mol) of propiolic acid were mixed and stirred at 70 °C for 6 h. After reaction completed, the product was washed under standard conditions. Organic layer was dried over Na2SO4. Solvent was distilled off. ϕ = 90%.
3. Results and Discussion
Limonene is a natural monoterpene that is widespread in nature. The technology of its isolation from the peel of citrus fruits as a waste product of the food industry is a well-studied and streamlined process [46,47]. It is interesting to note that citrus essential oils contain only d-limonene, while its isomer, l-limonene, is found in conifers. The commercial availability of limonene and its low toxicity predetermined its use as an alternative solvent to hexane or cyclohexane. In addition to this utilitarian use, attempts to use limonene for the synthesis of polymers as an alternative to crude oil and gas were reported. These reasons shaped our interest in limonene regarding the synthesis of dendrimers and JDs. JDs are often obtained using naturally occurring compounds with varying functionality as the parent branching site. Our interest in limonene as a branching point was guided by the possibility to selectively implement the hydrosilylation reaction, one of the main reactions of organosilicon chemistry that is used, in particular, for the synthesis of carbosilane and siloxane dendrimers. As we noted in the Introduction, limonene is involved in hydrosilylation selectively at the isoprenyl double bond, thus retaining the possibility of further functionalization of the cyclohexene double bond. This approach is efficient in the syntheses of amphiphilic dendrons, JDs, or dendronized polymers.
We have shown in a previous study that limonene-based dendrons with various peripheral groups can be obtained. This work, which is a logical continuation of that study, explores the ways to further functionalize the cyclohexene double bond and functionalize the peripheral substituents. These approaches would make it possible, in the future, to obtain dendrons with various natures and use the peripheral function for further modifications or to build more complex dendronized structures.
3.1. Epoxidation of Limonene-Based Carbosilane and Carbosilane-Siloxane Dendrons
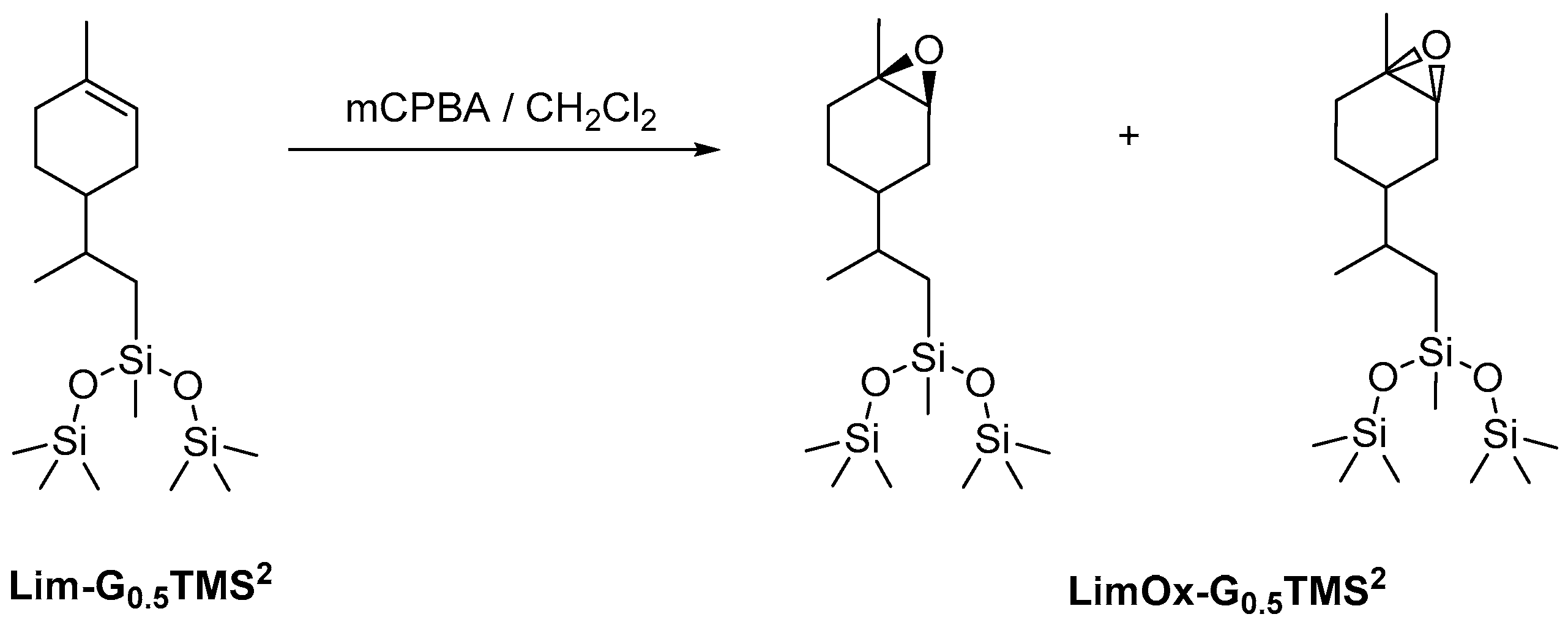
In the first stage, we studied the possibility of epoxidizing the cyclohexene double bond of limonene organosilicon derivatives. For a more focused examination, the product of limonene hydrosilylation with 1,1,1,3,5,5,5-heptamethyltrisiloxane was chosen as the model compound (Scheme 1). The siloxane derivative was chosen to enable the selection of epoxidation conditions under which the destruction of the siloxane bond does not occur, since this type of bond is known to be unstable in acidic and basic media and in the presence of oxidizing agents. The epoxidation of limonene is fairly well described in the literature [48,49,50]. Various reagents are used to perform this reaction: hydrogen peroxide, oxone, m-chloroperbenzoic acid, etc. In this work, we used m-chloroperbenzoic acid due to its high reactivity in the epoxidation reaction.
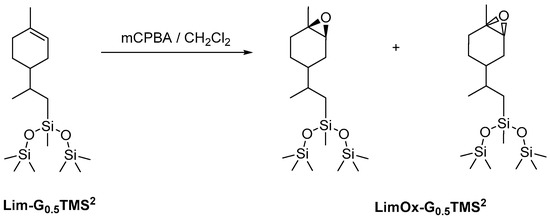
Scheme 1.
Epoxidation of trisiloxylimonene derivative using m-chloroperbenzoic acid.
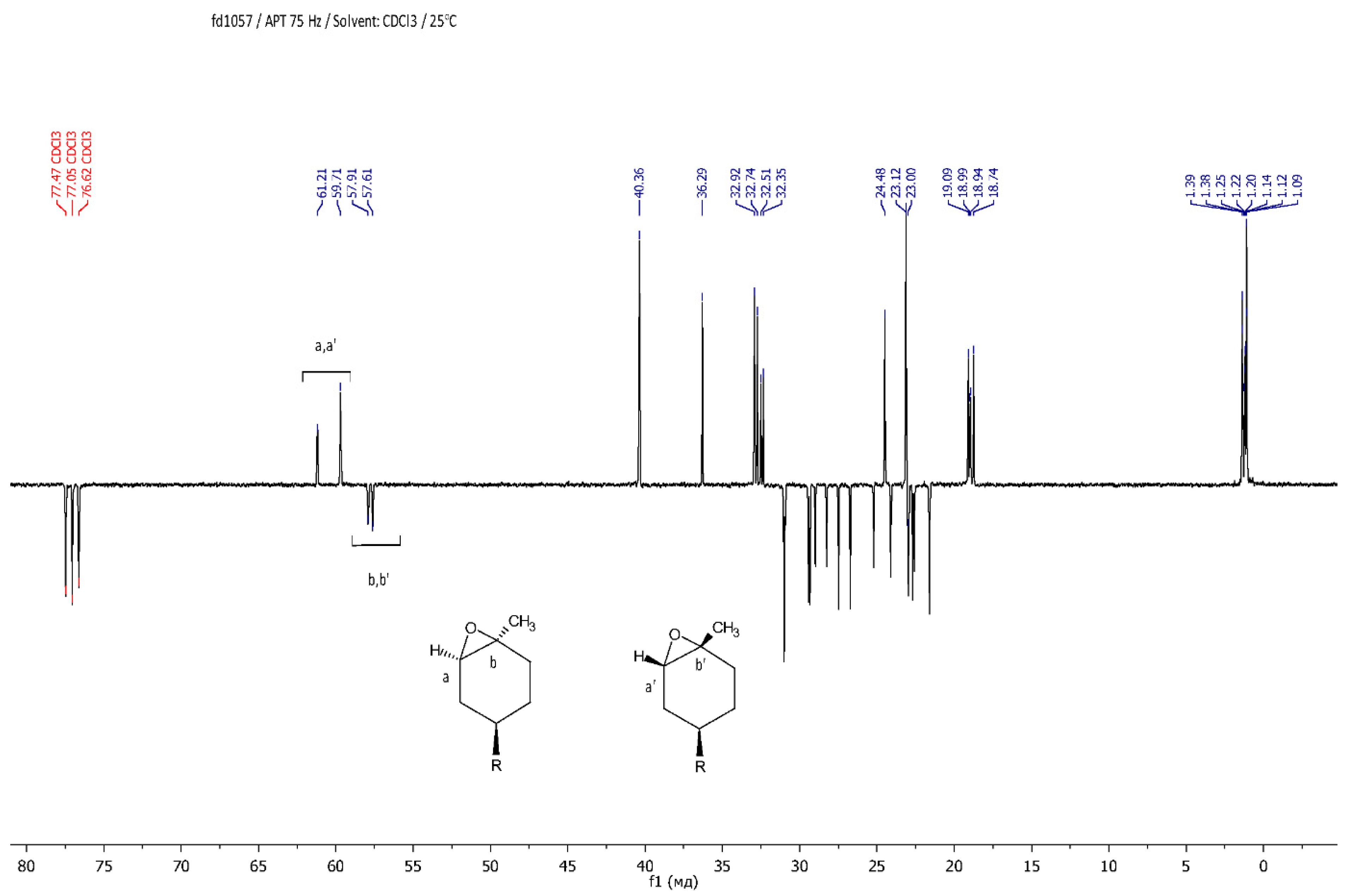
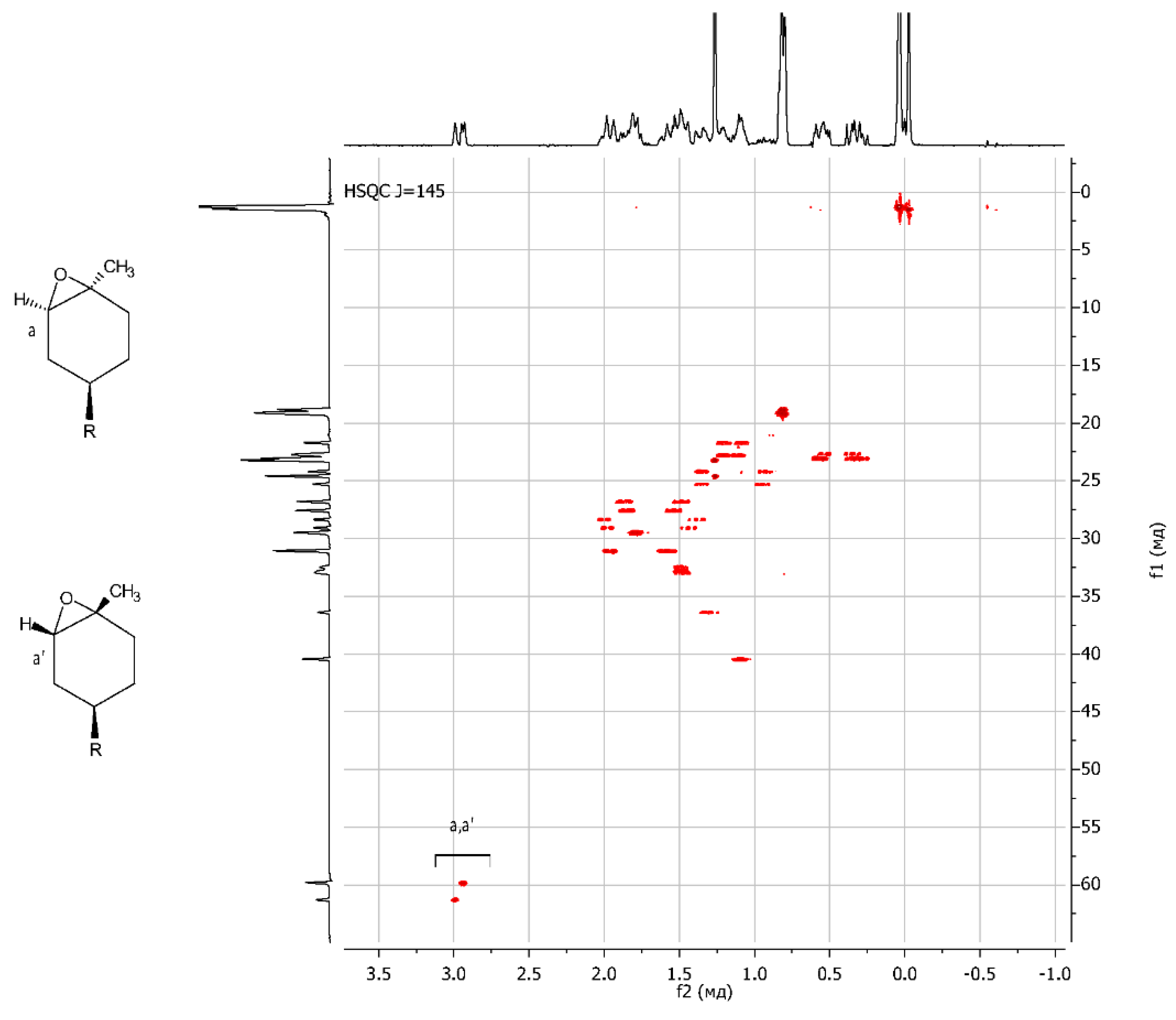
The resulting epoxy dendrons were mixtures of exo- and endo-isomers in a ratio of 1:1, as confirmed by NMR spectroscopy methods. Using NMR APT spectra, two signals for multiple carbon substitution (57.91 ppm and 57.61 ppm) and two signals for non-multiple carbon substitution (61.21 ppm and 59.71 ppm) were shown (Figure 1). The HSQC spectra have cross-peaks characterizing the C-H correlations at the oxirane ring, which makes it possible to attribute these signals in the carbon spectrum (Figure 2).
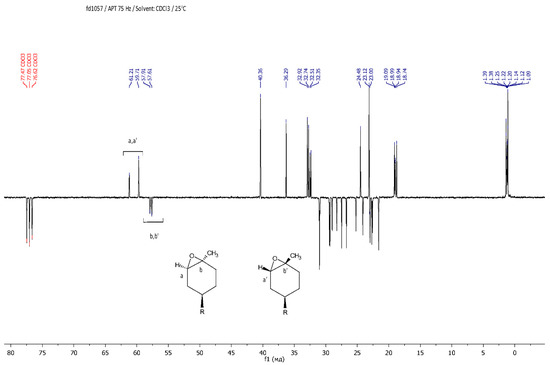
Figure 1.
APT NMR spectrum of LimOx-G0.5TMS2.
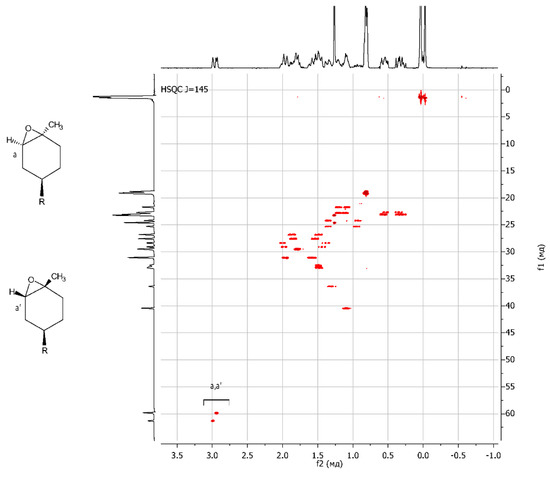
Figure 2.
{1H;13C} HSQC NMR spectrum of LimOx-G0.5TMS2.
After isolation of the reaction products, it was proved by NMR and GPC methods that epoxidation under the conditions we selected did not disrupt the siloxane bond.
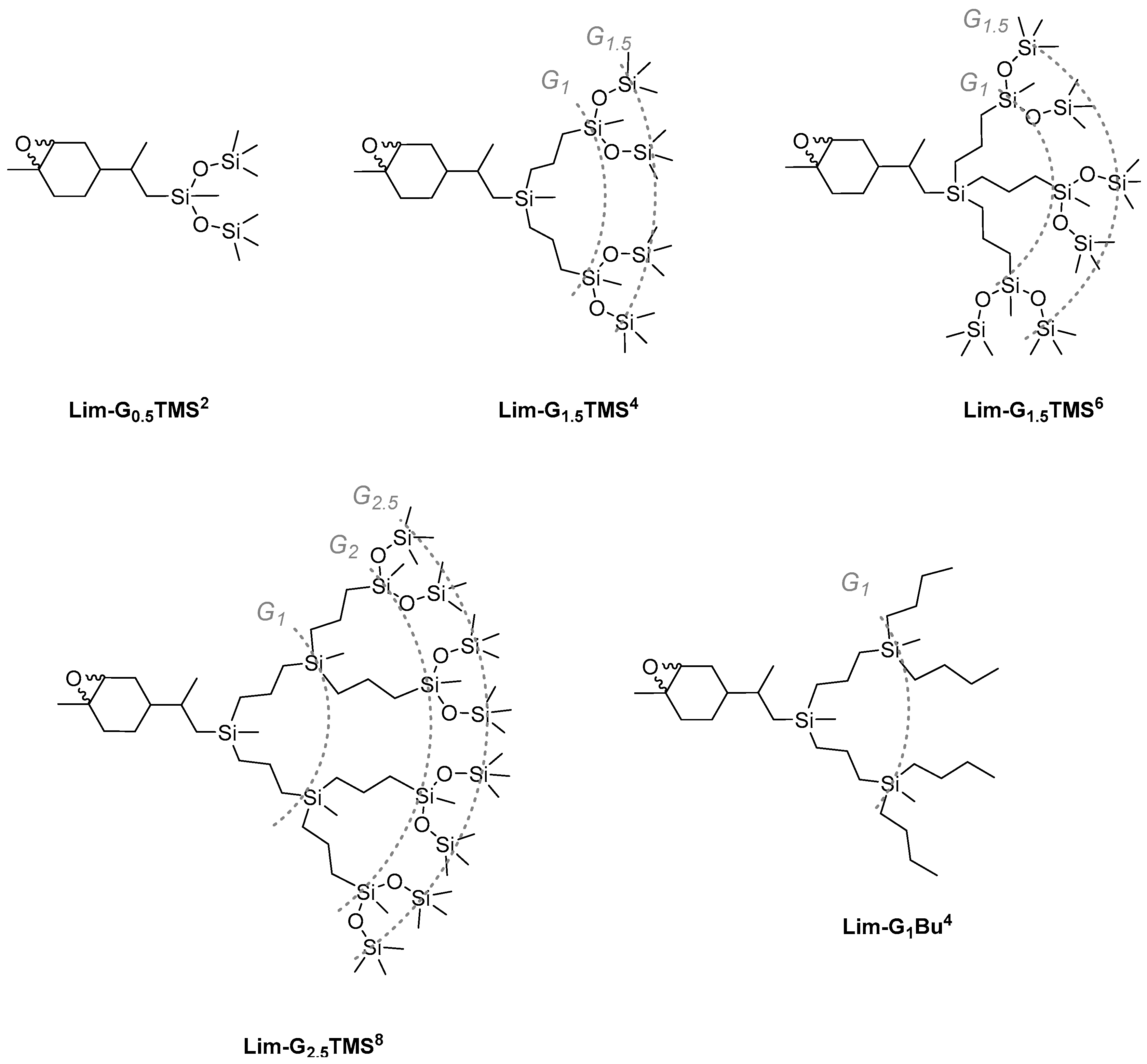
The epoxidation of cyclohexene double bonds in the corresponding limonene-based dendrons was performed under similar conditions (Scheme 2). In terms of the formal dendrimer nomenclature, the LimOx-G0.5TMS2 model compound can be considered as a generation-0.5 dendron. The figure shows the resulting carbosilane-siloxane dendrons of various generations: two-chain and three-chain generation-1.5 dendrons LimOx-G1.5TMS4 and LimOx-G1.5TMS6, and a two-chain generation-2.5 dendron LimOx-G2.5TMS8. To develop the synthesis of JDs further, we decided to test the epoxidation reaction under similar conditions on a completely carbosilane dendron. CDs are known to be more chemically stable than siloxane or carbosilane-siloxane ones. However, personal observations of the authors reveal that there are some reactions that efficiently occur on dendrons with a siloxane bond but nearly do not occur on carbosilane systems. All products were isolated in high yields, 89–99%. The structure and purity of the dendrons was proved by NMR spectroscopy and GPC (Supporting Information, SI1 and SI2).
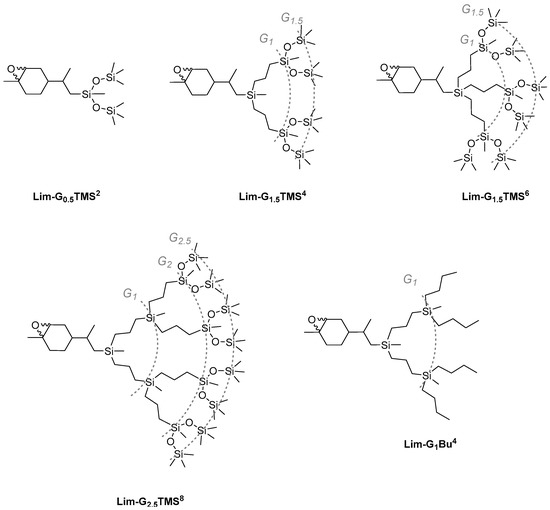
Scheme 2.
Carbosilane-siloxane dendrons based on limonene of different generations and different numbers of branches.
However, it was shown that epoxidation also occurred quantitatively on a generation-1 carbosilane dendron with peripheral butyl substituents and the corresponding mixture of Lim-G1Bu4 endo- and exo-epoxides was formed.
3.2. Opening of the Oxirane Ring in Limonene-Based Carbosilane and Carbosilane-Siloxane Dendrons
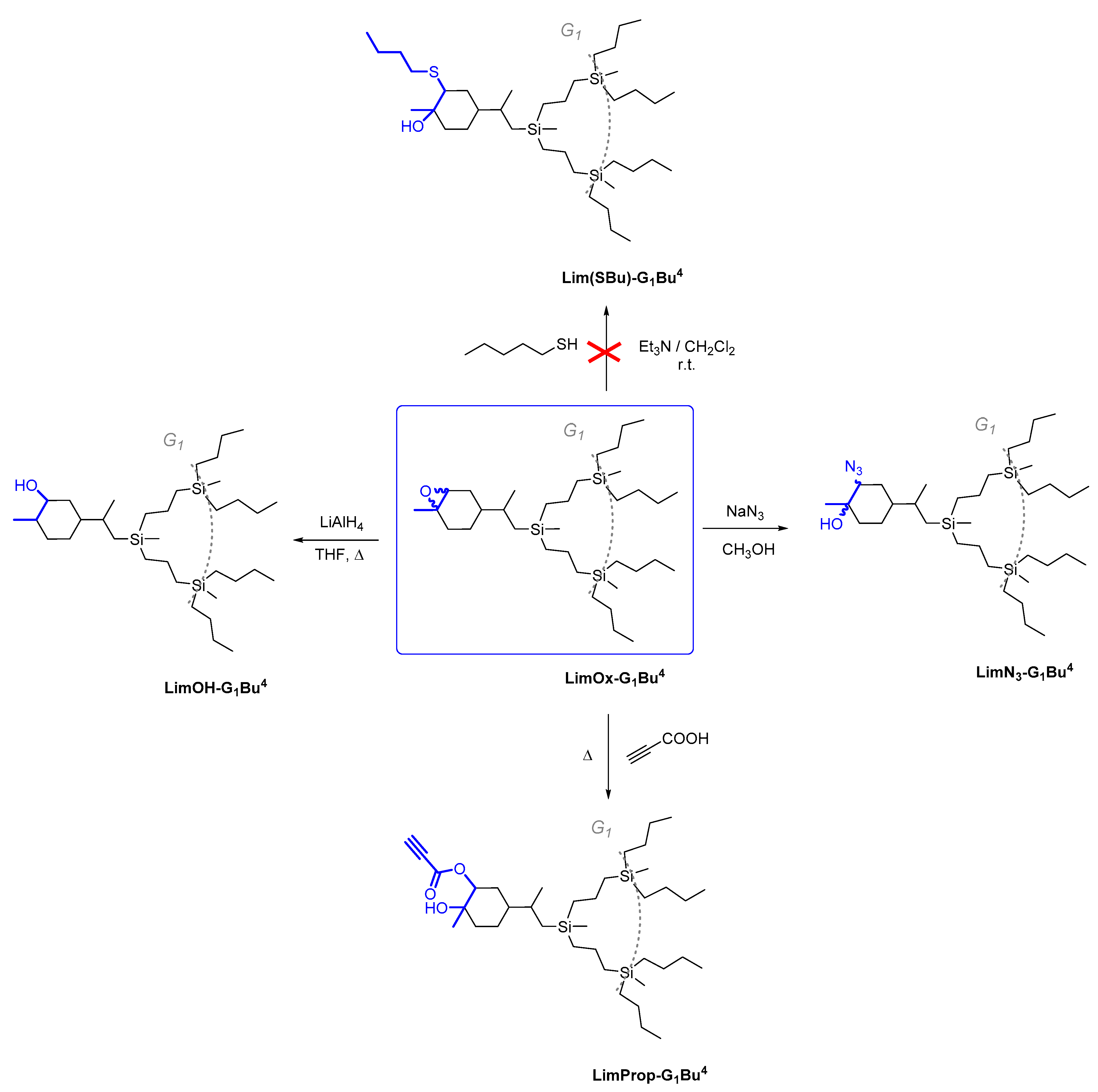
From the perspective of applied chemistry, the synthesis of dendrons with an oxirane ring at the focal point is of interest, primarily due to the possibility of its opening on treatment with both nucleophiles and electrophiles. The simplicity of oxirane ring opening along with the possibility of further incorporation of a wide range of functional groups offer innumerable options for further chemical transformations of the dendrons obtained.
Thus, in this work, we have shown the possibility of epoxide ring opening in dendrons with various nucleophiles that are of interest for further applications, including azide, thiolate, carboxylate, and hydride anions. The carbosilane-epoxidized dendron Lim-G1Bu4 was chosen as the model compound for these purposes. It was chosen for three reasons: first, because of the simpler NMR spectroscopic analysis of the products obtained upon oxirane ring opening; second, because the possible splitting of the siloxane bond in carbosilane-siloxane dendrons was prevented; and third, because it is CDs that are required in the subsequent synthesis of JDs.
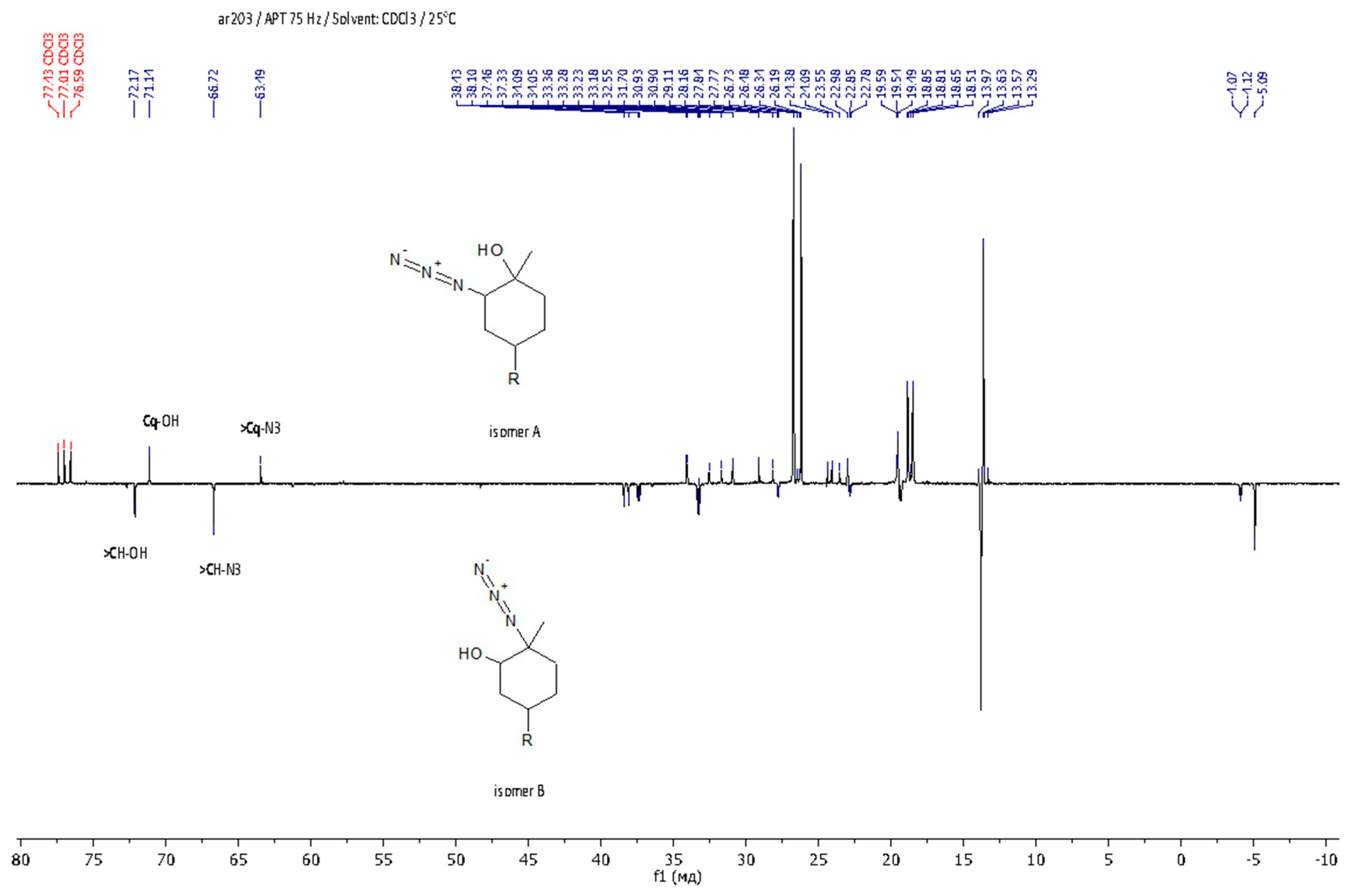
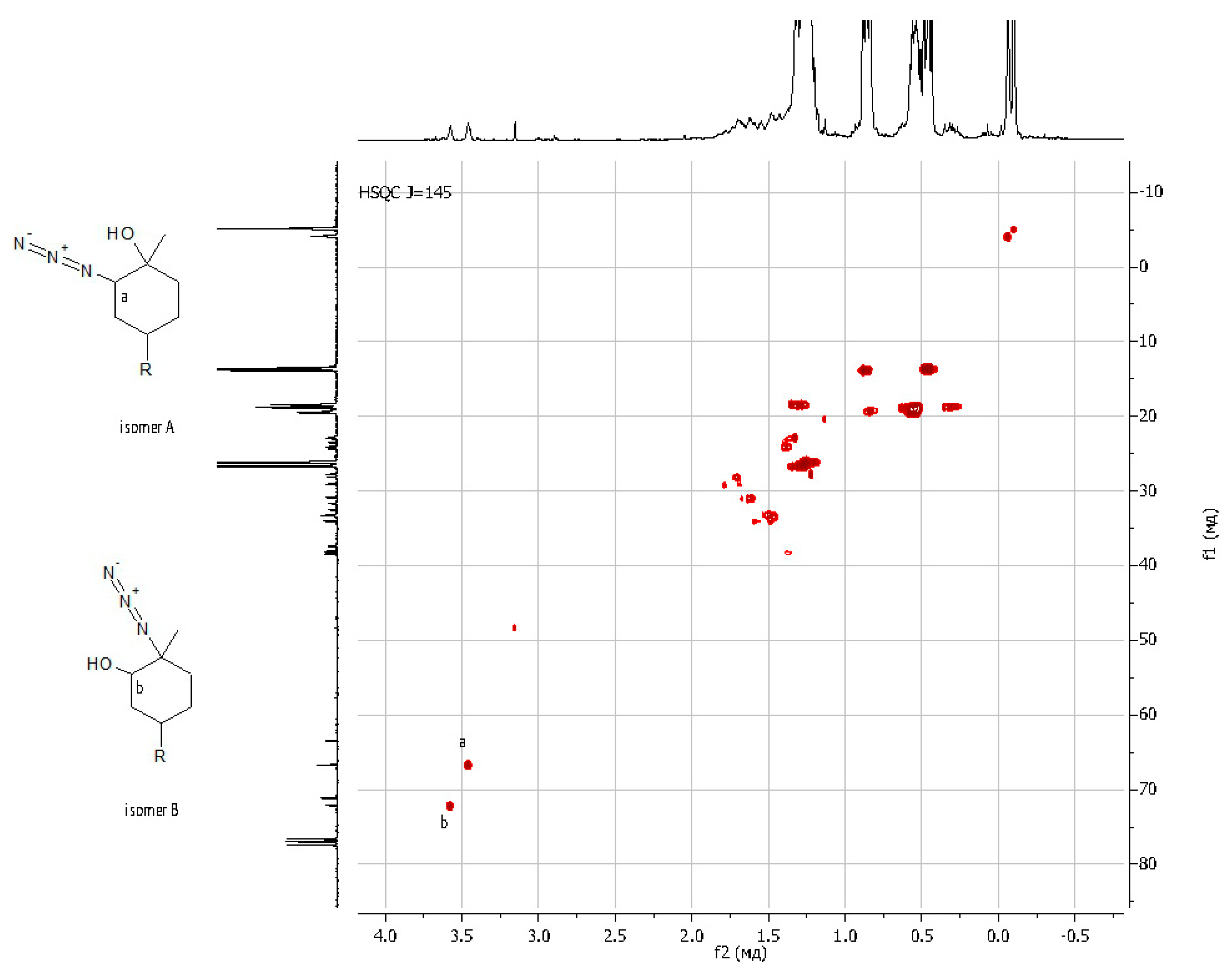
As regards further functionalization, the incorporation of the azide function is of great interest because it can be used for azide-alkyne cycloaddition and can be reduced to an amino group. The opening of the epoxide ring with sodium azide was performed under standard conditions by refluxing in methanol with sodium azide (Scheme 3, on the right) [51]. The progress of the reaction was monitored by 1H NMR spectroscopy through the disappearance of the characteristic signals of oxirane protons δ = 3.01 and 2.96 ppm. The yield of the product was 88%. It was shown that during the epoxidation of limonene dendrons, a mixture of regioisomers is formed in a ratio of 1:1. Using APT spectra, two signals of multiple carbon substitution (71.11 ppm and 63.19 ppm) and two signals of non-multiple carbon substitution (72.17 ppm and 66.72 ppm) were shown (Figure 3). The HSQC spectrum has cross-peaks, characterizing the C-H correlations for the azide group and for the hydroxyl group (Figure 4). According to the mechanism of this reaction, functional substituents (hydroxyl and azide groups) always take an axial position in the cyclohexane ring.
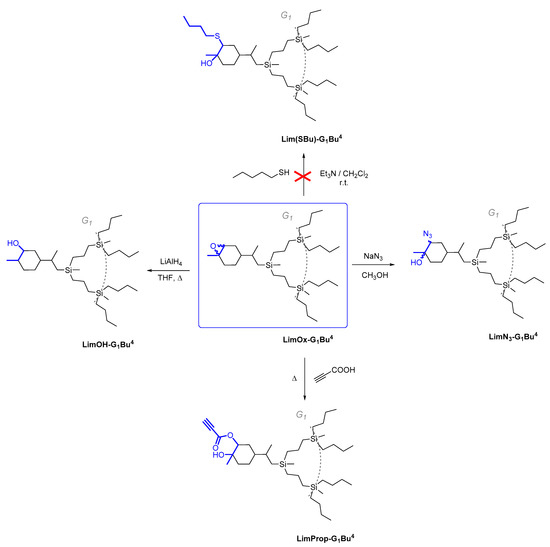
Scheme 3.
Cleavage of oxirane cycle of CDs based on epoxylimonene by different nucleophiles.
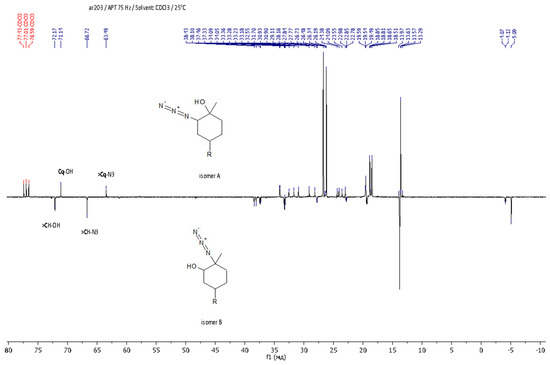
Figure 3.
APT NMR spectrum of LimN3-G1Bu4.
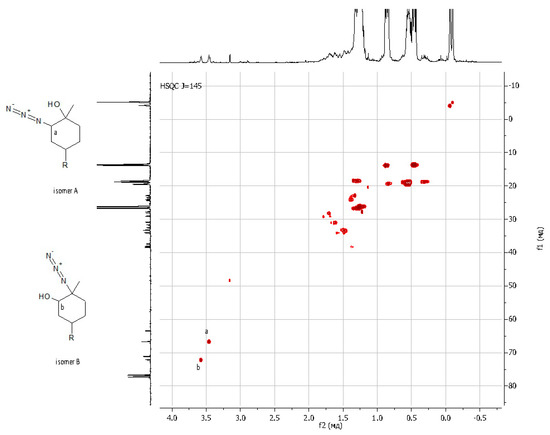
Figure 4.
{1H 13C} HSQC NMR spectrum of LimN3-G1Bu4.
The ethynyl group is yet another important function involved in the azide-alkyne cycloaddition reaction. Therefore, we made an attempt to perform oxirane ring opening with propargylic acid (Scheme 3, on bottom). The reaction was performed without a solvent with heating at 70 °C for 6 h until complete conversion. After isolation, the product yield was 90%.
We also studied the possibility of reducing the epoxy ring to an alcohol group. Reactions of this kind result in a secondary alcohol upon treatment with reducing agents such as metallic sodium or lithium aluminum hydride (Scheme 3, on the left).
Refluxing of the corresponding epoxide with lithium aluminum hydride was shown to result in complete opening of the oxirane ring. The product yield after isolation was 86%.
An attempt to perform the oxirane ring opening with thiolate (Scheme 3, on top) failed to yield a noticeable conversion according to 1H NMR spectra; hence, it is of no preparative value.
3.3. Functionalization of the Periphery of Limonene-Based Carbosilane Dendrons
As discussed in the previous section, CDs with a functional focal point are of great interest for further use in JDs, dendronized polymers, etc. To this end, many research teams, including ours, are actively developing the application of azide-alkyne cycloaddition as a convenient and versatile method for synthesizing complex macromolecules with various topologies. Therefore, the next stage of this work was to functionalize the peripheral groups of CDs with azide groups aimed at subsequent addition of hydrophilic or hydrophobic chains by azide-alkyne cycloaddition.
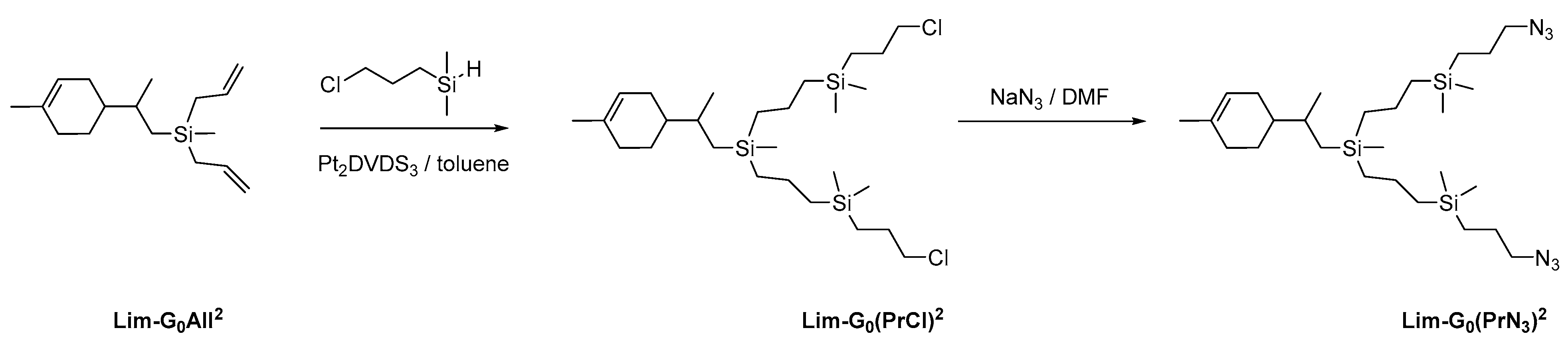
To obtain azide functional peripheral groups, we chose diallylsilyl limonene, which we obtained previously, as the model compound. For functionalization, the corresponding hydrosilane was first obtained from commercially available (3-chloropropyl)dimethylchlorosilane by the standard procedure, then hydrosilylation with diallylsilyl limonene was performed (Scheme 4). Hydrosilylation occurred with complete conversion in nearly quantitative yield. At the final stage, nucleophilic replacement of the chlorine atom by the azide group was performed. The yield of the final product at this stage reached 92%.
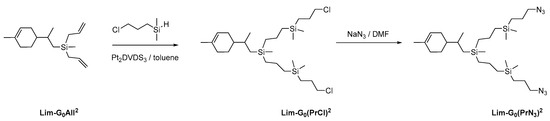
Scheme 4.
Strategy of synthesis of CDs with azidopropyl periphery branches.
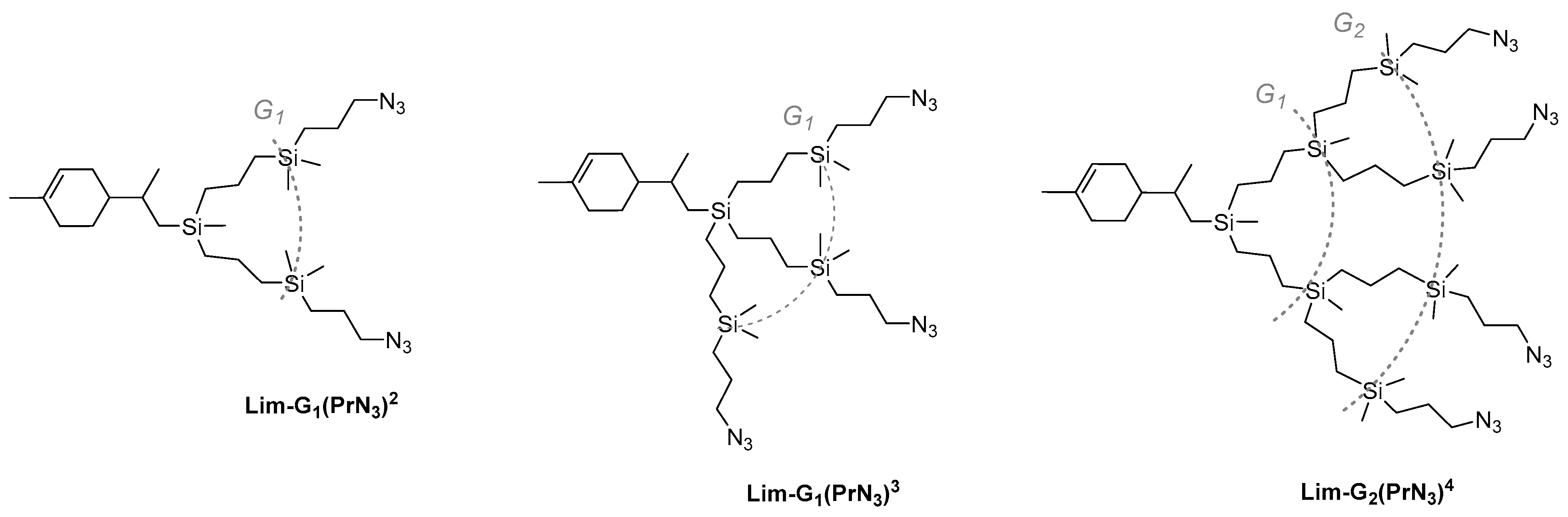
Based on the allyl-functional dendrons obtained previously, a library of CDs with peripheral azide groups of various generations and with various numbers of chains—two, three, or four—were obtained in a similar way (Scheme 5).
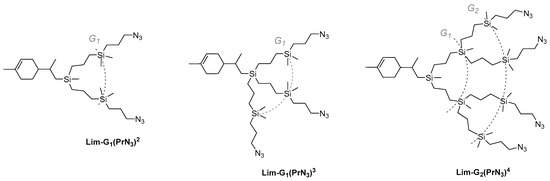
Scheme 5.
Structures of CDs with azidopropyl periphery branches of different generations and number of branches.
Azide-functional dendrons Lim-G1(PrN3)2 and Lim-G1(PrN3)2 were obtained from diallylsilyl- and triallylsilyllimonene, respectively; formally, from a nomenclature point of view, they can be considered as generation-1 dendrons. The Lim-G2(PrN3)4 dendron was obtained from the generation-1 dendron with four allyl functions. Thus, this approach makes it possible to obtain dendrons with a successively increasing number of azidopropyl chains and increase the generation of initial dendrons by one. It should be noted that dendrons are synthesized in high yields using simple methods of organic and organoelement chemistry. The structure and purity of the dendrons were estimated by NMR spectroscopy and GPC (Supporting Information, SI1 and SI2).
4. Conclusions
In this work, which is a logical continuation of previously published work addressing the synthesis of limonene-based carbosilane and carboxylane-siloxane dendrons, methods for the further functionalization of the cyclohexene double bond of limonene and peripheral chains of dendrons were shown.
Using carbosilane-siloxane dendrons as an example, we have shown that the epoxidation of the cyclohexene double bond of limonene can be performed under mild conditions, which makes it possible to perform this reaction without breaking the siloxane bond.
Using CDs as an example, various ways of oxirane ring opening to give functional dendrons at the focal point have been shown.
In addition, a simple and versatile procedure for the synthesis of azide-functional CDs on the periphery with a various number of chains is presented.
The approaches to the synthesis and functionalization of carbosilane and carbosilane-siloxane dendrons considered here should make it possible, in the future, to obtain JDs by azide-alkyne cycloaddition and other “click” reactions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13042121/s1, SI1: NMR spectra; SI2: GPC curves; SI3: GC curves.
Author Contributions
Experiment design and execution, manuscript writing/review, A.I.R. and F.V.D.; NMR experiments and analysis, G.V.C.; project administration, F.V.D. and A.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
All synthetic procedures were carried out with support by the Russian Science Foundation (RSF) (Project No. 22-13-00459) and investigations of structures were done with the aid of the Government of the Tula Region (Decree No. 899 of 30 December 2021) under Agreement No. 11 of 7 September 2022. NMR and GPC were performed with the financial support from Ministry of Science and Higher Education of the Russian Federation using the equipment of Collaborative Access Center “Center for Polymer Research” of ISPM RAS (FFSM-2021-0004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article and its Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaur, D.; Jain, K.; Mehra, N.K.; Kesharwani, P.; Jain, N.K. A review on comparative study of PPI and PAMAM dendrimers. J. Nanopart. Res. 2016, 18, 146. [Google Scholar] [CrossRef]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Astruc, D.; Chardac, F. Dendritic Catalysts and Dendrimers in Catalysis. Chem. Rev. 2001, 101, 2991–3024. [Google Scholar] [CrossRef] [PubMed]
- Roesler, R.; Har, B.J.N.; Piers, W.E. Synthesis and Characterization of (Perfluoroaryl)borane-Functionalized Carbosilane Dendrimers and Their Use as Lewis Acid Catalysts for the Hydrosilation of Acetophenone. Organometallics 2002, 21, 4300–4302. [Google Scholar] [CrossRef]
- Nager, M.; Becke, S.; Windisch, H.; Denninger, U. Noncoordinating Dendrimer Polyanions: Cocatalysts for the Metallocene-Catalyzed Olefin Polymerization. Angew. Chem. Int. Ed. 2001, 40, 1898–1902. [Google Scholar]
- Yamamoto, K.; Imaoka, T. Precision Synthesis of Subnanoparticles Using Dendrimers as a Superatom Synthesizer. Acc. Chem. Res. 2014, 47, 1127–1136. [Google Scholar] [CrossRef]
- Yamamoto, K.; Takanashi, K. Synthesis and functionality of dendrimer with finely controlled metal assembly. Polymer 2008, 49, 4033–4041. [Google Scholar] [CrossRef]
- Imaoka, T.; Kawana, Y.; Kurokawa, T.; Yamamoto, K. Macromolecular semi-rigid nanocavities for cooperative recognition of specific large molecular shapes. Nat. Commun. 2013, 4, 2581. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Otomo, A.; Nakahama, T.; Okuno, Y.; Mashiko, S. Dendrimers for optoelectronic applications. In Dendrimers V: Functional and Hyperbranched Building Blocks, Photophysical Properties, Applications in Materials and Life Sciences; Springer: Berlin/Heidelberg, Germany, 2003; Volume 228, p. 205. [Google Scholar]
- Cho, M.J.; Choi, D.H.; Sullivan, P.A.; Akelaitis, A.J.P.; Dalton, L.R. Recent progress in second-order nonlinear optical polymers and dendrimers. Prog. Polym. Sci. 2008, 33, 1013–1058. [Google Scholar] [CrossRef]
- Sullivan, P.A.; Aklelaitis, A.J.P.; Lee, S.K.; Mcgrew, G.; Lee, S.K.; Choi, D.H.; Dalton, L.R. Novel dendritic chromophores for electro-optics: Influence of binding mode and attachment flexibility on electro-optic behavior. Chem. Mater. 2006, 18, 344–351. [Google Scholar] [CrossRef]
- Sullivan, P.A.; Olbricht, B.C.; Akelaitis, A.J.P.; Mistry, A.A.; Liao, Y.; Dalton, L.R. Tri-component Diels–Alder polymerized dendrimer glass exhibiting large, thermally stable, electro-optic activity. J. Mater. Chem. 2007, 17, 2899–2903. [Google Scholar] [CrossRef]
- Albert, I.D.L.; Marks, T.J.; Ratner, M.A. Large molecular hyperpolarizabilities. Quantitative analysis of aromaticity and auxiliary donor–acceptor effects. J. Am. Chem. Soc. 1997, 119, 6575–6582. [Google Scholar] [CrossRef]
- Min, J.; Luponosov, Y.N.; Zhang, Z.-G.; Ponomarenko, S.A.; Ameri, T.; Li, Y.-F.; Brabec, C.J. Interface Design to Improve the Performance and Stability of Solution-Processed Small-Molecule Conventional Solar Cells. Adv. Energy Mater. 2014, 4, 1400816. [Google Scholar] [CrossRef]
- Min, J.; Luponosov, Y.N.; Baran, D.; Chvalun, S.N.; Shcherbina, M.A.; Bakirov, A.V.; Dmitryakov, P.V.; Peregudova, S.M.; Kausch-Busies, N.; Ponomarenko, S.A.; et al. Effects of oligothiophene π-bridge length on physical and photovoltaic properties of star-shaped molecules for bulk heterojunction solar cells. J. Mater. Chem. A 2014, 2, 16135–16147. [Google Scholar] [CrossRef]
- Luponosov, Y.; Min, J.; Solodukhin, A.N.; Bakirov, A.V.; Dmitryakov, P.V.; Shcherbina, M.A.; Peregudova, S.M.; Cherkaev, G.V.; Chvalun, S.N.; Brabec, C.J.; et al. Star-shaped D–π–A oligothiophenes with a tris(2-methoxyphenyl)amine core and alkyldicyanovinyl groups: Synthesis and physical and photovoltaic properties. J. Mater. Chem. C 2016, 4, 7061. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Huang, C.; Wang, L.; Wang, J.; Chen, J. Dendrimer-functionalized mesoporous silica as a reversed-phase/anion-exchange mixed-mode sorbent for solid phase extraction of acid drugs in human urine. J. Chromatogr. A 2015, 1392, 28–36. [Google Scholar] [CrossRef]
- Gerasimov, A.V.; Ziganshin, M.A.; Vandyukov, A.E.; Kovalenko, V.I.; Gorbatchuk, V.V.; Caminade, A.-M.; Majoral, J.-P. Specific vapor sorption properties of phosphorus-containing dendrimers. J. Colloid Interface Sci. 2011, 360, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.J.; Ametov, I.; Prestidge, C.A. Dendrimer adsorption on charged particulate surfaces. Asia-Pac. J. Chem. Eng. 2008, 3, 13–17. [Google Scholar]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Shen, J.; Li, Z.; Wu, Y.; Zhang, B.; Li, F. Dendrimer-based preparation of mesoporous alumina nanofibers by electrospinning and their application in dye adsorption. Chem. Eng. J. 2015, 264, 48–55. [Google Scholar] [CrossRef]
- Acosta, E.J.; Carr, C.S.; Simanek, E.E.; Shan, D.F. Engineering Nanospaces: Iterative Synthesis of Melamine-Based Dendrimers on Amine-Functionalized SBA-15 Leading to Complex Hybrids with Controllable Chemistry and Porosity. Adv. Mater. 2004, 16, 985–989. [Google Scholar] [CrossRef]
- Du, X.; Qiao, S.Z. Dendritic Silica Particles with Center-Radial Pore Channels: Promising Platforms for Catalysis and Biomedical Applications. Small 2015, 11, 392–413. [Google Scholar] [CrossRef] [PubMed]
- Van der Made, A.W.; van Leeuwen, P.W. Silane dendrimers. J. Chem. Soc. Chem. Commun. 1992, 1400–1401. [Google Scholar] [CrossRef]
- Zhou, L.L.; Hadjichristidis, N.; Toporowski, P.M.; Roovers, J. Synthesis and Properties of Regular Star Polybutadienes with 32 Arms. J. Rubber Chem. Technol. 1992, 65, 303–314. [Google Scholar] [CrossRef]
- Martínez-Olid, F.; Benito, J.M.; Flores, J.C.; de Jesús, E. Polymetallic Carbosilane Dendrimers Containing N,N’-Iminopyridine Chelating Ligands: Applications in Catalysis. Isr. J. Chem. 2009, 49, 99–108. [Google Scholar] [CrossRef]
- Eggeling, E.B.; Hovestad, N.J.; Jastrzebski, J.T.; Vogt, G.; van Koten, G. Phosphino carboxylic acid ester functionalized carbosilane dendrimers: Nanoscale ligands for the Pd-catalyzed hydrovinylation reaction in a membrane reactor. J. Org. Chem. 2000, 65, 8857–8865. [Google Scholar] [CrossRef]
- Rodríguez, L.-I.; Rossell, O.; Seco, M.; Grabulosa, A.; Muller, G.; Rocamora, M. Carbosilane Dendrimers Peripherally Functionalized with P-Stereogenic Monophosphines. Catalytic Behavior of Their Allylpalladium Complexes in the Asymmetric Hydrovinylation of Styrene. Organometallics 2006, 25, 1368–1376. [Google Scholar] [CrossRef]
- Andrés, R.; De Jesús, E.; Fierro, J.L.G.; Terreros, P. Bifunctional carbosilane dendrons for the immobilization of zirconocene catalysts on silica. New J. Chem. 2011, 35, 2203–2211. [Google Scholar] [CrossRef]
- Gutierrez-Ulloa, C.E.; Buyanova, M.Y.; Apartsin, E.K.; Venyaminova, A.G.; Javier de la Mata, F.; Valiente, M.; Gómez, R. Amphiphilic carbosilane dendrons as a novel synthetic platform toward micelle formation. Org. Biomol. Chem. 2017, 15, 7352–7364. [Google Scholar] [CrossRef]
- Heredero-Bermejo, I.; Hernández-Ros, J.M.; Sánchez-García, L.; Maly, M.; Verdú-Expósito, C.; Soliveri, J.; Javier de la Mata, F.; Copa-Patiño, J.L.; Pérez-Serrano, J.; Sánchez-Nieves, J.; et al. Ammonium and guanidine carbosilane dendrimers and dendrons as microbicides. Eur. Polym. J. 2018, 101, 159–168. [Google Scholar] [CrossRef]
- Barrios-Gumiel, A.; Sanchez-Nieves, J.; Pérez-Serrano, J.; Gómez, R.; de la Mata, F.J. PEGylated AgNP covered with cationic carbosilane dendrons to enhance antibacterial and inhibition of biofilm properties. Int. J. Pharm. 2019, 569, 118591. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Beltrán, C.; Ceña-Diez, R.; Sepúlveda-Crespo, D.; De la Mata, J.; Gómez, R.; Leal, M.; Muñoz-Fernández, M.A.; Jiménez, J.L. Carbosilane dendrons with fatty acids at the core as a new potential microbicide against HSV-2/HIV-1 co-infection. Nanoscale 2017, 9, 17263–17273. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, N.; Ahmadvand, S.; Ahmadi, S.; Fatahi, Y.; Dinarvandeh, R.; Bagherzadeh, M.; Rabiee, M.; Tahriri, M.; Tayebi, L.; Hamblini, M.R. Carbosilane dendrimers: Drug and gene delivery applications. J. Drug Deliv. Sci. Tech. 2020, 59, 101879. [Google Scholar] [CrossRef]
- Muzafarov, A.M.; Rebrov, E.A. Current trends in the chemistry of dendrimers. Polym. Sci. 2000, 42, 55–77. [Google Scholar]
- Muzafarov, A.M.; Gorbatsevich, O.B.; Rebrov, E.A.; Ignat’eva, G.M.; Chenskaya, T.B.; Myakushev, V.D.; Bulkin, A.F.; Papkov, V.S. Organosilicon dendrimers: Volume-growing polyallylcarbosilanes. Polym. Sci. 1993, 35, 1575–1580. [Google Scholar]
- Rebrov, E.A.; Leshchiner, I.D.; Muzafarov, A.M. Synthesis of Carbosilane Dendrimers with Variable Distance between Branching Nodes. Macromolecules 2012, 45, 8796–8804. [Google Scholar] [CrossRef]
- Lorenz, K.; Hölter, D.; Stühn, B.; Müllwupt, R.; Frey, H. A mesogen-functionalized carbosilane dendrimer: A dendritic liquid crystalline polymer. Adv. Mater. 1996, 8, 414–416. [Google Scholar] [CrossRef]
- Lorenz, K.; Mülhaupt, R.; Frey, H.; Rapp, U.; Mayer-Posner, F.J. Carbosilane-Based Dendritic Polyols. Macromolecules 1995, 28, 6657–6661. [Google Scholar] [CrossRef]
- Kim, C.; Hong, J.H. Carbosilane and Carbosiloxane Dendrimers. Molecules 2009, 14, 3719–3730. [Google Scholar] [CrossRef]
- Dvornic, P.R.; Owen, M.J. Silicon-Containing Dendritic Polymers; Springer: Cham, Switzerland, 2009. [Google Scholar]
- Drozdov, F.V.; Cherkaev, G.V.; Muzafarov, A.M. Synthesis of new functional siloxane derivatives of limonene. Part I: Combination of hydrosilylation and hydrothiolation reactions. J. Organomet. Chem. 2019, 880, 293–299. [Google Scholar] [CrossRef]
- Drozdov, F.V.; Tarasenkov, A.N.; Cherkaev, G.V.; Demchenko, N.V.; Buzin, M.I.; Leites, L.A.; Muzafarov, A.M. Synthesis and properties of prepolymers and their siloxane analogues by thiol-ene polyaddition of limonene with dithiols. Polym. Int. 2019, 68, 2017–2023. [Google Scholar] [CrossRef]
- Ryzhkov, A.I.; Drozdov, F.V.; Cherkaev, G.V.; Muzafarov, A.M. Synthesis of Carbosilane and Carbosilane-Siloxane Dendrons Based on Limonene. Polymers 2022, 14, 3279. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Lee, C.H.; Jun, C.H. Styrylsilane coupling reagents for immobilization of organic functional groups on silica and glass surfaces. Chem. Commun. 2018, 54, 9961–9964. [Google Scholar] [CrossRef]
- Wilbon, P.A.; Chu, F.; Tang, C. Progress in Renewable Polymers from Natural Terpenes, Terpenoids, and Rosin. Macromol. Rapid Commun. 2013, 34, 8–37. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Lomeli-Rodriguez, M.; Demma Carà, P.; Lopez-Sanchez, J.A.; Pagliaro, M. Limonene: A versatile chemical of the bioeconomy. Chem. Commun. 2014, 50, 15288–15296. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, L.; Foster, X.; Kaliaguine, S. Ultrasonic and Catalyst-Free Epoxidation of Limonene and Other Terpenes Using Dimethyl Dioxirane in Semibatch Conditions. ACS Sustain. Chem. Eng. 2018, 6, 12224–12231. [Google Scholar]
- Bihanic, C.; Stanovych, A.; Pelissier, F.; Grison, C. Putting Waste to Work: The Demonstrative Example of Pyrite Quarry Effluents Turned into Green Oxidative Catalysts. ACS Sustain. Chem. Eng. 2019, 7, 6223–6233. [Google Scholar] [CrossRef]
- Schutz, L.; Kazemi, F.; Mackenzie, E.; Bergeron, J.-V.; Gagnon, E.; Claverie, J.P. Trans-limonene dioxide, a promising bio-based epoxy monomer. J. Polym. Sci. 2021, 59, 321–328. [Google Scholar] [CrossRef]
- Cimarelli, C.; Fratoni, D.; Palmieri, G. Synthesis of new enantiopure trans-3,4-diaminocaranes from (+)-3-carene. Tetrahedron Asymmetry 2011, 22, 603–608. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).