Noble Metal-Based Heterogeneous Catalysts for Electrochemical Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Factors That Affect HER Performance
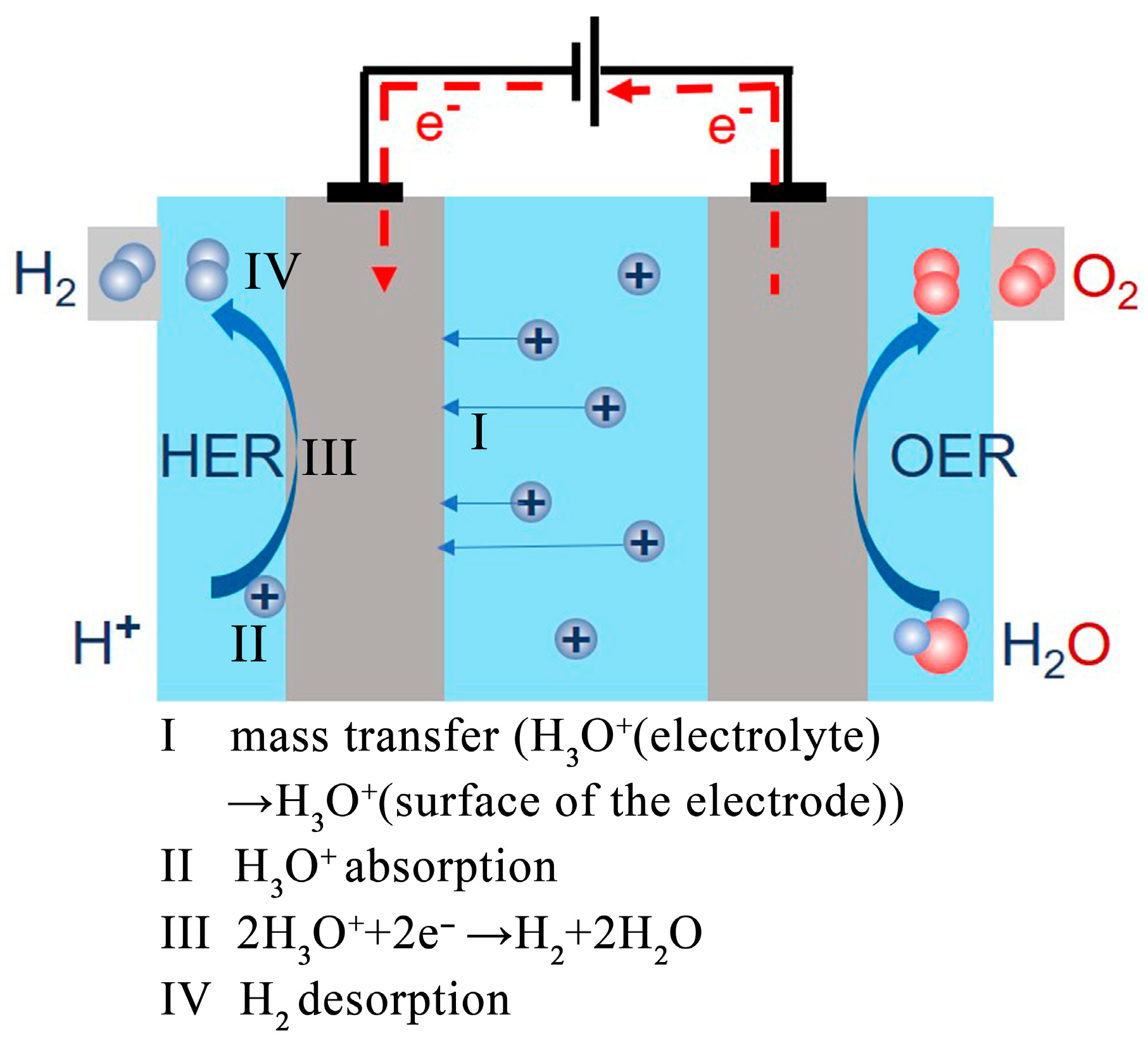
2.1. Mass Transfer Process
2.2. Adsorption and Desorption Process
2.3. Catalytic Process
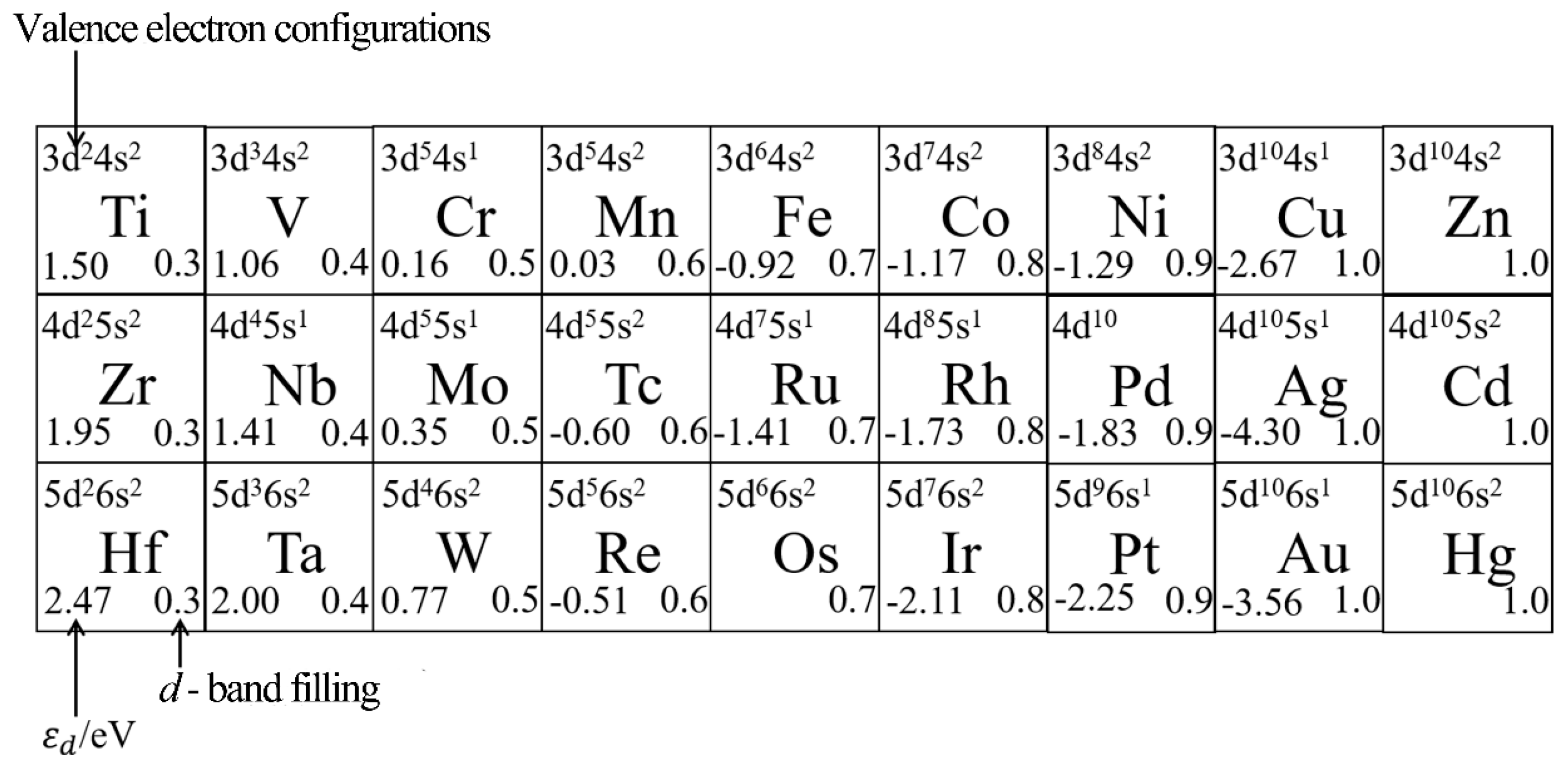
2.3.1. Electron Structure Effect
2.3.2. Surface Structure Effect
2.3.3. Geometric Structure Effect
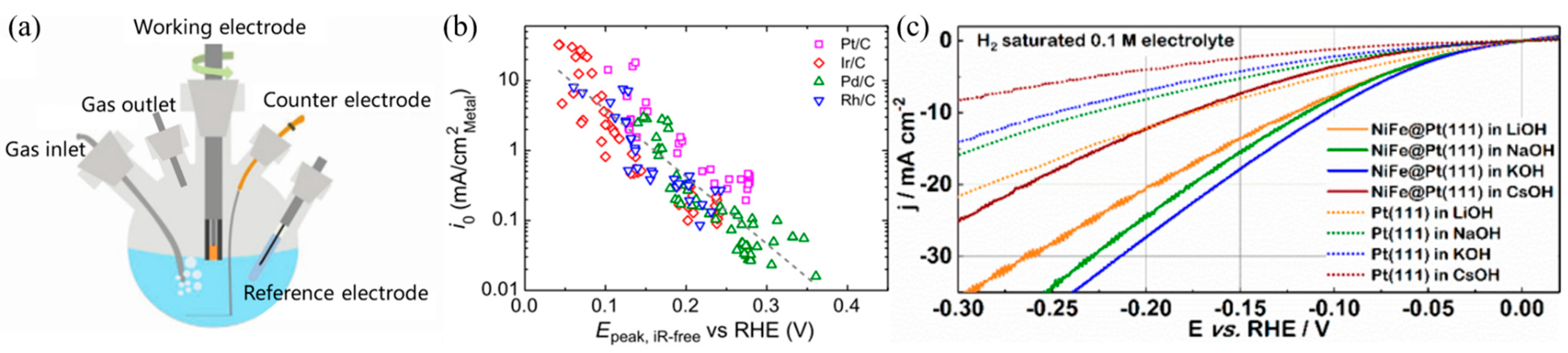
2.4. Influence of the Working Electrode
2.5. Influence of the Electrolyte
3. Catalytic Mechanism of the HER
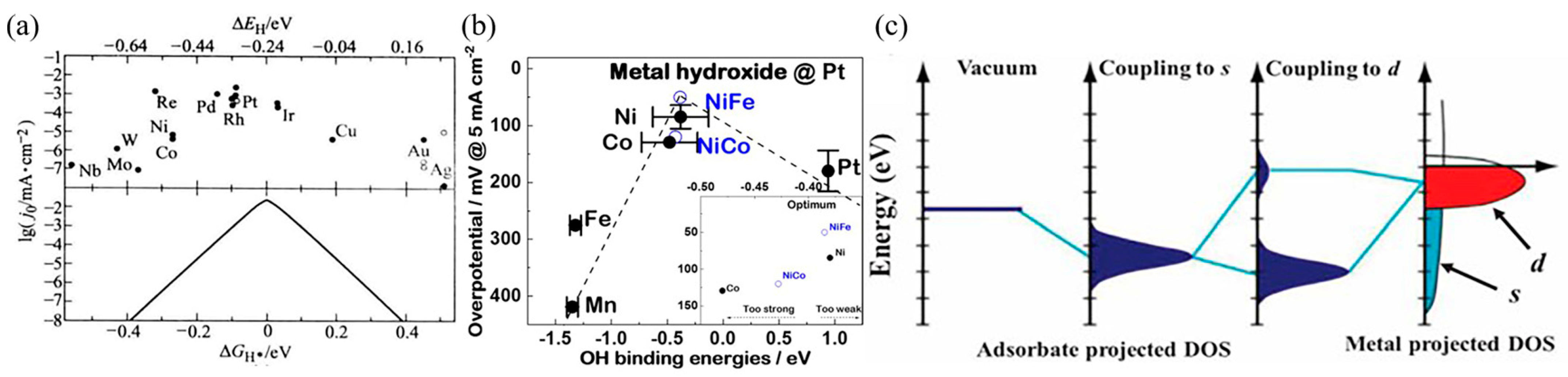
3.1. Volcano Plot
3.2. D-Band Center Theory
4. Noble Metal-Based Heterogeneous Catalysts
4.1. Alloying Strategy
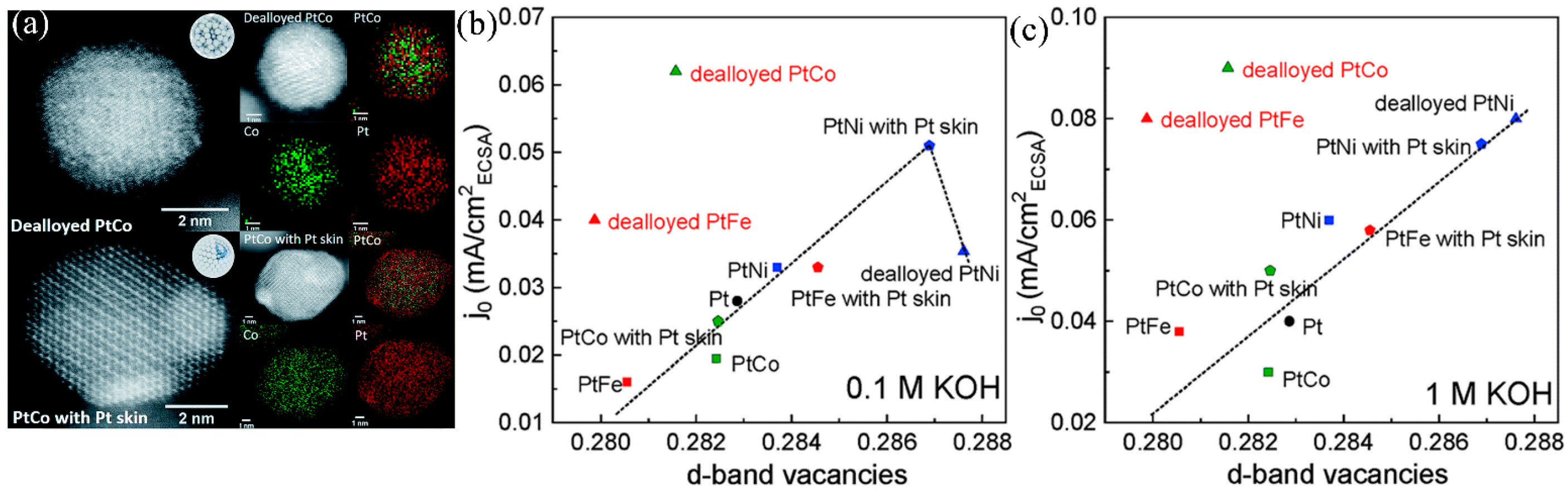
4.1.1. Pt-Base Alloy
4.1.2. Pd-Based Alloy
4.1.3. Other Pt Group Metal Alloys
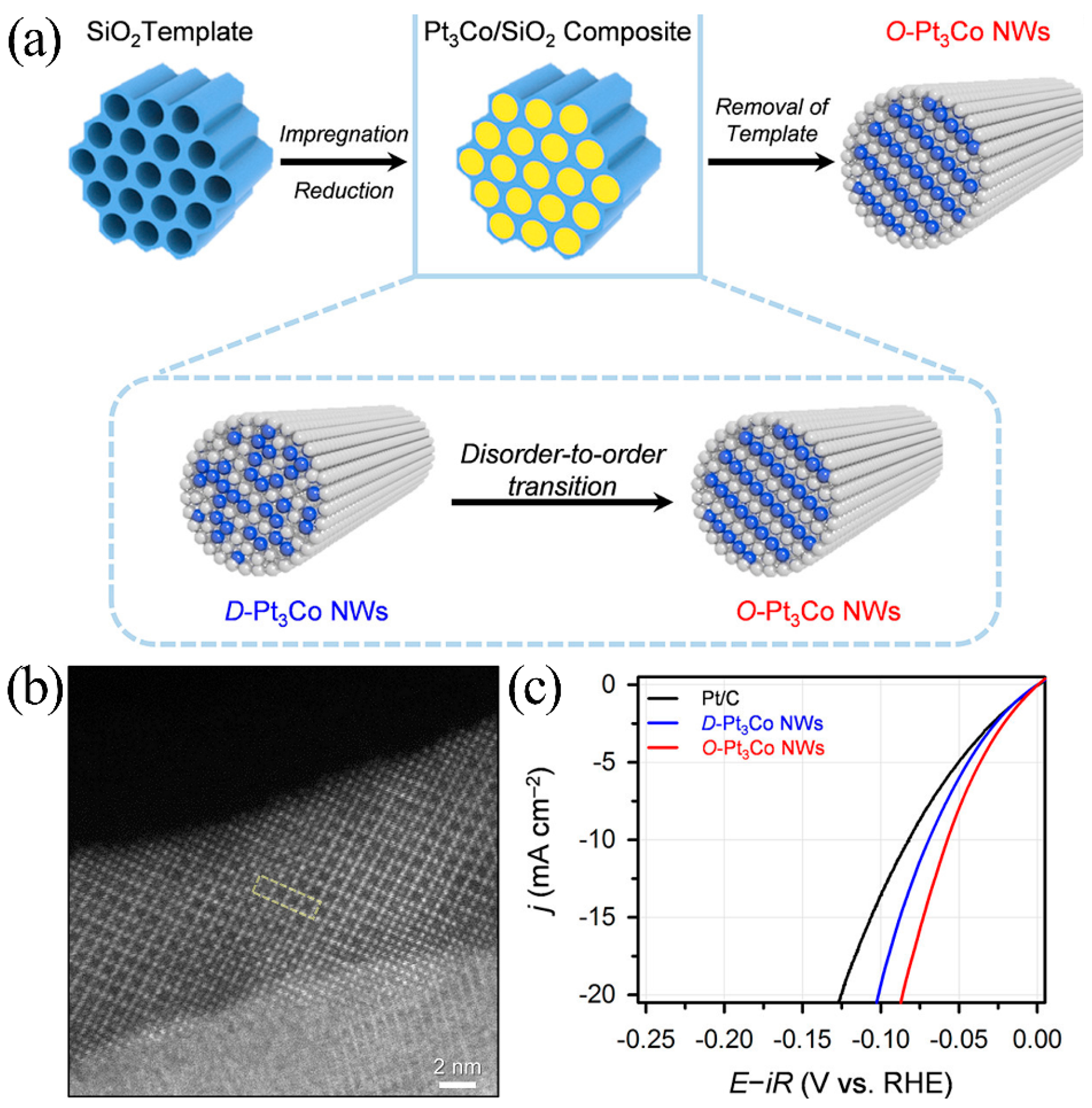
4.1.4. Intermetallic Compound
4.2. Interface Engineering
4.2.1. Metal/Metal Core–Shell Nanostructures
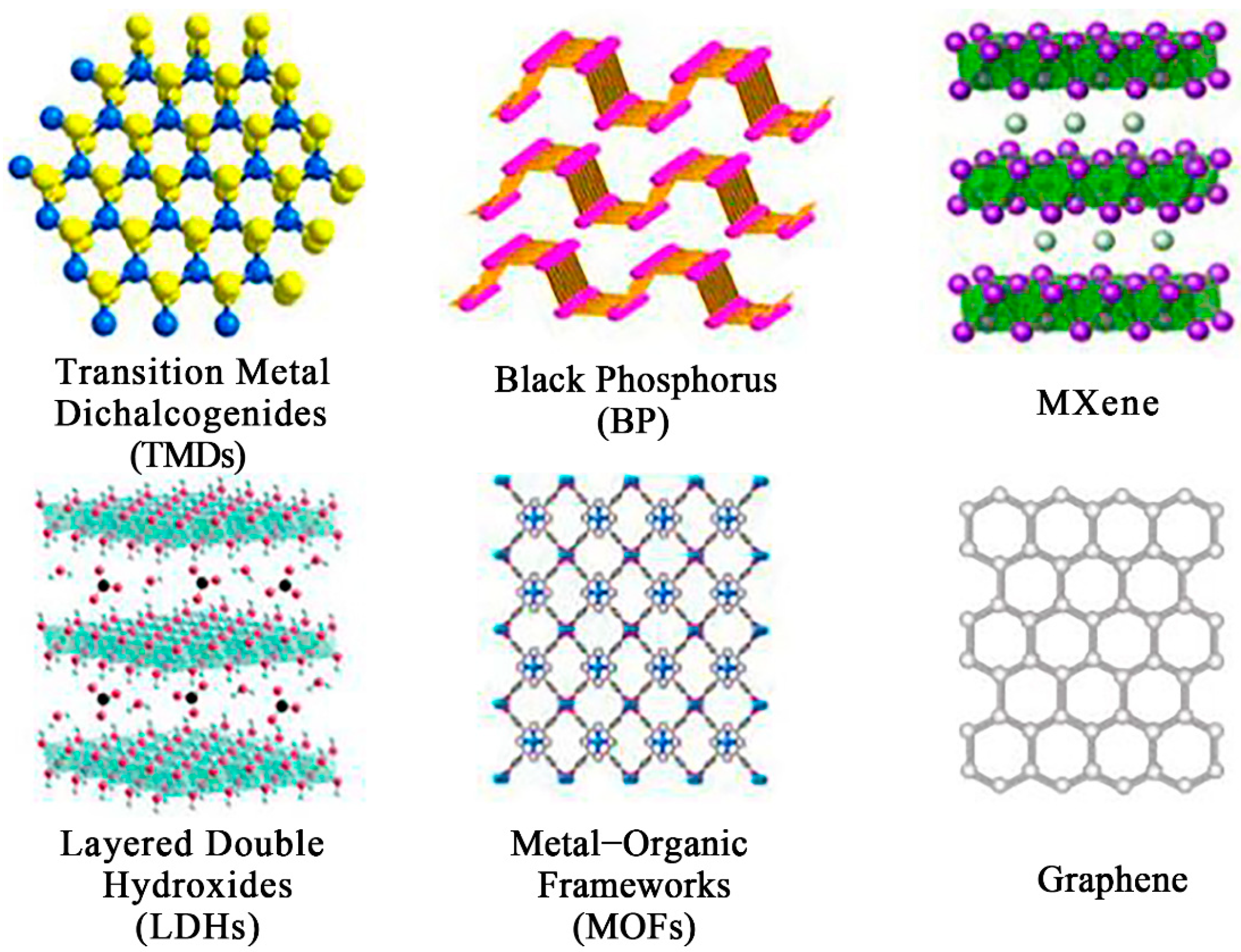
4.2.2. D Structure Hybrid Nanostructures Loaded with Noble Metals
- TMDs, Transition Metal Dichalcogenides
- BP, Black Phosphorus
- MXene
- LDHs, Layered Double Hydroxides
- MOFs, Metal–Organic Frameworks
- Other 2D materials
4.2.3. 1D/3D Structure Hybrid Nanostructures Loaded with Noble Metals
4.2.4. Single Atom Noble Metal Catalyst
5. Conclusions and Prospects
- Noble metals are expensive, and the preparation process of catalysts is complex. Although various effective noble metal–based catalysts with low metal loading capacity and small size have been developed, the high surface energy leads to the easy aggregation of metal atoms in the catalytic process, resulting in reduced catalytic activity, which makes it difficult to meet the commercial demand. Therefore, it is urgent to develop a simple synthesis method to prepare small monodisperse catalysts with favorable dispersion and high loading capacity.
- A thorough understanding of the HER mechanism is of great significance to adjusting catalytic activity and guiding the structural design of prospective catalysts. Although the DFT calculations have been used to predict reaction intermediates and active centers of catalysts and to design efficient HER catalysts, theoretical models are mostly simplifications of actual catalytic conditions. Especially in an alkaline medium, there is no consensus on the HER activity descriptor and action mechanism. At present, most of the catalyst characterization techniques used are situ techniques, which can only provide information before and after the measurement of the catalyst, while ignoring the evolution of the microstructure of the atomic layer on the catalyst surface and the reaction intermediates adsorbed on the catalyst surface during the process. Therefore, there is an urgent need to develop advanced in situ characterization techniques and theoretical simulations to accurately elucidate the reaction mechanism at the molecular level.
- The development of HER and OER bifunctional catalysts with high activity, selectivity and stability are necessary, which can reduce the costs and simplify setups. The efficiency of hydrogen production depends not only on the cathode catalytic efficiency but also on the anode catalytic efficiency in practical water electrolysis hydrogen production devices and applications. Most HER catalysts (e.g., Pt-based catalysts, chalcogenides, and phosphides) have better catalytic effects in acidic media, while most OER catalysts (e.g., transition metal oxides and (oxy)hydroxides) have better catalytic activity in alkaline media. Under the same experimental conditions, few catalysts can effectively catalyze both the HER and OER. When the two-electrode reactions are paired together in an electrolytic cell with the same electrolyte, the integration is incompatible, thus damping the catalytic performance. Therefore, the development of bifunctional catalysts with excellent HER and OER activities is a future research goal and opportunity.
- Establishing standardized measurements to compare the HER performance of different electrocatalysts will help to screen and optimize existing catalytic systems and also help to determine and compare the activity of newly developed electrocatalysts.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Niu, H.-J.; Yan, Y.; Jiang, S.; Liu, T.; Sun, T.; Zhou, W.; Guo, L.; Li, J. Interfaces decrease the alkaline hydrogen-evolution kinetics energy barrier on NiCoP/Ti3C2Tx MXene. ACS Nano 2022, 16, 11049–11058. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, P.; Shao, Q.; Huang, X. Metallic nanostructures with low dimensionality for electrochemical water splitting. Chem. Soc. Rev. 2020, 49, 3072–3106. [Google Scholar] [CrossRef] [PubMed]
- Palmer, G.; Roberts, A.; Hoadley, A.; Dargaville, R.; Honnery, D. Life-cycle greenhouse gas emissions and net energy assessment of large-scale hydrogen production via electrolysis and solar PV. Energy Environ. Sci. 2021, 14, 5113–5131. [Google Scholar] [CrossRef]
- Cai, P.; Li, Y.; Wang, G.; Wen, Z. Alkaline–acid Zn–H2O fuel cell for the simultaneous generation of hydrogen and electricity. Angew. Chem. Int. Ed. 2018, 130, 3974–3979. [Google Scholar] [CrossRef]
- Torella, J.P.; Gagliardi, C.J.; Chen, J.S.; Bediako, D.K.; Colón, B.; Way, J.C.; Silver, P.A.; Nocera, D.G. Efficient solar-to-fuels production from a hybrid microbial–water-splitting catalyst system. Proc. Natl. Acad. Sci. USA 2015, 112, 2337–2342. [Google Scholar] [CrossRef]
- Huang, B.; Wang, P.; Wang, L.; Yang, S.; Wu, D. Recent advances in ocean wave energy harvesting by triboelectric nanogenerator: An overview. Nanotechnol. Rev. 2020, 9, 716–735. [Google Scholar] [CrossRef]
- Feng, Y.; Lin, H.; Ho, S.L.; Yan, J.; Dong, J.; Fang, S.; Huang, Y. Overview of wind power generation in China: Status and development. Renew. Sustain. Energy Rev. 2015, 50, 847–858. [Google Scholar] [CrossRef]
- Shen, S.; Lin, Z.; Song, K.; Wang, Z.; Huang, L.; Yan, L.; Meng, F.; Zhang, Q.; Gu, L.; Zhong, W. Reversed active sites boost the intrinsic activity of graphene-like cobalt selenide for hydrogen evolution. Angew. Chem. Int. Ed. 2021, 133, 12468–12473. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Qin, Y.; Zhang, W.; Wang, L.; Luo, M.; Yang, H.; Guo, S. Recent advances on water-splitting electrocatalysis mediated by noble-metal-based nanostructured materials. Adv. Energy Mater. 2020, 10, 1903120. [Google Scholar] [CrossRef]
- Sápi, A.; Rajkumar, T.; Kiss, J.; Kukovecz, Á.; Kónya, Z.; Somorjai, G.A. Metallic nanoparticles in heterogeneous catalysis. Catal. Lett. 2021, 151, 2153–2175. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, Z.; Pan, Y.; Dai, R.; Wu, Y.; Zhuang, Z.; Wang, D.; Peng, Q.; Chen, C.; Li, Y. Functionalization of hollow nanomaterials for catalytic applications: Nanoreactor construction. Adv. Mater. 2019, 31, 1800426. [Google Scholar] [CrossRef]
- Feng, Y.; Guan, Y.; Zhang, H.; Huang, Z.; Li, J.; Jiang, Z.; Gu, X.; Wang, Y. Selectively anchoring Pt single atoms at hetero-interfaces of γ-Al2O3/NiS to promote the hydrogen evolution reaction. J. Mater. Chem. A 2018, 6, 11783–11789. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Liu, S.; Norouzi Banis, M.; Song, Z.; Li, J.; Yang, L.; Markiewicz, M.; Zhao, Y.; Li, R. Pt/Pd single-atom alloys as highly active electrochemical catalysts and the origin of enhanced activity. ACS Catal. 2019, 9, 9350–9358. [Google Scholar] [CrossRef]
- Wei, C.; Rao, R.R.; Peng, J.; Huang, B.; Stephens, I.E.; Risch, M.; Xu, Z.J.; Shao-Horn, Y. Recommended practices and benchmark activity for hydrogen and oxygen electrocatalysis in water splitting and fuel cells. Adv. Mater. 2019, 31, 1806296. [Google Scholar] [CrossRef]
- Zheng, J.; Sheng, W.; Zhuang, Z.; Xu, B.; Yan, Y. Universal dependence of hydrogen oxidation and evolution reaction activity of platinum-group metals on pH and hydrogen binding energy. Sci. Adv. 2016, 2, e1501602. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, X.; Jiang, X.; Song, S.; Huang, D.; Yu, L.; Zhang, Y.; Chen, S.; Wang, M.; Shen, Y. In situ growth of Ru nanoparticles on (Fe, Ni)(OH)2 to boost hydrogen evolution activity at high current density in alkaline media. Small Methods 2020, 4, 1900796. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, R.; Yu, Y.; Sun, Z.; Che, R.; Wei, B.; LaGrow, A.P.; Wang, Z.; Zhou, W. Interfacial optimization of PtNi octahedrons@Ti3C2 MXene with enhanced alkaline hydrogen evolution activity and stability. Appl. Catal. B 2021, 291, 120100. [Google Scholar] [CrossRef]
- Zhang, L.; Zhuang, L.; Liu, H.; Zhang, L.; Cai, R.; Chen, N.; Yang, X.; Zhu, Z.; Yang, D.; Yao, X. Beyond Platinum: Defects Abundant CoP3/Ni2P Heterostructure for Hydrogen Evolution Electrocatalysis. Small Sci. 2021, 1, 2000027. [Google Scholar] [CrossRef]
- Wang, G.; Wen, Z. Self-supported bimetallic Ni–Co compound electrodes for urea- and neutralization energy-assisted electrolytic hydrogen production. Nanoscale 2018, 10, 21087–21095. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, W.; Guo, W.H.; Yang, Y.X.; Lei, J.L.; Luo, H.Q.; Li, N.B. Macroporous array induced multiscale modulation at the surface/interface of Co(OH)2/NiMo self-supporting electrode for effective overall water splitting. Adv. Funct. Mater. 2021, 31, 2102117. [Google Scholar] [CrossRef]
- Ge, J.; Wei, P.; Wu, G.; Liu, Y.; Yuan, T.; Li, Z.; Qu, Y.; Wu, Y.; Li, H.; Zhuang, Z. Ultrathin palladium nanomesh for electrocatalysis. Angew. Chem. Int. Ed. 2018, 130, 3493–3496. [Google Scholar] [CrossRef]
- Mei, P.; Kim, J.; Kumar, N.A.; Pramanik, M.; Kobayashi, N.; Sugahara, Y.; Yamauchi, Y. Phosphorus-based mesoporous materials for energy storage and conversion. Joule 2018, 2, 2289–2306. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, C.; Chen, J.; Yan, Y.; Zakhidov, A.A.; Baughman, R.H.; Xu, L. Three-dimensionally ordered macro-/mesoporous Ni as a highly efficient electrocatalyst for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 11367–11375. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.C.; Bang, J.U.; Song, H. Precise tuning of porosity and surface functionality in Au@SiO2 nanoreactors for high catalytic efficiency. Chem. Mater. 2008, 20, 5839–5844. [Google Scholar] [CrossRef]
- Shinozaki, K.; Zack, J.W.; Richards, R.M.; Pivovar, B.S.; Kocha, S.S. Oxygen reduction reaction measurements on platinum electrocatalysts utilizing rotating disk electrode technique: I. Impact of impurities, measurement protocols and applied corrections. J. Electrochem. Soc. 2015, 162, F1144. [Google Scholar] [CrossRef]
- Liu, G.; Li, X.; Wang, M.; Wang, M.; Kim, J.Y.; Woo, J.Y.; Wang, X.; Lee, J.K. A study on anode diffusion layer for performance enhancement of a direct methanol fuel cell. Energy Convers. Manag. 2016, 126, 697–703. [Google Scholar] [CrossRef]
- Li, M.; Xia, Z.; Luo, M.; He, L.; Tao, L.; Yang, W.; Yu, Y.; Guo, S. Structural Regulation of Pd-Based Nanoalloys for Advanced Electrocatalysis. Small Sci. 2021, 1, 2100061. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Abild-Pedersen, F.; Studt, F.; Bligaard, T. Density functional theory in surface chemistry and catalysis. Proc. Natl. Acad. Sci. USA 2011, 108, 937–943. [Google Scholar] [CrossRef]
- Tan, W.; Xie, S.; Le, D.; Diao, W.; Wang, M.; Low, K.-B.; Austin, D.; Hong, S.; Gao, F.; Dong, L. Fine-tuned local coordination environment of Pt single atoms on ceria controls catalytic reactivity. Nat. Commun. 2022, 13, 7070. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, L.; Wen, Z.; Jiang, Q. Understanding electro-catalysis by using density functional theory. Phys. Chem. Chem. Phys. 2019, 21, 23782–23802. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-R.; Li, M.-X.; Li, S.-N.; Liu, Y.-J.; Chen, J.; Wang, Y. A review of energy and environment electrocatalysis based on high-index faceted nanocrystals. Rare Met. 2021, 40, 3406–3441. [Google Scholar] [CrossRef]
- Kang, J.; Qiu, X.; Hu, Q.; Zhong, J.; Gao, X.; Huang, R.; Wan, C.; Liu, L.-M.; Duan, X.; Guo, L. Valence oscillation and dynamic active sites in monolayer NiCo hydroxides for water oxidation. Nat. Catal. 2021, 4, 1050–1058. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, Y.; Li, F.; Hao, R.; Wang, S.; Guo, L. A generalized strategy for the synthesis of large-size ultrathin two-dimensional metal oxide nanosheets. Angew. Chem. Int. Ed. 2017, 56, 8766–8770. [Google Scholar] [CrossRef] [PubMed]
- Nai, J.; Tian, Y.; Guan, X.; Guo, L. Pearson’s principle inspired generalized strategy for the fabrication of metal hydroxide and oxide nanocages. J. Am. Chem. Soc. 2013, 135, 16082–16091. [Google Scholar] [CrossRef]
- Mao, D.; Wan, J.; Wang, J.; Wang, D. Sequential templating approach: A groundbreaking strategy to create hollow multishelled structures. Adv. Mater. 2019, 31, 1802874. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, K.; Hu, K.; Cai, C.; Li, H.; Li, H.; Herran, M.; Lu, Y.-R.; Chan, T.-S.; Ma, C.; et al. Attenuating metal-substrate conjugation in atomically dispersed nickel catalysts for electroreduction of CO2 to CO. Nat. Commun. 2022, 13, 6082. [Google Scholar] [CrossRef]
- Dalton, F. Ecs classics: Historical origins of the rotating ring-disk electrode. Electrochem. Soc. Interface 2016, 25, 50. [Google Scholar] [CrossRef]
- Schmidt, T.; Gasteiger, H.; Stäb, G.; Urban, P.; Kolb, D.; Behm, R. Characterization of high-surface-area electrocatalysts using a rotating disk electrode configuration. J. Electrochem. Soc. 1998, 145, 2354. [Google Scholar] [CrossRef]
- Hu, E.; Feng, Y.; Nai, J.; Zhao, D.; Hu, Y.; Lou, X.W.D. Construction of hierarchical Ni–Co–P hollow nanobricks with oriented nanosheets for efficient overall water splitting. Energy Environ. Sci. 2018, 11, 872–880. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Shao-Horn, Y. Electrocatalytic measurement methodology of oxide catalysts using a thin-film rotating disk electrode. J. Electrochem. Soc. 2010, 157, B1263. [Google Scholar] [CrossRef]
- Sheng, W.; Gasteiger, H.A.; Shao-Horn, Y. Hydrogen oxidation and evolution reaction kinetics on platinum: Acid vs alkaline electrolytes. J. Electrochem. Soc. 2010, 157, B1529. [Google Scholar] [CrossRef]
- Xue, S.; Haid, R.W.; Kluge, R.M.; Ding, X.; Garlyyev, B.; Fichtner, J.; Watzele, S.; Hou, S.; Bandarenka, A.S. Enhancing the hydrogen evolution reaction activity of platinum electrodes in alkaline media using nickel–iron clusters. Angew. Chem. Int. Ed. 2020, 59, 10934–10938. [Google Scholar] [CrossRef]
- Ye, R.; del Angel-Vicente, P.; Liu, Y.; Arellano-Jimenez, M.J.; Peng, Z.; Wang, T.; Li, Y.; Yakobson, B.I.; Wei, S.H.; Yacaman, M.J. High-performance hydrogen evolution from MoS2(1−x)Px solid solution. Adv. Mater. 2016, 28, 1427–1432. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yan, Z.; Liu, F.; Xu, W.; Cheng, F.; Chen, J. Self-supported transition-metal-based electrocatalysts for hydrogen and oxygen evolution. Adv. Mater. 2020, 32, 1806326. [Google Scholar] [CrossRef]
- Shen, X.; Li, H.; Zhang, Y.; Ma, T.; Li, Q.; Jiao, Q.; Zhao, Y.; Li, H.; Feng, C. Construction dual-regulated NiCo2S4@Mo-doped CoFe-LDH for oxygen evolution reaction at large current density. Appl. Catal. B 2022, 319, 121917. [Google Scholar] [CrossRef]
- Zhu, X.; Tang, C.; Wang, H.-F.; Li, B.-Q.; Zhang, Q.; Li, C.; Yang, C.; Wei, F. Monolithic-structured ternary hydroxides as freestanding bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 2016, 4, 7245–7250. [Google Scholar] [CrossRef]
- Durst, J.; Siebel, A.; Simon, C.; Hasché, F.; Herranz, J.; Gasteiger, H. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci. 2014, 7, 2255–2260. [Google Scholar] [CrossRef]
- Garlyyev, B.; Xue, S.; Pohl, M.D.; Reinisch, D.; Bandarenka, A.S. Oxygen electroreduction at high-index Pt electrodes in alkaline electrolytes: A decisive role of the alkali metal cations. ACS Omega 2018, 3, 15325–15331. [Google Scholar] [CrossRef]
- Xue, S.; Garlyyev, B.; Watzele, S.; Liang, Y.; Fichtner, J.; Pohl, M.D.; Bandarenka, A.S. Influence of alkali metal cations on the hydrogen evolution reaction activity of Pt, Ir, Au, and Ag electrodes in alkaline electrolytes. ChemElectroChem 2018, 5, 2326–2329. [Google Scholar] [CrossRef]
- Garlyyev, B.; Fichtner, J.; Piqué, O.; Schneider, O.; Bandarenka, A.S.; Calle-Vallejo, F. Revealing the nature of active sites in electrocatalysis. Chem. Sci. 2019, 10, 8060–8075. [Google Scholar] [CrossRef]
- Li, A.; Sun, Y.; Yao, T.; Han, H. Earth-abundant transition-metal-based electrocatalysts for water electrolysis to produce renewable hydrogen. Chem.-A Eur. J. 2018, 24, 18334–18355. [Google Scholar] [CrossRef]
- Parsons, R. The rate of electrolytic hydrogen evolution and the heat of adsorption of hydrogen. Trans. Faraday Soc. 1958, 54, 1053–1063. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Chang, K.-C.; Strmcnik, D.; Paulikas, A.P.; Hirunsit, P.; Chan, M.; Greeley, J.; Stamenkovic, V.; Markovic, N.M. Trends in activity for the water electrolyser reactions on 3d M (Ni, Co, Fe, Mn) hydr (oxy) oxide catalysts. Nat. Mater. 2012, 11, 550–557. [Google Scholar] [CrossRef]
- Cao, X.; Tan, Y.; Zheng, H.; Hu, J.; Chen, X.; Chen, Z. Effect of cobalt phosphide (CoP) vacancies on its hydrogen evolution activity via water splitting: A theoretical study. Phys. Chem. Chem. Phys. 2022, 24, 4644–4652. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.H.; Han, Y.; Chen, Y.; Jaroniec, M.; Qiao, S.-Z. High electrocatalytic hydrogen evolution activity of an anomalous ruthenium catalyst. J. Am. Chem. Soc. 2016, 138, 16174–16181. [Google Scholar] [CrossRef] [PubMed]
- Medford, A.J.; Vojvodic, A.; Hummelshøj, J.S.; Voss, J.; Abild-Pedersen, F.; Studt, F.; Bligaard, T.; Nilsson, A.; Nørskov, J.K. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. J. Catal. 2015, 328, 36–42. [Google Scholar] [CrossRef]
- Fan, L.; Ji, Y.; Wang, G.; Chen, J.; Chen, K.; Liu, X.; Wen, Z. High entropy alloy electrocatalytic electrode toward alkaline glycerol valorization coupling with acidic hydrogen production. J. Am. Chem. Soc. 2022, 144, 7224–7235. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Che, Z.; Zhao, S.; Sheng, X.; Han, M.; Bao, J. Concave octahedral Pd@PdPt electrocatalysts integrating core–shell, alloy and concave structures for high-efficiency oxygen reduction and hydrogen evolution reactions. J. Mater. Chem. A 2016, 4, 16690–16697. [Google Scholar] [CrossRef]
- Li, K.; Li, Y.; Wang, Y.; Ge, J.; Liu, C.; Xing, W. Enhanced electrocatalytic performance for the hydrogen evolution reaction through surface enrichment of platinum nanoclusters alloying with ruthenium in situ embedded in carbon. Energy Environ. Sci. 2018, 11, 1232–1239. [Google Scholar] [CrossRef]
- Wu, W.; Tang, Z.; Wang, K.; Liu, Z.; Li, L.; Chen, S. Peptide templated AuPt alloyed nanoparticles as highly efficient bi-functional electrocatalysts for both oxygen reduction reaction and hydrogen evolution reaction. Electrochim. Acta 2018, 260, 168–176. [Google Scholar] [CrossRef]
- Shao, F.-Q.; Zhu, X.-Y.; Wang, A.-J.; Fang, K.-M.; Yuan, J.; Feng, J.-J. One-pot synthesis of hollow AgPt alloyed nanocrystals with enhanced electrocatalytic activity for hydrogen evolution and oxygen reduction reactions. J. Colloid Interface Sci. 2017, 505, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kuang, Y.; Li, Y.; Jiang, M.; Cai, Z.; Pang, Y.; Chang, Z.; Sun, X. Synthesis and performance optimization of ultrathin two-dimensional CoFePt alloy materials via in situ topotactic conversion for the hydrogen evolution reaction. J. Mater. Chem. A 2019, 7, 9517–9522. [Google Scholar] [CrossRef]
- Chen, Q.; Cao, Z.; Du, G.; Kuang, Q.; Huang, J.; Xie, Z.; Zheng, L. Excavated octahedral Pt-Co alloy nanocrystals built with ultrathin nanosheets as superior multifunctional electrocatalysts for energy conversion applications. Nano Energy 2017, 39, 582–589. [Google Scholar] [CrossRef]
- Cao, Z.; Chen, Q.; Zhang, J.; Li, H.; Jiang, Y.; Shen, S.; Fu, G.; Lu, B.-A.; Xie, Z.; Zheng, L. Platinum-nickel alloy excavated nano-multipods with hexagonal close-packed structure and superior activity towards hydrogen evolution reaction. Nat. Commun. 2017, 8, 15131. [Google Scholar] [CrossRef]
- Huang, X.-Y.; You, L.-X.; Zhang, X.-F.; Feng, J.-J.; Zhang, L.; Wang, A.-J. L-proline assisted solvothermal preparation of Cu-rich rhombic dodecahedral PtCu nanoframes as advanced electrocatalysts for oxygen reduction and hydrogen evolution reactions. Electrochim. Acta 2019, 299, 89–97. [Google Scholar] [CrossRef]
- Li, Z.; Qi, Z.; Wang, S.; Ma, T.; Zhou, L.; Wu, Z.; Luan, X.; Lin, F.-Y.; Chen, M.; Miller, J.T. In situ formed Pt3Ti nanoparticles on a two-dimensional transition metal carbide (MXene) used as efficient catalysts for hydrogen evolution reactions. Nano Lett. 2019, 19, 5102–5108. [Google Scholar] [CrossRef]
- Wang, X.; He, Q.; Song, L.; Jaroniec, M.; Zheng, Y.; Qiao, S.-Z. Breaking the volcano-plot limits for Pt-based electrocatalysts by selective tuning adsorption of multiple intermediates. J. Mater. Chem. A 2019, 7, 13635–13640. [Google Scholar] [CrossRef]
- Zhang, L.; Roling, L.T.; Wang, X.; Vara, M.; Chi, M.; Liu, J.; Choi, S.-I.; Park, J.; Herron, J.A.; Xie, Z. Platinum-based nanocages with subnanometer-thick walls and well-defined, controllable facets. Science 2015, 349, 412–416. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, L.; Xu, G.-L.; Zhu, S.; Wang, Q.; Gu, M.; Zhang, X.; Sun, C.; Balbuena, P.B.; Amine, K. The role of Ru in improving the activity of Pd toward hydrogen evolution and oxidation reactions in alkaline solutions. ACS Catal. 2019, 9, 9614–9621. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, Q.; Chen, H.; Shao, M. Recent advances in palladium-based electrocatalysts for fuel cell reactions and hydrogen evolution reaction. Nano Energy 2016, 29, 198–219. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Yu, D.; Zhang, M.; Zhu, H.; Du, M. Carbon nanofiber-supported PdNi alloy nanoparticles as highly efficient bifunctional catalysts for hydrogen and oxygen evolution reactions. Electrochim. Acta 2017, 246, 17–26. [Google Scholar] [CrossRef]
- Jiang, P.; Huang, H.; Diao, J.; Gong, S.; Chen, S.; Lu, J.; Wang, C.; Sun, Z.; Xia, G.; Yang, K. Improving electrocatalytic activity of iridium for hydrogen evolution at high current densities above 1000 mA cm−2. Appl. Catal. B 2019, 258, 117965. [Google Scholar] [CrossRef]
- Jiang, P.; Chen, J.; Wang, C.; Yang, K.; Gong, S.; Liu, S.; Lin, Z.; Li, M.; Xia, G.; Yang, Y. Tuning the activity of carbon for electrocatalytic hydrogen evolution via an iridium-cobalt alloy core encapsulated in nitrogen-doped carbon cages. Adv. Mater. 2018, 30, 1705324. [Google Scholar] [CrossRef]
- Gong, S.; Wang, C.; Jiang, P.; Yang, K.; Lu, J.; Huang, M.; Chen, S.; Wang, J.; Chen, Q. O species-decorated graphene shell encapsulating iridium–nickel alloy as an efficient electrocatalyst towards hydrogen evolution reaction. J. Mater. Chem. A 2019, 7, 15079–15088. [Google Scholar] [CrossRef]
- Shan, J.; Ling, T.; Davey, K.; Zheng, Y.; Qiao, S.Z. Transition-metal-doped RuIr bifunctional nanocrystals for overall water splitting in acidic environments. Adv. Mater. 2019, 31, 1900510. [Google Scholar] [CrossRef]
- Zhao, Y.; Bai, J.; Wu, X.-R.; Chen, P.; Jin, P.-J.; Yao, H.-C.; Chen, Y. Atomically ultrathin RhCo alloy nanosheet aggregates for efficient water electrolysis in broad pH range. J. Mater. Chem. A 2019, 7, 16437–16446. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, W.; Chen, B.; Zhang, Q.; Cui, X.; Li, B.; Lai, Z.; Chen, Y.; Zhang, Z.; Gu, L. Synthesis of RuNi alloy nanostructures composed of multilayered nanosheets for highly efficient electrocatalytic hydrogen evolution. Nano Energy 2019, 66, 104173. [Google Scholar] [CrossRef]
- Li, J.; Sun, S. Intermetallic nanoparticles: Synthetic control and their enhanced electrocatalysis. Acc. Chem. Res. 2019, 52, 2015–2025. [Google Scholar] [CrossRef]
- Xiao, W.; Lei, W.; Gong, M.; Xin, H.L.; Wang, D. Recent advances of structurally ordered intermetallic nanoparticles for electrocatalysis. ACS Catal. 2018, 8, 3237–3256. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, J.M.; Ha, Y.; Woo, J.; Byun, A.; Shin, T.J.; Park, K.H.; Jeong, H.Y.; Kim, H.; Kim, J.Y.; et al. Activity origin and multifunctionality of Pt-based intermetallic nanostructures for efficient electrocatalysis. ACS Catal. 2019, 9, 11242–11254. [Google Scholar] [CrossRef]
- Ai, X.; Zou, X.; Chen, H.; Su, Y.; Feng, X.; Li, Q.; Liu, Y.; Zhang, Y.; Zou, X. Transition-metal–boron intermetallics with strong interatomic d–sp orbital hybridization for high-performance electrocatalysis. Angew. Chem. Int. Ed. 2020, 59, 3961–3965. [Google Scholar] [CrossRef] [PubMed]
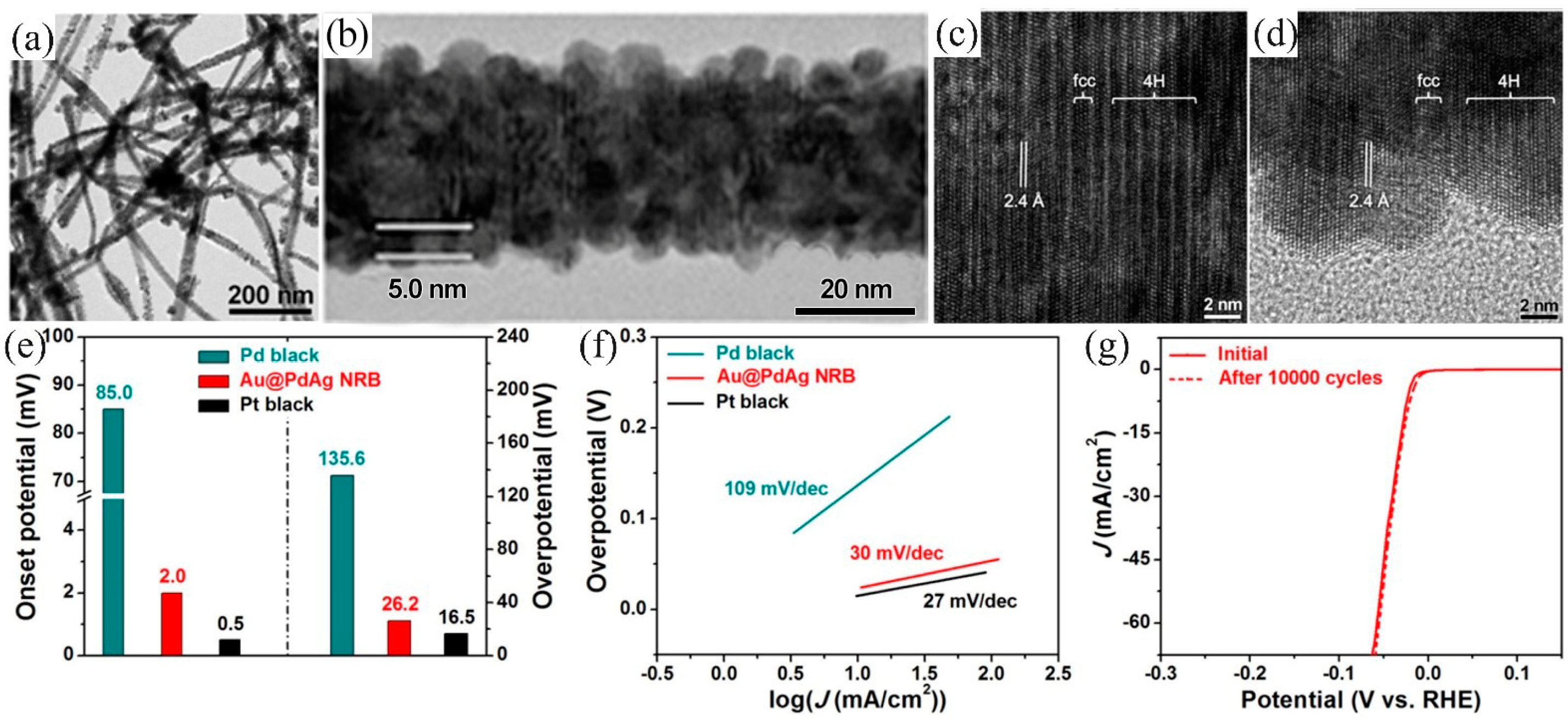
- Fan, Z.; Luo, Z.; Huang, X.; Li, B.; Chen, Y.; Wang, J.; Hu, Y.; Zhang, H. Synthesis of 4H/fcc noble multimetallic nanoribbons for electrocatalytic hydrogen evolution reaction. J. Am. Chem. Soc. 2016, 138, 1414–1419. [Google Scholar] [CrossRef]
- Luo, Y.; Luo, X.; Wu, G.; Li, Z.; Wang, G.; Jiang, B.; Hu, Y.; Chao, T.; Ju, H.; Zhu, J.; et al. Mesoporous Pd@Ru core–shell nanorods for hydrogen evolution reaction in alkaline solution. ACS Appl. Mater. Interfaces 2018, 10, 34147–34152. [Google Scholar] [CrossRef]
- Huang, X.; Zeng, Z.; Bao, S.; Wang, M.; Qi, X.; Fan, Z.; Zhang, H. Solution-phase epitaxial growth of noble metal nanostructures on dispersible single-layer molybdenum disulfide nanosheets. Nat. Commun. 2013, 4, 1444. [Google Scholar] [CrossRef]
- Cui, C.; Cheng, R.; Zhang, H.; Zhang, C.; Ma, Y.; Shi, C.; Fan, B.; Wang, H.; Wang, X. Ultrastable MXene@Pt/SWCNTs’ nanocatalysts for hydrogen evolution reaction. Adv. Funct. Mater. 2020, 30, 2000693. [Google Scholar] [CrossRef]
- Mahmood, J.; Li, F.; Jung, S.-M.; Okyay, M.S.; Ahmad, I.; Kim, S.-J.; Park, N.; Jeong, H.Y.; Baek, J.-B. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction. Nat. Nanotechnol. 2017, 12, 441–446. [Google Scholar] [CrossRef]
- Nazir, R.; Fageria, P.; Basu, M.; Gangopadhyay, S.; Pande, S. Decoration of Pd and Pt nanoparticles on a carbon nitride (C3N4) surface for nitro-compounds reduction and hydrogen evolution reaction. New J. Chem. 2017, 41, 9658–9667. [Google Scholar] [CrossRef]
- Kweon, D.H.; Okyay, M.S.; Kim, S.-J.; Jeon, J.-P.; Noh, H.-J.; Park, N.; Mahmood, J.; Baek, J.-B. Ruthenium anchored on carbon nanotube electrocatalyst for hydrogen production with enhanced Faradaic efficiency. Nat. Commun. 2020, 11, 1278. [Google Scholar] [CrossRef]
- Xiu, L.; Pei, W.; Zhou, S.; Wang, Z.; Yang, P.; Zhao, J.; Qiu, J. Multilevel hollow MXene tailored Low-Pt catalyst for efficient hydrogen evolution in full-pH range and seawater. Adv. Funct. Mater. 2020, 30, 1910028. [Google Scholar] [CrossRef]
- Yang, J.; Chen, B.; Liu, X.; Liu, W.; Li, Z.; Dong, J.; Chen, W.; Yan, W.; Yao, T.; Duan, X.; et al. Efficient and robust hydrogen evolution: Phosphorus nitride imide nanotubes as supports for anchoring single ruthenium sites. Angew. Chem. Int. Ed. 2018, 57, 9495–9500. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Feng, Y.; Wan, J.; Yang, X.; Fang, L.; Gu, X.; Liu, R.; Huang, Z.; Li, J.; Luo, J.; et al. Ganoderma-like MoS2/NiS2 with single platinum atoms doping as an efficient and stable hydrogen evolution reaction catalyst. Small 2018, 14, 1800697. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yan, Y.; Wu, Z.; Jiang, Y.; Li, X.; Xu, Q.; Yang, X.; Zhang, H.; Yang, D. Facile synthesis of Pd@Ru nanoplates with controlled thickness as efficient catalysts for hydrogen evolution reaction. CrystEngComm 2018, 20, 4230–4236. [Google Scholar] [CrossRef]
- Li, Y.; Pei, W.; He, J.; Liu, K.; Qi, W.; Gao, X.; Zhou, S.; Xie, H.; Yin, K.; Gao, Y. Hybrids of PtRu nanoclusters and black phosphorus nanosheets for highly efficient alkaline hydrogen evolution reaction. ACS Catal. 2019, 9, 10870–10875. [Google Scholar] [CrossRef]
- Yan, Q.; Yan, P.; Wei, T.; Wang, G.; Cheng, K.; Ye, K.; Zhu, K.; Yan, J.; Cao, D.; Li, Y. A highly efficient and durable water splitting system: Platinum sub-nanocluster functionalized nickel–iron layered double hydroxide as the cathode and hierarchical nickel–iron selenide as the anode. J. Mater. Chem. A 2019, 7, 2831–2837. [Google Scholar] [CrossRef]
- Guo, C.; Jiao, Y.; Zheng, Y.; Luo, J.; Davey, K.; Qiao, S.-Z. Intermediate modulation on noble metal hybridized to 2D metal-organic framework for accelerated water electrocatalysis. Chem 2019, 5, 2429–2441. [Google Scholar] [CrossRef]
- Tymoczko, A.; Kamp, M.; Rehbock, C.; Kienle, L.; Cattaruzza, E.; Barcikowski, S.; Amendola, V. One-step synthesis of Fe–Au core–shell magnetic-plasmonic nanoparticles driven by interface energy minimization. Nanoscale Horiz. 2019, 4, 1326–1332. [Google Scholar] [CrossRef]
- Kukovecz, Á.; Kordás, K.; Kiss, J.; Kónya, Z. Atomic scale characterization and surface chemistry of metal modified titanate nanotubes and nanowires. Surf. Sci. Rep. 2016, 71, 473–546. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Vasileff, A.; Jiao, Y.; Chen, S.; Song, L.; Zheng, B.; Zheng, Y.; Qiao, S.-Z. Strain effect in bimetallic electrocatalysts in the hydrogen evolution reaction. ACS Energy Lett. 2018, 3, 1198–1204. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.-e.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Zhang, H. Ultrathin Two-Dimensional Nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef]
- Li, X.; Du, D.; Zhang, Y.; Xing, W.; Xue, Q.; Yan, Z. Layered double hydroxides toward high-performance supercapacitors. J. Mater. Chem. A 2017, 5, 15460–15485. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Yi, L.; Liu, Y.; Chen, K.; Shao, P.; Wen, Z. Ultrafine Ru nanoparticles confined in 3D nitrogen-doped porous carbon nanosheet networks for alkali-acid Zn-H2 hybrid battery. Appl. Catal. B 2021, 280, 119412. [Google Scholar] [CrossRef]
- Lin, L.; Sherrell, P.; Liu, Y.; Lei, W.; Zhang, S.; Zhang, H.; Wallace, G.G.; Chen, J. Engineered 2D transition metal dichalcogenides—A vision of viable hydrogen evolution reaction catalysis. Adv. Energy Mater. 2020, 10, 1903870. [Google Scholar] [CrossRef]
- Yi, L.; Ji, Y.; Shao, P.; Chen, J.; Li, J.; Li, H.; Chen, K.; Peng, X.; Wen, Z. Scalable synthesis of tungsten disulfide nanosheets for alkali-acid electrocatalytic sulfion recycling and H2 generation. Angew. Chem. Int. Ed. 2021, 60, 21550–21557. [Google Scholar] [CrossRef]
- Lin, Y.; Pan, Y.; Zhang, J. In-situ grown of Ni2P nanoparticles on 2D black phosphorus as a novel hybrid catalyst for hydrogen evolution. Int. J. Hydrogen. Energy 2017, 42, 7951–7956. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D.; Huang, H.; Yang, N.; Yu, B.; Wen, M.; Wang, X.; Chu, P.K.; Yu, X.-F. In-plane black phosphorus/dicobalt phosphide heterostructure for efficient electrocatalysis. Angew. Chem. Int. Ed. 2018, 57, 2600–2604. [Google Scholar] [CrossRef]
- He, R.; Hua, J.; Zhang, A.; Wang, C.; Peng, J.; Chen, W.; Zeng, J. Molybdenum disulfide–black phosphorus hybrid nanosheets as a superior catalyst for electrochemical hydrogen evolution. Nano Lett. 2017, 17, 4311–4316. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Xiong, D.; Li, X.; Bai, Z.; Lu, S. Recent advances in layered Ti3C2Tx MXene for electrochemical energy storage. Small 2018, 14, 1703419. [Google Scholar] [CrossRef] [PubMed]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.-Y.; Wong, Y.-T.; Yuan, S.; Liu, Y.; Tsang, M.-K.; Yang, Z.; Huang, H.; Wong, W.-T.; Hao, J. Universal strategy for HF-free facile and rapid synthesis of two-dimensional MXenes as multifunctional energy materials. J. Am. Chem. Soc. 2019, 141, 9610–9616. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Gao, G.; Li, F.-T.; Ma, T.-Y.; Du, A.; Qiao, S.-Z. Ti3C2 MXene co-catalyst on metal sulfide photo-absorbers for enhanced visible-light photocatalytic hydrogen production. Nat. Commun. 2017, 8, 13907. [Google Scholar] [CrossRef]
- Long, X.; Wang, Z.; Xiao, S.; An, Y.; Yang, S. Transition metal based layered double hydroxides tailored for energy conversion and storage. Mater. Today 2016, 19, 213–226. [Google Scholar] [CrossRef]
- Liu, Y.; Teng, X.; Mi, Y.; Chen, Z. A new architecture design of Ni–Co LDH-based pseudocapacitors. J. Mater. Chem. A 2017, 5, 24407–24415. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Y.; Dong, J.; He, C.-T.; Yin, H.; An, P.; Zhao, K.; Zhang, X.; Gao, C.; Zhang, L.; et al. Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy 2016, 1, 16184. [Google Scholar] [CrossRef]
- Jin, S.; Son, H.-J.; Farha, O.K.; Wiederrecht, G.P.; Hupp, J.T. Energy transfer from quantum dots to metal–organic frameworks for enhanced light harvesting. J. Am. Chem. Soc. 2013, 135, 955–958. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Cheng, Q.; Hu, C.; Wang, G.; Zou, Z.; Yang, H.; Dai, L. Carbon-defect-driven electroless deposition of Pt atomic clusters for highly efficient hydrogen evolution. J. Am. Chem. Soc. 2020, 142, 5594–5601. [Google Scholar] [CrossRef]
- Li, J.; Zheng, G. One-dimensional earth-abundant nanomaterials for water-splitting electrocatalysts. Adv. Sci. 2017, 4, 1600380. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, J.; Li, H.; Cai, P.; Li, Y.; Wen, Z. Ru-RuO2/CNT hybrids as high-activity pH-universal electrocatalysts for water splitting within 0.73 V in an asymmetric-electrolyte electrolyzer. Nano Energy 2019, 61, 576–583. [Google Scholar] [CrossRef]
- Kumar, S.; Sahoo, P.K.; Satpati, A.K. Insight into the catalytic performance of HER catalysis of noble metal/3D-G nanocomposites. Electrochim. Acta 2020, 333, 135467. [Google Scholar] [CrossRef]
- Wang, Y.; Su, H.; He, Y.; Li, L.; Zhu, S.; Shen, H.; Xie, P.; Fu, X.; Zhou, G.; Feng, C.; et al. Advanced electrocatalysts with single-metal-atom active sites. Chem. Rev. 2020, 120, 12217–12314. [Google Scholar] [CrossRef]
- Wei, Z.-X.; Zhu, Y.-T.; Liu, J.-Y.; Zhang, Z.-C.; Hu, W.-P.; Xu, H.; Feng, Y.-Z.; Ma, J.-M. Recent advance in single-atom catalysis. Rare Met. 2021, 40, 767–789. [Google Scholar] [CrossRef]
- Li, S.; Xu, Y.; Wang, H.; Teng, B.; Liu, Q.; Li, Q.; Xu, L.; Liu, X.; Lu, J. Tuning the CO2 hydrogenation selectivity of rhodium single-atom catalysts on zirconium dioxide with alkali ions. Angew. Chem. Int. Ed. 2023, e202218167. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Sun, Q.; He, Q.; Ji, H.; He, X. Boosting hydrogenation properties of Pt single-atom catalysts via tailoring the electronic structures by coordination number regulation. Chem. Eng. J. 2023, 455, 140808. [Google Scholar] [CrossRef]

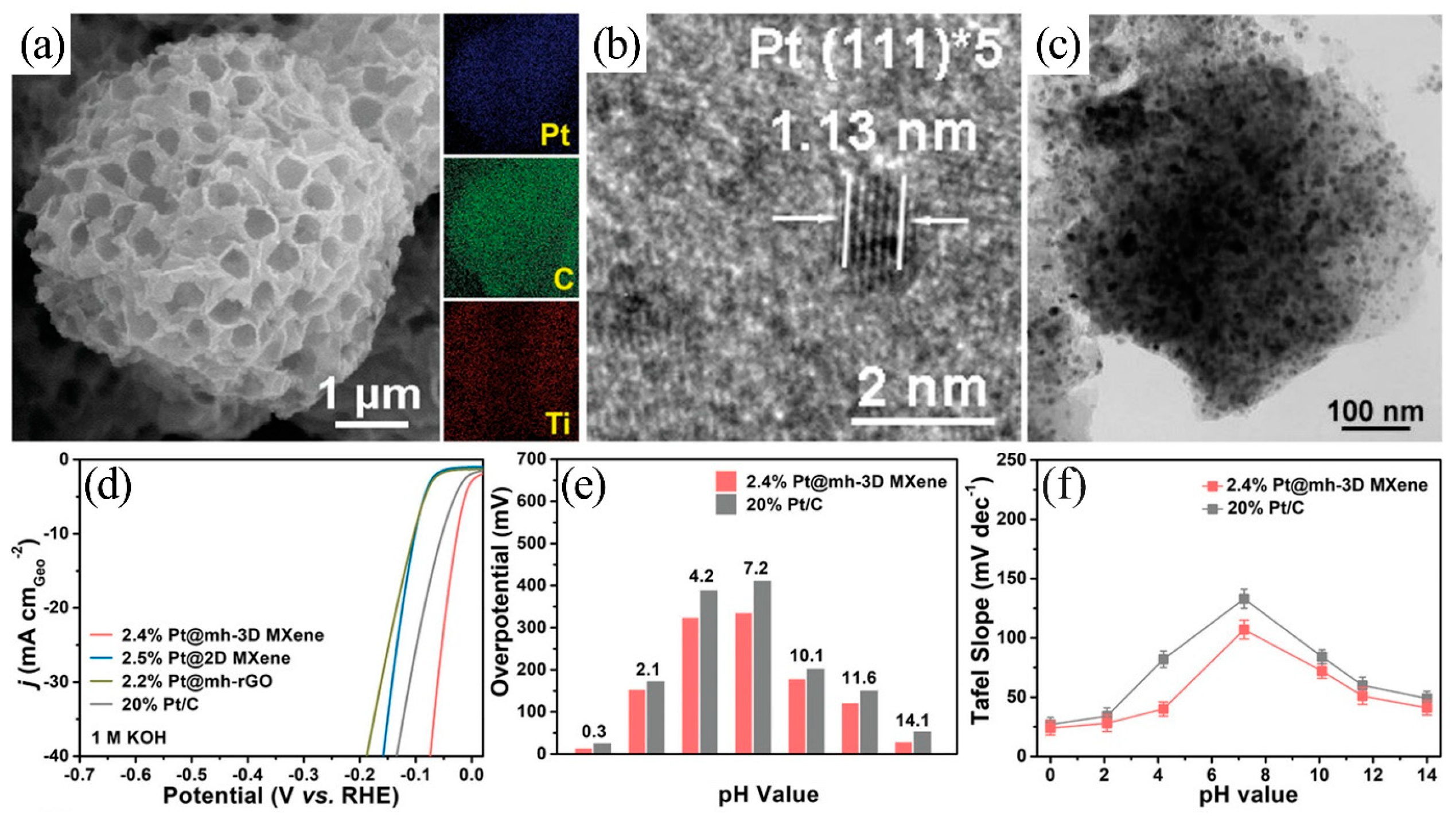
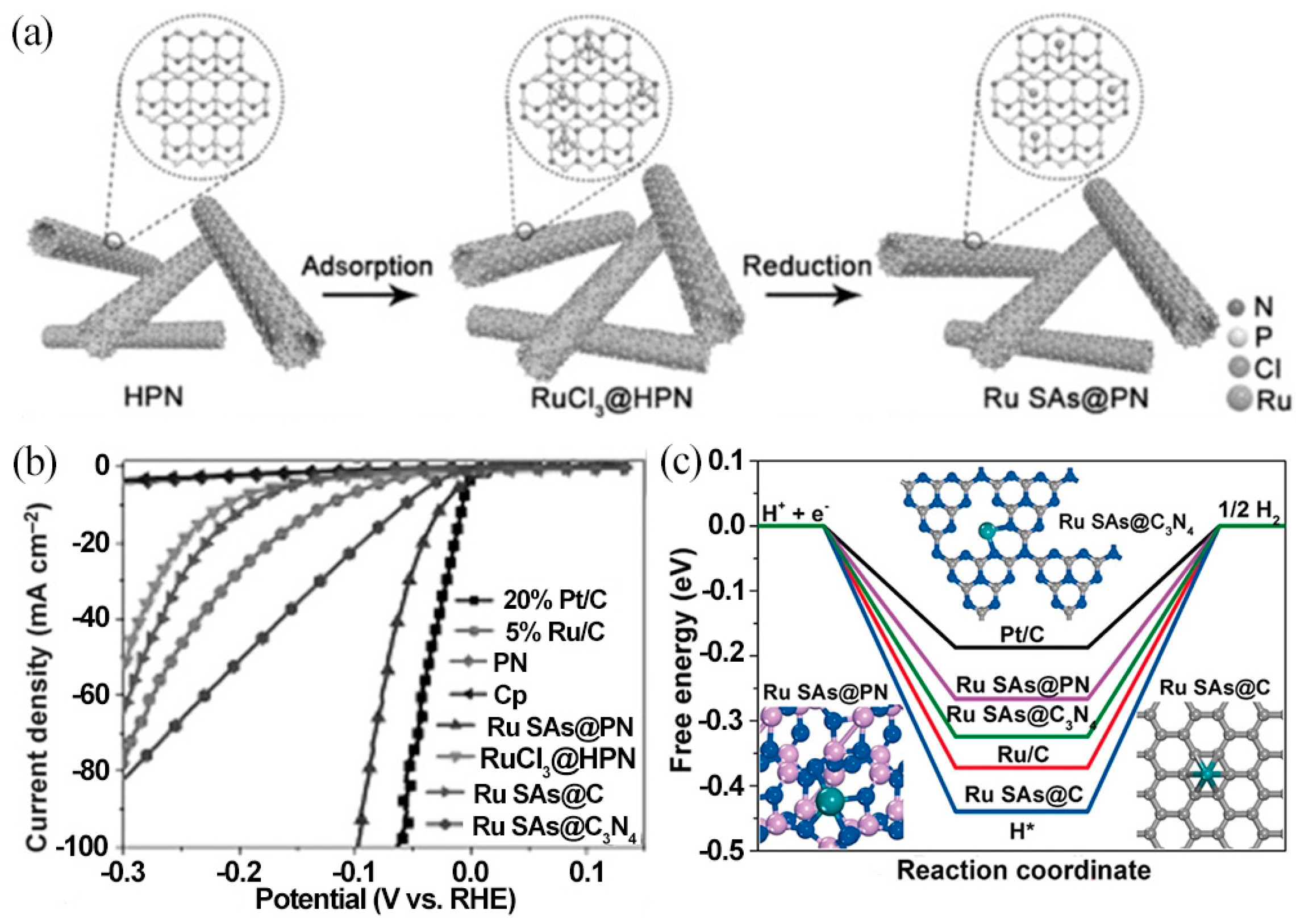
| Catalyst | Electrolyte | Overpotential (η10/mV) | Tafel Slope (mV·dec−1) | Ref. |
|---|---|---|---|---|
| Pd@PdPt | 0.5 M H2SO4 | 39 | 38 | [59] |
| PtRu | 0.5 M H2SO4 | 19.7 | 27.2 | [60] |
| H-AgPt NCs a | 0.5 M H2SO4 | 51 | 40 | [62] |
| PdNi | 0.5 M H2SO4 | 55 | 57 | [72] |
| RhCo ANAs a | 0.5 M H2SO4 | 12.4 | 30.7 | [77] |
| RuB | 0.5 M H2SO4 | 22 | 30.7 | [82] |
| Pt3Ti | 0.1 M HClO4 | 32.7 | 32.3 | [67] |
| Co-RuIr | 0.1 M HClO4 | 14 | 31.1 | [76] |
| Au33Pt67 | 0.1 M KOH | 171 | 81 | [61] |
| PtCo | 0.1 M KOH | η20 = 76.2 | 76 | [64] |
| O-Pt3Co-NWs a | 0.1 M KOH | 56.1 | 30 | [81] |
| Pt24Cu76 NFs a | 0.5 M KOH | 18 | 52 | [66] |
| CoFe–Pt1% | 1 M KOH | 18 | 29 | [63] |
| Pd3Ru | 1 M KOH | 42 | - | [70] |
| IrFe@NC a | 1 M KOH | η1000 = 850 | 30 | [73] |
| RuNi | 1 M KOH | 15 | 28 | [78] |
| Catalyst | Electrolyte | Overpotential (η10/mV) | Tafel Slope (mV·dec−1) | Ref. |
|---|---|---|---|---|
| 4H/fcc Au@PdAg NRBs a | 0.5 M H2SO4 | 26.2 | 30 | [83] |
| Pd@Ru NRs a | 0.5 M H2SO4 | 37 | 33 | [84] |
| Pt-MoS2 | 0.5 M H2SO4 | - | 40 | [85] |
| Pt–MXene–CNTs a | 0.5 M H2SO4 | 62 | 66.6 | [86] |
| Ru@C2N | 0.5 M H2SO4 | 22 | 30 | [87] |
| C3N4/Pd | 0.5 M H2SO4 | - | 116 | [88] |
| C3N4/Pt | 0.5 M H2SO4 | - | 71 | [88] |
| Ru@MWCNT a | 0.5 M H2SO4 | 13 | 27 | [89] |
| Pt@2D MXene | 0.5 M H2SO4 | 80 | 66.6 | [90] |
| Pt@3D MXene | 0.5 M H2SO4 | 13 | 24.2 | [90] |
| Ru SAs@PN a | 0.5 M H2SO4 | 24 | 38 | [91] |
| Pt@MoS2/NiS2 | 0.5 M H2SO4 | 34 | 40 | [92] |
| Pt/NiS@Al2O3 | 0.5 M H2SO4 | 34 | 35 | [14] |
| Pd@Ru NPs a | 0.1 M KOH | 41 | 36 | [93] |
| Pd@Ru NRs a | 1 M KOH | 30 | 30 | [84] |
| PtRu NCs/BP a | 1 M KOH | 22 | 19 | [94] |
| Pt–NiFe LDH/CC a | 1 M KOH | 28 | 39 | [95] |
| Pt/Ni-MOF | 1 M KOH | 25 | 42.1 | [96] |
| Ru@C2N | 1 M KOH | 17 | 38 | [87] |
| Ru@MWCNT a | 1 M KOH | 17 | 27 | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, H.; Wang, Q.; Huang, C.; Zhang, M.; Yan, Y.; Liu, T.; Zhou, W. Noble Metal-Based Heterogeneous Catalysts for Electrochemical Hydrogen Evolution Reaction. Appl. Sci. 2023, 13, 2177. https://doi.org/10.3390/app13042177
Niu H, Wang Q, Huang C, Zhang M, Yan Y, Liu T, Zhou W. Noble Metal-Based Heterogeneous Catalysts for Electrochemical Hydrogen Evolution Reaction. Applied Sciences. 2023; 13(4):2177. https://doi.org/10.3390/app13042177
Chicago/Turabian StyleNiu, Huajie, Qingyan Wang, Chuanxue Huang, Mengyang Zhang, Yu Yan, Tong Liu, and Wei Zhou. 2023. "Noble Metal-Based Heterogeneous Catalysts for Electrochemical Hydrogen Evolution Reaction" Applied Sciences 13, no. 4: 2177. https://doi.org/10.3390/app13042177
APA StyleNiu, H., Wang, Q., Huang, C., Zhang, M., Yan, Y., Liu, T., & Zhou, W. (2023). Noble Metal-Based Heterogeneous Catalysts for Electrochemical Hydrogen Evolution Reaction. Applied Sciences, 13(4), 2177. https://doi.org/10.3390/app13042177






