Abstract
Bioactive plant chemicals are considered to be rich and useful for creating nanomaterials. The current work investigated the biosynthesis of silver nanoparticles (AgNPs) using ethanolic flaxseed extract as an efficient reducing factor. The production of AgNPs was verified by color-shifting observation of the mixture of silver nitrate (AgNO3) from yellow to a reddish suspension after the addition of the extract and by evaluating it by UV–visible inspection. Additionally, FTIR spectrum was used to support the identification of functional groups. The morphology and structure of AgNPs were assessed using scanning electron microscopy (SEM), and X-ray diffraction (XRD) examinations, which revealed spherical AgNPs with a diameter of 46.98 ± 12.45 nm and a crystalline structure. The zeta potential (ZP) and dynamic light scattering (DLS) measurements of AgNPs revealed values of −44.5 mV and 231.8 nm, respectively, suggesting appropriate physical stability. The antibacterial activity of AgNPs was investigated against Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus, and Streptococcus pyogenes, while the antioxidant effect was investigated using the DPPH technique. These obtained AgNPs could potentially be used as efficient antibacterial and antioxidant nanomaterials.
1. Introduction
Nanoparticles are a topic of great interest because of the many applications they have in a variety of fields, such as biology, optics, catalysis, pharmaceutics and health, agriculture, and the industrial sectors [1,2,3,4,5,6,7,8]. These NPs are produced using a variety of physical and chemical procedures; however, these methods have some disadvantages, including high costs and the requirement for high temperatures and pressures [9]. Additionally, chemicals used in chemical synthesis processes, such as capped and reductants, have an influence on both the environment and researchers [10]. Recent developments in nanobiotechnology and the eco-friendly production of nanoparticles using plants have received more attention [11,12,13,14]. In comparison to chemical and physical methods, biosynthesis processes are quick, easy, cheap, and, most importantly, efficient and environmentally friendly [15]. In practice, the extract’s constituents act as potential capping and reducing agents, limit the over-growth of nanoparticles in colloidal synthesis and prevent their excessive accumulation. These molecules could also affect and improve the characteristics of the resulting nanoparticles by enhancing the performance of these molecules to make them more suitable for a variety of applications [16]. Biogenic synthesis of nanoparticles, particularly plant-based nanoparticle synthesis, appears to be a more efficient process because it does not use risky or dangerous chemicals, as utilized in organic synthesis, to generate nanoparticles [17].
AgNPs have attracted more attention than other nanoparticles due to their unique surface plasmon resonance [18]. Silver nanoparticles are used in antibacterial activities, sensors, electrical conductivity, and water purification in addition to their catalytic properties. Furthermore, significant antiangiogenesis, anti-inflammatory, antiplatelet, and antiviral activity are demonstrated by silver nanoparticles [19,20,21,22]. Interesting plants with pharmacological characteristics may be helpful in the creation of green AgNPs. Flaxseed (Linumusitatissimum L), often known as the flax plant, is a member of the Linaceae family. Flaxseed has garnered a lot of attention in the world of food and health research due to its possible medical advantages. Flaxseeds also contain proteins, soluble and insoluble fibers, omega-3 fatty acids, short-chain polyunsaturated fatty acids, phytoestrogens lignans, and a variety of antioxidants [23,24,25,26]. Flaxseed offers several functional advantages in addition to its nutritional benefits, such as antimicrobial activity, and the capacity to emulsify, bind water, and alter viscosity [27,28,29]. The bioactive components of flaxseeds have a variety of functions, including anti-inflammatory (reducing the synthesis of the pro-inflammatory cytokines IL-6 and TNF-) and antiplatelet (inhibiting the anticoagulant prostacyclin) effects. The mucilage from soluble flaxseeds has antioxidant properties, regulates digestive functions, safeguards the liver, and reduces the risk of cardiovascular diseases. The antibacterial activity of flaxseeds may be related to their long-chain unsaturated fatty acids, particularly linolenic and linoleic acids, lignans, and phenolic acids. The most common application of flaxseeds is as a dietary supplement to reduce cholesterol or restore normal gastrointestinal function [30]. Therefore, the objective of this research was to produce AgNPs using flaxseed extract in an environmentally friendly manner. The biosynthesized nanoparticles were physicochemically evaluated by UV–Vis, FTIR, XRD, DSL-zeta potential, SEM, and TEM. The antioxidant capacity has been studied using the DPPH free radical scavenging technique. The ability of the produced AgNPs to inhibit the growth of certain prevalent pathogenic bacteria that are antibiotic-resistant was also evaluated, Gram-negative, such as Escherichia coli and Klebsiella pneumonia, in addition to Gram-positive bacteria, including Staphylococcus aureus and Streptococcus pyogenes.
2. Materials and Methods
2.1. Extract of Flaxseed and Green Synthesis of Silver NPs
The plant was taxonomically identified as flaxseed (Linumusitatissimum L.), which was recently confirmed at the Department of Plant and Gardening/College of Agriculture, Al-Qadisiyah University, Iraq. The plant extraction method reported was performed following recently reported literature [31], with only a few adjustments. Briefly, flaxseed extract was produced by boiling 50 mL of deionized water with 3 g of finely ground flaxseed. After cooling, Whatman No. 1 filter paper was used to filter the extract. To produce flaxseed-silver nanoparticles Fs-AgNPs, 10 mL of extract (filtrate) was combined with 6 mL of made previously 1 mM AgNO3 solution, and the mixture was left to stand for about 5 h until the solution turned red color, which is a clear indication that AgNPs were being formed with flaxseed. Because the flaxseed extract contains antioxidants, the Ag ions reductions were confirmed by producing the solution’s distinctive red color.
2.2. Characterization of Green Synthesized AgNPs
To determine the optimum parameters for the synthesis of AgNPs from flaxseed extract, several factors that affect the synthesis of AgNPs were studied, including the amount of flaxseed extract, the amount of AgNO3 solution, reaction time, pH, and temperature. Green fabricated AgNPs were tested using UV–Vis spectroscopy. The functional groups of the AgNPs were investigated using FT-IR analysis in the 400–4000 cm−1 range. Dynamic light scattering and zeta potential were used to determine the size and surface charge of the AgNPs; on the other hand, SEM was used to investigate the size and morphology of the AgNPs. A prepared sample of AgNPs was subjected to a scanning electron microscope (Carl Zeiss, Jena, Germany) at 20 kV (length of 3 × 3 cm) to examine the morphological characteristics of AgNPs at different magnifications. In addition, the crystalline form of the powdered AgNPs was evaluated by XRD measurement.
2.3. Antioxidants Evaluation
The antioxidant capacity of the AgNPs and flaxseed extract was evaluated using the DPPH free radical scavenging technique, with ascorbic acid serving as the positive control. In all, 1 mL of biosynthesized AgNP solution was diluted with methanol to acquire different concentrations (10–100 µg/mL), then mixed with 2 mL of DPPH solution (0.1 mM), and the standard solution of ascorbic acid was used as a positive control. The mixture was then agitated and kept at room temperature in the dark. The measurements of radical scavenging activity were determined by measuring the absorbance (A) of each sample with a UV–visible spectrophotometer at 517 nm, after 1 h of incubation. Calculating the percentage inhibition (I%) using the following equation:
2.4. Antibacterial Activity
The bacterial strains include Gram-negative, such as Escherichia coli and Klebsiella pneumonia, in addition to Gram-positive bacteria, including Staphylococcus aureus and Streptococcus pyogenes, were provided by the Laboratory of Bacteriology, Zoonotic Disease Unit, College of Veterinary Medicine, University of Al-Qadisiyah. AgNPs’ antibacterial effect was evaluated using the disk-diffusion tests [32]. Mueller–Hinton agar plates were quickly sterilized and allowed to culture the bacterial strains. First, sterile glass rods were used to inoculate the plates with 35 µL of suspensions of the bacteria with a concentration of about 105 cfu/mL. In all, 100 mg of the dry AgNPs was dissolved in 1 mL of distilled water to create the AgNP suspension. After being thoroughly cleaned, the disks were placed on the agar plates and immersed in the nanoparticle suspension. Finally, distilled water and AgNO3 solution were utilized as negative and positive controls in addition to flaxseed extract.
2.5. Statistical Analysis
One-way ANOVA was used to evaluate the differences between the groups, and Tukey’s test and the Student’s t-test were used to compare the groups. Test results were presented as the mean standard error of the three replicated as-say-specific mean values.
3. Results and Discussion
3.1. UV–Vis Spectroscopy
An essential and effective technique for the comprehensive categorization of synthesized nanoparticles is UV–vis spectroscopy. It is also used to examine AgNPs’ stability and manufacturing process [33]. Due to the distinct optical characteristics of AgNPs, they strongly interact with specific light wavelengths [34]. Moreover, UV–Vis spectroscopy is said to be quick, easy, and simple to use. It is quick to measure different types of NPs and is both sensitive and selective for them. Additionally, colloidal suspension particle characterization does not require calibration [35].
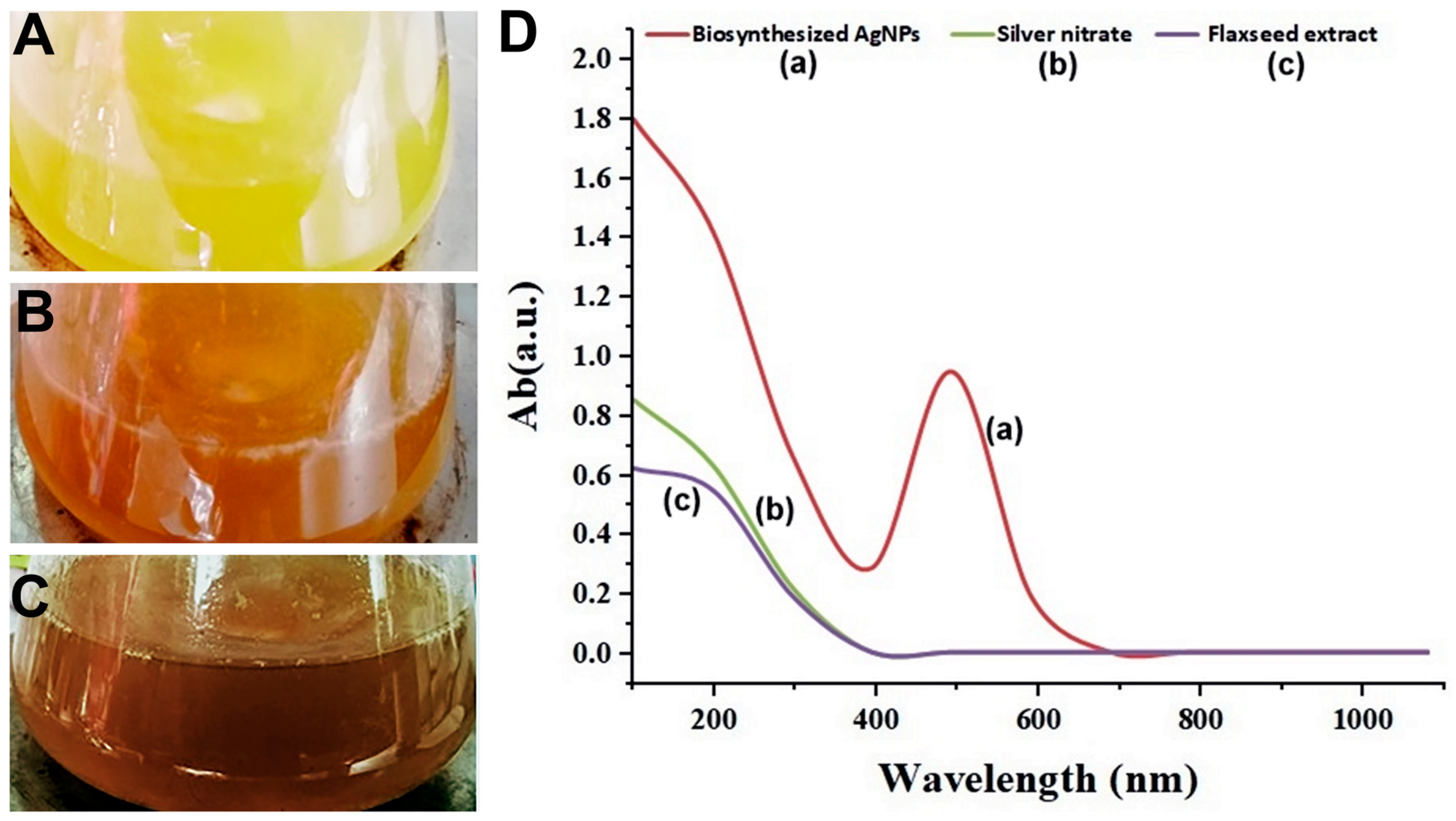
According to the reports, since the valence band and conduction band are so close together in AgNPs, electrons can move freely between them. An SPR absorption band is formed when the electrons of silver nanoparticles collectively oscillate in resonance with the light wave, which is caused by the free electrons discussed here [36]. The chemical environment, particle size, and dielectric medium all play a role in the absorption of AgNPs [37]. When the extract was incorporated into the AgNO3 solution, the color gradually changed from yellow to reddish-brown due to the reduction of silver salts Ag1+ to Ag0 by the bioactive compound present in the flaxseed extract.
The successful production of AgNPs was shown by the identification of the surface plasmon resonance which generates a single broad peak at 530 nm after 25 min (Figure 1).
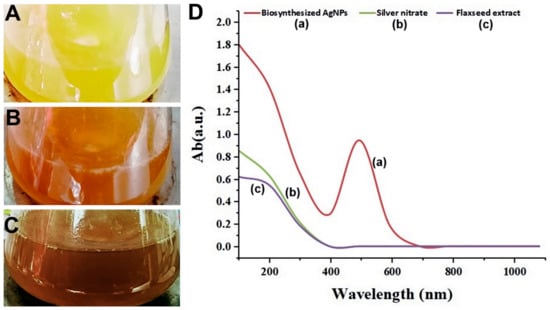
Figure 1.
Shows the color change patterns of silver nitrate (AgNO3) (A), flaxseed extract (B), and biosynthesized AgNPs (C). As well as (D). Illustrates the UV–Vis spectrophotometer measurement of the biosynthesized AgNPs (a), AgNO3 (b), and flax-seed extract (c).
According to a study by Nandi, I., and Ghosh, M., sesame husk, rice bran, and flaxseed are good fiber sources that can be added to a variety of foods (2015) [38]. It has been noted that flaxseed contains phytochemicals such as phenolic acids, cinnamic acids, flavonoids, and lignins that can act as reducing agents due to its capacity to donate electrons, the phenolic group enhances the transformation of silver nitrate into AgNPs [39], whereas proteins and several other phytochemicals act as capping agents for AgNPs [40]. The generation of the SPR peak is affected by variables such as size, shape, and particles produced. Furthermore, the variations in absorbance peak are directly correlated with changes in nanoparticle concentration. More AgNPs are produced as a result, which leads the peak intensity to increase over time as the extracted content becomes active [41]. When assessing these synthesized NPs, it is important to take into account several variables that impact the release rates of Ag ions. These variables primarily include particle size and environmental variables, including pH, temperature, time, radiation, dissolved oxygen, and capping agents [42].
However, in most cases, the bioreduction of silver ions to create silver nanocrust by biocompounds derived from a biological source of plant or organism is rarely achieved.
Generally, flavonoids, membrane proteins, NAD (P)+ reductases, dehydrogenases, citric acid, polyphenols, and secondary metabolites are examples of reducing agents [43], whereas extracellular proteins, enzymes, peptides, and tannic acids are examples of capping agents [44].
3.2. XRD Assay
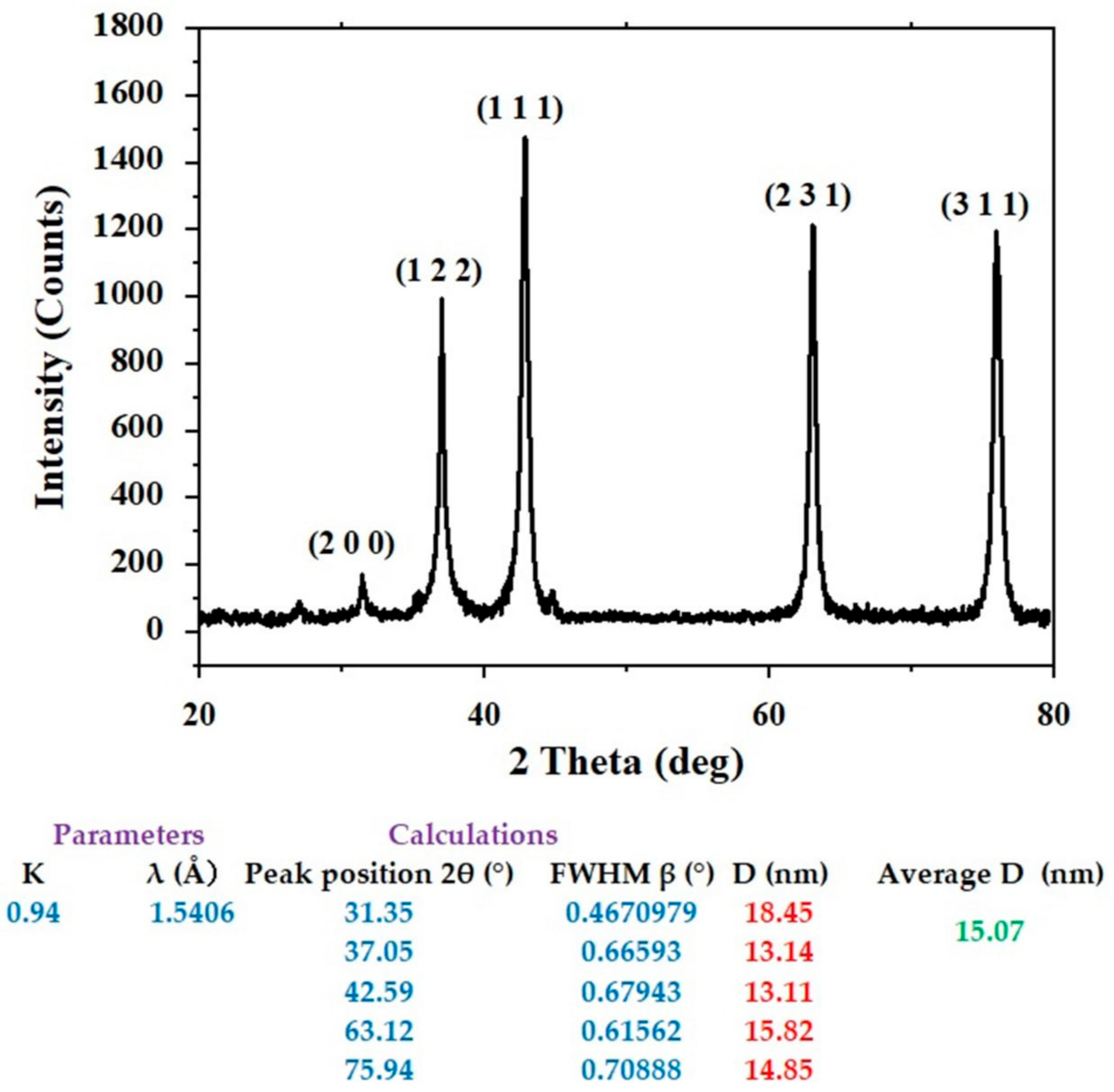
Figure 2 displays the XRD results, which demonstrate the crystal structure of AgNPs produced using flaxseed extract. It can be attributed the planes (1 1 1), (2 0 0), (2 2 0), and (3 1 1) to the diffraction peaks at 2 of 38.06, 44.24, 64.45, and 77.61, respectively, which indicated the face-centered cubic (fcc) structure of flaxseed-mediated silver nanoparticles as reported in the reference (JCPDS: file No-087–0720). The diameters of particle size of silver nanoparticles were determined using Scherrer’s Equation:
where D is the crystallite size, k is the shape factor, which is assumed to be 0.89 for silver, X is the X-ray wavelength (1.5406 A), is the half-height width of the XRD peak, and θ is the diffraction angle.
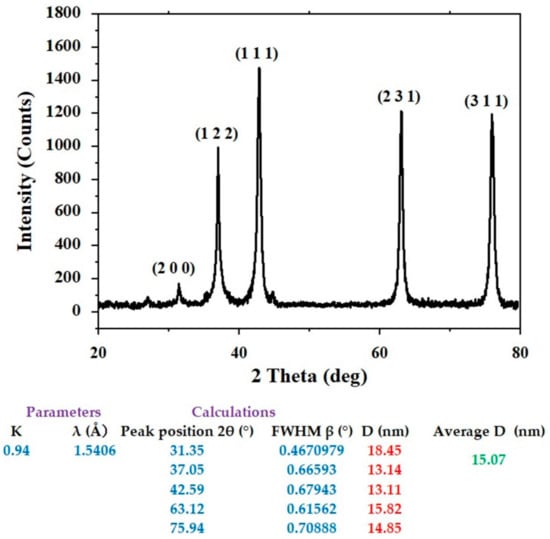
Figure 2.
XRD of the biosynthesized AgNPs.
AgNPs’ predicted average crystallite size was estimated to be around 15.07 nm. Researchers can follow the guidelines of green chemistry and use less energy for synthesis by operating at temperatures, pressures, and pH that are close to those found in the ambient environment.
This method can improve nanoparticles in some ways, including crystallinity, stability, size, and shape. Biomolecules (large macromolecules such as proteins, polysaccharides, lipids, and nucleic acids, and small natural molecules such as primary and secondary metabolites) produced by living organisms can convert the positive oxidation state of silver ion Ag+ into the zero-oxidation state of atoms Ag0 and form the nanosilver particles in the biosynthesis of colloidal dispersions of AgNPs [18].
3.3. Zeta Potential and DLS Characterization
Zeta potential and DLS measurement of nanoparticles are appropriate for characterizing nanoparticles and identifying the particle size and surface charge of the fabricated AgNPs.
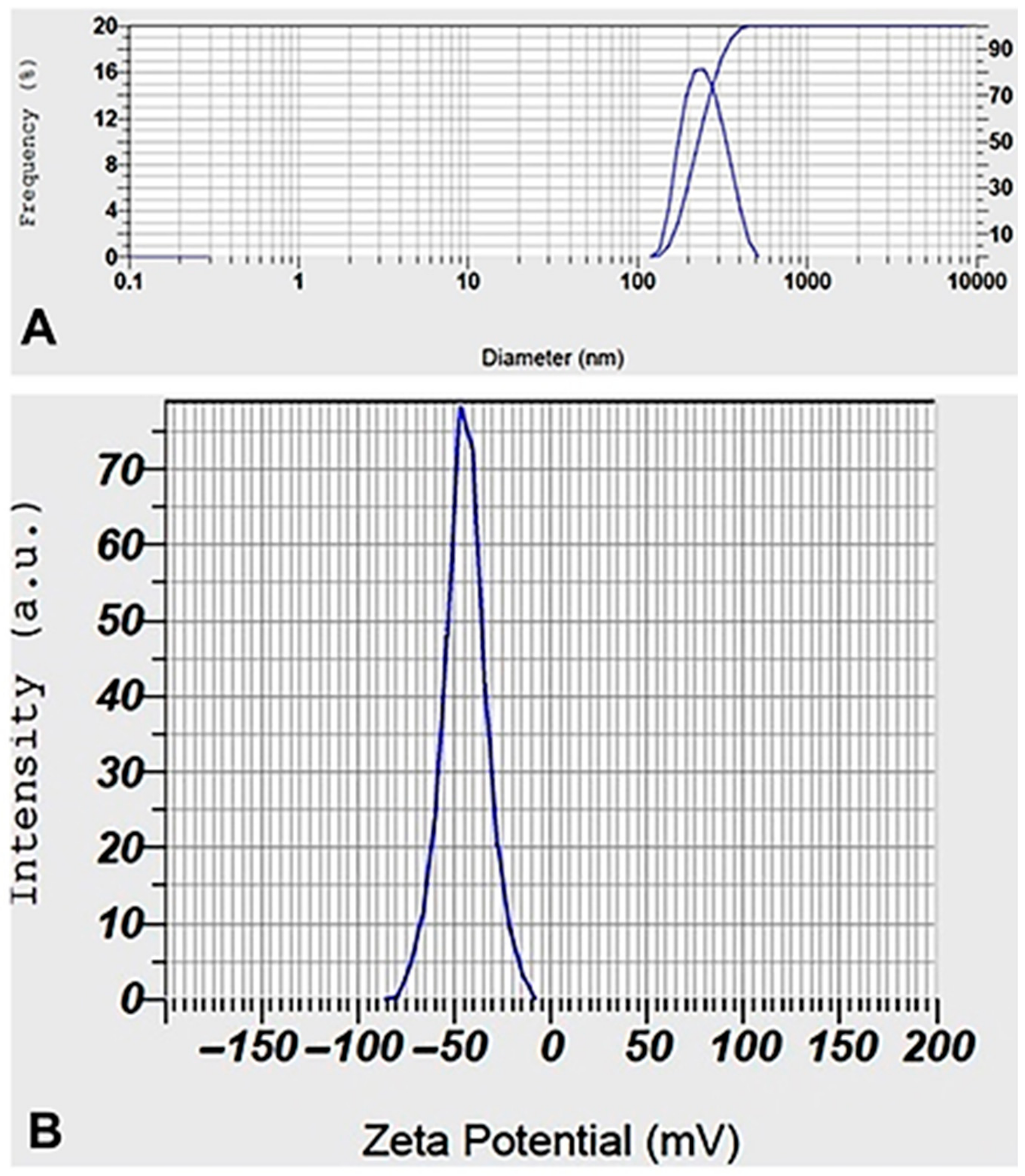
Figure 3a shows the AgNPs’ hydrodynamic size distribution as determined by DLS. AgNPs’ average size was determined to be 231.8 nm. Zeta potential measurements were used to test the colloidal stability of the AgNPs, as shown in Figure 3b, where the obtained value of −44.5 mV indicates a high level of stability. Because of the formation of a hydrodynamic sphere around particles in aqueous media, which leads to a larger interpretation of the particle diameter when analyzing the scattered light in a DLS measurement, the mean size of the nanoparticles obtained by DLS tends to be larger than the values obtained from electron microscopy. It is believed that the zeta potential values reported above 30 mV in magnitude demonstrate that the nanoparticle sample is basically stable concerning colloidal stability [45].
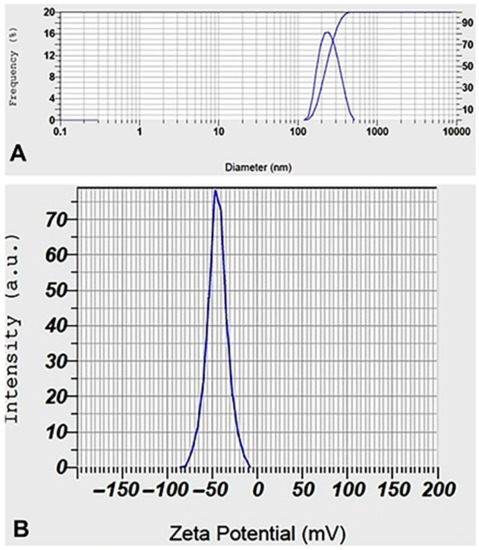
Figure 3.
DLS (A) and Zeta potential (B) distribution of biosynthesized AgNPs.
3.4. FTIR Assay
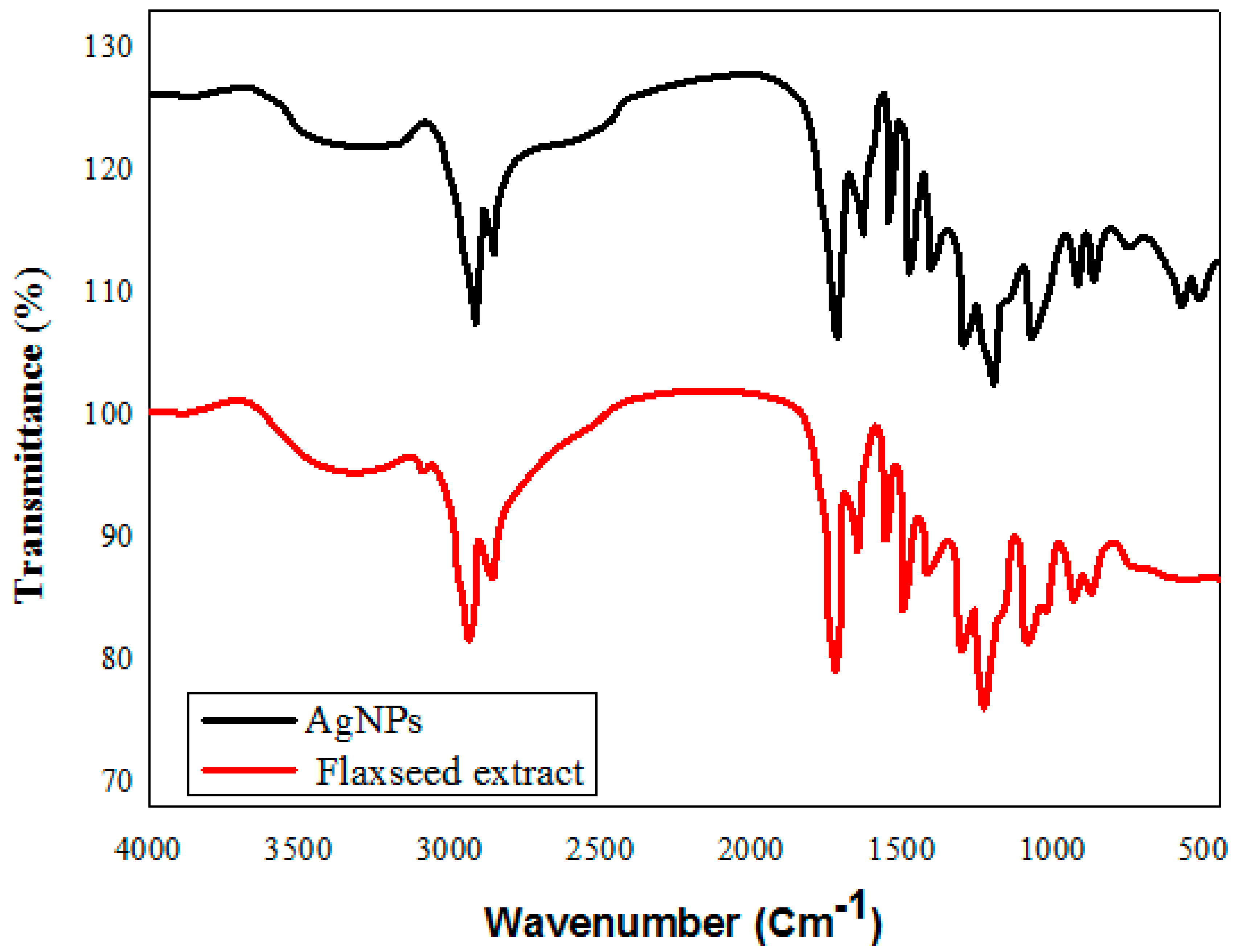
FTIR spectra of the flaxseed extract revealed a variety of distinctive IR absorption peaks at wave numbers of 3400 cm−1, 2928 cm−1, 1742 cm−1, and 1020 cm−1 in the aromatic and fingerprint areas of the IR band (Figure 4). The broad peak at 3400 cm−1 results from -OH groups, the 3010 cm−1 peak from aromatic C-H bonds, and the 1743 cm−1 and 1030 cm−1 wave-number peaks result from the presence of C = O (carbonyl groups) and C-O stretching vibrations of alcohols, carboxylic acids, ester, or ethers of biomolecules found in the flaxseed. The interaction of metal ions with the various functional groups presents accounts for the minor displacement in the peaks of the FTIR spectrum of the NPs relative to the extracted curve. The peak for silver oxide, which was seen at 518 cm−1 and 446 cm−1, is in close accord with the FTIR for AgNPs reported by Chettri, Prajwal, et al. [46]. Plant extracts are favored over microbial creation of nanoparticles because of their low synthesis rate, rapidity, high efficiency, and low toxicity. Synthesis of AgNPs from plant extract is likely to occur via an enzymatic process, the same as that used by microbes. Due to the presence of a complex of diverse antioxidant metabolites in plant cells that prevent oxidation and damage to biological components, the chemicals used for the stabilization and final capping of the nanoparticles must be distinct from those used for microbes. This means that biomolecules, including enzymes, glycosides, and saponins, can help to stabilize the nanoparticle. The literature suggests that when metal salts are added to a herb extract, silver ions bind to water-soluble chemicals through -OH and -COOH groups.
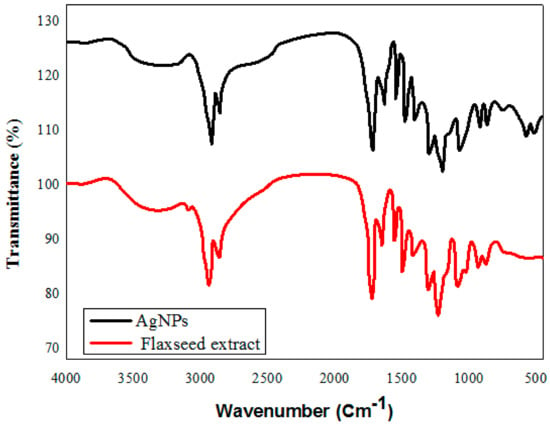
Figure 4.
FT-IR spectroscopic analysis of green synthesized silver nanoparticles, and flaxseed extract.
3.5. Morphology of the AgNPs
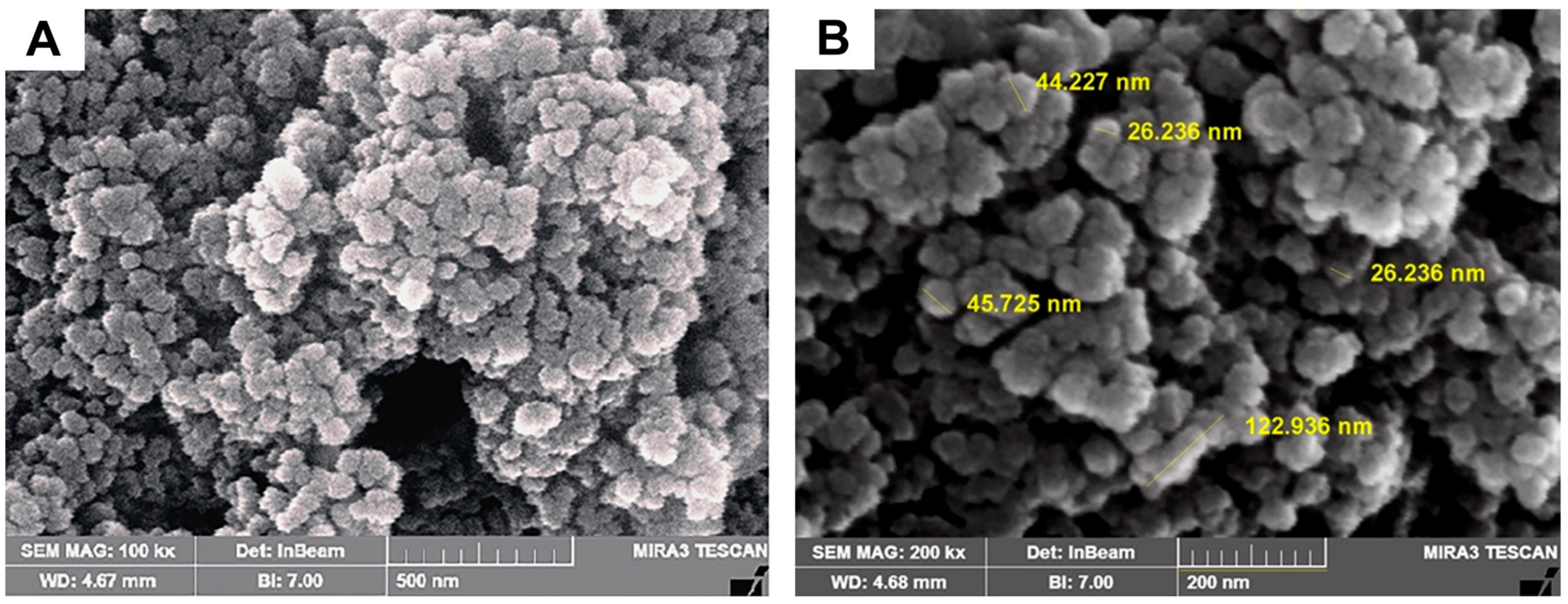
The SEM image was captured to examine surface characteristics and determine the size of AgNPs produced at the optimal concentration ratio of flaxseed extract and AgNO3 mixture. The image that was acquired is shown in Figure 5, and the presence of white color dots in the image demonstrated the synthesis of AgNPs. The particles were spherical in shape and had an average size of 46.98 ± 12.45 nm. The capping reagent employed to stabilize the NPs did not interact even in the collected aggregates, proving that only the extracts were responsible for the reduction. The agglomeration of smaller NPs resulted in the formation of some bigger AgNP aggregates that can be seen in the SEM image.
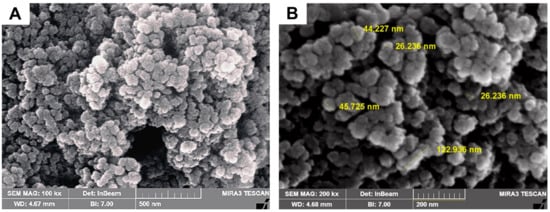
Figure 5.
SEM images of green synthesized AgNPs 0.2% flaxseed extract as illustrated in (A) low and (B) high SEM magnification SEM images, respectively.
3.6. Antioxidant Activity
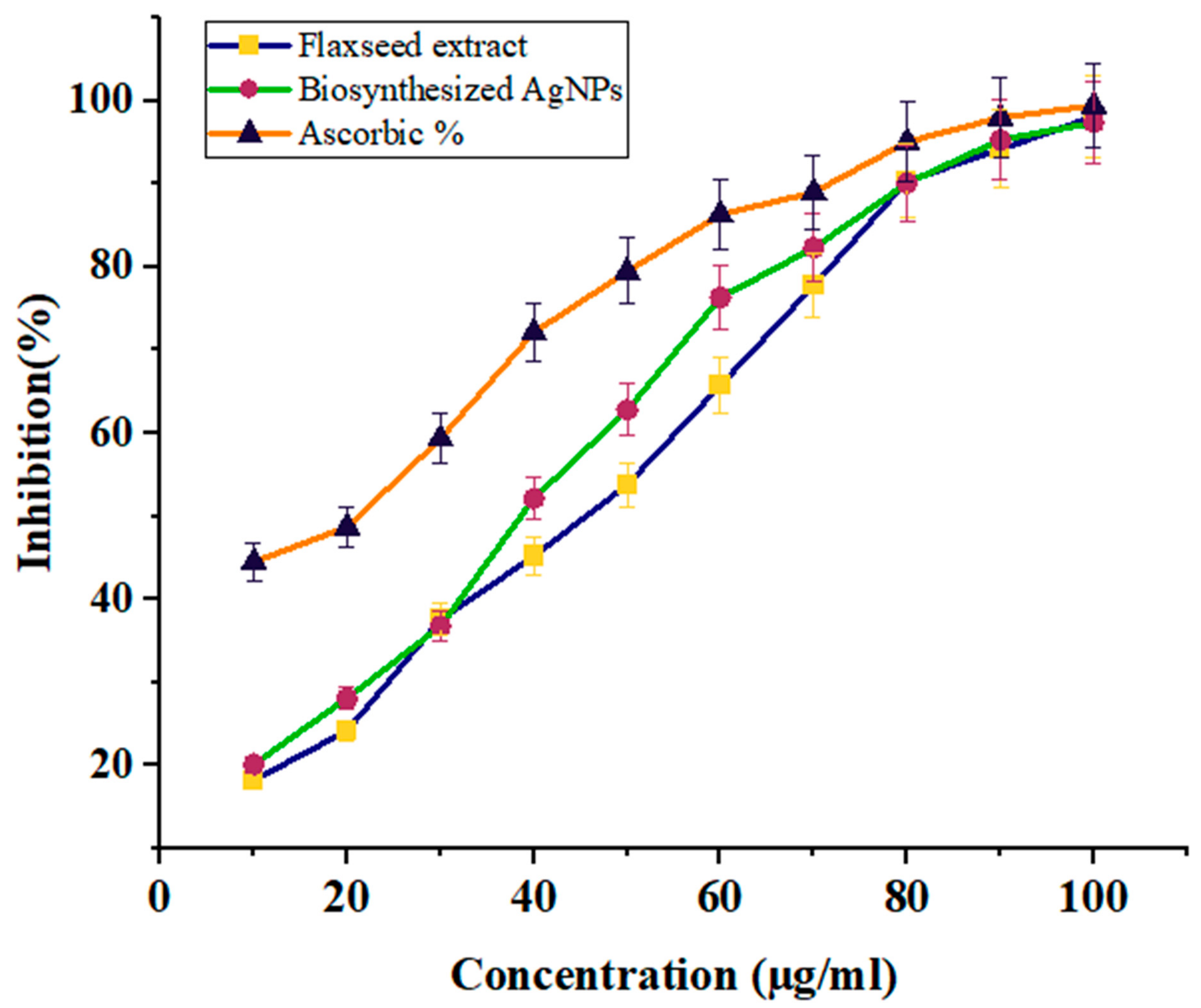
The antioxidant activity of flaxseed extract and biosynthesized AgNPs was evaluated using the DPPH method and ascorbic acid was used as a standard reference. The effect of various green synthesized AgNPs concentrations on the DPPH free radical inhibitory is shown in Table 1 and Figure 6. Our results indicate that both flaxseed extract and manufactured AgNPs are effective scavengers of free radicals. However, AgNPs outperformed flaxseed extract in terms of DPPH scavenging efficacy. The AgNPs and extract of flaxseed both increased their DPPH activity dose-dependently. Green-produced AgNPs exhibited scavenging activity ranging from 17% to 97% at doses of 10–100 µg/mL, with an average IC50 value of 44.56 ± 0.02 µg/mL. The antioxidant activity was significantly less than that of conventional ascorbic acid. Our findings are consistent with those of Yangqing He, et al., who investigated the antioxidant activity of AgNPs using Alpinia katsumadai seed extract [47].

Table 1.
IC50 values of flaxseed extract, AgNPs, and ascorbic acid.
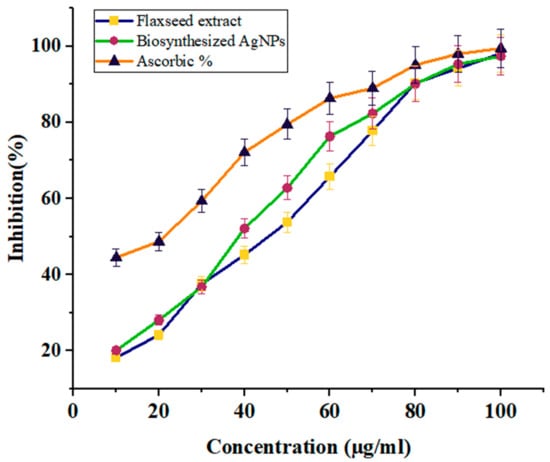
Figure 6.
The graph represents the percentage of Antioxidant (DPPH) scavenging activity of green synthesized silver nanoparticles and flaxseed ethanolic extract.
There is a difference in the antioxidant potential of flaxseed extract and AgNPs, as demonstrated in Table 1 and Figure 6, and both samples directly reacted and inhibited a variety of DPPH free radicals, and the scavenging activity raised as the concentration of the tested sample increased (46.38 ± 2.16%, 53.8 ± 2.22%, and 98.12 ± 1.31% at 20, 50, and 100 µg/mL) with flaxseed extract and (28.09 ± 0.95%, 62.81 ± 2.02%, and 97.4 ± 0.92% at 20, 50, and 100 µg/mL) with AgNPs. Furthermore, AgNPs had the best antioxidant activity; whose IC50 was 44.56 ± 0.02 μg/mL compared to 82 ± 0.079 μg/mL for flaxseed extract.
Concerning antioxidant capacity, the biosynthesized AgNPs made from flaxseed extract exceeded the extract, and this difference may be explained by the chemical makeup of the various samples used in the experiment [48]. Previous investigations have shown that flaxseed extract has substantial antioxidant activity [49]. The presence of a complex of diverse antioxidant metabolites in plant cells that prevent oxidation and damage to biological components, such as flavonoid and phenolic compounds, may be related to the antioxidant capacity of AgNPs. These compounds formed a coat over the silver nanoparticle and gave it a spherical morphology with a larger surface area after the physicochemical interaction of Ag ions with the functional groups of the plant extract [50].
3.7. Antimicrobial Activity
In this study, the antimicrobial activity results of inhibition zones induced by distilled water, flaxseed extract, AgNO3, and AgNPs are shown in Table 2. The positive control (AgNO3) presented antibacterial activity against E. coli, K. pneumoniae, S. aureus, and S. pyogenes. However, the biosynthesized AgNPs exhibit stronger antibacterial activity than AgNO3 and flaxseed extract with values of 8.9 ± 1.08 and 8.7 ± 1.04 for Gram-positive (S. aureus and S. pyogenes) bacteria and 11.0 ± 0.42, 10.8 ± 1.02 for Gram-negative (E. coli and K. pneumoniae) bacteria, respectively (Table 2). Gram-positive bacteria have a thick peptidoglycan layer in their cell walls, whereas Gram-negative bacteria have a thinner one. In a related investigation, it was discovered that the bactericidal effects of AgNPs made by using Alpinia galangal rhizome extract were more efficient against Gram-negative bacteria. The biosynthesized AgNPs often exhibit better antibacterial effects against Gram-negative bacteria because of the nature of the peptidoglycan layer in their cell walls [51]. A recent study suggests that decreasing the rate of agglomeration by including supporting components in the form of nanocomposites is an effective way to increase the antibacterial activity of AgNPs [52]. The mechanism by which AgNPs affect bacteria is yet to be understood. One theory proposes that AgNPs can bind to the cell membrane surface and alter the permeability and respiration properties of the membrane. It is simple to conceive that a particle’s interactions rely on how accessible its surface area is. Because of this, smaller particles have greater antibacterial activity than larger particles because of their large surface area [53]. Several investigations, including those using a wide variety of plant extracts, suggest that AgNPs may physically interact with the cell surfaces of various bacteria. The biological effects of AgNP occur through several different pathways. Intracellular adhesion, penetration, and disruption of organelles and biomolecules [54], as well as oxidative stress, are essential regulators of many bacterial functions. AgNPs were found attached to and deposited on the cell surface of bacteria, especially Gram-negative bacteria. AgNPs can enter Gram-negative bacteria cells through a water-filled channel, called a porin, in the outer membrane. The primary role of porins is the passive transport across the membrane of hydrophilic molecules of varying sizes and charges. Because silver ions are less likely to penetrate the thicker cell wall of Gram-positive bacteria, their action from AgNPs is more pronounced in Gram-negative bacteria. Since the negative charge of lipopolysaccharides encourages AgNP adherence, it is likely that they also strengthen the cell walls of Gram-negative bacteria, making them more vulnerable to silver nanoparticles [55]. Some studies have suggested that the ability of silver nanoparticles to adhere to the bacterial cell wall is due to an electrostatic interaction between the positively charged silver ions and the negatively charged surface of the cell membrane caused by the carboxyl, phosphate, and amino groups in the cell wall. Because of their electrostatic attraction to one another, silver nanoparticles can cross the cell membrane and alter its molecular composition and permeability. This causes the proton motive force (PMF) to dissipate and the membrane to degrade [56]. New biological applications, such as therapeutic drugs, diagnostics, antimicrobial substances, medication delivery systems, bio-imaging devices, labeling reagents, cancer medicines, etc., have been developed as a result of the development of new substances with synergistic effects, particularly naturally derived chemicals mixed with NPs [57]. Plant extracts are preferred over microbial creation of nanoparticles because of their low synthesis rate, rapidity, high efficiency, and viability. Synthesis of AgNPs from plant extract is likely to occur by an enzymatic process, the same as that used by microbes, due to the presence of a complex of diverse antioxidant metabolites in plant cells that prevent oxidation and damage to biological components.

Table 2.
Antimicrobial activity of flaxseed extract and green synthesized AgNPs.
The search for new sources of antimicrobial drugs has increased in recent years to tackle pathogenic infections with resistance to existing drugs. This has led to substantial research on medicinal plants, and plant-mediated nanomaterials with antimicrobial properties, as they contain a wide variety of bioactive compounds with well-known therapeutic properties. The effectiveness of green-fabricated nanoparticles exhibits great activity against a wide range of food-borne pathogens, including Salmonella, Bacillus, Escherichia coli, Shigella, proteus, as well as Staphylococcus species [58].
4. Conclusions
The development of eco-friendly green techniques for creating nanoparticles is a crucial step in the progress of nanotechnology as a dependable, environmentally sustainable method for producing a large variety of biocompatible materials and nanomaterials, including metal/metal oxide nanomaterials and nanocomposites. Therefore, green synthesis is viewed as a crucial technique to minimize the adverse effects related to the conventional methods of synthesis for nanoparticles that are frequently used in laboratories and industry. The green synthesis method offers a fast, safe, and affordable alternative when compared to other available physical and chemical approaches. Antibiotic-resistant bacteria are a global problem, but silver nanoparticles may provide a way to combat this problem. Making silver nanoparticles through synthesis provides access to a vast new realm of possibilities. The biosynthesis of nanoparticles using reducing and capping agents derived from biological sources such as plants, plant extracts, and microbes has gained a great deal of attention during the past ten years. Since no harmful or poisonous chemicals are used in natural green synthesis, it is also very cost-effective. When trying to create better treatments for bacterial infections, knowing how an antibacterial system works is a crucial first step. In general, nanoparticles have proven to be an effective replacement for antibiotic therapy and combinational therapy due to their astounding potential to address the issue of antibiotic-resistant microorganisms [59,60]. In conclusion, silver nanoparticles (AgNPs) are a product with potential uses in medicine and hygiene, and their eco-friendly synthesis can open the way for these applications. According to the findings of our research, flaxseed extract was a powerful reducing and capping agent for the production of AgNPs. Analytical methods such as FTIR, SEM, XRD, Zeta potential, and DLS were used to characterize the produced AgNPs. The spherical and triangular shapes of AgNPs, which have a size of roughly 46.9812.45 nm, were discovered using SEM research. According to the antibacterial tests, flaxseed extract shows promising antioxidant activity as well as antibacterial activity against both Gram-positive and Gram-negative bacteria.
Author Contributions
Conceptualization and methodology, A.K.A. and W.J.A.-K.; formal analysis, A.A.A., H.A.-K. and M.A.; investigation and data curation, S.A. and G.M.S.; validation, Y.K.; visualization, original draft preparation, S.A., Y.K. and W.J.A.-K.; writing—review and editing, S.A., G.M.S., A.K.A. and A.A.A.; supervision, A.A.A., S.A. and G.M.S.; project administration, A.K.A. and W.J.A.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to the Department of Medical Laboratory Sciences, College of Applied Medical Sciences, University of Bisha, Bisha, Saudi Arabia; College of Agriculture, University of Misan, Mayasn, Iraq; Department of Internal and Preventive Medicine, College of Veterinary Medicine, University of Al-Qadisiyah, Al-Diwaniyah, Iraq; and Division of Biotechnology, Department of Applied Sciences, University of Technology, Baghdad, Iraq for their technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Safat, S.; Buazar, F.; Albukhaty, S.; Matroodi, S. Enhanced sunlight photocatalytic activity and biosafety of marine-driven synthesized cerium oxide nanoparticles. Sci. Rep. 2021, 11, 14734. [Google Scholar] [CrossRef] [PubMed]
- Ragab, H.; Algethami, N.; Elamin, N.Y.; Asnag, G.; Rajeh, A.; Alzahrani, H.S. An insight into the influence of Ag/Se nanoparticles on the structural, optical, and electrical properties of Cs/PAM nanocomposites films as application in electrochemical devices. J. Mol. Struct. 2022, 1267, 133619. [Google Scholar] [CrossRef]
- Axet, M.; Philippot, K. Catalysis with colloidal ruthenium nanoparticles. Chem. Rev. 2020, 120, 1085–1145. [Google Scholar] [CrossRef] [PubMed]
- Albukhaty, S.; Naderi-Manesh, H.; Tiraihi, T.; Sakhi Jabir, M. Poly-l-lysine-coated superparamagnetic nanoparticles: A novel method for the transfection of pro-BDNF into neural stem cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, S125–S132. [Google Scholar] [CrossRef]
- Jabir, M.; Sahib, U.I.; Taqi, Z.; Taha, A.; Sulaiman, G.; Albukhaty, S.; Al-Shammari, A.; Alwahibi, M.; Soliman, D.; Dewir, Y.H. Linalool-loaded glutathione-modified gold nanoparticles conjugated with CALNN peptide as apoptosis inducer and NF-κB translocation inhibitor in SKOV-3 cell line. Int. J. Nanomed. 2020, 15, 9025. [Google Scholar] [CrossRef]
- Al-Kaabi, W.J.; Albukhaty, S.; Al-Fartosy, A.J.M.; Al-Karagoly, H.K.; Al-Musawi, S.; Sulaiman, G.M.; Dewir, Y.H.; Alwahibi, M.S.; Soliman, D.A. Development of Inula graveolens (L.) Plant Extract Electrospun/Polycaprolactone Nanofibers: A Novel Material for Biomedical Application. Appl. Sci. 2021, 11, 828. [Google Scholar] [CrossRef]
- Abdelmigid, H.M.; Morsi, M.M.; Hussien, N.A.; Alyamani, A.A.; Alhuthal, N.A.; Albukhaty, S. Green Synthesis of Phosphorous-Containing Hydroxyapatite Nanoparticles (nHAP) as a Novel Nano-Fertilizer: Preliminary Assessment on Pomegranate (Punica granatum L.). Nanomaterials 2022, 12, 1527. [Google Scholar] [CrossRef]
- Zhou, K.; Zhou, X.; Liu, J.; Huang, Z. Application of magnetic nanoparticles in petroleum industry: A review. J. Pet. Sci. Eng. 2020, 188, 106943. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Alhujaily, M.; Albukhaty, S.; Yusuf, M.; Mohammed, M.K.A.; Sulaiman, G.M.; Al-Karagoly, H.; Alyamani, A.A.; Albaqami, J.; AlMalki, F.A. Recent Advances in Plant-Mediated Zinc Oxide Nanoparticles with Their Significant Biomedical Properties. Bioengineering 2022, 9, 541. [Google Scholar] [CrossRef]
- Yadi, M.; Mostafavi, E.; Saleh, B.; Davaran, S.; Aliyeva, I.; Khalilov, R.; Nikzamir, M.; Nikzamir, N.; Akbarzadeh, A.; Panahi, Y.; et al. Current developments in green synthesis of metallic nanoparticles using plant extracts: A review. Artif. Cells Nanomed. Biotechnol. 2018, 46, S336–S343. [Google Scholar] [CrossRef]
- Karagoly, H.; Rhyaf, A.; Naji, H.; Albukhaty, S.; AlMalki, F.A.; Alyamani, A.A.; Albaqami, J.; Aloufi, S. Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract. Green Process. Synth. 2022, 11, 254–265. [Google Scholar] [CrossRef]
- Jihad, M.A.; Noori, F.T.M.; Jabir, M.S.; Albukhaty, S.; AlMalki, F.A.; Alyamani, A.A. Polyethylene Glycol Functionalized Graphene Oxide Nanoparticles Loaded with Nigella sativa Extract: A Smart Antibacterial Therapeutic Drug Delivery System. Molecules 2021, 26, 3067. [Google Scholar] [CrossRef]
- Alyamani, A.A.; Albukhaty, S.; Aloufi, S.; AlMalki, F.A.; Al-Karagoly, H.; Sulaiman, G.M. Green Fabrication of Zinc Oxide Nanoparticles Using Phlomis Leaf Extract: Characterization and In Vitro Evaluation of Cytotoxicity and Antibacterial Properties. Molecules 2021, 26, 6140. [Google Scholar] [CrossRef]
- Mahmood, R.I.; Kadhim, A.A.; Ibraheem, S.; Albukhaty, S.; Mohammed-Salih, H.S.; Abbas, R.H.; Al-Karagoly, H. Biosynthesis of copper oxide nanoparticles mediated annona muricata as cytotoxic and apoptosis inducer factor in breast cancer cell lines. Sci. Rep. 2022, 12, 16165. [Google Scholar] [CrossRef]
- Mohamad, N.A.N.; Arham, N.A.; Jai, J.; Hadi, A. Plant extract as reducing agent in synthesis of metallic nanoparticles: A review. Adv. Mat. Res. 2014, 832, 350–355. [Google Scholar] [CrossRef]
- Wang, C.; Mathiyalagan, R.; Kim, Y.J.; Castro-Aceituno, V.; Singh, P.; Ahn, S.; Wang, D.; Yang, D.C. Rapid green synthesis of silver and gold nanoparticles using Dendropanax morbifera leaf extract and their anticancer activities. Int. J. Nanomed. 2016, 11, 3691–3701. [Google Scholar]
- Delgado-Beleño, Y.; Martinez-Nuñez, C.E.; Cortez-Valadez, M.; Flores-López, N.S.; Flores-Acosta, M. Optical properties of silver, silver sulfide and silver selenide nanoparticles and antibacterial applications. Mater. Res. Bull. 2018, 99, 385–392. [Google Scholar] [CrossRef]
- Higa, A.M.; Mambrini, G.P.; Hausen, M.; Strixino, F.T.; Leite, F.L. Ag-nanoparticle-based nano-immunosensor for anti-glutathione s-transferase detection. Biointerface Res. Appl. Chem. 2016, 6, 1053–1058. [Google Scholar]
- Qiu, C.; Bennet, K.E.; Tomshine, J.R.; Hara, S.; Ciubuc, J.D.; Schmidt, U.; Durrer, W.G.; McIntosh, M.B.; Eastman, M.; Manciu, F.S. Ultrasensitive detection of neurotransmitters by surface enhanced raman spectroscopy for biosensing applications. Biointerface Res. Appl. Chem. 2017, 7, 1921–1926. [Google Scholar]
- Gonzalez, C.; Rosas-Hernandez, H.; Ramirez-Lee, M.A.; Salazar-Garcia, S.; Ali, S.F. Role of silver nanoparticles (AgNPs) on the cardiovascular system. Arch. Toxicol. 2016, 90, 493–511. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed]
- Kajla, P.; Sharma, A.; Sood, D.R. Flaxseed-a potential functional food source. J. Food Sci. Technol. 2015, 52, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [PubMed]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Akhilender Naidu, K.; Shang, X.; Keum, Y.-S. Omega−3 Polyunsaturated Fatty Acids (PUFAs): Emerging Plant and Microbial Sources, Oxidative Stability, Bioavailability, and Health Benefits—A Review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef]
- Halligudi, N. Pharmacological properties of flax seeds: A Review. Hygeia JD. Med. 2012, 4, 70–77. [Google Scholar]
- Mueed, A.; Shibli, S.; Korma, S.A.; Madjirebaye, P.; Esatbeyoglu, T.; Deng, Z. Flaxseed Bioactive Compounds: Chemical Composition, Functional Properties, Food Applications and Health Benefits-Related Gut Microbes. Foods 2022, 11, 3307. [Google Scholar] [CrossRef]
- Dzuvor, C.K.O.; Taylor, J.T.; Acquah, C.; Pan, S.; Agyei, D. Bioprocessing of functional ingredients from flaxseed. Molecules 2018, 23, 2444. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Shavandi, A.; Jodjaja, T.; Birch, J.; Teh, S.; Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Saeedi, P.; Bekhit, A.A. Flaxseed: Composition, detoxification, utilization, and opportunities. Biocatal. Agric. Biotechnol. 2018, 13, 129–152. [Google Scholar] [CrossRef]
- Shakir, K.F.; Madhusudhan, B. Hypocholesterolemic and hepatoprotective effects of flaxseed chutney: Evidence from animal studies. Indian J. Clin. Biochem. 2007, 22, 117. [Google Scholar] [CrossRef]
- Al-Radadi, N.S. Green Biosynthesis of Flaxseed Gold Nanoparticles (Au-NPs) as Potent Anti-cancer Agent against Breast Cancer Cells. J. Saudi Chem. Soc. 2021, 25, 101243. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad, M.B.; Jazayeri, S.D.; Shabanzadeh, P.; Sangpour, P.; Jahangirian, H.; Gharayebi, Y. Investigation of antibacterial properties silver nanoparticles prepared via green method. Chem. Cent. J. 2012, 6, 73. [Google Scholar] [CrossRef]
- Rao, Y.S.; Kotakadi, V.S.; Prasad, T.N.V.K.V.; Reddy, A.V.; Gopal, D.V.R.S. Green synthesis and spectral characterization of silver nanoparticles from Lakshmi tulasi (Ocimum sanctum) leaf extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 103, 156–159. [Google Scholar]
- Sulaiman, G.M.; Ali, E.H.; Jabbar, I.I.; Saleem, A.H. Synthesis, characterization, antibacterial and cytotoxic effects of silver nanoparticles. Dig. J. Nanomater. Biostructures 2014, 9, 787–796. [Google Scholar]
- Baalousha, M.; Stolpe, B.; Lead, J.R. Flow field-flow fractionation for the analysis and characterization of natural colloids and manufactured nanoparticles in environmental systems: A critical review. J. Chromatogr. A 2011, 1218, 4078–4103. [Google Scholar] [CrossRef]
- Desai, R.; Mankad, V.; Gupta, S.K.; Jha, P.K. Size distribution of silver nanoparticles: UV-visible spectroscopic assessment. Nanosci. Nanotech. Lett. 2012, 4, 30–34. [Google Scholar] [CrossRef]
- Vu, N.K.; Zille, A.; Oliveira, F.R.; Carneiro, N.; Souto, A.P. Effect of Particle Size on Silver Nanoparticle Deposition onto Dielectric Barrier Discharge (DBD) Plasma Functionalized Polyamide Fabric. Plasma Process. Polym. 2013, 10, 285–296. [Google Scholar] [CrossRef]
- Nandi, I.; Ghosh, M. Studies on functional and antioxidant property of dietary fibre extracted from defatted sesame husk, rice bran and flaxseed. Bioact. Carbohydr. Diet. Fibre 2015, 5, 129–136. [Google Scholar] [CrossRef]
- Gunathilake, T.; Akanbi, T.O.; Suleria, H.A.R.; Nalder, T.D.; Francis, D.S.; Barrow, C.J. Seaweed Phenolics as Natural Antioxidants, Aquafeed Additives, Veterinary Treatments and Cross-Linkers for Microencapsulation. Mar. Drugs 2022, 20, 445. [Google Scholar] [CrossRef]
- Balčiūnaitienė, A.; Liaudanskas, M.; Puzerytė, V.; Viškelis, J.; Janulis, V.; Viškelis, P.; Griškonis, E.; Jankauskaitė, V. Eucalyptus globulus and Salvia officinalis Extracts Mediated Green Synthesis of Silver Nanoparticles and Their Application as an Antioxidant and Antimicrobial Agent. Plants 2022, 11, 1085. [Google Scholar] [CrossRef]
- Khane, Y.; Benouis, K.; Albukhaty, S.; Sulaiman, G.M.; Abomughaid, M.M.; Al Ali, A.; Aouf, D.; Fenniche, F.; Khane, S.; Chaibi, W.; et al. Green Synthesis of Silver Nanoparticles Using Aqueous Citrus limon Zest Extract: Characterization and Evaluation of Their Antioxidant and Antimicrobial Properties. Nanomaterials 2022, 12, 2013. [Google Scholar] [CrossRef] [PubMed]
- Prosposito, P.; Mochi, F.; Ciotta, E.; Casalboni, M.; De Matteis, F.; Venditti, I.; Fontana, L.; Testa, G.; Fratoddi, I. Hydrophilic silver nanoparticles with tunable optical properties: Application for the detection of heavy metals in water. Beilstein J. Nanotechnol. 2016, 7, 1654–1661. [Google Scholar] [CrossRef]
- Batra, P.; Sharma, A.K. Anti-cancer potential of flavonoids: Recent trends and future perspectives. Biotech 2013, 3, 439–459. [Google Scholar] [CrossRef] [PubMed]
- Pochapski, D.J.; Santos, C.C.D.; Leite, G.W.; Pulcinelli, S.H.; Santilli, C.V. Zeta Potential and Colloidal Stability Predictions for Inorganic Nanoparticle Dispersions: Effects of Experimental Conditions and Electrokinetic Models on the Interpretation of Results. Langmuir 2021, 37, 13379–13389. [Google Scholar] [CrossRef] [PubMed]
- Tho, N.T.M.; An, T.N.M.; Tri, M.D.; Sreekanth, T.V.M.; Lee, J.-S.; Nagajyothi, P.C.; Lee, K.D. Green synthesis of silver nanoparticles using Nelumbo nucifera seed extract and its antibacterial activity. Acta Chim. Slov. 2013, 60, 673–678. [Google Scholar]
- Chettri, P.; Vendamani, V.S.; Tripathi, A.; Singh, M.K.; Pathak, A.P.; Tiwari, A. Green synthesis of silver nanoparticle-reduced graphene oxide using Psidium guajava and its application in SERS for the detection of methylene blue. Appl. Surf. Sci. 2017, 406, 312–318. [Google Scholar] [CrossRef]
- He, Y.; Wei, F.; Ma, Z.; Zhang, H.; Yang, Q.; Yao, B.; Huang, Z.; Li, J.; Zeng, C.; Zhang, Q. Green synthesis of silver nanoparticles using seed extract of Alpinia katsumadai, and their antioxidant, cytotoxicity, and antibacterial activities. RSC Adv. 2017, 7, 39842–39851. [Google Scholar] [CrossRef]
- Chinnasamy, G.; Chandrasekharan, S.; Bhatnagar, S. Biosynthesis of silver nanoparticles from Melia azedarach: Enhancement of antibacterial, wound healing, antidiabetic and antioxidant activities. Int. J. Nanomed. 2019, 14, 9823–9836. [Google Scholar] [CrossRef]
- Anwar, F.; Przybylski, R. Effect of solvents extraction on total phenolics and antioxidant activity of extracts from flaxseed (Linun usitatissimum L.). Acta Sci. Pol. Technol. Aliment. 2012, 11, 293–301. [Google Scholar]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef]
- Bhakya, S.; Muthukrishnan, S.; Sukumaran, M.; Muthukumar, M. Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl. Nanosci. 2016, 6, 755–766. [Google Scholar] [CrossRef]
- Cui, J.; Liang, Y.; Yang, D.; Liu, Y. Facile fabrication of rice husk based silicon dioxide nanospheres loaded with silver nanoparticles as a rice antibacterial agent. Sci. Rep. 2016, 6, 21423. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef]
- Tavares, T.D.; Antunes, J.C.; Padrão, J.; Ribeiro, A.I.; Zille, A.; Amorim, M.T.P.; Ferreira, F.; Felgueiras, H.P. Activity of Specialized Biomolecules against Gram-Positive and Gram-Negative Bacteria. Antibiotics 2020, 9, 314. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Ibraheem, D.R.; Hussein, N.N.; Sulaiman, G.M.; Mohammed, H.A.; Khan, R.A.; Al Rugaie, O. Ciprofloxacin-Loaded Silver Nanoparticles as Potent Nano-Antibiotics against Resistant Pathogenic Bacteria. Nanomaterials 2022, 12, 2808. [Google Scholar] [CrossRef]
- Murali, M.; Kalegowda, N.; Gowtham, H.G.; Ansari, M.A.; Alomary, M.N.; Alghamdi, S.; Shilpa, N.; Singh, S.B.; Thriveni, M.C.; Aiyaz, M.; et al. Plant-Mediated Zinc Oxide Nanoparticles: Advances in the New Millennium towards Understanding Their Therapeutic Role in Biomedical Applications. Pharmaceutics 2021, 13, 1662. [Google Scholar] [CrossRef]
- Wang, S.; Gao, Y.; Jin, Q.; Ji, J. Emerging antibacterial nanomedicine for enhanced antibiotic therapy. Biomater. Sci. 2020, 8, 6825–6839. [Google Scholar] [CrossRef]
- Roy, A.; Bulut, O.; Some, S.; Kumar Mandal, A.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).