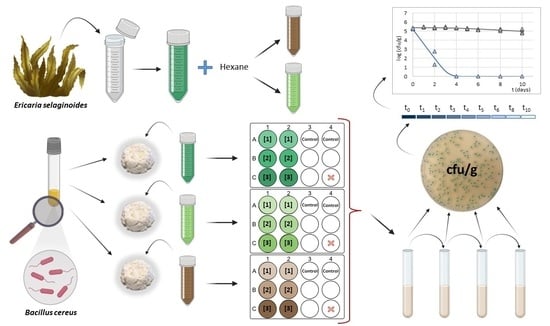
Challenge Test in Catalan “Mató” Fresh Cheese to Assess the Antimicrobial Activity of Ericaria selaginoides Extracts against Bacillus cereus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Macroalgae Collection
2.2. Macroalgae Extract Processing
2.2.1. Preparation of Crude Extracts
2.2.2. Two-Phase Extraction Procedure
2.2.3. Preparation of Extracts for Application in Fresh Cheese
2.3. Challenge Test—Experimental Design
2.3.1. Bacterial Strains and Culture Conditions
2.3.2. Laboratory-Scale Fresh Cheese Model
2.4. Analytical Determinations
2.5. Statistical Analysis of Data
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bottone, E.J. Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev. 2010, 23, 382–398. [Google Scholar] [CrossRef]
- From, C.; Pukall, R.; Schumann, P.; Hormazábal, V.; Granum, P.E. Toxin-producing ability among Bacillus spp. outside the Bacillus cereus group. Appl. Environ. Microbiol. 2005, 71, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, R.; Jessberger, N.; Ehling-Schulz, M.; Märtlbauer, E.; Granum, P.E. The food poisoning toxins of Bacillus cereus. Toxins 2021, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, D.; Rosell, C.M.; Martinez, A. Risk of Bacillus cereus in relation to rice and derivatives. Foods 2021, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- EFSA. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e06406. [Google Scholar] [CrossRef]
- Choi, W.; Kim, S.S. Outbreaks, germination, and inactivation of Bacillus cereus in food products: A review. J. Food Prot. 2020, 83, 1480–1487. [Google Scholar] [CrossRef]
- Griffiths, M.W.; Schraft, H. Bacillus cereus food poisoning. In Foodborne Diseases: Third Edition; Dodd, C.E.R., Aldsworth, T., Stein, R.A., Cliver, D.O., Riemann, H.P., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 395–405. [Google Scholar] [CrossRef]
- Nicholson, W.L.; Munakata, N.; Horneck, G.; Melosh, H.J.; Setlow, P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 2000, 64, 548–572. [Google Scholar] [CrossRef]
- Becker, H.; Schaller, G.; von Wiese, W.; Terplan, G. Bacillus cereus in infant foods and dried milk products. Int. J. Food Microbiol. 1994, 23, 1–15. [Google Scholar] [CrossRef]
- Di Pinto, A.; Bonerba, E.; Bozzo, G.; Ceci, E.; Terio, V.; Tantillo, G. Occurrence of potentially enterotoxigenic Bacillus cereus in infant milk powder. Eur. Food Res. Technol. 2013, 237, 275–279. [Google Scholar] [CrossRef]
- Lücking, G.; Stoeckel, M.; Atamer, Z.; Hinrichs, J.; Ehling-Schulz, M. Characterization of aerobic spore-forming bacteria associated with industrial dairy processing environments and product spoilage. Int. J. Food Microbiol. 2013, 166, 270–279. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Bacillus cereus and Other Bacillus spp. In Bad Bug Book: Foodborne Pathogenic Microorganisms and Natural Toxins Handbook; International Medical Publishing: London, UK, 2012; pp. 92–95. [Google Scholar]
- Montone, A.M.I.; Capuano, F.; Mancusi, A.; Di Maro, O.; Peruzy, M.F.; Proroga, Y.T.R.; Cristiano, D. Exposure to Bacillus cereus in water buffalo mozzarella cheese. Foods 2020, 9, 1899. [Google Scholar] [CrossRef]
- Adame-Gómez, R.; Muñoz-Barrios, S.; Castro-Alarcón, N.; Leyva-Vázquez, M.A.; Toribio-Jiménez, J.; Ramírez-Peralta, A. Prevalence of the strains of Bacillus cereus group in artisanal Mexican cheese. Foodborne Pathog. Dis. 2020, 17, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, S.; Mulargia, A.F.; Pisano, B.; Tuveri, P.; Palmas, F. Incidence and biochemical characteristics of Bacillus flora in Sardinian dairy products. Int. J. Food Microbiol. 1997, 38, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Ehling-Schulz, M.; Messelhäusser, U.; Granum, P.E. Bacillus cereus in milk and dairy production. In Rapid Detection, Characterization, and Enumeration of Foodborne Pathogens; Hoorfar, J., Ed.; ASM Press: Washington DC, USA, 2011. [Google Scholar] [CrossRef]
- Statista Research Department. Global per Capita Consumption of Cheese 2020 by Country. 2021. Available online: https://www.statista.com/statistics/527195/consumption-of-cheese-per-capita-worldwide-country/ (accessed on 26 July 2022).
- European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, 50, 1–26. [Google Scholar]
- Bondi, M.; Lauková, A.; De Niederhausern, S.; Messi, P.; Papadopoulou, C. Natural preservatives to improve food quality and safety. J. Food Qual. 2017, 2017, 090932. [Google Scholar] [CrossRef]
- Shi, C.; Maktabdar, M. Lactic Acid Bacteria as biopreservation against spoilage molds in dairy products–A review. Front. Microbiol. 2022, 12, 819684. [Google Scholar] [CrossRef] [PubMed]
- Kilinç, B.; Cirik, S.; Turan, G.; Tekogul, H.; Koru, E. Seaweeds for food and industrial applications. Chem. Age India 2013, 33, 475–482. [Google Scholar]
- Barbosa, M.; Valentão, P.; Andrade, P.B. Bioactive compounds from macroalgae in the new millennium: Implications for neurodegenerative diseases. Mar. Drugs 2014, 12, 4934–4972. [Google Scholar] [CrossRef]
- Silva, A.; Silva, S.A.; Carpena, M.; Garcia-Oliveira, P.; Gullón, P.; Barroso, M.F.; Prieto, M.A.; Simal-Gandara, J. Macroalgae as a source of valuable antimicrobial compounds: Extraction and applications. Antibiotics 2020, 9, 642. [Google Scholar] [CrossRef]
- Biris-Dorhoi, E.S.; Michiu, D.; Pop, C.R.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae—A sustainable source of chemical compounds with biological activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef]
- Martínez, M.A.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Maximiliano, J.E.; Rodríguez, J.L.; Martínez-Larrañaga, M.R.; Anadón, A.; Peteiro, C.; Rubiño, S.; et al. Brown marine algae Gongolaria baccata extract protects Caco-2 cells from oxidative stress induced by tert-butyl hydroperoxide. Food Chem. Toxicol. 2021, 156, 112460. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Rubiño, S.; Peteiro, C.; Aymerich, T.; Hortós, M. Brown macroalgae (Phaeophyceae): A valuable reservoir of antimicrobial compounds in North coasts of Spain. Mar. Drugs 2022, 20, 775. [Google Scholar] [CrossRef]
- Cabral, E.M.; Oliveira, M.; Modala, J.R.M.; Curtin, J.; Tiwari, B.K.; García-Vaquero, M. Antimicrobials from seaweeds for food applications. Mar. Drugs 2021, 19, 211. [Google Scholar] [CrossRef] [PubMed]
- Molinari-Novoa, E.A.; Guiry, M.D. Reinstatement of the genera Gongolaria Boehmer and Ericaria Stackhouse (Sargassaceae, Phaeophyceae). Notulae Algarum 2020, 172, 1–10. [Google Scholar]
- Orellana, S.; Hernandez, M.; Sanson, M. Diversity of Cystoseira sensu lato (Fucales, Phaeophyceae) in the eastern Atlantic and Mediterranean based on morphological and DNA evidence, including Carpodesmia gen. emend. and Treptacantha gen. emend. Eur. J. Phycol. 2019, 54, 447–465. [Google Scholar] [CrossRef]
- Fletcher, R.L. Seaweeds of the British Isles; Fucophyceae (Phaeophyceae) Part 1.; British Museum (Natural History): London, UK, 1987; Volume 3. [Google Scholar]
- Gómez Garreta, A.; Barceló Martí, M.C.; Gallardo García, T.; Pérez-Ruzafa, I.M.; Ribera Sigual, M.A.; Rull Lluch, J. Flora Phycológica Ibérica. 1. Fucales; Universidad de Murcia, Servicio de Publicaciones: Murcia, Spain, 2000. [Google Scholar]
- Rubiño, S.; Peteiro, C.; Aymerich, T.; Hortós, M. Major lipophilic pigments in Atlantic seaweeds as valuable food ingredients: Analysis and assessment of quantification methods. Food Res. Int. 2022, 159, 111609. [Google Scholar] [CrossRef]
- European Parliament; Council of the European Union. Directive 2009/32/EC of the European Parliament and of the Council of 23 April 2009 on the approximation of the laws of the Member States on extraction solvents used in the production of foodstuffs and food ingredients. Off. J. Eur. Union 2009, 141, 3–11. [Google Scholar]
- CLSI Standard M07; Methods for Dilution Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically. Approved standard, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- Jofré, A.; Latorre-Moratalla, M.L.; Garriga, M.; Bover-Cid, S. Domestic refrigerator temperatures in Spain: Assessment of its impact on the safety and shelf-life of cooked meat products. Food Res. Int. 2019, 126, 108578. [Google Scholar] [CrossRef]
- EURL Lm. Technical Guidance Document on Challenge Tests and Durability Studies for Assessing Shelf-Life of Ready-to-Eat Foods Related to Listeria monocytogenes; Version 4 of 1 July 2021; Anses – Food Safety Laboratory: Maisons-Alfort, France, 2021. [Google Scholar]
- Abourriche, A.; Charrouf, M.; Berrada, M.; Bennamara, A.; Chaib, N.; Francisco, C. Antimicrobial activities and cytotoxicity of the brown alga Cystoseira tamariscifolia. Fitoterapia 1999, 70, 611–614. [Google Scholar] [CrossRef]
- Salvador, N.; Garreta, A.G.; Lavelli, L.; Ribera, M.A. Antimicrobial activity of Iberian macroalgae. Sci. Mar. 2007, 71, 101–114. [Google Scholar] [CrossRef]
- Karima, S.; Fatiha, B. Study of the antimicrobial activity of four Algerian marin algae species. In Microbes in Applied Research; World Scientific: Singapore, 2012; pp. 578–581. [Google Scholar]
- Ainane, T.; Abourriche, A.; Kabbaj, M.; Elkouali, M.; Bennamara, A.; Charrouf, M.; Lemrani, M. Biological activities of extracts from seaweed Cystoseira tamariscifolia: Antibacterial activity, antileishmanial activity and cytotoxicity. J. Chem. Pharm. Res. 2014, 6, 607–611. [Google Scholar]
- De La Fuente, G.; Pinteus, S.; Silva, J.; Alves, C.; Pedrosa, R. Antioxidant and antimicrobial potential of six fucoids from the Mediterranean Sea and the Atlantic. Ocean. J. Sci. Food. Agric. 2022, 102, 5568–5575. [Google Scholar] [CrossRef]
- Moroney, N.C.; O’Grady, M.N.; O’Doherty, J.V.; Kerry, J.P. Effect of a brown seaweed (Laminaria digitata) extract containing laminarin and fucoidan on the quality and shelf-life of fresh and cooked minced pork patties. Meat Sci. 2013, 94, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Sineiro, J.; Amado, I.R.; Franco, D. Influence of natural extracts on the shelf life of modified atmosphere-packaged pork patties. Meat Sci. 2014, 96, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-J.; Choi, J.-S.; Song, E.-J.; Lee, S.-Y.; Kim, K.-B.-W.-R.; Kim, S.-J.; Yoon, S.-Y.; Lee, S.-J.; Park, N.-B.; Jung, J.-Y. Effect of Myagropsis myagroides extracts on shelf-life and quality of bread. Korean J. Food Sci. Technol. 2010, 42, 50–55. [Google Scholar]
- Jung, K.-I.; Choi, Y.-J.; Cho, E.-K. Effect of Ecklonia cava hot water extracts on shelf-life and quality of muffin. J. Korean Soc. Food Sci. Nutr. 2010, 39, 1672–1677. [Google Scholar] [CrossRef]
- Kim, M.-J.; Kim, K.-B.-W.-R.; Lee, C.-J.; Kwak, J.-H.; Kim, D.-H.; SunWoo, C.; Jung, S.-A.; Kang, J.-Y.; Kim, H.-J.; Choi, J.-S. Effect of Sargassum sagamianum extract on shelf-life and improved quality of morning bread. Korean J. Food Sci. Technol. 2011, 43, 723–728. [Google Scholar] [CrossRef]
- O’Sullivan, A.M.; O’Callaghan, Y.C.; O’Grady, M.N.; Waldron, D.S.; Smyth, T.J.; O’Brien, N.M.; Kerry, J.P. An examination of the potential of seaweed extracts as functional ingredients in milk. Int. J. Dairy Technol. 2014, 67, 182–193. [Google Scholar] [CrossRef]
- O’Sullivan, A.M.; O’Grady, M.N.; O’Callaghan, Y.C.; Smyth, T.J.; O’Brien, N.M.; Kerry, J.P. Seaweed extracts as potential functional ingredients in yogurt. Innov. Food Sci. Emerg. Technol. 2016, 37, 293–299. [Google Scholar] [CrossRef]
- Bhowmick, S.; Mazumdar, A.; Moulick, A.; Adam, V. Algal metabolites: An inevitable substitute for antibiotics. Biotechnol. Adv. 2020, 43, 107571. [Google Scholar] [CrossRef] [PubMed]
- Shannon, E.; Abu-Ghannam, N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Zhang, W.; Smid, S.D. Phlorotannins: A review on biosynthesis, chemistry and bioactivity. Food Biosci. 2021, 39, 100832. [Google Scholar] [CrossRef]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, K.; Iwamura, Y.; Shibata, T.; Hirayama, I.; Nakamura, T. Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J. Antimicrob. Chemother. 2002, 50, 889–893. [Google Scholar] [CrossRef]
- Deyab, M.A.; Abou-Dobara, M.I. Antibacterial activity of some marine algal extracts against most nosocomial bacterial infections. Egypt. J. Exp. Biol. 2013, 9, 281–286. [Google Scholar]
- Sivagnanam, S.P.; Yin, S.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Biological properties of fucoxanthin in oil recovered from two brown seaweeds using supercritical CO2 extraction. Mar. Drugs 2015, 13, 3422–3442. [Google Scholar] [CrossRef]
- Rajeshkumar, S. Phytochemical constituents of fucoidan (Padina tetrastromatica) and its assisted AgNPs for enhanced antibacterial activity. IET Nanobiotechnol. 2017, 11, 292–299. [Google Scholar] [CrossRef]
- Khan, F.; Jeong, G.J.; Khan, M.S.A.; Tabassum, N.; Kim, Y.M. Seaweed-Derived phlorotannins: A review of multiple biological roles and action mechanisms. Mar. Drugs 2022, 20, 384. [Google Scholar] [CrossRef]
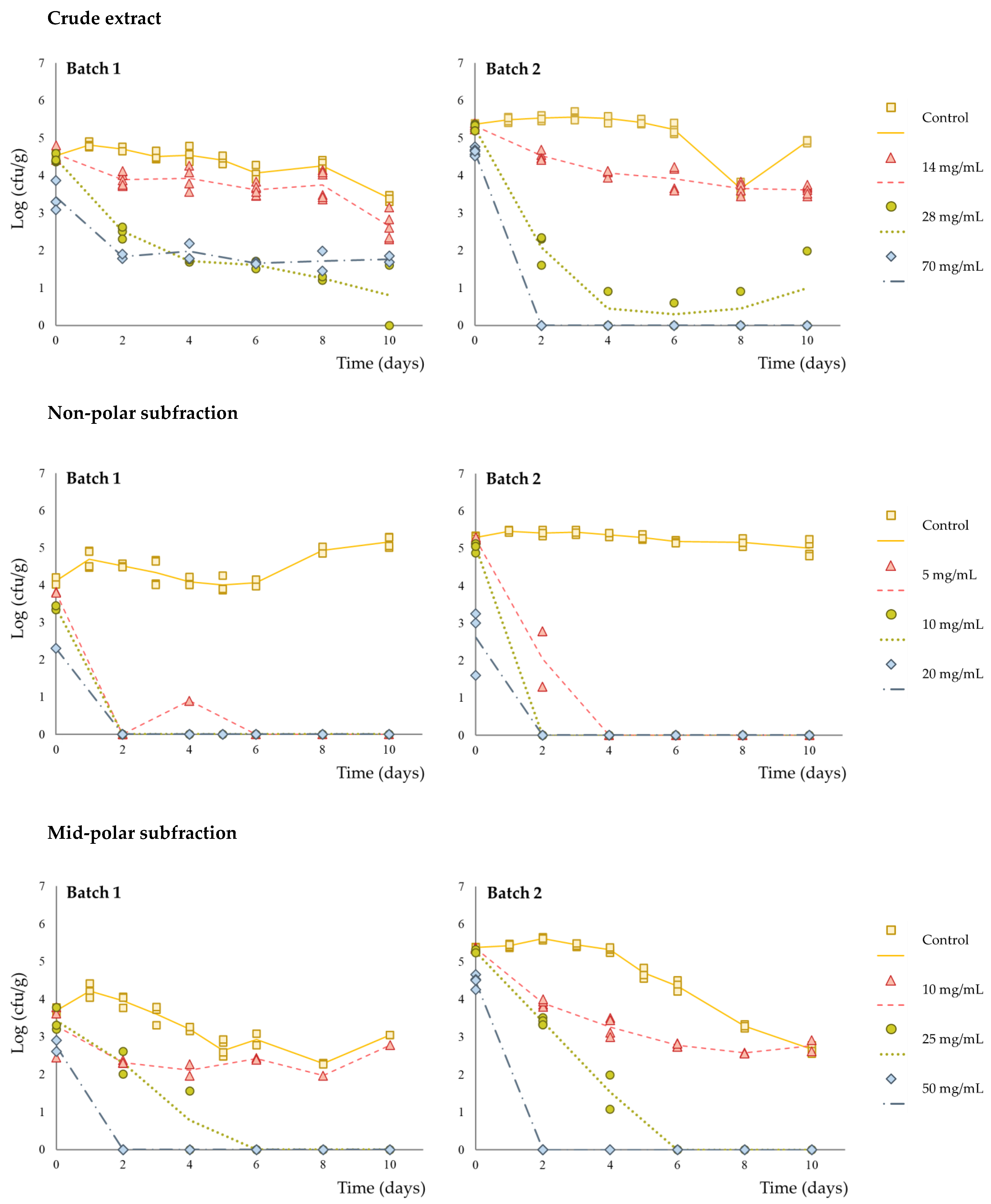
| Crude extract | |||
|---|---|---|---|
| 14 mg/g | 28 mg/g | 70 mg/g | |
| Initial log reduction a | <0.5 | <0.5 | 0.92 ± 0.27 |
| Inactivation potential b | 1.81 ± 0.11 | 4.05 ± 0.45 | 4.06 ± 1.84 |
| Non-polar subfraction | |||
| 5 mg/g | 10 mg/g | 20 mg/g | |
| Initial log reduction a | <0.5 | <0.5 | 2.24 ± 0.61 |
| Inactivation potential b | 4.70 ± 0.83 | 4.70 ± 0.83 | 4.70 ± 0.83 |
| Mid-polar subfraction | |||
| 10 mg/g | 25 mg/g | 50 mg/g | |
| Initial log reduction a | <0.5 | <0.5 | 0.91 ± 0.04 |
| Inactivation potential b | 1.76 ± 1.20 | 4.53 ± 1.19 | 4.53 ± 1.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubiño, S.; Aymerich, T.; Peteiro, C.; Bover-Cid, S.; Hortós, M. Challenge Test in Catalan “Mató” Fresh Cheese to Assess the Antimicrobial Activity of Ericaria selaginoides Extracts against Bacillus cereus. Appl. Sci. 2023, 13, 2207. https://doi.org/10.3390/app13042207
Rubiño S, Aymerich T, Peteiro C, Bover-Cid S, Hortós M. Challenge Test in Catalan “Mató” Fresh Cheese to Assess the Antimicrobial Activity of Ericaria selaginoides Extracts against Bacillus cereus. Applied Sciences. 2023; 13(4):2207. https://doi.org/10.3390/app13042207
Chicago/Turabian StyleRubiño, Susana, Teresa Aymerich, César Peteiro, Sara Bover-Cid, and María Hortós. 2023. "Challenge Test in Catalan “Mató” Fresh Cheese to Assess the Antimicrobial Activity of Ericaria selaginoides Extracts against Bacillus cereus" Applied Sciences 13, no. 4: 2207. https://doi.org/10.3390/app13042207
APA StyleRubiño, S., Aymerich, T., Peteiro, C., Bover-Cid, S., & Hortós, M. (2023). Challenge Test in Catalan “Mató” Fresh Cheese to Assess the Antimicrobial Activity of Ericaria selaginoides Extracts against Bacillus cereus. Applied Sciences, 13(4), 2207. https://doi.org/10.3390/app13042207