Assessing the Features of Tomato Pomace Powder in Suspensions
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Physico-Chemical Analysis of the Tomato Pomace Powder
2.2.1. Total Soluble Solid Content
2.2.2. Titratable Acidity
2.2.3. pH Value
2.2.4. Water Activity
2.2.5. Pectin Soluble in Water
2.3. Hydration Properties of the Tomato Pomace Powder
2.4. Color Evaluation
2.5. Rheological Measurements
2.5.1. Dynamic Oscillatory Measurements
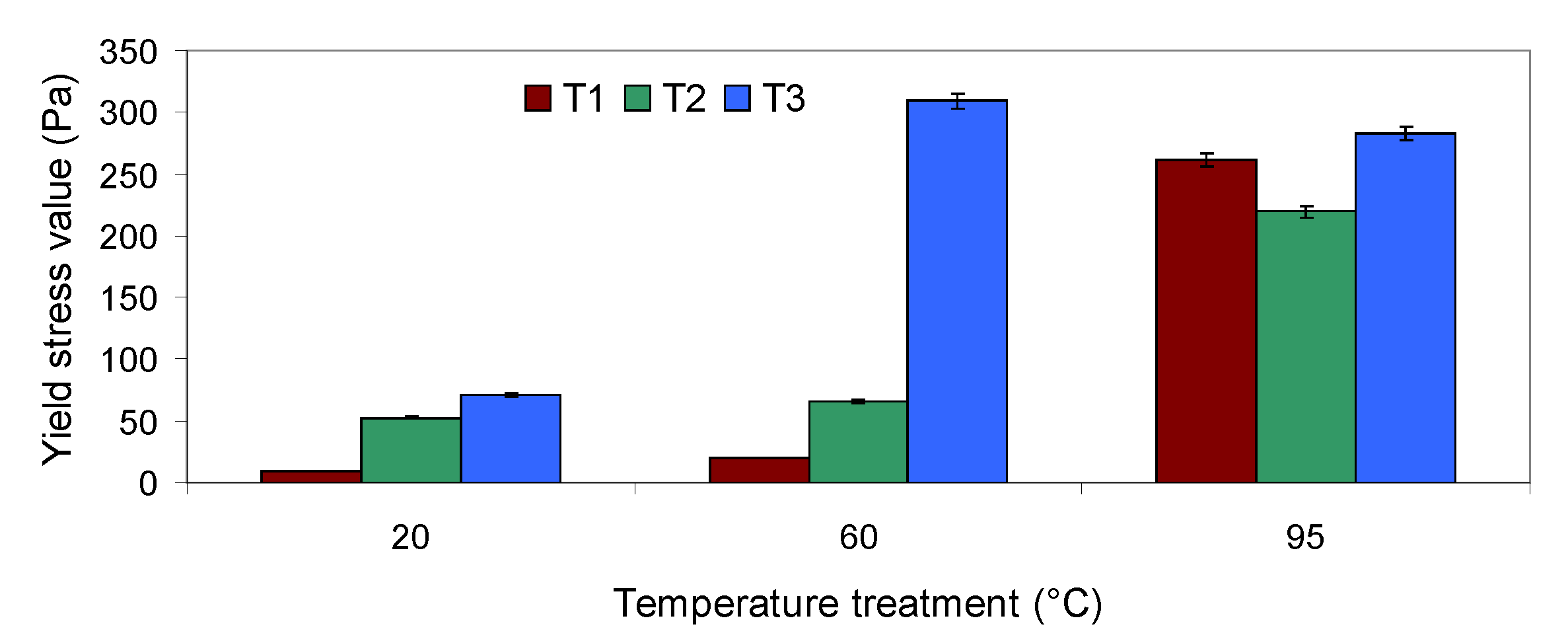
2.5.2. Yield Stress of Tomato Pomace Suspension Measurements
2.6. Data Analysis
3. Results and Discussion
3.1. Tomato Pomace Powder Characterization
3.2. Physico-Chemical Properties of Tomato Pomace Suspensions
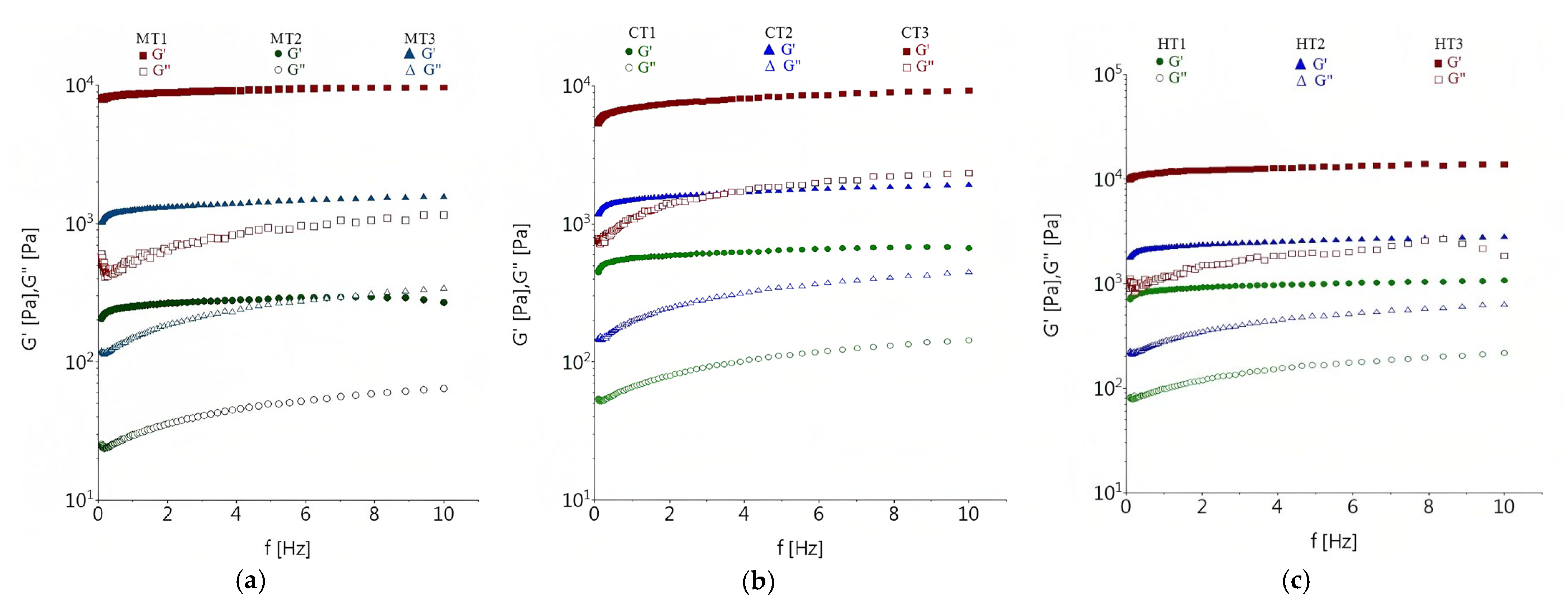
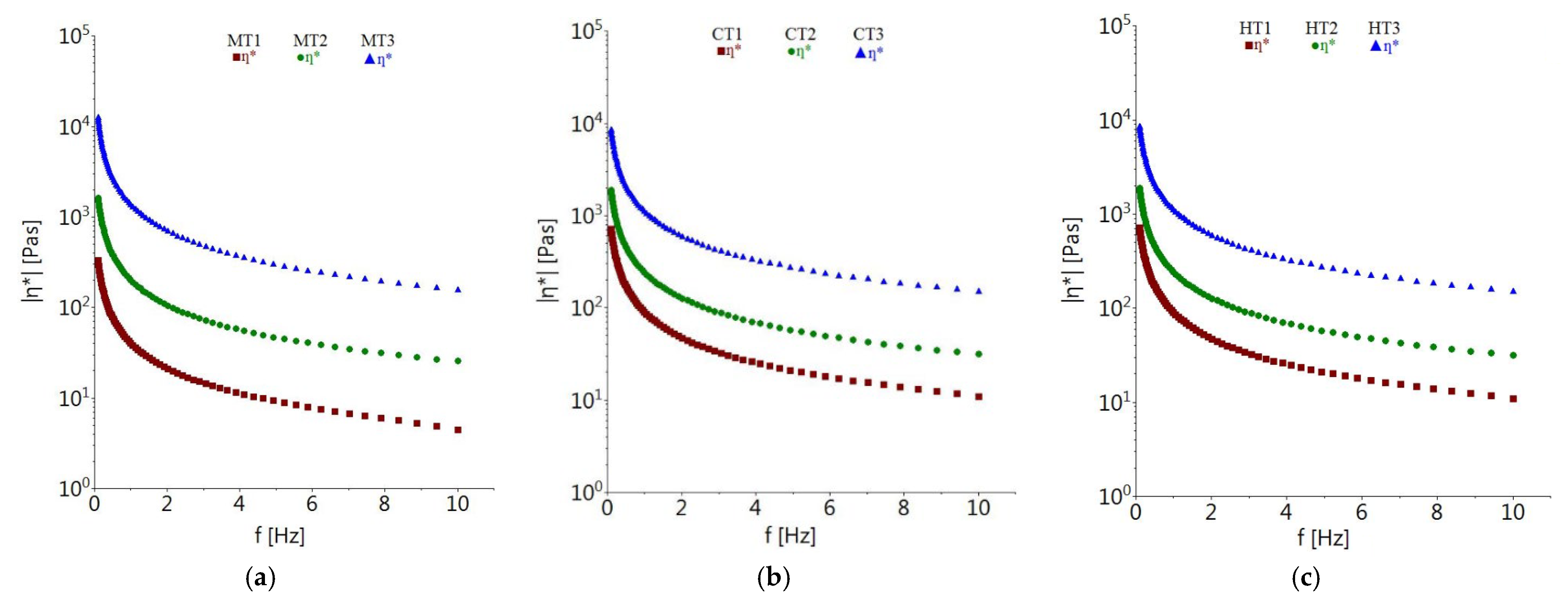
3.3. Dynamic Rheological Properties of Tomato Pomace Powder Suspension
3.3.1. Effect of Concentration and Temperature on Dynamic Rheological Properties
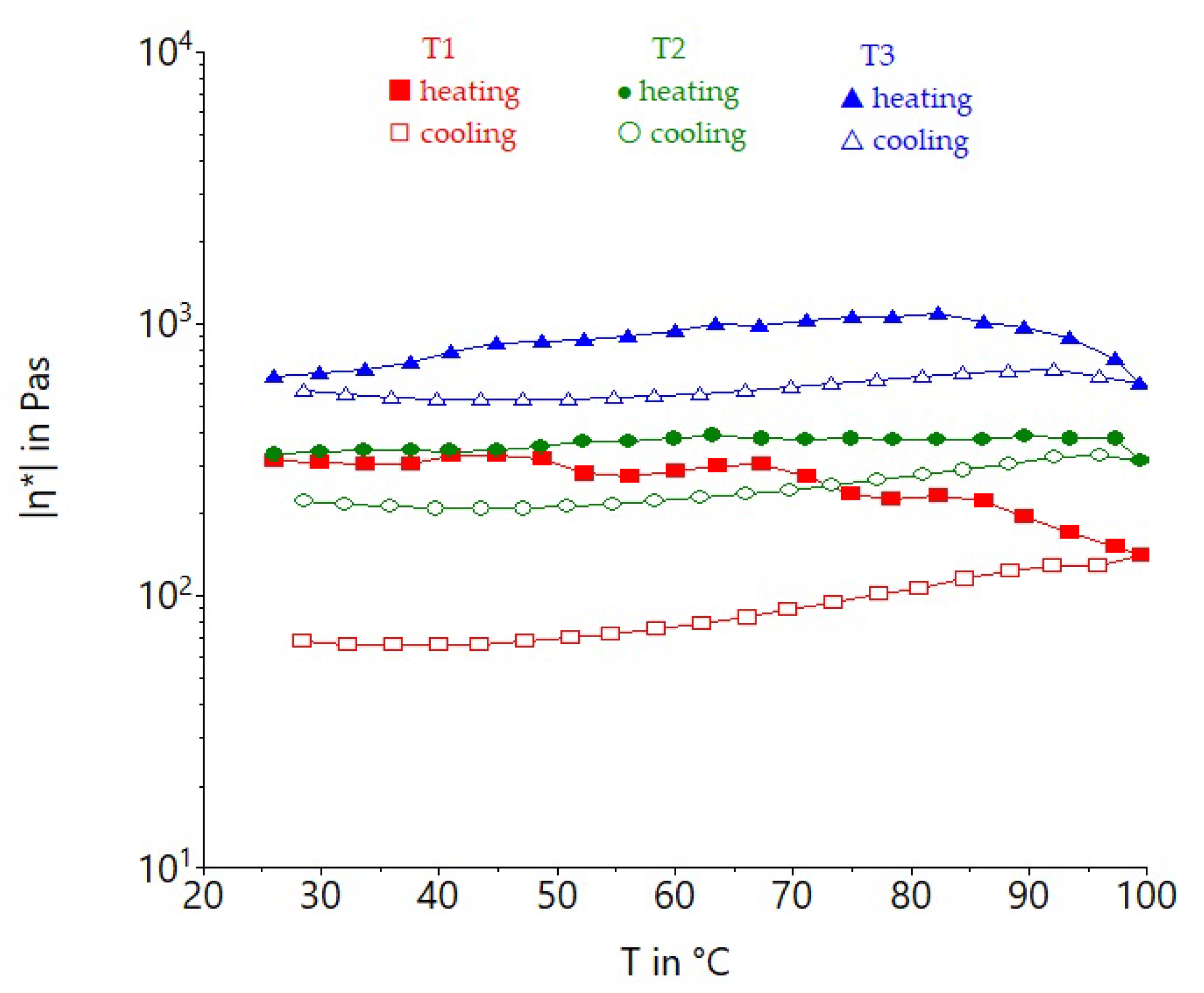
3.3.2. Effect of Non-Isothermal Heating on Tomato Pomace Powder Suspension
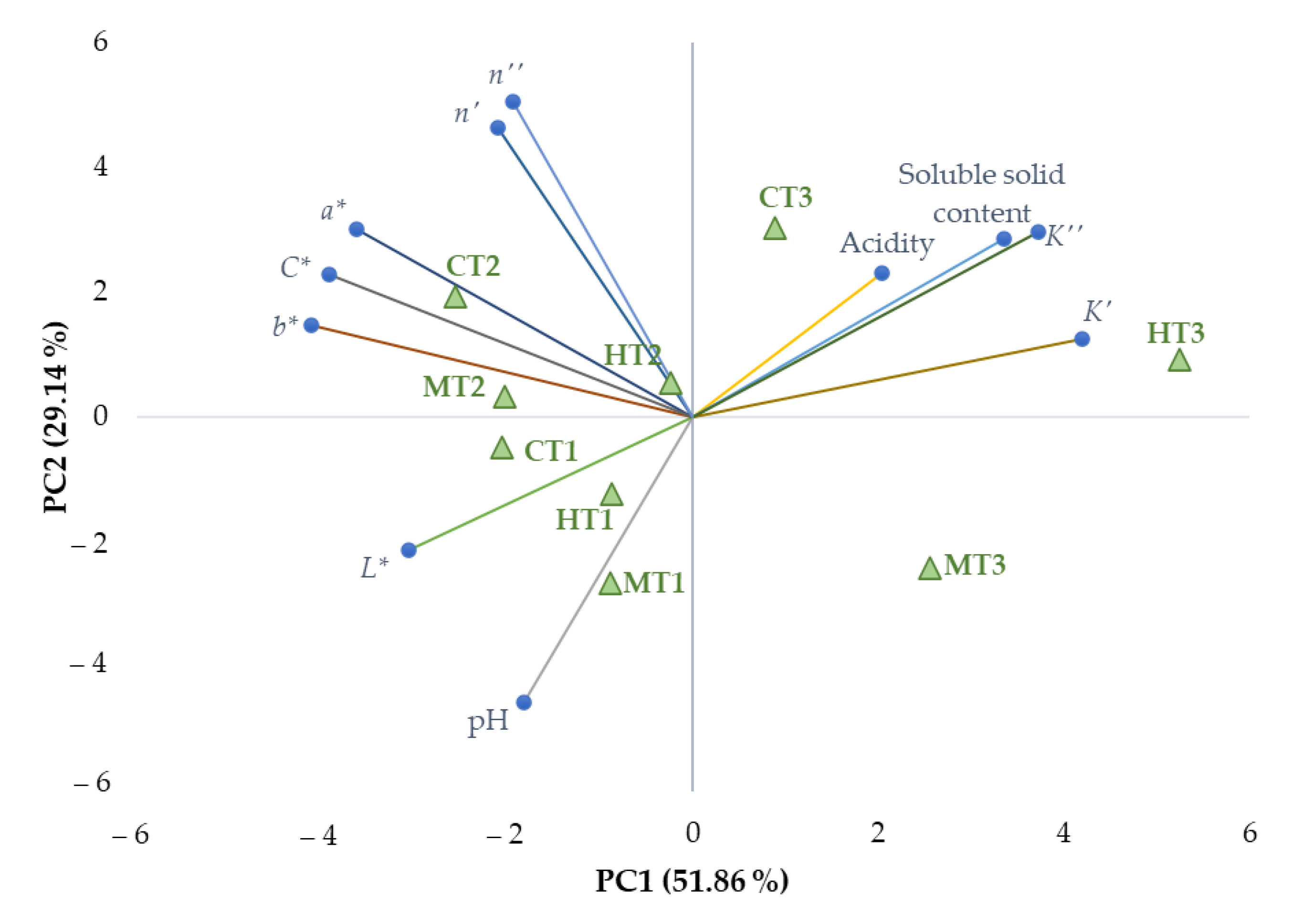
3.4. Relationships between Evaluated Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blum, A.; Monir, M.; Wirsansky, I.; Ben-Arzi, S. The beneficial effects of tomatoes. Eur. J. Intern. Med. 2005, 16, 402–404. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, R.; Sharopov, F.; Namiesnik, J.; Roointan, A.; Kamle, M.; Kumar, P.; Martins, N.; Sharifi-Rad, J. Beneficial effects and potential risks of tomato consumption for human health: An overview. Nutrition 2019, 62, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.P.S.; Paswan, S.; Srivastava, S. Tomato—A natural medicine and its health benefits. J. Pharmacogn. Phytochem. 2012, 1, 33–43. [Google Scholar]
- Duma, M.; Alsina, I.; Dubova, L.; Erdberga, I. Chemical composition of tomatoes depending on the stage of ripening. Chem. Technol. 2015, 66, 24–28. [Google Scholar] [CrossRef]
- Pinela, J.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Nutritional composition and antioxidant activity of four tomato (Lycopersicon esculentum L.) farmer’varieties in Northeastern Portugal homegardens. Food Chem. Toxicol. 2012, 50, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Bergougnoux, V. The history of tomato: From domestication to biopharming. Biotechnol. Adv. 2014, 32, 170–189. [Google Scholar] [CrossRef] [PubMed]
- Torbica, A.; Belović, M.; Mastilović, J.; Kevrešan, Ž.; Pestorić, M.; Škrobot, D.; Hadnađev, T.D. Nutritional, rheological, and sensory evaluation of tomato ketchup with increased content of natural fibres made from fresh tomato pomace. Food Bioprod. Process. 2016, 98, 299–309. [Google Scholar] [CrossRef]
- Pourahmadi, Z.; Mahboob, S.; Saedisomeolia, A.; Reykandeh, M.T. The effect of tomato juice consumption on antioxidant status in overweight and obese females. Women Health 2015, 55, 795–804. [Google Scholar] [CrossRef]
- Atanasova, V.K.; Gatseva, P.D. Natural Beverages and Their Role as Functional Foods. In Natural Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 37–71. [Google Scholar]
- Giovannucci, E. Tomatoes, tomato-based products, lycopene, and cancer: Review of the epidemiologic literature. J. Natl. Cancer Inst. 1999, 91, 317–331. [Google Scholar] [CrossRef]
- Islam, J.; Kabir, Y. Effects and mechanisms of antioxidant-rich functional beverages on disease prevention. In Functional and Medicinal Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 157–198. [Google Scholar]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable valorisation of tomato pomace: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Lu, S.; Chen, S.; Li, H.; Paengkoum, S.; Taethaisong, N.; Meethip, W.; Surakhunthod, J.; Sinpru, B.; Sroichak, T.; Archa, P.; et al. Sustainable Valorization of Tomato Pomace (Lycopersicon esculentum) in Animal Nutrition: A Review. Animals 2022, 12, 3294. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.A.; Borba, B.C.; Pereira, V.A.; Reis, M.G.; Caliari, M.; Brooks, M.S.-L.; Ferreira, T.A.P.C. Characterization of tomato processing by-product for use as a potential functional food ingredient: Nutritional composition, antioxidant activity and bioactive compounds. Int. J. Food Sci. Nutr. 2019, 70, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Sogi, D.S.; Garg, S.K.; Bawa, A.S. Flotation-cum-sedimentation system for skin and seed separation from tomato pomace. J. Food Eng. 2005, 71, 341–344. [Google Scholar] [CrossRef]
- Corrêa-Filho, L.C.; Lourenço, S.C.; Duarte, D.F.; Moldão-Martins, M.; Alves, V.D. Microencapsulation of tomato (Solanum lycopersicum L.) pomace ethanolic extract by spray drying: Optimization of process conditions. Appl. Sci. 2019, 9, 612. [Google Scholar] [CrossRef]
- Knoblich, M.; Anderson, B.; Latshaw, D. Analyses of tomato peel and seed byproducts and their use as a source of carotenoids. J. Sci. Food Agric. 2005, 85, 1166–1170. [Google Scholar] [CrossRef]
- Del Valle, M.; Cámara, M.; Torija, M. Chemical characterization of tomato pomace. J. Sci. Food Agric. 2006, 86, 1232–1236. [Google Scholar] [CrossRef]
- Ho, K.K.H.Y.; Ferruzzi, M.G.; Liceaga, A.M.; San Martín-González, M.F. Microwave-assisted extraction of lycopene in tomato peels: Effect of extraction conditions on all-trans and cis-isomer yields. LWT—Food Sci. Technol. 2015, 62, 160–168. [Google Scholar] [CrossRef]
- Kehili, M.; Kammlott, M.; Choura, S.; Zammel, A.; Zetzl, C.; Smirnova, I.; Allouche, N.; Sayadi, S. Supercritical CO2 extraction and antioxidant activity of lycopene and β-carotene-enriched oleoresin from tomato (Lycopersicum esculentum L.) peels by-product of a Tunisian industry. Food Bioprod. Process. 2017, 102, 340–349. [Google Scholar] [CrossRef]
- Shao, D.; Atungulu, G.G.; Pan, Z.; Yue, T.; Zhang, A.; Chen, X. Separation methods and chemical and nutritional characteristics of tomato pomace. Trans. ASABE 2013, 56, 261–268. [Google Scholar] [CrossRef]
- Oleszek, M.; Kowalska, I.; Bertuzzi, T.; Oleszek, W. Phytochemicals Derived from Agricultural Residues and Their Valuable Properties and Applications. Molecules 2023, 28, 342. [Google Scholar] [CrossRef]
- Fuentes, E.; Carle, R.; Astudillo, L.; Guzmán, L.; Gutiérrez, M.; Carrasco, G.; Palomo, I. Antioxidant and antiplatelet activities in extracts from green and fully ripe tomato fruits (Solanum lycopersicum) and pomace from industrial tomato processing. Evid.-Based Complement. Altern. Med. 2013, 2013, 867578. [Google Scholar] [CrossRef] [PubMed]
- Elbadrawy, E.; Sello, A. Evaluation of nutritional value and antioxidant activity of tomato peel extracts. Arab. J. Chem. 2016, 9, S1010–S1018. [Google Scholar] [CrossRef]
- Wang, Q.; Xiong, Z.; Li, G.; Zhao, X.; Wu, H.; Ren, Y. Tomato peel powder as fat replacement in low-fat sausages: Formulations with mechanically crushed powder exhibit higher stability than those with airflow ultra-micro crushed powder. Eur. J. Lipid Sci. Technol. 2016, 118, 175–184. [Google Scholar] [CrossRef]
- Savadkoohi, S.; Farahnaky, A. Dynamic rheological and thermal study of the heat-induced gelation of tomato-seed proteins. J. Food Eng. 2012, 113, 479–485. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Capocasale, M. Physicochemical composition of tomato seed oil for an edible use: The effect of cultivar. Int. Food Res. J. 2016, 23, 583–591. [Google Scholar]
- Kalogeropoulos, N.; Chiou, A.; Pyriochou, V.; Peristeraki, A.; Karathanos, V.T. Bioactive phytochemicals in industrial tomatoes and their processing byproducts. LWT—Food Sci. Technol. 2012, 49, 213–216. [Google Scholar] [CrossRef]
- Akanbi, C.T.; Adeyemi, R.S.; Ojo, A. Drying characteristics and sorption isotherm of tomato slices. J. Food Eng. 2006, 73, 157–163. [Google Scholar] [CrossRef]
- Bhat, M.A.; Hafiza, A. Physico-chemical characteristics of cookies prepared with tomato pomace powder. J. Food Process. Technol. 2016, 7, 543. [Google Scholar]
- Belović, M.; Torbica, A.; Lijaković, I.P.; Tomić, J.; Lončarević, I.; Petrović, J. Tomato pomace powder as a raw material for ketchup production. Food Biosci. 2018, 26, 193–199. [Google Scholar] [CrossRef]
- Belović, M.; Pajić-Lijaković, I.; Torbica, A.; Mastilović, J.; Pećinar, I. The influence of concentration and temperature on the viscoelastic properties of tomato pomace dispersions. Food Hydrocoll. 2016, 61, 617–624. [Google Scholar] [CrossRef]
- Gould, W.A. Tomato Production, Processing and Technology; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 184569614X. [Google Scholar]
- Altan, A.; Maskan, M. Rheological behavior of pomegranate (Punica granatum L.) juice and concentrate. J. Texture Stud. 2005, 36, 68–77. [Google Scholar] [CrossRef]
- Hassen, Y.; Gebre, H.; Haile, A. Effects of Pre-Heating and concentration temperatures on Physico-Chemical quality of semi concentrated tomato (Solanum lycopersicum) paste. J. Food Process. Technol 2019, 10, 1000795. [Google Scholar]
- Sowbhagya, H.B.; Suma, P.F.; Mahadevamma, S.; Tharanathan, R.N. Spent residue from cumin—A potential source of dietary fiber. Food Chem. 2007, 104, 1220–1225. [Google Scholar] [CrossRef]
- Moelants, K.R.N.; Cardinaels, R.; Van Buggenhout, S.; Van Loey, A.M.; Moldenaers, P.; Hendrickx, M.E. A review on the relationships between processing, food structure, and rheological properties of plant-tissue-based food suspensions. Compr. Rev. Food Sci. Food Saf. 2014, 13, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Lapasin, R.; Pricl, S. Rheology of Industrial Polysaccharides: Theory and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 9781461521853. [Google Scholar]
- Barbana, C.; El-Omri, A. Viscometric behavior of reconstituted tomato concentrate. Food Bioprocess Technol. 2012, 5, 209–215. [Google Scholar] [CrossRef]
- Yoo, B.; Rao, M.A. Effect of unimodal particle size and pulp content on rheological properties of tomato puree. J. Texture Stud. 1994, 25, 421–436. [Google Scholar] [CrossRef]
- Koocheki, A.; Ghandi, A.; Razavi, S.M.A.; Mortazavi, S.A.; Vasiljevic, T. The rheological properties of ketchup as a function of different hydrocolloids and temperature. Int. J. Food Sci. Technol. 2009, 44, 596–602. [Google Scholar] [CrossRef]
- Ibarz, A.; Garvin, A.; Costa, J. Rheological behaviour of sloe (Prunus spinosa) fruit juices. J. Food Eng. 1996, 27, 423–430. [Google Scholar] [CrossRef]
- Sharoba, A.M.; Senge, B.; El-Mansy, H.A.; Bahlol, H.; Blochwitz, R. Chemical, sensory and rheological properties of some commercial German and Egyptian tomato ketchups. Eur. Food Res. Technol. 2005, 220, 142–151. [Google Scholar] [CrossRef]
- Bayod, E.; Willers, E.P.; Tornberg, E. Rheological and structural characterization of tomato paste and its influence on the quality of ketchup. LWT—Food Sci. Technol. 2008, 41, 1289–1300. [Google Scholar] [CrossRef]
- Rydzak, L.; Kobus, Z.; Nadulski, R.; Wilczyński, K.; Pecyna, A.; Santoro, F.; Sagan, A.; Starek-Wójcicka, A.; Krzywicka, M. Analysis of selected physicochemical properties of commercial apple juices. Processes 2020, 8, 1457. [Google Scholar] [CrossRef]
- Maskan, M.; Göǧüş, F. Effect of sugar on the rheological properties of sunflower oil–water emulsions. J. Food Eng. 2000, 43, 173–177. [Google Scholar] [CrossRef]
- Gleissle, W.; Hochstein, B. Validity of the Cox–Merz rule for concentrated suspensions. J. Rheol. 2003, 47, 897–910. [Google Scholar] [CrossRef]
- Rao, M.A.; Cooley, H.J. Rheological behavior of tomato pastes in steady and dynamic shear. J. Texture Stud. 1992, 23, 415–425. [Google Scholar] [CrossRef]
- Moelants, K.; Cardinaels, R.; Jolie, R.P.; Verrijssen, T.A.J.; Van Buggenhout, S.; Van Loey, A.M.; Moldenaers, P.; Hendrickx, M.E. Rheology of concentrated tomato-derived suspensions: Effects of particle characteristics. Food Bioprocess Technol. 2014, 7, 248–264. [Google Scholar] [CrossRef]
- Quemada, D. Rheological modelling of complex fluids. I. The concept of effective volume fraction revisited. Eur. Phys. J.—Appl. Phys. 1998, 1, 119–127. [Google Scholar] [CrossRef]
- Moelants, K.; Jolie, R.P.; Palmers, S.K.J.; Cardinaels, R.; Christiaens, S.; Van Buggenhout, S.; Van Loey, A.M.; Moldenaers, P.; Hendrickx, M.E. The effects of process-induced pectin changes on the viscosity of carrot and tomato sera. Food Bioprocess Technol. 2013, 6, 2870–2883. [Google Scholar] [CrossRef]
- Day, L.; Xu, M.; Øiseth, S.K.; Lundin, L.; Hemar, Y. Dynamic rheological properties of plant cell-wall particle dispersions. Colloids Surf. B Biointerfaces 2010, 81, 461–467. [Google Scholar] [CrossRef]
- Schijvens, E.; Van Vliet, T.; Van Dijk, C. Effect of processing conditions on the composition and rheological properties of applesauce. J. Texture Stud. 1998, 29, 123–143. [Google Scholar] [CrossRef]
- Fei, X.; Jones, O.G.; Reuhs, B.L.; Campanella, O.H. Soluble pectin acts as a particle stabilizer of tomato suspensions: The impact on tomato products rheological characterization. LWT 2021, 139, 110508. [Google Scholar] [CrossRef]
- Cámara Hurtado, M.; Greve, L.C.; Labavitch, J.M. Changes in cell wall pectins accompanying tomato (Lycopersicon esculentum Mill.) paste manufacture. J. Agric. Food Chem. 2002, 50, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Beresovsky, N.; Kopelman, I.J.; Mizrahi, S. The role of pulp interparticle interaction in determining tomato juice viscosity. J. Food Process. Preserv. 1995, 19, 133–146. [Google Scholar] [CrossRef]
- Tornberg, E. Influence of fibers and particle size distribution on food rheology. In Advances in Food Rheology and Its Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 177–208. [Google Scholar]
| Sample | pH | Acidity (% Citric Acid) | Soluble Solid Content (°Brix) |
|---|---|---|---|
| MT1 | 4.23 ± 0.01 e | 0.352 ± 0.01 a | 1.22 ± 0.03 a |
| MT2 | 4.22 ± 0.01 cd | 0.357 ± 0.01 b | 1.77 ± 0.06 c |
| MT3 | 4.22 ± 0.02 bcd | 0.366 ± 0.00 c | 1.97 ± 0.06 d |
| CT1 | 4.21 ± 0.01 abc | 0.374 ± 0.01 d | 1.13 ± 0.06 a |
| CT2 | 4.21 ± 0.01 abc | 0.381 ± 0.00 e | 1.52 ± 0.08 b |
| CT3 | 4.20 ± 0.00 a | 0.391 ± 0.01 f | 2.20 ± 0.05 e |
| HT1 | 4.23 ± 0.02 de | 0.413 ± 0.00 g | 1.23 ± 0.03 a |
| HT2 | 4.22 ± 0.02 bcd | 0.422 ± 0.01 h | 1.60 ± 0.10 b |
| HT3 | 4.20 ± 0.01 ab | 0.426 ± 0.00 i | 2.28 ± 0.03 e |
| Samples | L* | a* | b* | C* | ΔE |
|---|---|---|---|---|---|
| MT1 | 32.88 ± 0.46 b | 17.37 ± 0.72 ab | 16.9 ± 0.62 b | 24.23 ± 0.94 abc | - |
| MT2 | 32.45 ± 0.40 b | 20.13 ± 0.56 de | 19.14 ± 0.22 cd | 27.78 ± 0.56 de | - |
| MT3 | 32.33 ± 0.42 b | 16.44 ± 0.84 a | 16.5 ± 0.55 b | 23.29 ± 0.98 ab | - |
| CT1 | 33.04 ± 0.75 b | 19.21 ± 0.98 cd | 18.73 ± 1.45 cd | 26.83 ± 1.71 de | 1.79 ± 0.93 c |
| CT2 | 32.03 ± 0.70 ab | 21.05 ± 0.53 e | 20.13 ± 0.67 d | 29.13 ± 0.85 e | 1.49 ± 0.86 e |
| CT3 | 32.52 ± 0.05 b | 18.58 ± 0.03 bcd | 17.41 ± 0.01 bc | 25.46 ± 0.02 bcd | 1.79 ± 0.18 c |
| HT1 | 32.40 ± 0.05 b | 18.25 ± 0.03 bc | 17.80 ± 0.03 bc | 25.50 ± 0.07 bcd | 1.51 ± 0.63 d |
| HT2 | 32.01 ± 0.41 ab | 18.56 ± 0.06 bcd | 17.79 ± 0.50 bc | 25.71 ± 0.12 cd | 2.13 ± 0.57 b |
| HT3 | 30.96 ± 0.10 a | 16.56 ± 0.43 a | 14.68 ± 0.49 a | 22.13 ± 0.33 a | 2.41 ± 0.52 a |
| Sample | ||||||
|---|---|---|---|---|---|---|
| K′ (Pa sn′) | n′ | R2 | K″ (Pa sn″) | n″ | R2 | |
| MT1 | 251.031 ± 1.057 a | 0.073 ± 0.002 b | 0.987 | 32.711 ± 0.274 a | 0.218 ± 0.002 b | 0.906 |
| MT2 | 1258.864 ± 6.288 b | 0.083 ± 0.003 cd | 0.990 | 165.410 ± 0.601 b | 0.244 ± 0.004 c | 0.925 |
| MT3 | 8678.545 ± 29.673 c | 0.044 ± 0.002 c | 0.978 | 611.086 ± 5.035 c | 0.192 ± 0.002 bc | 0.865 |
| CT1 | 562.535 ± 2.951 d | 0.083 ± 0.002 cd | 0.991 | 72.712 ± 0.603 d | 0.228 ± 0.002 d | 0.922 |
| CT2 | 1507.565 ± 8.846 e | 0.095 ± 0.002 e | 0.993 | 217.505 ± 1.119 e | 0.257 ± 0.003 e | 0.945 |
| CT3 | 6984.136 ± 24.118 f | 0.110 ± 0.004 de | 0.992 | 1163.265 ± 6.306 f | 0.272 ± 0.003 d | 0.965 |
| HT1 | 870.74 ± 3.821 h | 0.081 ± 0.002 a | 0.996 | 109.245 ± 0.876 g | 0.223 ± 0.002 a | 0.916 |
| HT2 | 2240.471 ± 11.857 g | 0.089 ± 0.002 f | 0.993 | 311.078 ± 1.516 h | 0.245± 0.004 f | 0.928 |
| HT3 | 11,619.090 ± 49.471 i | 0.068 ± 0.002 b | 0.988 | 1278.633 ± 5.788 i | 0.216 ± 0.002 b | 0.864 |
| Parameter | pH | Acidity | SS Content | L* | a* | b* | C* | K′ | n′ | K″ | n″ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | ||||||||||
| Acidity | −0.802 ** | 1 | |||||||||
| SS content | −0.419 * | 0.275 | 1 | ||||||||
| L* | 0.478 * | −0.580 ** | −0.552 ** | 1 | |||||||
| a* | 0.179 | −0.205 | −0.300 | 0.201 | 1 | ||||||
| b* | 0.338 | −0.359 | −0.478 * | 0.340 | 0.933 ** | 1 | |||||
| C* | 0.262 | −0.287 | −0.394 * | 0.274 | 0.984 ** | 0.983 ** | 1 | ||||
| K′ | −0.495 ** | 0.368 | 0.883 ** | −0.569 ** | −0.599 ** | −0.719 ** | −0.669 ** | 1 | |||
| n′ | −0.158 | 0.183 | −0.047 | 0.118 | 0.657 ** | 0.500 ** | 0.588 ** | −0.350 | 1 | ||
| K″ | −0.567 ** | 0.452 * | 0.910 ** | −0.529 ** | −0.431 * | −0.623 ** | −0.535 ** | 0.922 ** | 0.023 | 1 | |
| n″ | −0.142 | 0.111 | 0.110 | 0.049 | 0.701 ** | 0.531 ** | 0.627 ** | −0.259 | 0.963 ** | 0.090 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begliţa, V.; Ungureanu-Iuga, M.; Mironeasa, S. Assessing the Features of Tomato Pomace Powder in Suspensions. Appl. Sci. 2023, 13, 2235. https://doi.org/10.3390/app13042235
Begliţa V, Ungureanu-Iuga M, Mironeasa S. Assessing the Features of Tomato Pomace Powder in Suspensions. Applied Sciences. 2023; 13(4):2235. https://doi.org/10.3390/app13042235
Chicago/Turabian StyleBegliţa, Victoria, Mădălina Ungureanu-Iuga, and Silvia Mironeasa. 2023. "Assessing the Features of Tomato Pomace Powder in Suspensions" Applied Sciences 13, no. 4: 2235. https://doi.org/10.3390/app13042235
APA StyleBegliţa, V., Ungureanu-Iuga, M., & Mironeasa, S. (2023). Assessing the Features of Tomato Pomace Powder in Suspensions. Applied Sciences, 13(4), 2235. https://doi.org/10.3390/app13042235









