The Effect of Custom Insoles on Muscle Activity in Diabetic Individuals with Neuropathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
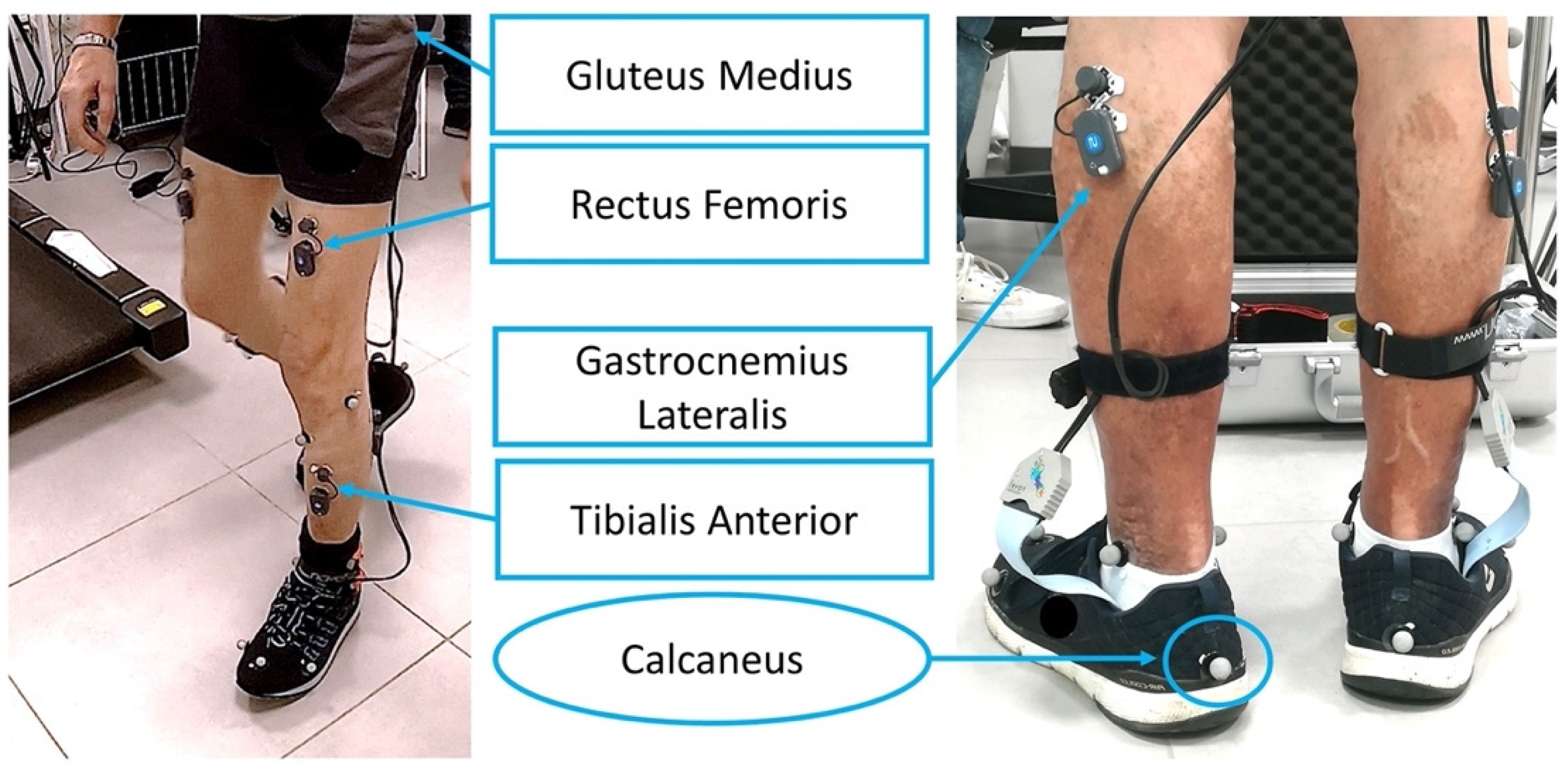
2.2. Acquisition Set Up
2.3. Custom Insoles
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
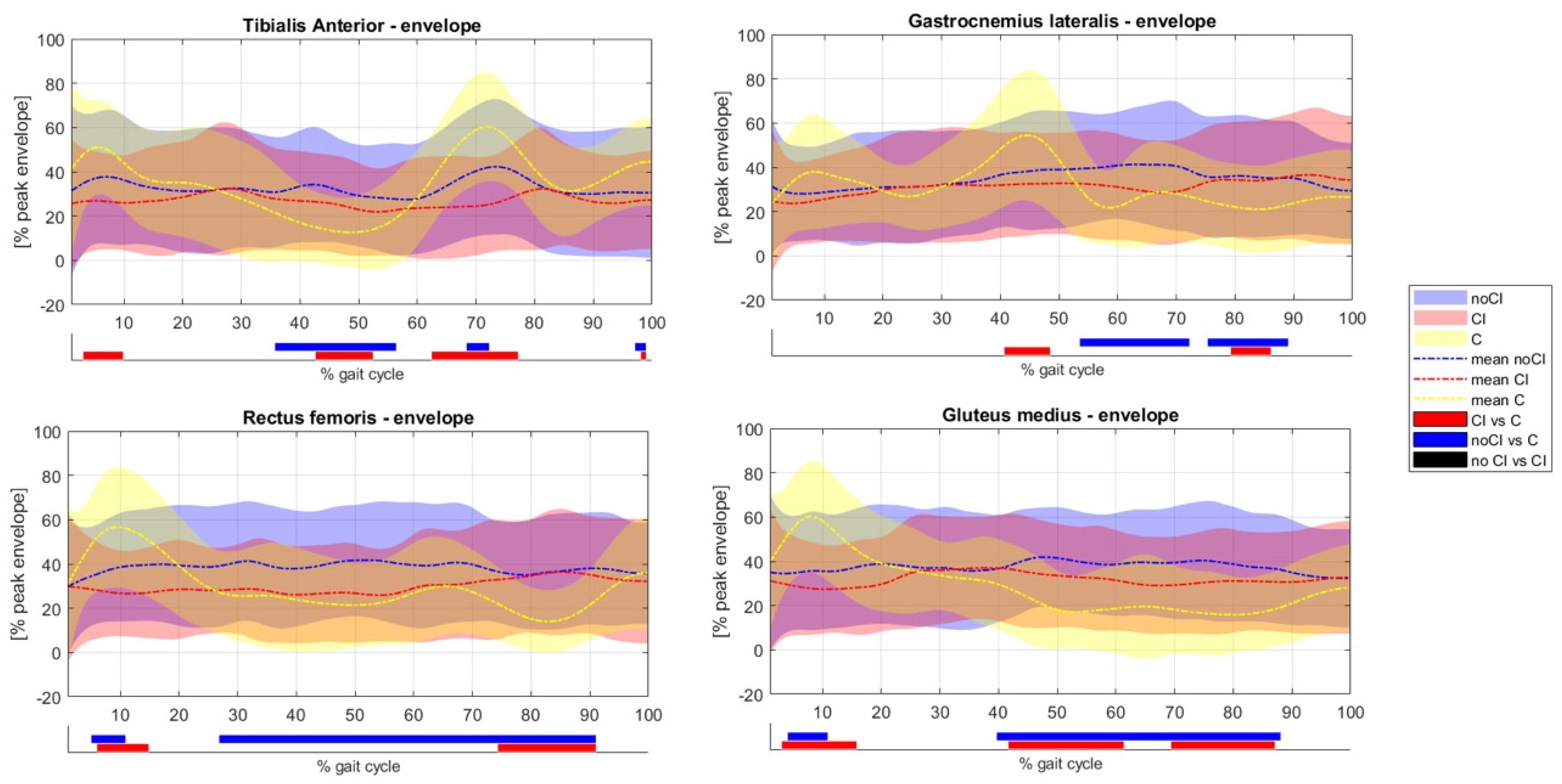
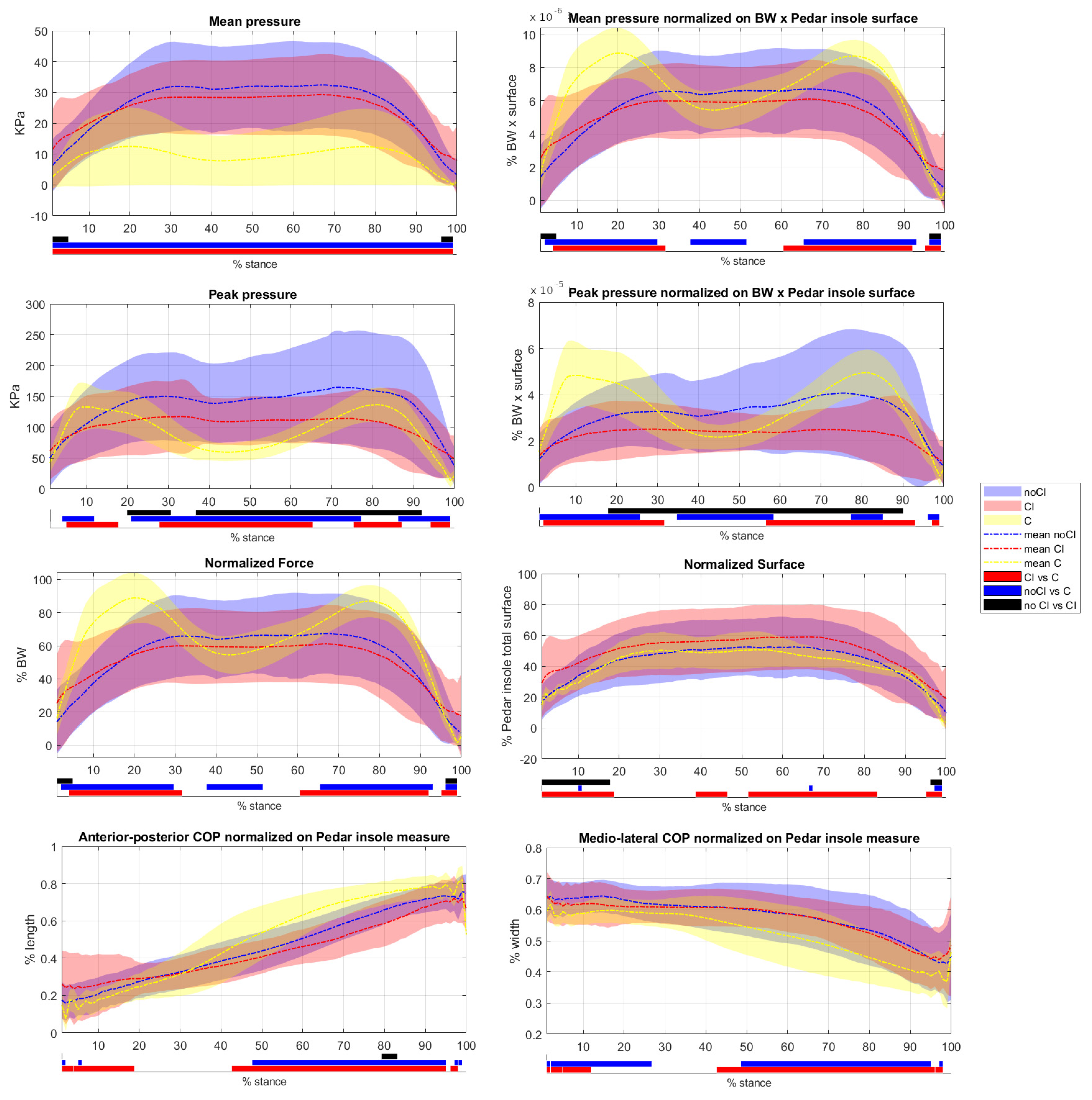
3.1. Comparison with Healthy Subjects
3.2. Comparison between CI and NoCI Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Available online: https://www.who.int (accessed on 18 January 2023).
- Hicks, C.W.; Selvin, E. Epidemiology of Peripheral Neuropathy and Lower Extremity Disease in Diabetes. Curr. Diab. Rep. 2019, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- IDF_Diabetic_Foot_Z_Card_Final Pages 1-2—Flip PDF Download|FlipHTML5. Available online: https://fliphtml5.com/ivngc/bfvm/basic (accessed on 2 February 2023).
- Introduction: Standards of Medical Care in Diabetes—2021|Diabetes Care|American Diabetes Association. Available online: https://diabetesjournals.org/care/article/44/Supplement_1/S1/30961/Introduction-Standards-of-Medical-Care-in-Diabetes (accessed on 18 January 2023).
- Diabetic Foot Ulcers|SpringerLink. Available online: https://link.springer.com/article/10.2165/00003495-200666070-00003 (accessed on 12 July 2022).
- Collings, R.; Freeman, J.; Latour, J.M.; Paton, J. Footwear and insole design features for offloading the diabetic at risk foot—A systematic review and meta-analyses. Endocrinol. Diabetes Metab. 2020, 4, e00132. [Google Scholar] [CrossRef] [PubMed]
- Heuch, L.; Streak Gomersall, J. Effectiveness of offloading methods in preventing primary diabetic foot ulcers in adults with diabetes: A systematic review. JBI Evid. Synth. 2016, 14, 236. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, F.; Li, X.; Hu, J.; He, Y.; Zhang, J. Characteristics of Plantar Pressure Distribution in Diabetes with or without Diabetic Peripheral Neuropathy and Peripheral Arterial Disease. J. Healthc. Eng. 2022, 2022, 2437831. [Google Scholar] [CrossRef]
- Sawacha, Z.; Gabriella, G.; Cristoferi, G.; Guiotto, A.; Avogaro, A.; Cobelli, C. Diabetic gait and posture abnormalities: A biomechanical investigation through three dimensional gait analysis. Clin. Biomech. (Bristol Avon) 2009, 24, 722–728. [Google Scholar] [CrossRef]
- Sawacha, Z.; Sartor, C.D.; Yi, L.C.; Guiotto, A.; Spolaor, F.; Sacco, I.C.N. Clustering classification of diabetic walking abnormalities: A new approach taking into account intralimb coordination patterns. Gait Posture 2020, 79, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Sawacha, Z.; Spolaor, F.; Guarneri, G.; Contessa, P.; Carraro, E.; Venturin, A.; Avogaro, A.; Cobelli, C. Abnormal muscle activation during gait in diabetes patients with and without neuropathy. Gait Posture 2012, 35, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Piatkowska, W.; Spolaor, F.; Guiotto, A.; Guarneri, G.; Avogaro, A.; Sawacha, Z. EMG analysis across different tasks improves prevention screenings in diabetes: A cluster analysis approach. Med. Biol. Eng. Comput. 2022, 60, 1659–1673. [Google Scholar] [CrossRef]
- Gomes, A.A.; Onodera, A.N.; Otuzi, M.E.I.; Pripas, D.; Mezzarane, R.A.; Sacco, I.C.N. Electromyography and kinematic changes of gait cycle at different cadences in diabetic neuropathic individuals. Muscle Nerve 2011, 44, 258–268. [Google Scholar] [CrossRef]
- Jones, A.D.; De Siqueira, J.; Nixon, J.E.; Siddle, H.J.; Culmer, P.R.; Russell, D.A. Plantar shear stress in the diabetic foot: A systematic review and meta-analysis. Diabet. Med. 2022, 39, e14661. [Google Scholar] [CrossRef]
- Butugan, M.K.; Sartor, C.D.; Watari, R.; Martins, M.C.S.; Ortega, N.R.S.; Vigneron, V.A.M.; Sacco, I.C.N. Multichannel EMG-based estimation of fiber conduction velocity during isometric contraction of patients with different stages of diabetic neuropathy. J. Electromyogr. Kinesiol. 2014, 24, 465–472. [Google Scholar] [CrossRef]
- Monteiro, R.L.; Sartor, C.D.; Ferreira, J.S.S.P.; Dantas, M.G.B.; Bus, S.A.; Sacco, I.C.N. Protocol for evaluating the effects of a foot-ankle therapeutic exercise program on daily activity, foot-ankle functionality, and biomechanics in people with diabetic polyneuropathy: A randomized controlled trial. BMC Musculoskelet. Disord. 2018, 19, 400. [Google Scholar] [CrossRef]
- Tran, M.M.; Haley, M.N. Does exercise improve healing of diabetic foot ulcers? A systematic review. J. Foot Ankle Res. 2021, 14, 19. [Google Scholar] [CrossRef]
- Guidelines on Offloading Foot Ulcers in Persons with Diabetes (IWGDF 2019 Update)—Bus—2020—Diabetes/Metabolism Research and Reviews—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/dmrr.3274 (accessed on 17 January 2023).
- Mcintosh, C.; Halford, G. The importance of effective offloading and footwear for the diabetic foot “Diabetic foot ulcers are frequently associated with adverse outcomes, including limb and/or life threatening infection and lower extremity amputation”. Wounds Essent. 2014, 9, 2. [Google Scholar]
- Lin, T.-L.; Sheen, H.-M.; Chung, C.-T.; Yang, S.-W.; Lin, S.-Y.; Luo, H.-J.; Chen, C.-Y.; Chan, I.-C.; Shih, H.-S.; Sheu, W.H.-H. The effect of removing plugs and adding arch support to foam based insoles on plantar pressures in people with diabetic peripheral neuropathy. J. Foot Ankle Res. 2013, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, P.A.; Jarl, G.; Gooday, C.; Viswanathan, V.; Caravaggi, C.F.; Armstrong, D.G.; Bus, S.A. Effectiveness of offloading interventions to heal foot ulcers in persons with diabetes: A systematic review. Diabetes Metab. Res. Rev. 2020, 36, e3275. [Google Scholar] [CrossRef] [PubMed]
- Bus, S.A.; van Netten, J.J.; Lavery, L.A.; Monteiro-Soares, M.; Rasmussen, A.; Jubiz, Y.; Price, P.E.; International Working Group on the Diabetic Foot IWGDF. Guidance on the prevention of foot ulcers in at-risk patients with diabetes. Diabetes Metab. Res. Rev. 2016, 32 (Suppl. 1), 16–24. [Google Scholar] [CrossRef] [PubMed]
- Moisan, G.; Robb, K.; Mainville, C.; Blanchette, V. Effects of foot orthoses on the biomechanics of the lower extremities in adults with and without musculoskeletal disorders during functional tasks: A systematic review. Clin. Biomech. 2022, 95, 105641. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Lin, G.; Wang, M.-J.J. Evaluating insole design with joint motion, plantar pressure and rating of perceived exertion measures. Work 2012, 41 (Suppl. 1), 1114–1117. [Google Scholar] [CrossRef]
- Creaby, M.W.; May, K.; Bennell, K.L. Insole effects on impact loading during walking. Ergonomics 2011, 54, 665–671. [Google Scholar] [CrossRef]
- Bus, S.A.; van Deursen, R.W.; Armstrong, D.G.; Lewis, J.E.A.; Caravaggi, C.F.; Cavanagh, P.R.; International Working Group on the Diabetic Foot (IWGDF). Footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in patients with diabetes: A systematic review. Diabetes Metab. Res. Rev. 2016, 32, 99–118. [Google Scholar] [CrossRef] [Green Version]
- Jackson, L.; Binning, J.; Potter, J. Plantar pressures in rheumatoid arthritis using prefabricated metatarsal padding. J. Am. Podiatr. Med. Assoc. 2004, 94, 239–245. [Google Scholar] [CrossRef]
- Barani, Z.; Haghpanahi, M.; Katoozian, H. Three dimensional stress analysis of diabetic insole: A finite element approach. Technol. Health Care 2005, 13, 185–192. [Google Scholar] [CrossRef]
- Ghassemi, A.; Mossayebi, A.R.; Jamshidi, N.; Naemi, R.; Karimi, M.T. Manufacturing and finite element assessment of a novel pressure reducing insole for Diabetic Neuropathic patients. Australas Phys. Eng. Sci. Med. 2015, 38, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Wang, M.-J. Ergonomic evaluation and field validation of the insole padding system. Hum. Factors Ergon. Manuf. Serv. Ind. 2022, 32, 228–236. [Google Scholar] [CrossRef]
- Whitley, E.; Ball, J. Statistics review 4: Sample size calculations. Crit. Care 2002, 6, 335–341. [Google Scholar] [CrossRef]
- Blanc, Y.; Dimanico, U. Electrode Placement in Surface Electromyography (sEMG) “Minimal Crosstalk Area” (MCA). Open Rehabil. J. 2010, 3, 110–126. [Google Scholar] [CrossRef]
- Romanato, M.; Piatkowska, W.; Spolaor, F.; To, D.-K.; Volpe, D.; Sawacha, Z. Different perspectives in understanding muscle functions in Parkinson’s disease through surface electromyography: Exploring multiple activation patterns. J. Electromyogr. Kinesiol. 2022, 64, 102658. [Google Scholar] [CrossRef]
- Ciniglio, A.; Guiotto, A.; Spolaor, F.; Sawacha, Z. The Design and Simulation of a 16-Sensors Plantar Pressure Insole Layout for Different Applications: From Sports to Clinics, a Pilot Study. Sensors 2021, 21, 1450. [Google Scholar] [CrossRef] [PubMed]
- Pataky, T.C.; Vanrenterghem, J.; Robinson, M.A. Zero- vs. one-dimensional, parametric vs. non-parametric, and confidence interval vs. hypothesis testing procedures in one-dimensional biomechanical trajectory analysis. J. Biomech. 2015, 48, 1277–1285. [Google Scholar] [CrossRef]
- Worsley, K.J.; Taylor, J.E.; Tomaiuolo, F.; Lerch, J. Unified univariate and multivariate random field theory. NeuroImage 2004, 23, S189–S195. [Google Scholar] [CrossRef] [PubMed]
- Albert, S.; Rinoie, C. Effect of custom orthotics on plantar pressure distribution in the pronated diabetic foot. J. Foot Ankle Surg. 1994, 33, 598–604. [Google Scholar]
- Bus, S.A.; Ulbrecht, J.S.; Cavanagh, P.R. Pressure relief and load redistribution by custom-made insoles in diabetic patients with neuropathy and foot deformity. Clin. Biomech. 2004, 19, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Lott, D.J.; Hastings, M.K.; Commean, P.K.; Smith, K.E.; Mueller, M.J. Effect of footwear and orthotic devices on stress reduction and soft tissue strain of the neuropathic foot. Clin. Biomech. 2007, 22, 352–359. [Google Scholar] [CrossRef]
- Owings, T.M.; Woerner, J.L.; Frampton, J.D.; Cavanagh, P.R.; Botek, G. Custom therapeutic insoles based on both foot shape and plantar pressure measurement provide enhanced pressure relief. Diabetes Care 2008, 31, 839–844. [Google Scholar] [CrossRef]
- Nouman, M.; Dissaneewate, T.; Leelasamran, W.; Chatpun, S. The insole materials influence the plantar pressure distributions in diabetic foot with neuropathy during different walking activities. Gait Posture 2019, 74, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Fernando, M.; Crowther, R.; Lazzarini, P.; Sangla, K.; Cunningham, M.; Buttner, P.; Golledge, J. Biomechanical characteristics of peripheral diabetic neuropathy: A systematic review and meta-analysis of findings from the gait cycle, muscle activity and dynamic barefoot plantar pressure. Clin. Biomech. 2013, 28, 831–845. [Google Scholar] [CrossRef]
- Almurdhi, M.M.; Reeves, N.D.; Bowling, F.L.; Boulton, A.J.M.; Jeziorska, M.; Malik, R.A. Reduced Lower-Limb Muscle Strength and Volume in Patients With Type 2 Diabetes in Relation to Neuropathy, Intramuscular Fat, and Vitamin D Levels. Diabetes Care 2016, 39, 441–447. [Google Scholar] [CrossRef]
- Andreassen, C.S.; Jakobsen, J.; Andersen, H. Muscle Weakness: A Progressive Late Complication in Diabetic Distal Symmetric Polyneuropathy. Diabetes 2006, 55, 806–812. [Google Scholar] [CrossRef]
- Andersen, H.; Nielsen, S.; Mogensen, C.E.; Jakobsen, J. Muscle Strength in Type 2 Diabetes. Diabetes 2004, 53, 1543–1548. [Google Scholar] [CrossRef]
- Favretto, M.A.; Andreis, F.R.; Cossul, S.; Negro, F.; Oliveira, A.S.; Marques, J.L.B. Differences in motor unit behavior during isometric contractions in patients with diabetic peripheral neuropathy at various disease severities. J. Electromyogr. Kinesiol. 2023, 68, 102725. [Google Scholar] [CrossRef]
- Andreassen, C.S.; Jakobsen, J.; Flyvbjerg, A.; Andersen, H. Expression of neurotrophic factors in diabetic muscle--relation to neuropathy and muscle strength. Brain 2009, 132, 2724–2733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreassen, C.S.; Jensen, J.M.; Jakobsen, J.; Ulhøj, B.P.; Andersen, H. Striated muscle fiber size, composition, and capillary density in diabetes in relation to neuropathy and muscle strength. J. Diabetes 2014, 6, 462–471. [Google Scholar] [CrossRef]
- Matos, M.; Mendes, R.; Silva, A.B.; Sousa, N. Physical activity and exercise on diabetic foot related outcomes: A systematic review. Diabetes Res. Clin. Pract. 2018, 139, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.A.; Sartor, C.D.; João, S.M.A.; Sacco, I.C.N.; Bernik, M.M.S. Efeitos da intervenção fisioterapêutica nas respostas sensoriais e funcionais de diabéticos neuropatas. Fisioter. E Pesqui. 2007, 14, 14–21. [Google Scholar] [CrossRef]
- Sartor, C.D.; Hasue, R.H.; Cacciari, L.P.; Butugan, M.K.; Watari, R.; Pássaro, A.C.; Giacomozzi, C.; Sacco, I.C.N. Effects of strengthening, stretching and functional training on foot function in patients with diabetic neuropathy: Results of a randomized controlled trial. BMC Musculoskelet. Disord. 2014, 15, 137. [Google Scholar] [CrossRef]
- Mousavi, E.; Zamanian, Z.; Hadadi, M.; Sobhani, S. Investigating the effect of custom-made insoles and exercises on lower limb and back discomfort in assembly-line workers in a rubber tire factory: A randomized controlled trial. Hum. Factors Ergon. Manuf. Serv. Ind. 2019, 29, 478–484. [Google Scholar] [CrossRef]
- Moisan, G.; Descarreaux, M.; Cantin, V. Biomechanical effects of foot orthoses with and without a lateral bar in individuals with cavus feet during comfortable and fast walking. PLoS ONE 2021, 16, e0248658. [Google Scholar] [CrossRef]
- Dingwell, J.B.; Ulbrecht, J.S.; Boch, J.; Becker, M.B.; O’Gorman, J.T.; Cavanagh, P.R. Neuropathic gait shows only trends towards increased variability of sagittal plane kinematics during treadmill locomotion. Gait Posture 1999, 10, 21–29. [Google Scholar] [CrossRef]

| Age (Mean ± st.dev) | Weight (Mean ± st.dev) | Height (Mean ± st.dev) | BMI (Mean ± st.dev) | Risk Classification [3] | Years of Disease | |||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||||
| PN | 61.7 (±12) | 93 (±20) * | 1.73 (0.07) | 30 (±5) * | 80% | 10% | 10% | 27.6 (±13) |
| C | 61.2 (±5.07) | 70 (±17) | 1.68 (12) | 24 (±3) | N.a | N.a | ||
| Subject | Prescription | Production Method | Insole Material | Insole Cover Material | Insole Shape | Produced Insole Picture |
|---|---|---|---|---|---|---|
| 1 | custom with arch supports | CAD/CAM | Biomech mt197 | Diapod | Ecosanit |  |
| 2 | custom, multilayer | casting | POD 50 + stress balance under the hallux + heel rigid reinforcement in Duroform | PPT perforated | Ecosanit |  |
| 3 | custom, total contact | CAD/CAM | Orthoshock | Drypod | Normal |  |
| 4 | custom with medial arch support, supination heel wedge | CAD/CAM | Orthoextreme 35sh | Diapod | Normal |  |
| 5 | custom, casting unloaded, unload 1st right toe, wrap heel, toe support left | casting | Pod 50 + sunfiber | Diapod | Ecosanit |  |
| 6 | custom with insole with reinforcement base | CAD/CAM | Pod 50 | Diapod | Ecosanit |  |
| 7 | casting | Pod 50 + sunfiber | Diapod | Ecosanit |  | |
| 8 | custom | casting | Pod 50 | Diapod | Ecosanit |  |
| 9 | custom, wrapping, with right heel discharge and lateral wedge | casting | Pod 50 | Diapod | Ecosanit |  |
| 10 | custom with plantar arch support | casting | Pod 50 | Diapod | Ecosanit |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spolaor, F.; Guiotto, A.; Ciniglio, A.; Sawacha, Z. The Effect of Custom Insoles on Muscle Activity in Diabetic Individuals with Neuropathy. Appl. Sci. 2023, 13, 2326. https://doi.org/10.3390/app13042326
Spolaor F, Guiotto A, Ciniglio A, Sawacha Z. The Effect of Custom Insoles on Muscle Activity in Diabetic Individuals with Neuropathy. Applied Sciences. 2023; 13(4):2326. https://doi.org/10.3390/app13042326
Chicago/Turabian StyleSpolaor, Fabiola, Annamaria Guiotto, Alfredo Ciniglio, and Zimi Sawacha. 2023. "The Effect of Custom Insoles on Muscle Activity in Diabetic Individuals with Neuropathy" Applied Sciences 13, no. 4: 2326. https://doi.org/10.3390/app13042326









