Application of Thermochemical Method to Determine the Crystallinity Degree of Cellulose Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- ➢
- Pure chemical-grade cotton cellulose (CC) of Hercules, Inc. (Wilmington, DE, USA).
- ➢
- Microcrystalline cellulose (MCC) prepared by treatment of CC with boiling 2.5 M HCl for 1 h at the acid-to-CC ratio of 20, followed by washing and drying.
- ➢
- Mercerized cotton cellulose (CCM) prepared by treatment of CC with 6 M NaOH for 1 h at room temperature and alkali-to-CC ratio of 20, followed by washing and drying.
- ➢
- Bleached Kraft pine chemical pulp (KP) of Weyerhaeuser, further refined by treatment with 2 M NaOH for 1 h at room temperature and alkali-to-pulp ratio of 20, followed by washing and drying.
- ➢
- Bleached high-pure sulfite spruce pulp (SP) of Weyerhaeuser Co. (Seattle, WA, USA).
- ➢
- Mercerized sulfite pulp (SPM) prepared by treatment of SP with 6 M NaOH for 1 h at room temperature and alkali-to-pulp ratio of 20, followed by washing and drying.
- ➢
- Viscose rayon fibers (VF) of Rayonier, Inc. (Wildlight, FL, USA).
2.2. Methods
2.2.1. Characterization
2.2.2. WAXS
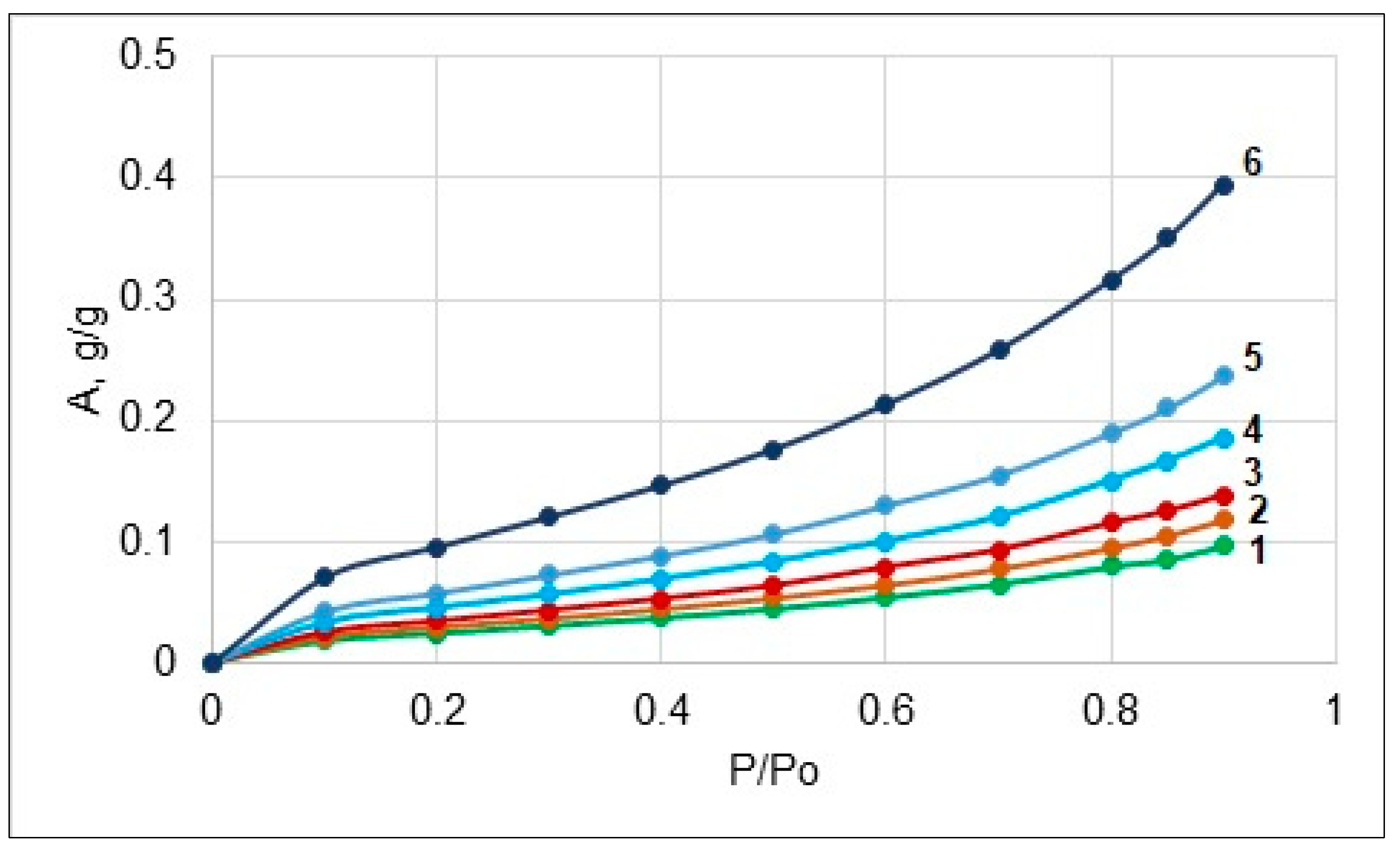
2.2.3. Sorption of Water Vapor
2.2.4. Enthalpy of Wetting
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. 2005, 44, 2–37. [Google Scholar] [CrossRef]
- Ioelovich, M. Cellulose: Nanostructured Natural Polymer; LAP: Saarbrücken, Germany, 2014; p. 88. [Google Scholar]
- Ioelovich, M. Models of supramolecular structure and properties of cellulose. Polymer Sci. Ser. A 2016, 58, 925–943. [Google Scholar] [CrossRef]
- Belgacem, M.N.; Pizzi, A. Lignocellulosic Fibers and Wood Handbook: Renewable Materials for Today’s Environment; Scrivener Publishing LLC: Beverly, MA, USA, 2016; 704p. [Google Scholar]
- Jardim, J.M.; Hart, P.W.; Lucia, L.A.; Jameel, H.; Chang, H.M. The effect of the Kraft pulping process, wood species, and pH on lignin recovery from black liquor. Fibers 2022, 10, 16. [Google Scholar] [CrossRef]
- Potůček, F.; Gurung, B.; Hájkov, K. Soda pulping of rapeseed straw. Cell. Chem. Technol. 2014, 48, 683–691. [Google Scholar]
- Wildschut, J.; Smit, A.T.; Reith, J.H.; Huijgen, W. EtOH-based organosolv fractionation of wheat straw for the production of lignin and enzymatically digestible cellulose. Bioresour. Technol. 2013, 135, 58–66. [Google Scholar] [CrossRef]
- Ioelovich, M. Cellulose: Structure and Properties Relationships. In Handbook of Sustainable Polymers: Structure and Chemistry; Thakur, V.K., Thakur, M.K., Eds.; Pan Stanford Publishing: Singapore, 2016; pp. 200–264. [Google Scholar]
- Maier, G.; Zipper, P.; Stubičar, M.; Schurz, J. Amorphization of different cellulose samples by ball milling. Cellul. Chem. Technol. 2005, 39, 167–177. [Google Scholar]
- Halonen, H.; Larsson, P.T.; Iversen, T. Mercerized cellulose biocomposites: A study of influence of mercerization on cellulose supramolecular structure, water retention value and tensile properties. Cellulose 2013, 20, 57–65. [Google Scholar] [CrossRef]
- Mittal, A.; Katahira, R.; Himmel, M.E.; Johnson, D.K. Effects of alkaline or liquid ammonia treatment on crystalline cellulose: Changes in crystalline structure and effects on enzymatic digestibility. Biotechnol. Biofuels 2011, 4, 41–57. [Google Scholar] [CrossRef]
- Duchemin, B.; Le Corre, D.; Leray, N.; Dufresne, A.; Staiger, M.P. All-cellulose composites based on microfibrillated cellulose and filter paper via a NaOH-Urea solvent system. Cellulose 2016, 23, 593–609. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Terinte, N.; Ibbett, R.; Schuster, K.C. Overview on native cellulose and microcrystalline cellulose I structure studied by X-ray diffraction (WAXD): Comparison between measurement techniques. Lenzing. Ber. 2011, 89, 118–131. [Google Scholar]
- Rongpipi, S.; Ye, D.; Gomez, E.D.; Gomez, E.W. Progress and opportunities in the characterization of cellulose—An important regulator of cell wall growth and mechanics. Front. Plant Sci. 2019, 9, 1–28. [Google Scholar] [CrossRef]
- Madhushani, W.H.; Priyadarshana, R.W.I.B.; Ranawana, S.R.W.; Senarathna, K.G.C.; Kaliyadasa, P.E. Determining the crystallinity index of cellulose in chemically and mechanically extracted banana fiber for the synthesis of nanocellulose. J. Nat. Fibers 2022, 19, 7973–7981. [Google Scholar] [CrossRef]
- Leong, S.L.; Tiong, S.I.X.; Siva, S.P.; Ahamed, F.; Chan, C.H.; Lee, C.L.; Chew, I.M.L.; Ho, Y.K. Morphological control of cellulose nanocrystals via sulfuric acid hydrolysis based on sustainability considerations: An overview of the governing factors and potential challenges. J. Environ. Chem. Eng 2022, 10, 1–20. [Google Scholar]
- Li, Y.Y.; Bai, Y.R.; Zhang, X.Q.; Liu, X.; Dai, Z.; Jiang, Y.L.; Yan, Y.B.; Liu, Z.Q.; Ma, M.G. Preparation, properties and mechanism of anionic and cationic cellulose nanocrystals/waterborne polyurethane composite films. Bioresources 2023, 18, 447–464. [Google Scholar] [CrossRef]
- Schmidt, L.D. The Heat of Wetting of Cellulose; University Press: Minneapolis, MN, USA, 1936; 57p. [Google Scholar]
- Wahba, M.; Nashed, S. Change with temperature of the heat of wetting of dry cellulose in water, and its bearing on the specific heat of the adsorbed water and of the swollen cellulose. Nature 1950, 166, 998–1000. [Google Scholar] [CrossRef]
- Varga, K.; Schädel, U.; Nilsson, H.; Persson, O.; Schuster, K. Measuring the heat of wetting of textile fibres by reaction calorimetry. Fibres Text. East. Eur. 2007, 15, 59–63. [Google Scholar]
- Prusov, A.N.; Prusova, S.M.; Radugin, M.V.; Zakharov, A.G. Interrelation between the crystallinity of polysaccharides and water absorption. J. Phys. Chemistry 2014, 88, 813–818. [Google Scholar] [CrossRef]
- Mizutani, C.; Tsuj, Y.; Bertoniere, N. Effect of fiber structure on heat of wetting of cotton and regenerated cellulosic fibers. Text. Res. J. 1999, 69, 559–564. [Google Scholar] [CrossRef]
- Standard TAPPI Method T-203; Alpha-, Beta- and Gamma-Cellulose in Pulp. TAPPI: Peachtree Corners, GA, USA, 2009.
- Hindi, S.S. Microcrystalline cellulose: The inexhaustible treasure for pharmaceutical industry. Nanosci. Nanotech. Res. 2017, 4, 17–24. [Google Scholar]
- Revol, J.F.; Dietrich, A.; Goring, D.A.I. Effect of mercerization on the crystallite size and crystallinity index in cellulose from different sources. Can. J. Chem. 1987, 65, 1724–1725. [Google Scholar] [CrossRef]
- Ioelovich, M.; Leykin, A.; Figovsky, O. Study of cellulose paracrystallinity. Bioresources 2010, 5, 1393–1407. [Google Scholar]
- Ioelovich, M.; Aripov, K. Comparative study of supramolecular structure of cellulose in cotton fibers of Gossypium hirsutum and Hossypium barbadense. Cell. Chem. Technol. 2020, 54, 635–641. [Google Scholar]
- Harjunen, P.; Lehto, V.P.; Koivisto, M.; Levonen, E.; Paronen, P.; Järvinen, K. Determination of amorphous content of lactose samples by solution calorimetry. Drug Dev. Ind. Pharm. 2004, 30, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Fulchiron, R.; Gouanvé, F. Water sorption and mechanical properties of cellulosic derivative fibers. Polymers 2022, 14, 2836. [Google Scholar] [CrossRef]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal crystallinity index revisited by the simulation of X-ray diffraction patterns of cotton cellulose Iβ and cellulose II. Carbohydr. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; Nijenhuis, K. Properties of Polymers: Correlations with Chemical Structure; Elsevier: Amsterdam, The Netherlands, 2009; 1004p. [Google Scholar]
- Ioelovich, M. Models of water vapor sorption by hydrophilic polymers. Adv. Res. Org. Inorg. Chem. 2022, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bhama, I.P.; Sreenivasan, S.; Chidambareswaran, P.K.; Patil, N.B. Crystallization of amorphous cellulose. Text. Res. J. 1984, 54, 732–735. [Google Scholar] [CrossRef]
- Ago, M.; Endo, T.; Hirotsu, T. Crystalline transformation of native cellulose from cellulose I to cellulose II polymorph by a ball milling method with a specific amount of water. Cellulose 2004, 11, 163–167. [Google Scholar] [CrossRef]
- Krässig, H. Cellulose: Structure, Accessibility and Reactivity; Gordon and Breach Publishers: Amsterdam, The Netherlands, 1996; 376p. [Google Scholar]

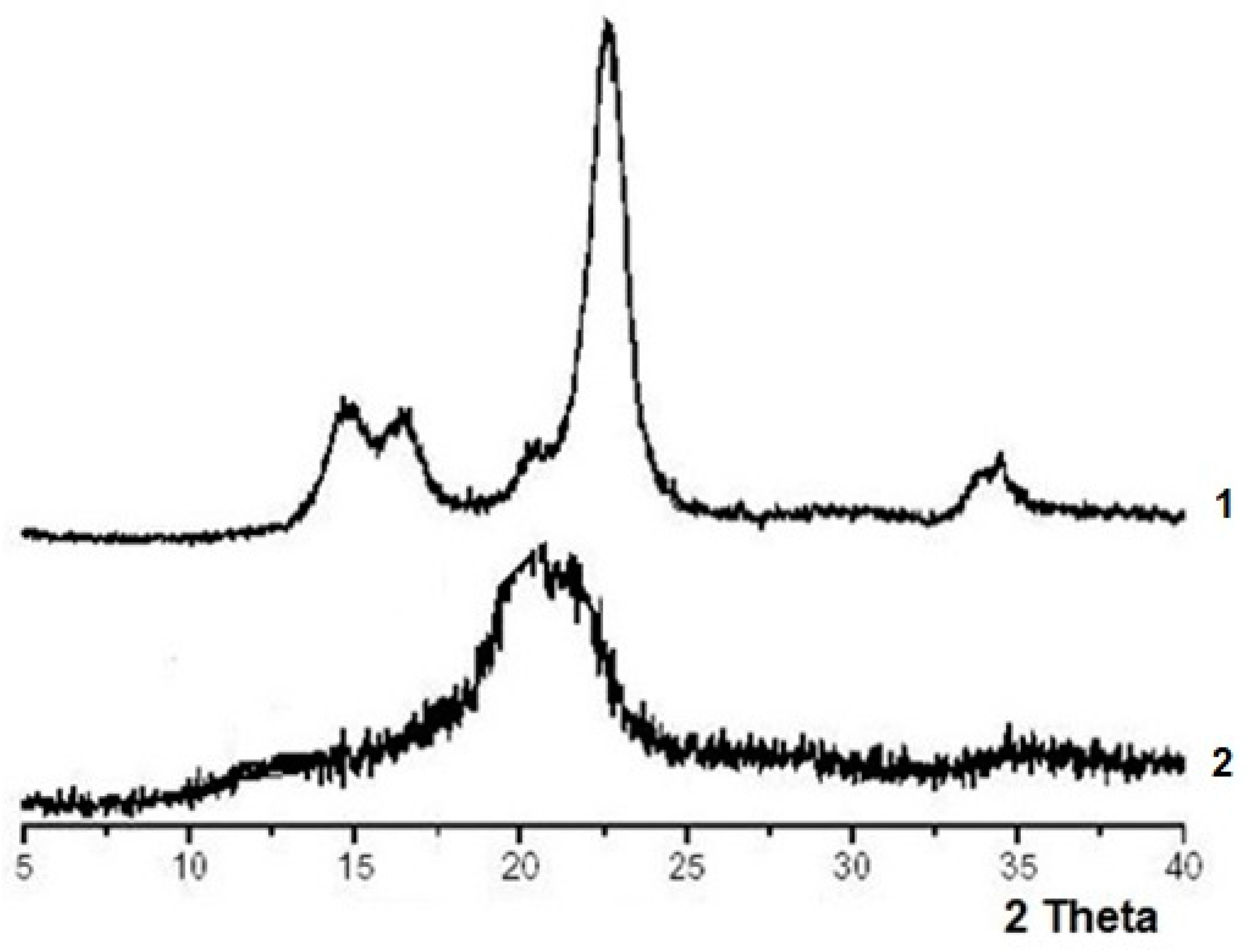


| Sample | *CrA | α-Cellulose, % | DP | CrI |
|---|---|---|---|---|
| CC | CI | 98 ± 0.3 | 2700 ± 120 | 0.83 ± 0.02 |
| MCC | CI | 88 ± 0.5 | 170 ± 30 | 0.92 ± 0.01 |
| CCM | CII | 99 ± 0.2 | 2100 ± 110 | 0.68 ± 0.02 |
| KP | CI | 97 ± 0.2 | 1200 ± 80 | 0.78 ± 0.02 |
| SP | CI | 94 ± 0.4 | 1100 ± 90 | 0.75 ± 0.03 |
| SPM | CII | 98 ± 0.3 | 960 ± 60 | 0.66 ± 0.03 |
| VF | CII | - | 250 ± 30 | 0.54 ± 0.02 |
| Sample | Ao, g/g | −ΔHw, J/g |
|---|---|---|
| CC | 0.144 ± 0.002 | 46.5 ± 0.2 |
| MCC | 0.125 ± 0.003 | 42.2 ± 0.2 |
| CCM | 0.226 ± 0.005 | 75.6 ± 0.5 |
| KP | 0.174 ± 0.003 | 58.5 ± 0.3 |
| SP | 0.183 ± 0.004 | 62.1 ± 0.4 |
| SPM | 0.236 ± 0.005 | 78.9 ± 0.3 |
| VF | 0.310 ± 0.004 | 104.2 ± 0.5 |
| AC | 0.500 * | 168 * |
| Sample | Y | X | CrI | RD, % |
|---|---|---|---|---|
| CC | 0.28 ± 0.01 | 0.72 ± 0.01 | 0.83 ± 0.02 | 15 |
| MCC | 0.25 ± 0.01 | 0.75 ± 0.01 | 0.92 ± 0.01 | 23 |
| CCM | 0.45 ± 0.01 | 0.55 ± 0.01 | 0.68 ± 0.02 | 24 |
| KP | 0.35 ± 0.01 | 0.65 ± 0.01 | 0.78 ± 0.02 | 20 |
| SP | 0.37 ± 0.01 | 0.63 ± 0.01 | 0.75 ± 0.03 | 19 |
| SPM | 0.47 ± 0.01 | 0.53 ± 0.01 | 0.66 ± 0.03 | 24 |
| VF | 0.62 ± 0.01 | 0.38 ± 0.01 | 0.54 ± 0.02 | 42 |
| Properties | Symbol | Zc | Za |
|---|---|---|---|
| Specific volume, cm3/g | Vs | 0.617 | 0.694 |
| Moisture content (%) at standard RH = 65% | Aw | 0 | 23 |
| Accessibility for deuterium | AD | 0.14 | 1.14 |
| Sorption of alkali from 1 M NaOH, mmol/g | AA | 0 | 3.1 |
| Hydrolysability (%) after treatment with boiling 2.5 M HCl, 1 h | H | 0 | 40 |
| Properties | CC | VF | ||
|---|---|---|---|---|
| Calc | Exp | Calc | Exp | |
| Vs, cm3/g | 0.638 | 0.640 | 0.665 | 0.661 |
| *Gs, g/cm3 | 1.57 | 1.56 | 1.50 | 1.51 |
| Aw, % | 6.4 | 6.5 | 14.3 | 14.1 |
| AD | 0.42 | 0.43 | 0.76 | 0.75 |
| AA, mmol/g | 0.87 | 0.86 | 1.92 | 1.94 |
| H, % | 11 | 10 | 25 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioelovich, M. Application of Thermochemical Method to Determine the Crystallinity Degree of Cellulose Materials. Appl. Sci. 2023, 13, 2387. https://doi.org/10.3390/app13042387
Ioelovich M. Application of Thermochemical Method to Determine the Crystallinity Degree of Cellulose Materials. Applied Sciences. 2023; 13(4):2387. https://doi.org/10.3390/app13042387
Chicago/Turabian StyleIoelovich, Michael. 2023. "Application of Thermochemical Method to Determine the Crystallinity Degree of Cellulose Materials" Applied Sciences 13, no. 4: 2387. https://doi.org/10.3390/app13042387




