1. Introduction
The return to nature and rediscovery of its resources have become a key trend in contemporary industry, science, and consumption. The efforts to reemploy the natural properties of long-forgotten plants (such as milk thistle and borage) aim to popularise such materials in accordance with the European’s environmentally friendly policies. This is important because these plants have valuable properties facilitating the correct functioning of the human body in medicine and biocosmetics [
1]. It has been reported that γ-linolenic acid contained in borage oil is effective against atopic dermatitis [
2]. The research demonstrated that children who had worn undershirts coated with borage oil for 2 weeks showed improvements in their erythema and itch, which were statistically significant. Transepidermal water loss from the back was decreased. The undershirts coated with borage oil were found to be statistically effective, and had no side effects for children with mild atopic dermatitis [
2]. Milk thistle has been used to treat various diseases for years. Many studies suggest a positive role for milk thistle in liver protection and regeneration [
3,
4,
5]. Das and Mukherje [
3] showed that the administration of an adequate amount of silybin restores the oxidative–antioxidant balance and reduces inflammation biomarkers and immunomodulation in induced liver oxidative stress in rats. Kwon et al. [
4] found that the silymarin in milk thistle promotes hepatic glutathione production, which enhances antioxidant defences in mouse liver models. The health effects of milk thistle have also been proven in human studies. Lucena et al. [
5] revealed a statistically significant reduction in lipid peroxidation in patients with alcoholic cirrhosis after the administration of silymarin from the dry extract of the fruit of the
Silybum marianum plant [
5].
There have been a growing number of dietary, cosmetic, and pharmaceutical breakthroughs relying on the bioactive substances present in plants rich in polyunsaturated fatty acids from the n-3 and n-6 families, as well as γ-linolenic acid (GLA), the prostaglandin precursor, with many tracing back to the two plants mentioned above. In the case of milk thistle and borage, the industrial-grade material consists primarily of seeds and the oils extracted from the same [
6]. The most valuable oil is obtained in the process of cold pressing. With such treatment, both borage (
Borago officinalis L.) and milk thistle (
Silybum marianum L. Gaertn) can be a source of a number of valuable compounds with proven medicinal and cosmetic benefits. Such compounds are necessary ingredients of a healthy human diet that cannot be synthesised, and therefore should or even must be obtained from plant sources [
7]. However, milk thistle seed oil is considered one of the most valuable products of natural origin due to its multiple and complex healing properties. Milk thistle seed oil has also been documented as a potential natural source of vitamin E and has often been recommended as a favourable edible oil [
8].
Milk thistle has a number of confirmed health benefits, verified by documented research results that list it among alternative medicines facilitating the treatment of liver- and bile-related ailments [
9]. Its anti-inflammatory properties allow it to be used as an ointment applied on pruritic, psoriatic, exsiccated, burned, or frostbitten skin [
10].
Oils obtained from the two plants are a rich source of lipophilic compounds, tocopherols (vitamin E), counted as some of the most potent natural antioxidants, carotenoids, tocochromanols, phytosterols, and squalene, showing significant health benefits, as well as sterols (cholesterol, campesterol, stigmasterol, and sitosterol) [
11], sugars (arabinose, rhamnose, xylose, and glucose), and flavonoids, contributing to protection of the liver against toxins [
12].
Given the growing interest in products based on borage and milk thistle oils, it is prudent to expand our knowledge on the chemical composition and biological activity of the plants. Hence, the aim of this study was a comparative analysis of their seeds and the oils extracted from them in terms of selected primary qualitative parameters. To this end, seeds were examined for the content of fat and protein, and the oils were studied in terms of raw fat, protein, water (H2O), phosphorus (P), acid number (AN), peroxide value (PV), and free fatty acid content (FFA). The analysis included the composition of fatty acids, the content of sterols and squalene, tocopherols and tocotrienols, total chlorophyll and carotenoids, oil colour, and total polyphenols, as well as antioxidative activity (DPPH). Moreover, a qualitative analysis was conducted on the two oils using FTIR spectroscopy as a non-invasive, fast, and efficient method of determining general differences in this type of organic sample.
3. Results
Table 1 presents the results of the analysis of the seeds’ basic chemical compositions. They revealed typical, not particularly varied moisture content of 6.55 and 6.61%, respectively, for the milk thistle and borage seeds. The analysis of fat content returned 34.34% in the borage seeds. This was 24.6% higher than the corresponding value for milk thistle seeds (25.90%). Less differentiation was observed in terms of the protein content, with the values recorded as 24.80% (borage seeds) and 29.49% (milk thistle seeds), which corresponded to a difference of 19% between the analysed samples (
Table 1).
The table below (
Table 2) presents the results recorded in terms of the fatty acid content in the analysed oils. Seven distinct fatty acids were identified in the milk thistle oil, and nine in the borage oil. Both samples were characterised by significant levels of polyunsaturated fatty acids n-6, including linolic acid (18:2n-6) (ALA). However, statistically significant differences were also observed. The content of linolic acid in borage oil was 26% higher than the corresponding value in milk thistle oil. The second most prominent acid identified was oleic acid (C18:1n9c). Its content also varied statistically significantly between the samples, with the value obtained for milk thistle oil exceeding the corresponding result for borage oil by 28%. Furthermore, the borage oil sample contained 19.79% of γ-linolenic acid (C18:3n6) and 2.90% of arachidic acid (C20:0). The latter two acids were not found in the milk thistle oil sample. The content of the remaining acids in the respective oils also varied to a lesser or greater degree.
Values such as the peroxide value (form of primary oxidation products), water content, and acid number (free fatty acids), as significant to the rancidification process, are key parameters considered for the purposes of quality analyses in both industrial and scientific contexts. The parameters determine the durability of the product, and consequently the economic viability of the oil.
Table 3 presents some of the most important chemical parameters influencing the quality of oils used in the fuel and oil manufacturing industries. In both analysed samples, most of the measurements returned higher values in the oil extracted from milk thistle seeds. The water content in the milk thistle oil was 14.7% higher as compared to the borage oil. The content of phosphorus was 30% higher, AN 24.5% higher, PV 32.9% higher, and FFA 63.4% higher (5.2 and 1.9%). In terms of the oxidative stability parameter, the measured time was 4.53 and 4.28 h, respectively, which corresponded to a 5.5% higher value for milk thistle oil. In terms of the other parameters, such as PV or NJ, their levels observed for milk thistle oil were lower, respectively, by 32.93 and 13.34% compared to borage oil.
Table 4,
Table 5 and
Table 6 present the parameters responsible for the characteristic colour of the oils, as well as their antioxidative properties (
Table 7). The content of total chlorophyll in borage oil was 26.17% (3.63 mg·kg
−1) higher than the value for milk thistle oil. Furthermore, significant differences were observed in terms of carotenoid content as the value recorded for borage oil was 18% lower than that for milk thistle oil (
Table 4).
The polyphenol content varied by 71.3% between the respective samples, with the higher level of 7.18 mgGAE·g−1 oil recorded for milk thistle oil. The corresponding value for borage oil was 4.19 mgGAE·g−1 oil.
The content of flavonoids in the analysed oils was 4.45 mg·kg−1 in milk thistle oil, which was significantly higher than in the case of borage oil (by 12.37%).
Tocopherols are important lipophilic compounds with antioxidative properties (
Table 5). The concentration of tocochromanols varied by 224.5% between the analysed oils. The content of α-tocopherol (vitamin E), i.e., the most bioactive of the same, was positively verified in milk thistle oil (275.7 mg·kg
−1) but was not present in borage oil. β-tocopherol was not detected in either of the samples. The content of γ-tocopherol was significantly higher in borage oil, exceeding the corresponding amount in the other sample by 34.62%. The lowest values were observed for δ-tocopherols and were the same in both samples (14.23 mg·kg
−1). The free radical scavenging capacity of the studied oils determined using the common DPPH method was 1.83 and 1.54 mgTrolox·100 g
−1, respectively, in milk thistle and borage oil, i.e., the discrepancy between samples amounted to 18.83%.
Table 6 presents the colour parameters of the analysed oils. The value of the L* coordinate for oil obtained from milk thistle seeds was approximately ten units lower relative to borage oil, signifying that the latter was considerably lighter. The values of the a* and b* colour coordinates, as well as the chroma value (c), were all higher for milk thistle oil, signifying that the colour could be described as more “lively”. On the other hand, the hue (h) coordinate was higher for borage oil, i.e., the hues of the respective oils were considerably different.
The table below (
Table 7) lists the basic steroid compounds present in plants. The content of total sterols varied between the two analysed oil samples. The level thereof recorded for borage oil (261.33 mg·100 g
−1 oil) was 33% lower than in the case of milk thistle oil (347.52 mg·100 g
−1 oil). The highest specific content was observed for β-sitosterol, Δ
5-campestanol, Δ
7-stigmasterol, and Δ
5-stigmasterol, with the differences between the two oils estimated at 11.85%, 252%, 88.94%, and 177.30%, respectively. The lowest content or a complete absence of the relevant compounds in the milk thistle sample was recorded for clerosterol, Δ
5-avenasterol, brassicasterol (0 mg·100 g
−1 oil), and Δ
7-avenasterol (0 mg·100 g
−1 oil). In the case of borage oil, the presence of campestanol, clerosterol, sitostanol, Δ
5-24-stigmastadienol, and cholesterol was not detected.
The content of squalene also varied significantly between the analysed oils. It was 78.23 mg·100 g−1 oil in milk thistle oil, which was 97% higher than the corresponding value for borage oil (39.76 mg·100 g−1 oil).
The conducted analysis of Pearson’s linear correlation coefficient confirmed our expectations and revealed a close dependence between the factors considered for both oils (
Table 8). There was a strong, both positive and negative, correlation between the content of phosphorus, C16:0 acid, acid number, peroxide value, iodine number, free fatty acids, and oxidative stability coefficient relative to the content of tocopherols δ, γ, α. Moreover, the same was also observed relative to sterols, polyphenols, DPPH, flavonoids, squalene, carotenoids, and chlorophylls, i.e., the group of antioxidative compounds.
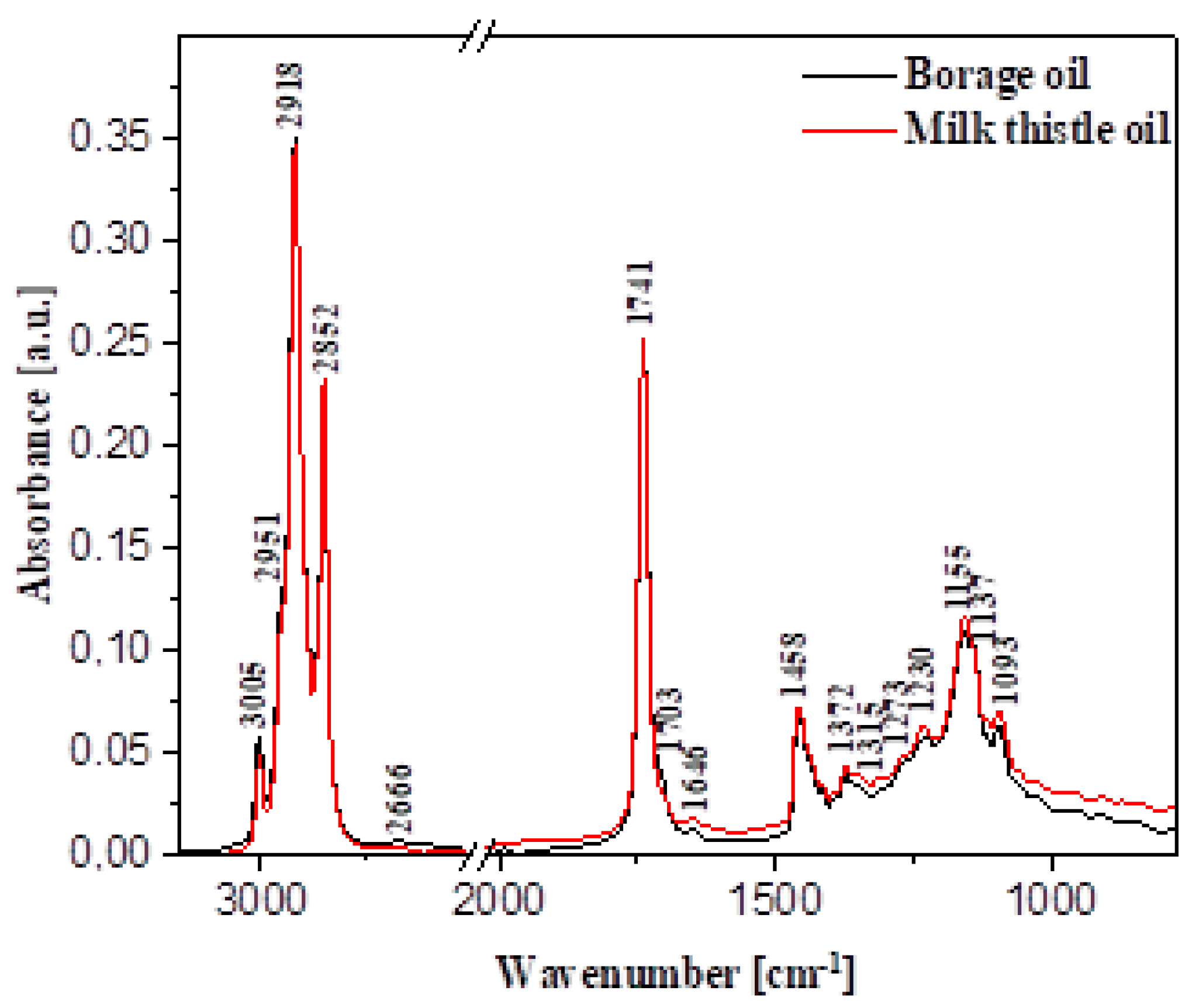
Figure 1 presents the FTIR spectra measured for the respective oil samples obtained from borage (black line) and milk thistle (red line). For the sake of easier analysis and comparison, the spectra were normalised at the same intensity with the maximum at ~1741 cm
−1. The samples were placed on the crystal and analysed in a neutral gas atmosphere (as described in the
Section 2).
Table 9 below illustrates the characteristic bands observed for the analysed oil samples attributed to the vibrations of specific functional groups based on an extensive literature review. It should be noted that the infrared spectra of the analysed oil samples contained clear bands that could be relatively easily attributed to specific vibrations of particular functional groups characteristic of certain food ingredients. Most edible vegetable oils are composed primarily of a variety of triglyceride fractions. Differences in this respect are primarily related to the degree and form of unsaturation of acyl groups involved, and the lengths of particular chains. There is currently a constantly growing number of worldwide publications reporting studies wherein FTIR spectroscopy was used in the analysis of various vegetable oils, e.g., rape oils. It should be noted, however, that despite the above, some bands prove notoriously difficult to decisively associate with specific groups.
Table 9 below provides a fairly detailed breakdown of spectral frequencies with the most important band enhancements, as well as the corresponding functional groups involved. As a general note, it should be mentioned that, in this type of biological sample, many bands tend to overlap, which renders their association with specific vibrations considerably more difficult.
The general characteristics of the presented oil samples reveal rather intensive vibrations of the methylene group, usually located within the spectral range of 1350 to 1175 cm
−1. In this case, these correspond to the stretching vibrations of the -C-H bond of the -CH3 group and deformation vibrations occurring in the same group (located at ~1160 cm
−1, in our case, as an enhancement of the main band with the maximum at 1372cm
−1). The stretching vibrations of the ester bond ν(C-O) include two combined asymmetrical vibrations—in this case, originating from the C-C(=O)-O and O-C-C groups. The intensity of the former is significantly higher, which is typical of most oily samples. The bands are usually located in the region of ~1300 (C-C(=O)-O) and ~1000 cm
−1. In our case, the same were located at 1315 and 1093 cm
−1. At the same time, the bands corresponding to saturated esters, i.e., C-C(=O)-O are also found between 1240 and 1160 cm
−1—in our case, at approximately 1230 cm
−1. Meanwhile, vibrations of this type corresponding to unsaturated esters tend to be located at considerably lower frequencies. The characteristic O-C-O band associated with primary alcohols tends to appear within the range from 1090 to 1020 cm
−1, and was present in our case at 1093 cm
−1. For secondary alcohols, the band is typically found with the maximum at ~1100 cm
−1; in the described case, it was present as an enhancement in the band with the light maximum at 1137 cm
−1. Both mentioned ester types are present in triglyceride molecules. In turn, the band with the maximum at approximately 1230 cm
−1 is often associated exclusively with the out-of-plane deformation vibrations originating from the methylene group. Further regions represented in
Table 9 and
Figure 1 correspond to other vibrations characteristic of food ingredients. Firstly, there is the region with the maximum at ~1458 cm
−1, and, secondly, at ~1315 cm
−1 (as band widening,
Figure 1). The former vibrations (~1458 cm
−1) originate from the methyl groups in the aliphatic chains of the analysed oils. The band with the maximum at ~905 cm
−1 present in both oil samples can be attributed to the stretching vibrations of
cis-substituted olefin groups, which may be additionally enhanced by the vibrations of the vinyl group.
The infrared spectra of the analysed oil samples were fairly similar, albeit not without certain discrepancies that seem fairly characteristic and therefore analytically useful.
Another characteristic area of vibrations corresponds to the band maximum at ~1741 cm−1 and originates from the stretching vibrations of the C=O group. On the shorter wavelength side, this band is enhanced with the maximum at ~1703 cm−1 corresponding to the vibrations of another carbonyl group, this one present primarily in acid groups. In turn, the band with the maximum at ~1646 cm−1 represents the stretching vibrations of the -C=C- group (cis-transformation). A very characteristic region is also found in the band with the maximum at 1458 cm−1 (already mentioned above), originating from the deformation vibrations of -C-H groupings in -CH2 and -CH3 groups (scissor vibrations).
Moving on to longer-wavelength regions, one should also mention the =C-H stretching vibrations (
trans-transformation) with the maximum at ~3066 cm
−1 (
Table 9), originating from the vibrations of triglyceride fractions. In turn, for the stretching vibrations of =C-H in the
cis-configuration, characteristic vibrations are observed with the maximum at ~3005 cm
−1 (
Figure 1 and
Table 9). The bands with the maxima at 2951 and 2852 cm
−1 correspond to the stretching vibrations of –C-H in -CH
3, CH
2, present in the aliphatic groups in triglycerides.
In the case of the stretching vibrations of the C=O and the corresponding spectral band, a slight enhancement is present on the short-wavelength side at ~1703 cm−1, which can be attributed to the formation of a hydrogen bond between C=O…H-O- groups.
The observed spectral differences correlate very well with the differences in the fatty acid profile presented in
Table 2 and described in the first part of the paper.
4. Discussion
The water content measured in borage seeds (
Table 1) was not different from the value reported by Borowy and Kapłan [
37] or results obtained for four other oilseeds cultivated in Poland [
38]. Considerably higher moisture content was reported by Abdel Samed et al. [
39] in their study on seeds originating from 2005 and 2006 harvests in Cairo. The content of lipids (
Table 1) and γ-linolenic acid (GLA) and linolic acid (LA) (
Table 2) in the seeds was very similar to the values obtained by Borowy and Kapłan [
37] in their study on borage seeds harvested in the south-eastern region of Poland in 2017. Similar results were also reported by Zadernowski et al. [
6] in a study wherein the research material consisted of borage seeds originating from Northern Poland. As we compared our results with those reported in other countries, we observed that the lipid levels were high relative to the GLA content [
40]. Similar conclusions were reached by Galwey and Shirlin [
41], who emphasised the negative correlation between the two components. Discrepancies in terms of lipid and GLA content can be related to differences in borage genotypes [
42], times of sowing and harvest [
43,
44], seed maturity [
43], or overall growth conditions, such as rainfall or air temperature [
44]. As suggested by Berti et al. [
43], the content of oil in borage seeds increases under the conditions of physiological seed maturity.
The content of protein in the analysed seeds (
Table 1) was slightly higher than corresponding results reported by Abdel Samed et al. [
39] and reached 22.8%, which was comparable to the results obtained for borage seeds harvested in South-Eastern Poland [
37]. In a study on flax, rape, and sunflower seeds, Budzyński and Zając [
38] observed and reported similar protein content, as opposed to soya seeds, where the protein content was significantly lower.
Our results in the study on the chemical properties of milk thistle seeds were consistent with those reported by other researchers. Aziz et al. [
45] studied various milk thistle cultivars originating from three cities in Pakistan and were able to demonstrate that samples from Islamabad contained more protein (blue and white cultivars: 30.09 and 27.60%) and moisture (blue and white cultivars: 6.27 and 6.90%) as compared to seeds from other cities. Very low moisture content (approximately 1%) compared to other contemporary results was reported by Malekzadeh et al. [
46], likely due to factors related to climate change and the fact that the milk thistle was grown in a sandy area. On the other hand, observations made by Mahmoud et al. [
47] coincide with ours in terms of fat content in milk thistle seeds. The researchers studied two Egyptian cultivars (wild and cultivated) and the values that they obtained were within the range of 28.53–29.68%. Moreover, they analysed true protein (25.25%), total carbohydrates (38.16%), and crude fibre (29.95%). The seeds of wild plants acquired greater amounts of lipids and crude fibre and less content of carbohydrates and proteins. The amino acids were similar in both wild and cultivated plants but their content varied [
47]. Perez et al. [
48] reported the content of oil in seeds of Asteraceae family plants as 25%.
Based on the analyses conducted for the two oils, we were able to identify seven fatty acids in borage oil and nine in milk thistle oil. The former contained the following acids: palmitic (C16:0), palmitoleic (C16:1), stearic (C18:0), oleic (C18:1 n-9), linolic (C18:2 n-6), linolenic (C18:3), and arachidic (C20:0). Other authors identified nine fatty acids in oil obtained from this plant [
46,
49]. The oil analysed in our study did not contain eicosenoic acid (C20:1), whose presence had been reported in milk thistle oil by Malekzadeh et al. [
40]. Similarly to results published by other authors, the content of linolenic acid was dominant in our study at or above 47.84%.
In a study conducted by Tasset-Cuevas et al. [
50] on cold-pressed borage oil, the reported content of linolic acid (C18:2) was 36.6%, i.e., less than observed in our study. At the same time, the content of γ-linolenic acid (C18:3) was 21.1% and was higher than measured in our case (0.22%). The results are comparable to those reported by other authors [
51].
By comparing our results with those obtained by Zadernowski et al. [
6] for oils pressed from borage seeds and oils obtained by means of hexane extraction, it was concluded that the basic physicochemical properties characterising “virgin” oils can vary, but do not exceed the reference values posited in the Codex Alimentarius [
52]. The AN values in their pressed oils were significantly lower, while the results obtained for hexane-extracted oil were identical. The oils obtained by the cited authors were characterised by a significantly lower (by approximately 20 gJ
2·100 g
−1 oil) iodine number, regardless of the method of extraction. At the same time, the peroxide value measured for their oil extracted in a hydraulic press was similar to that recorded in our study. Results in terms of the oxidative stability of borage seed oil were also published by Sensidoni et al. [
53]. In their study, they analysed refined, solvent-extracted oil, as well as two carbon-dioxide-extracted oils (pressure 25.3 MPa, temperature 45 °C; and pressure 30.4 MPa, temperature 55 °C). The induction times measured in their study varied within the range of 4.5 h (CO
2-extracted oil at 45 °C) to 9.0 h (refined oil). The value reported for oil extracted with carbon dioxide was similar to that observed in our study. A considerably longer raw borage oil induction time of 11.17 h was reported by Szterka et al. [
54]. Their conductometric analysis of the total volatile lipid compounds formed during hydroxide degradation was performed using a Methrom Rancimat device (Herisau, Switzerland) by subjecting oil samples to an airflow of 10 L∙h
−1 at 100 °C. The discrepancies in terms of the oxidative stability of oils reported by different authors may be due to the methods of oil extraction used or other parameters significant to the Rancimat test.
The results of measurements conducted for the analysed milk thistle oil were partially inconsistent with the literature data. Bryś et al. [
55] demonstrated that the acid and peroxide values of cold-pressed oil were 2.44 mgKOH·g
−1 and 5.84 mmolO
2·kg
−1, respectively. In turn, Fathi-Achachlouei and Azadmard-Damirchi [
49], in a study on the impact of microwave heating on milk thistle seeds of the Iranian ecotype on the properties of oil extracted therefrom, reported that the acid and peroxide values were within the range of 4.24–2.16 mgKOH·g
−1 and 5.11–2.09 meqO
2·kg
−1, while the iodine number was 107–99 gI
2·100 g
−1, with the values decreasing as a result of thermal processing. A study conducted on oils from milk thistle seeds harvested in three different regions of Tunisia (Zaghouan, Bizerte, and Sousse) [
56] returned results similar to our own in terms of the acid value (oil from Bizerte seeds: 5.48 mgKOH·g
−1), peroxide value (oil from Sousse seeds: 4.20 meqO
2·kg
−1), and iodine number (oil from Zaghouan seeds: 118.32 gI
2·100 g
−1). The Rancimat test results reported by the authors varied from our value only in one instance, with the reported stability of milk thistle seed oil expressed as an oxidation induction time of 5.83, 8.75, and 4.55 h, respectively, for oils extracted from Bizerte, Zaghouan, and Sousse seeds. The oxidative stability registered in our study was lower than reported by Parry et al. [
8] (13.3 h) and Dabbour et al. [
57] (55.7 h) for oils obtained from
Silybum marianum L. seeds at 80 °C and under an airflow of 7 and 20 l·h
−1, respectively. The lower values of oil oxidative stability may be explained by differences in terms of the adopted measurement parameters.
Based on the available literature, differences in terms of surfactant ingredients and oil composition lead to varied responses to water content and the formation of various physical surfactant structures in vegetable oil [
58]. The resulting effects may be positive, but, in most cases, the presence of phospholipids in vegetable oils triggers oxidation, negatively affecting the smell and taste of the product [
58].
In the course of refining vegetable oil, phosphorus, as well as other active ingredients such as fatty acids, monoglycerides, and diglycerides, are removed in the so-called degumming process [
59]. However, in cold-pressed oil, the presence of phosphorus affects oxidation and contributes to the formation of colloidal aggregates and sediments [
60]. The eventual content of phosphorus in vegetable oil depends on the type of seeds, conditions of their storage, moisture content, and processing method. As follows from numerous publications, seeds of, e.g., rape or soya contain considerably more phosphorus than the oils analysed in this study. As posited by Rotkiewicz and Konopka [
61], the level of this element can range from 132 to 224 mg·kg
−1 depending on the initial moisture content in the seeds. In his research, McCormick [
62] demonstrated that the content of phosphorus in soybean oil can be within the range of 448–1286 mg·kg
−1.
The iodin number indicates the degree of fatty acids’ saturation and the oil’s oxidative stability, which in turn influences the economic viability of such products [
63]. The rancidification of oils poses potential health risks, including an increased risk of cancer and inflammation, due to the formation of toxic and reactive oxidation products [
64]. The iodine number measured for the analysed oil samples was within the range of typical values for commercially available seed oil products—118–123 g I
2·100 g
−1 [
65].
In the fuel industry, it is accepted that the desirable level of FFA and water content as well as the acid number should be below, respectively, 0.06% wt., 0.5% wt., and 1 mg KOH·g
−1 oil [
66]. As posited by Ma and Hanna [
67], the presence of water in raw oil material can reduce the FAME conversion ratio and cause saponification during transesterification reactions. Depending on the particular biological, meteorological, agricultural, and handling factors, the content of fatty acids (FFA) can vary considerably. As observed by Casas-Cardoso et al. [
51], the content of FFA in borage oil can correspond to any value between 2.7 and 10.5% depending on the method of obtaining the oil. One should aim to minimise the presence of free fatty acids in the oil as they reduce the product’s commercial value. The purification of raw borage or milk thistle oil is important not only due to customer preferences but also due to its indisputable impact on the economic viability of production [
23,
68]. As follows from our results, the analysed oil samples were within the commercial ranges. Specifically, the content of free fatty acids in milk thistle and borage oils was, respectively, 5.2 and 1.9%.
Another parameter analysed in our study pertained to a group of compounds described as antioxidants. They increase the durability of fats by preventing rancidification resulting from the oxidation of unsaturated fatty acids. One group of primary natural antioxidants is composed of tocopherols. Due to their high bioactivity, tocopherols and tocotrienols are usually generally described as vitamin E and associated with the effects of dilating blood vessels and inhibiting blood coagulation. The antioxidative properties of oils are inherited from their chemical components, such as polyunsaturated fatty acids, sterols, tocopherols, and polyphenols [
69]. The content of sterols may be influenced by the geographical location of cultivation, differences between cultivars, and the maturity of fruit [
70]. In the analysed milk thistle and borage oils, the content of sterols was measured at 347.52 mg·kg
−1 oil and 261.33 mg·100 g
−1 oil, respectively. The results were similar to those published by other authors [
71], who estimated the general content of sterols in vegetable oils as 338.46–340.37 mg·100 g
−1 oil. In a study on different cultivars of milk thistle, Fathi-Achachlouei and Azadmard-Damirchi [
49] described the level of β-sitosterol in seed oil as ranging from 617.9 to 689.4 mg·100 g
−1 oil and for ∆
7-sterol from 386.5 to 403.0 mg·100 g
−1 oil. In a study on borage oil, Phillips et al. [
72] demonstrated that the general content of sterols was 1098 mg·100 g
−1 oil, while, in palm oil, it was 58 mg·100 g
−1 oil.
Tocopherols are present in oils, in varying quantities, in four primary forms: α, β, γ, δ. Depending on the form, they show varying levels of antioxidative activity. Of all the tocochromanols, α-tocopherol (vit. E) shows 100%, β-tocopherol 50%, and γ-tocopherol 25%. Scientific evidence suggests that the content of tocopherols and phenols varies depending on the process of oil extraction employed [
73,
74]. Khan and Shahidi [
73] observed that borage oil obtained through the process of cold pressing contained over 711 mg·kg
−1 of tocopherol. In the cold-pressed borage seed oil analysed in the present study, the content of total tocopherol was 108 mg·kg
−1, and in the milk thistle seed oil, it was 3541.45 mg·kg
−1. Senanayake and Shahidi [
74] analysed borage oil available on the market and reported tocopherol content at the level of 1976 mg·kg
−1. As for studies on milk thistle oils conducted by other researchers, the products tended to have higher content of α-tocopherol as compared to other types of tocopherols (275.70 mg·kg
−1) [
49]. As follows from the literature data, similar amounts of this ingredient are found in sunflower oil [
8].
The remaining substances include carotenoids and chlorophylls—both responsible for the characteristic colour of oils. The content of carotenoid and chlorophyll pigments in all vegetable oils depends on the type and maturity of the raw material, as well as the technology used for the extraction and refinement of the oil [
75]. Both chlorophylls and carotenoids serve important physiological functions in living plants. Chlorophylls are involved in photosynthesis, while carotenoids protect plants against oxidation. The content of the ingredients may vary depending on the particular type of oil and the method of its production (refined, not refined). As posited by Mahoney et al. [
76], the content of chlorophyll in oils can range from 25 mg·kg
−1 in sesame oil to 3807 mg·kg
−1 in unrefined hemp oil. In refined oils, this ranges from 5mg·kg
−1 in rape oil to 58.5 mg·kg
−1 in soybean oil. As for the content of carotenoids, in unrefined oils, it ranges from 30 mg·kg
−1 in sesame seed oil to 3639 mg·kg
−1 in pumpkin seed oil. In analyses conducted by Rokosik et al. [
77], some oils, including milk thistle oil, showed chlorophyll content higher than measured in the presently tested oil (2.68 mg·kg
−1), namely 4.82 mg·kg
−1. In another study, Rahimi et al. [
78] demonstrated that weather and environmental conditions influenced the content of chlorophylls and carotenoids in borage oil. At 3.96 mg·kg
−1, their result was comparable to our own.
The literature provides very little data on the content of phytosterol oil. However, based on what is available, we know that phytosterols (plant sterols) are secondary components of vegetable oils and constitute a considerable portion of the non-saponifiable substances [
49,
79]. They contribute to reducing cholesterol levels in human blood serum [
75], as well as improving the oxidative and thermal stability and overall durability of vegetable oils [
80]. As evidenced by scientific research, the consumption of 1.5–3 g of plant sterols for a period of 2–3 weeks can lower the plasma levels of cholesterol by 7–12.5% [
81]. Similarly to other reports, the dominant form was β-sitosterol, followed by ∆
7-sterol [
49].
Generally speaking, phospholipids, tocopherols, sterols, hydrocarbons (squalene), carotenoids, chlorophylls, and other compounds acting as pigments are very desirable dietarily [
82]. Squalene, as the main component of polyunsaturated lipids on the skin’s surface, protects skin cells against oxidative damage caused by free radicals, while also providing moisturising and softening effects.
By comparing the FTIR spectra measured for the analysed borage and milk thistle oil samples, we observed slight discrepancies in a number of spectral regions that primarily reflected differences in the two oils’ compositions. We could clearly identify slight intensity changes at 3005 cm−1, which confirmed differences in terms of fatty acid content between the oils. The differences were also visible in the spectral fingerprint region, particularly between 1380 and 1100 cm−1. Below 1100 cm−1, we also observed discrepancies in terms of spectral intensity, although the bands were not particularly well separated and sharp. Nonetheless, the clear change in intensity may suggest, particularly in this spectral region, certain bridge connection differences between individual structural units in fatty acids. In the fingerprint region, the highest impact on the band intensity discrepancies may be attributed to the different content of fatty acids such as C18:1n9c or C18:2n-6, while, in the longer-wavelength region, the same are primarily associated with C18:3n6 and C16:0. The content of other compounds, e.g., chlorophylls, likely had a lesser impact on the discrepancies observed in the infrared spectra.