The Diatom Cylindrotheca closterium and the Chlorophyll Breakdown Product Pheophorbide a for Photodynamic Therapy Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgae Culture
2.2. Chemical Extraction
2.3. Cells
2.4. Antibody
2.5. In Vitro Cell Viability
2.6. RNA Extraction, Retrotranscription and PCR Array
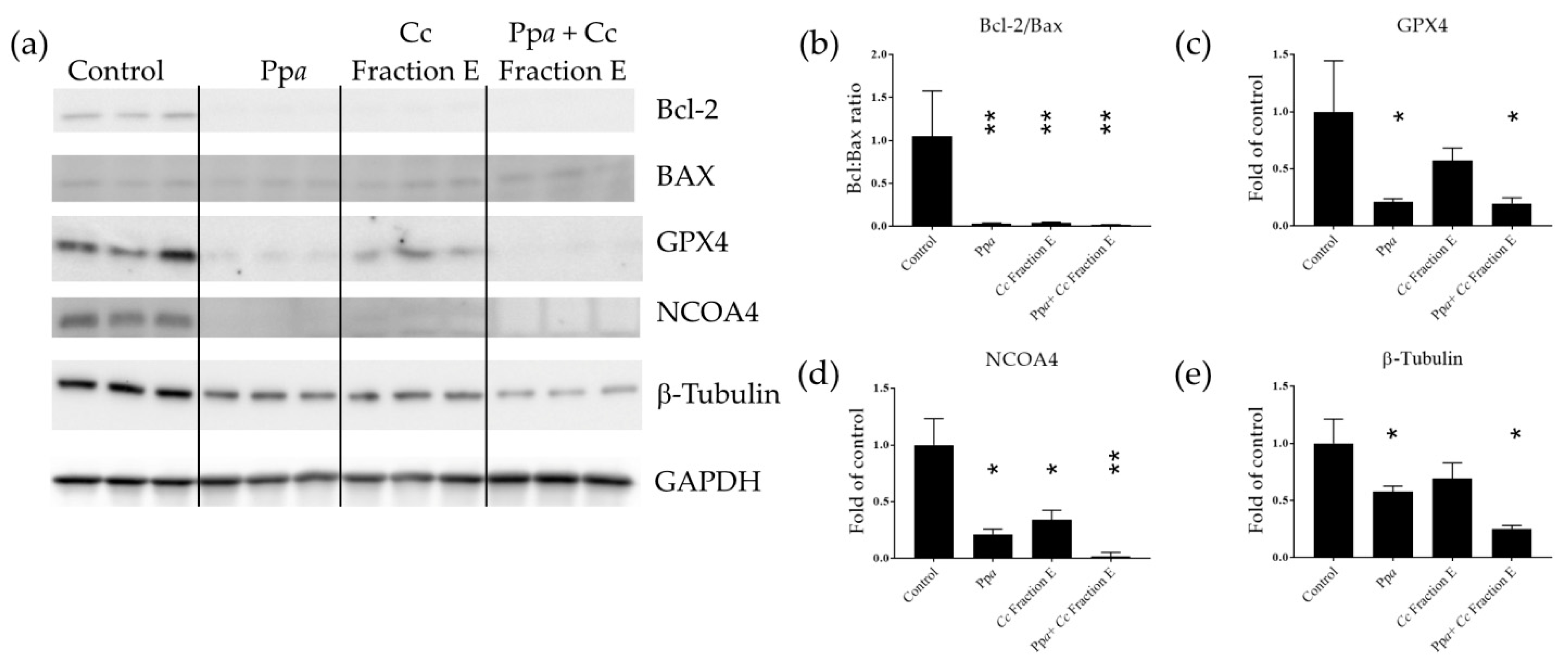
2.7. Protein Extraction and Western Blotting
3. Results
3.1. Anti-Proliferative Activity of Microalgal Extracts
3.2. Pheophorbide a Activity against Melanoma Cells
3.3. Mechanism of Action at Gene and Protein Level
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dobson, J.; de Queiroz, G.F.; Golding, J.P. Photodynamic Therapy and Diagnosis: Principles and Comparative Aspects. Vet. J. 2018, 233, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Wachowska, M.; Muchowicz, A.; Firczuk, M.; Gabrysiak, M.; Winiarska, M.; Wańczyk, M.; Bojarczuk, K.; Golab, J. Aminolevulinic Acid (ALA) as a Prodrug in Photodynamic Therapy of Cancer. Molecules 2011, 16, 4140–4164. [Google Scholar] [CrossRef]
- Saide, A.; Lauritano, C.; Ianora, A. Pheophorbide a: State of the Art. Mar. Drugs 2020, 18, 257. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.M.-K.; Chan, J.Y.-W.; Au, S.W.-N.; Kong, S.-K.; Tsui, S.K.-W.; Waye, M.M.-Y.; Mak, T.C.-W.; Fong, W.-P.; Fung, K.-P. Pheophorbide a, an Active Compound Isolated from Scutellaria Barbata, Possesses Photodynamic Activities by Inducing Apoptosis in Human Hepatocellular Carcinoma. Cancer Biol. Ther. 2006, 5, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.M.-K.; Liu, X.-Z.; Zhang, D.-M.; Fong, W.-P.; Fung, K.-P. Pheophorbide a Based Photodynamic Therapy Induces Apoptosis via Mitochondrial-Mediated Pathway in Human Uterine Carcinosarcoma. Cancer Biol. Ther. 2009, 8, 533–539. [Google Scholar] [CrossRef]
- Bui-Xuan, N.-H.; Tang, P.M.-K.; Wong, C.-K.; Fung, K.-P. Photo-Activated Pheophorbide-a, an Active Component of Scutellaria Barbata, Enhances Apoptosis via the Suppression of ERK-Mediated Autophagy in the Estrogen Receptor-Negative Human Breast Adenocarcinoma Cells MDA-MB-231. J. Ethnopharmacol. 2010, 131, 95–103. [Google Scholar] [CrossRef]
- Hoi, S.W.-H.; Wong, H.M.; Chan, J.Y.-W.; Yue, G.G.L.; Tse, G.M.-K.; Law, B.K.-B.; Fong, W.P.; Fung, K.P. Photodynamic Therapy of Pheophorbide a Inhibits the Proliferation of Human Breast Tumour via Both Caspase-Dependent and -Independent Apoptotic Pathways in In Vitro and In Vivo Models. Phytother. Res. 2012, 26, 734–742. [Google Scholar] [CrossRef]
- Ahn, M.Y.; Yoon, H.-E.; Kwon, S.-M.; Lee, J.; Min, S.-K.; Kim, Y.-C.; Ahn, S.-G.; Yoon, J.-H. Synthesized Pheophorbide A-Mediated Photodynamic Therapy Induced Apoptosis and Autophagy in Human Oral Squamous Carcinoma Cells. J. Oral Pathol. Med. 2013, 42, 17–25. [Google Scholar] [CrossRef]
- Cho, M.; Park, G.-M.; Kim, S.-N.; Amna, T.; Lee, S.; Shin, W.-S. Glioblastoma-Specific Anticancer Activity of Pheophorbide a from the Edible Red Seaweed Grateloupia Elliptica. J. Microbiol. Biotechnol. 2014, 24, 346–353. [Google Scholar] [CrossRef]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef]
- Romano, G.; Costantini, M.; Sansone, C.; Lauritano, C.; Ruocco, N.; Ianora, A. Marine Microorganisms as a Promising and Sustainable Source of Bioactive Molecules. Mar. Environ. Res. 2017, 128, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Saide, A.; Martínez, K.A.; Ianora, A.; Lauritano, C. Unlocking the Health Potential of Microalgae as Sustainable Sources of Bioactive Compounds. Int. J. Mol. Sci. 2021, 22, 4383. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Ianora, A. Marine Organisms with Anti-Diabetes Properties. Mar. Drugs 2016, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- Martínez Andrade, K.; Lauritano, C.; Romano, G.; Ianora, A. Marine Microalgae with Anti-Cancer Properties. Mar. Drugs 2018, 16, 165. [Google Scholar] [CrossRef]
- Martínez, K.A.; Lauritano, C.; Druka, D.; Romano, G.; Grohmann, T.; Jaspars, M.; Martín, J.; Díaz, C.; Cautain, B.; de la Cruz, M.; et al. Amphidinol 22, a New Cytotoxic and Antifungal Amphidinol from the Dinoflagellate Amphidinium Carterae. Mar. Drugs 2019, 17, 385. [Google Scholar] [CrossRef]
- Martínez, K.A.; Saide, A.; Crespo, G.; Martín, J.; Romano, G.; Reyes, F.; Lauritano, C.; Ianora, A. Promising Antiproliferative Compound From the Green Microalga Dunaliella Tertiolecta Against Human Cancer Cells. Front. Mar. Sci. 2022, 9, 778108. [Google Scholar] [CrossRef]
- Riccio, G.; Lauritano, C. Microalgae with Immunomodulatory Activities. Mar. Drugs 2020, 18, 2. [Google Scholar] [CrossRef]
- Giordano, D.; Costantini, M.; Coppola, D.; Lauritano, C.; Núñez Pons, L.; Ruocco, N.; di Prisco, G.; Ianora, A.; Verde, C. Biotechnological Applications of Bioactive Peptides From Marine Sources. In Advances in Microbial Physiology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 73, pp. 171–220. ISBN 978-0-12-815190-7. [Google Scholar]
- Brillatz, T.; Lauritano, C.; Jacmin, M.; Khamma, S.; Marcourt, L.; Righi, D.; Romano, G.; Esposito, F.; Ianora, A.; Queiroz, E.F.; et al. Zebrafish-Based Identification of the Antiseizure Nucleoside Inosine from the Marine Diatom Skeletonema Marinoi. PLoS ONE 2018, 13, e0196195. [Google Scholar] [CrossRef]
- Lauritano, C.; Martín, J.; de la Cruz, M.; Reyes, F.; Romano, G.; Ianora, A. First Identification of Marine Diatoms with Anti-Tuberculosis Activity. Sci. Rep. 2018, 8, 2284. [Google Scholar] [CrossRef]
- Riccio, G.; Ruocco, N.; Mutalipassi, M.; Costantini, M.; Zupo, V.; Coppola, D.; de Pascale, D.; Lauritano, C. Ten-Year Research Update Review: Antiviral Activities from Marine Organisms. Biomolecules 2020, 10, 1007. [Google Scholar] [CrossRef]
- Muller-Feuga, A. The Role of Microalgae in Aquaculture: Situation and Trends. J. Appl. Phycol. 2000, 12, 527–534. [Google Scholar] [CrossRef]
- Hossain, N.; Mahlia, T.M.I.; Saidur, R. Latest Development in Microalgae-Biofuel Production with Nano-Additives. Biotechnol. Biofuels 2019, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Orejuela-Escobar, L.; Gualle, A.; Ochoa-Herrera, V.; Philippidis, G.P. Prospects of Microalgae for Biomaterial Production and Environmental Applications at Biorefineries. Sustainability 2021, 13, 3063. [Google Scholar] [CrossRef]
- Pulz, O.; Gross, W. Valuable Products from Biotechnology of Microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Eryalçın, K.M. Nutritional Value and Production Performance of the Rotifer Brachionus Plicatilis Müller, 1786 Cultured with Different Feeds at Commercial Scale. Aquac. Int. 2019, 27, 875–890. [Google Scholar] [CrossRef]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a Future Food Source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef]
- Gouveia, L.; Coutinho, C.; Mendonça, E.; Batista, A.P.; Sousa, I.; Bandarra, N.M.; Raymundo, A. Functional Biscuits with PUFA-Ω3 from Isochrysis Galbana. J. Sci. Food Agric. 2008, 88, 891–896. [Google Scholar] [CrossRef]
- Fradique, M.; Batista, A.P.; Nunes, C.M.; Gouveia, L.; Bandarra, N.M.; Raymundo, A. Isochrysis Galbana and Diacronema Vlkianum Biomass Incorporation in Pasta Products as PUFA’s Source. LWT—Food Sci. Technol. 2013, 50, 312–319. [Google Scholar] [CrossRef]
- Matos, J.; Afonso, C.; Cardoso, C.; Serralheiro, M.L.; Bandarra, N.M. Yogurt Enriched with Isochrysis Galbana: An Innovative Functional Food. Foods 2021, 10, 1458. [Google Scholar] [CrossRef]
- Lauritano, C.; Helland, K.; Riccio, G.; Andersen, J.H.; Ianora, A.; Hansen, E. Lysophosphatidylcholines and Chlorophyll-Derived Molecules from the Diatom Cylindrotheca Closterium with Anti-Inflammatory Activity. Mar. Drugs 2020, 18, 166. [Google Scholar] [CrossRef]
- Pasquet, V.; Morisset, P.; Ihammouine, S.; Chepied, A.; Aumailley, L.; Berard, J.-B.; Serive, B.; Kaas, R.; Lanneluc, I.; Thiery, V.; et al. Antiproliferative Activity of Violaxanthin Isolated from Bioguided Fractionation of Dunaliella Tertiolecta Extracts. Mar. Drugs 2011, 9, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals: Proceedings—1st Conference on Culture of Marine Invertebrate Animals Greenport; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. ISBN 978-1-4615-8714-9. [Google Scholar]
- Keller, M.D.; Selvin, R.C.; Claus, W.; Guillard, R.R.L. Media for the Culture of Oceanic Ultraphytoplankton1,2. J. Phycol. 1987, 23, 633–638. [Google Scholar] [CrossRef]
- Ribalet, F.; Wichard, T.; Pohnert, G.; Ianora, A.; Miralto, A.; Casotti, R. Age and Nutrient Limitation Enhance Polyunsaturated Aldehyde Production in Marine Diatoms. Phytochemistry 2007, 9, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Cutignano, A.; Nuzzo, G.; Ianora, A.; Luongo, E.; Romano, G.; Gallo, C.; Sansone, C.; Aprea, S.; Mancini, F.; D’Oro, U.; et al. Development and Application of a Novel SPE-Method for Bioassay-Guided Fractionation of Marine Extracts. Mar. Drugs 2015, 13, 5736–5749. [Google Scholar] [CrossRef]
- Riccio, G.; Nuzzo, G.; Zazo, G.; Coppola, D.; Senese, G.; Romano, L.; Costantini, M.; Ruocco, N.; Bertolino, M.; Fontana, A.; et al. Bioactivity Screening of Antarctic Sponges Reveals Anticancer Activity and Potential Cell Death via Ferroptosis by Mycalols. Mar. Drugs 2021, 19, 459. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of Apoptosis in Health and Disease: The Balancing Act of BCL-2 Family Proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Korsmeyer, S.J.; Shutter, J.R.; Veis, D.J.; Merry, D.E.; Oltvai, Z.N. Bcl-2/Bax: A Rheostat That Regulates an Anti-Oxidant Pathway and Cell Death. Semin. Cancer Biol. 1993, 4, 327–332. [Google Scholar]
- Rapozzi, V.; Miculan, M.; Xodo, L. Evidence That Photoactivated Pheophorbide a Causes in Human Cancer Cells a Photodynamic Effect Involving Lipid Peroxidation. Cancer Biol. Ther. 2009, 8, 1318–1327. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.; Huang, Z.; Lin, Z.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, Present and Future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Santana-Codina, N.; Mancias, J.D. The Role of NCOA4-Mediated Ferritinophagy in Health and Disease. Pharmaceuticals 2018, 11, 114. [Google Scholar] [CrossRef]
- Ryu, M.-S.; Zhang, D.; Protchenko, O.; Shakoury-Elizeh, M.; Philpott, C.C. PCBP1 and NCOA4 Regulate Erythroid Iron Storage and Heme Biosynthesis. J. Clin. Investig. 2017, 127, 1786–1797. [Google Scholar] [CrossRef] [PubMed]
- Raisova, M.; Hossini, A.M.; Eberle, J.; Riebeling, C.; Wieder, T.; Sturm, I.; Daniel, P.T.; Orfanos, C.E.; Geilen, C.C. The Bax/Bcl-2 Ratio Determines the Susceptibility of Human Melanoma Cells to CD95/Fas-Mediated Apoptosis. J. Investig. Dermatol. 2001, 117, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Shekoohi, S.; Rajasekaran, S.; Patel, D.; Yang, S.; Liu, W.; Huang, S.; Yu, X.; Witt, S.N. Knocking out Alpha-Synuclein in Melanoma Cells Dysregulates Cellular Iron Metabolism and Suppresses Tumor Growth. Sci. Rep. 2021, 11, 5267. [Google Scholar] [CrossRef] [PubMed]
- Shailaja, V.L.; Christina, V.S.; Mohanapriya, C.D.; Sneha, P.; Lakshmi Sundaram, R.; Magesh, R.; George Priya Doss, C.; Gnanambal, K.M.E. A Natural Anticancer Pigment, Pheophytin a, from a Seagrass Acts as a High Affinity Human Mitochondrial Translocator Protein (TSPO) Ligand, in Silico, to Reduce Mitochondrial Membrane Potential (∆ψmit) in Adenocarcinomic A549 Cells. Phytomedicine 2019, 61, 152858. [Google Scholar] [CrossRef]
- Chen, C.-Y. The Anti-Cancer and Anti-Metastasis Effects of Phytochemical Constituents from Leucaena Leucocephala. Biomed. Res. 2017, 28, 2893–2897. [Google Scholar]
- Donia, M.; Kjeldsen, J.W.; Svane, I.M. The Controversial Role of TNF in Melanoma. OncoImmunology 2016, 5, e1107699. [Google Scholar] [CrossRef]
- Puimège, L.; Libert, C.; Van Hauwermeiren, F. Regulation and Dysregulation of Tumor Necrosis Factor Receptor-1. Cytokine Growth Factor Rev. 2014, 25, 285–300. [Google Scholar] [CrossRef]
- Dika, E.; Patrizi, A.; Lambertini, M.; Manuelpillai, N.; Fiorentino, M.; Altimari, A.; Ferracin, M.; Lauriola, M.; Fabbri, E.; Campione, E.; et al. Estrogen Receptors and Melanoma: A Review. Cells 2019, 8, 1463. [Google Scholar] [CrossRef]
- Weinberg, A.; Jin, G.; Sieg, S.; McCormick, T. The Yin and Yang of Human Beta-Defensins in Health and Disease. Front. Immunol. 2012, 3, 294. [Google Scholar] [CrossRef]
- O’Neil, D.A.; Porter, E.M.; Elewaut, D.; Anderson, G.M.; Eckmann, L.; Ganz, T.; Kagnoff, M.F. Expression and Regulation of the Human β-Defensins HBD-1 and HBD-2 in Intestinal Epithelium. J. Immunol. 1999, 163, 6718–6724. [Google Scholar] [CrossRef]
- Scola, N.; Gambichler, T.; Saklaoui, H.; Bechara, F.G.; Georgas, D.; Stücker, M.; Gläser, R.; Kreuter, A. The Expression of Antimicrobial Peptides Is Significantly Altered in Cutaneous Squamous Cell Carcinoma and Precursor Lesions. Br. J. Dermatol. 2012, 167, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Donald, C.D.; Sun, C.Q.; Lim, S.D.; Macoska, J.; Cohen, C.; Amin, M.B.; Young, A.N.; Ganz, T.A.; Marshall, F.F.; Petros, J.A. Cancer-Specific Loss of β-Defensin 1 in Renal and Prostatic Carcinomas. Lab. Investig. 2003, 83, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Young, A.N.; Amin, M.B.; Moreno, C.S.; Lim, S.D.; Cohen, C.; Petros, J.A.; Marshall, F.F.; Neish, A.S. Expression Profiling of Renal Epithelial Neoplasms: A Method for Tumor Classification and Discovery of Diagnostic Molecular Markers. Am. J. Pathol. 2001, 158, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Yoo, B.K.; Kim, H.-S.; Gilmore, H.L.; Lee, Y.; Lee, H.; Kim, S.-J.; Letterio, J.; Lee, H. Amyloid-β Precursor Protein Promotes Cell Proliferation and Motility of Advanced Breast Cancer. BMC Cancer 2014, 14, 928. [Google Scholar] [CrossRef] [PubMed]
- Hamura, R.; Shirai, Y.; Shimada, Y.; Saito, N.; Taniai, T.; Horiuchi, T.; Takada, N.; Kanegae, Y.; Ikegami, T.; Ohashi, T.; et al. Suppression of Lysosomal Acid Alpha-Glucosidase Impacts the Modulation of Transcription Factor EB Translocation in Pancreatic Cancer. Cancer Sci. 2021, 112, 2335–2348. [Google Scholar] [CrossRef] [PubMed]
- Schmit, K.; Michiels, C. TMEM Proteins in Cancer: A Review. Front. Pharmacol. 2018, 9, 1345. [Google Scholar] [CrossRef]
- Matarrese, P.; Ascione, B.; Ciarlo, L.; Vona, R.; Leonetti, C.; Scarsella, M.; Mileo, A.M.; Catricalà, C.; Paggi, M.G.; Malorni, W. Cathepsin B Inhibition Interferes with Metastatic Potential of Human Melanoma: An in Vitro and in Vivo Study. Mol. Cancer 2010, 9, 207. [Google Scholar] [CrossRef]
- McDowell, S.H.; Gallaher, S.A.; Burden, R.E.; Scott, C.J. Leading the Invasion: The Role of Cathepsin S in the Tumour Microenvironment. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2020, 1867, 118781. [Google Scholar] [CrossRef]
- McGrogan, B.T.; Gilmartin, B.; Carney, D.N.; McCann, A. Taxanes, Microtubules and Chemoresistant Breast Cancer. Biochim. Biophys. Acta BBA-Rev. Cancer 2008, 1785, 96–132. [Google Scholar] [CrossRef]
- Prüser, T.F.; Braun, P.G.; Wiacek, C. Microalgae as a Novel Food. Ernahrungs Umsch. 2021, 68, 78–85. [Google Scholar] [CrossRef]

| Species | Abb | Class | Growth Media | Sampling Location | Concentration at the End of the Stationary Phase |
|---|---|---|---|---|---|
| Asterionellopsis glacialis (RCC1712) | Ag | Fragilariophyceae | F/2 | English Channel | 105 |
| Chlamydomonas sp. (CCMP2536) | CspA | Chlorophyceae | F/2-Si | Mediterranean Sea (Almeria harbor) | 106 |
| Chlamydomonas sp. (CCMP225) | CspB | Chlorophyceae | F/2-Si | Nantucket Sound | 106 |
| Cylindrotheca closterium | Cc | Bacillariophyceae | F/2 | Mediterranean Sea (Adriatic Sea) | 4 × 105 |
| Dunaliella tertiolecta (CCMP1320) | Dt | Chlorophyceae | F/2-Si | Unknown | 2 × 106 |
| Ditylum brightwellii | Db | Mediophyceae | F/2 | Mediterranean Sea | 105 |
| Trieres mobiliensis | Om | Coscinodiscophyceae | F/2 | Mediterranean Sea | 105 |
| Pseudo-nitzchia arenysensis | Pa | Bacillariophyceae | F/2 | Mediterranean Sea | 3 × 105 |
| Pseudo-nitzchia fraudulenta | Pf | Bacillariophyceae | F/2 | Mediterranean Sea | 3 × 105 |
| Rhinomonas reticulata (CCAP 995/2) | Rr | Cryptophyceae | F/2 | Mediterranean Sea | 105 |
| Scrippsiellaacuminata | St | Dinophyceae | K | Mediterranean Sea | 104 |
| UniGEne | RefSeq | Symbol | Description | Fold | SD |
|---|---|---|---|---|---|
| Genes Up-Regulated by Pheophorbide a Treatment plus Photo-Activation | |||||
| Hs.632469 | NM_020387 | RAB25 | RAB25, member RAS oncogene family | 3.84 | 0.00082 |
| Hs.241570 | NM_000594 | TNF | Tumor necrosis factor | 3.06 | 0.00033 |
| Hs.744830 | NM_000125 | ESR1 | Estrogen receptor 1 | 2.44 | 0.00021 |
| Hs.643440 | NM_002361 | MAG | Myelin associated glycoprotein | 2.38 | 0.00017 |
| Hs.32949 | NM_005218 | DEFB1 | Defensin, beta 1 | 2.20 | 0.00019 |
| Hs.2007 | NM_000639 | FASLG | Fas ligand (TNF superfamily, member 6) | 2.02 | 0.00019 |
| Genes down-regulated by Pheophorbide a treatment plus photo-activation | |||||
| Hs.434980 | NM_000484 | APP | Amyloid beta (A4) precursor protein | –3.48 | 0.10040 |
| Hs.189782 | NM_018202 | TMEM57 | Transmembrane protein 57 | –2.80 | 0.00419 |
| Hs.1437 | NM_000152 | GAA | Glucosidase, alpha; acid | –2.47 | 0.00531 |
| Hs.269027 | NM_014568 | GALNT5 | UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 5 (GalNAc-T5) | –2.41 | 0.00010 |
| Hs.171844 | NM_006505 | PVR | Poliovirus receptor | –2.07 | 0.02680 |
| Genes up-regulated by Fraction E plus photo-activation | |||||
| Hs.744830 | NM_000125 | ESR1 | Estrogen receptor 1 | 5.59 | 0.00049 |
| Hs.472860 | NM_001250 | CD40 | CD40 molecule, TNF receptor superfamily member 5 | 4.17 | 0.00028 |
| Hs.241570 | NM_000594 | TNF | Tumor necrosis factor | 3.75 | 0.00040 |
| Hs.592244 | NM_000074 | CD40LG | CD40 ligand | 2.30 | 0.00019 |
| Hs.32949 | NM_005218 | DEFB1 | Defensin, beta 1 | 2.30 | 0.00020 |
| Hs.87236 | NM_012188 | FOXI 1 | Forkhead box I1 | 2.30 | 0.00019 |
| Hs.856 | NM_000619 | IFNG | Interferon, gamma | 2.30 | 0.00021 |
| Hs.700350 | NM_000207 | INS | Insulin | 2.30 | 0.00020 |
| Hs.519680 | NM_001145805 | IRGM | Immunity-related GTPase family, M | 2.30 | 0.00090 |
| Hs.592068 | NM_020655 | JPH3 | Junctophilin 3 | 2.30 | 0.00022 |
| Hs.553833 | NM_001004467 | OR10J3 | Olfactory receptor, family 10, subfamily J, member 3 | 2.30 | 0.00020 |
| Hs.442337 | NM_176823 | S100A7A | S100 calcium-binding protein A7A | 2.30 | 0.00020 |
| Hs.632469 | NM_020387 | RAB25 | RAB25, member RAS oncogene family | 2.14 | 0.00046 |
| Hs.2007 | NM_000639 | FASLG | Fas ligand (TNF superfamily, member 6) | 2.09 | 0.00020 |
| Genes down-regulated by Fraction E plus photo-activation | |||||
| Hs.591834 | NM_003844 | TNFRSF10A | Tumor necrosis factor receptor superfamily, member 10a | –4.35 | 0.00046 |
| Hs.434980 | NM_000484 | APP | Amyloid beta (A4) precursor protein | –2.73 | 0.13269 |
| Hs.1437 | NM_000152 | GAA | Glucosidase, alpha; acid | –2.59 | 0.00507 |
| Hs.189782 | NM_018202 | TMEM57 | Transmembrane protein 57 | –2.19 | 0.00537 |
| Hs.643120 | NM_000875 | IGF1R | Insulin-like growth factor 1 receptor | –2.15 | 0.02531 |
| Genes up-regulated by Pheophorbide a and Fraction E treatment plus photo-activation | |||||
| Hs.241570 | NM_000594 | TNF | Tumor necrosis factor | 3.75 | 0.00040 |
| Hs.744830 | NM_000125 | ESR1 | Estrogen receptor 1 | 3.67 | 0.00032 |
| Hs.592068 | NM_020655 | JPH3 | Junctophilin 3 | 2.55 | 0.00022 |
| Hs.592244 | NM_000074 | CD40LG | CD40 ligand | 2.19 | 0.00024 |
| Hs.32949 | NM_005218 | DEFB1 | Defensin, beta 1 | 2.19 | 0.00019 |
| Hs.87236 | NM_012188 | FOXI 1 | Forkhead box I1 | 2.19 | 0.00019 |
| Hs.856 | NM_000619 | IFNG | Interferon, gamma | 2.19 | 0.00132 |
| Hs.700350 | NM_000207 | INS | Insulin | 2.19 | 0.00019 |
| Hs.519680 | NM_001145805 | IRGM | Immunity-related GTPase family, M | 2.19 | 0.00021 |
| Hs.553833 | NM_001004467 | OR10J3 | Olfactory receptor, family 10, subfamily J, member 3 | 2.19 | 0.00009 |
| Hs.442337 | NM_176823 | S100A7A | S100 calcium binding protein A7A | 2.19 | 0.000019 |
| Genes down-regulated by Pheophorbide a and Fraction E treatment plus photo-activation | |||||
| Hs.434980 | NM_000484 | APP | Amyloid beta (A4) precursor protein | –9.80 | 0.03694 |
| Hs.1437 | NM_000152 | GAA | Glucosidase, alpha; acid | –4.91 | 0.00267 |
| Hs.269027 | NM_014568 | GALNT5 | UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 5 (GalNAc-T5) | –4.39 | 0.00055 |
| Hs.189782 | NM_018202 | TMEM57 | Transmembrane protein 57 | –4.24 | 0.00277 |
| Hs.591834 | NM_003844 | TNFRSF10A | Tumor necrosis factor receptor superfamily, member 10a | –3.72 | 0.00042 |
| Hs.171844 | NM_006505 | PVR | Poliovirus receptor | –3.70 | 0.01497 |
| Hs.643120 | NM_000875 | IGFR1 | Insulin-like growth factor 1 receptor | –2.58 | 0.02103 |
| Hs.713833 | NM_001065 | TNFRSF1A | Tumor necrosis factor receptor superfamily, member 1A | –2.54 | 0.01441 |
| Hs.181301 | NM_004079 | CTSS | Cathepsin S | –2.46 | 0.00208 |
| Hs.520898 | NM_001908 | CTSB | Cathepsin B | –2.17 | 0.01346 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saide, A.; Riccio, G.; Ianora, A.; Lauritano, C. The Diatom Cylindrotheca closterium and the Chlorophyll Breakdown Product Pheophorbide a for Photodynamic Therapy Applications. Appl. Sci. 2023, 13, 2590. https://doi.org/10.3390/app13042590
Saide A, Riccio G, Ianora A, Lauritano C. The Diatom Cylindrotheca closterium and the Chlorophyll Breakdown Product Pheophorbide a for Photodynamic Therapy Applications. Applied Sciences. 2023; 13(4):2590. https://doi.org/10.3390/app13042590
Chicago/Turabian StyleSaide, Assunta, Gennaro Riccio, Adrianna Ianora, and Chiara Lauritano. 2023. "The Diatom Cylindrotheca closterium and the Chlorophyll Breakdown Product Pheophorbide a for Photodynamic Therapy Applications" Applied Sciences 13, no. 4: 2590. https://doi.org/10.3390/app13042590
APA StyleSaide, A., Riccio, G., Ianora, A., & Lauritano, C. (2023). The Diatom Cylindrotheca closterium and the Chlorophyll Breakdown Product Pheophorbide a for Photodynamic Therapy Applications. Applied Sciences, 13(4), 2590. https://doi.org/10.3390/app13042590









