Analysis of Unexpected Leaks in AISI 316L Stainless Steel Pipes Used for Water Conduction in a Port Area
Abstract
:1. Introduction
1.1. General Background
1.2. Case Background
2. Experimental Approach
- -
- Visual analysis of inner and outer faces of the sample, in order to find any other evidence of damage that is not detected yet.
- -
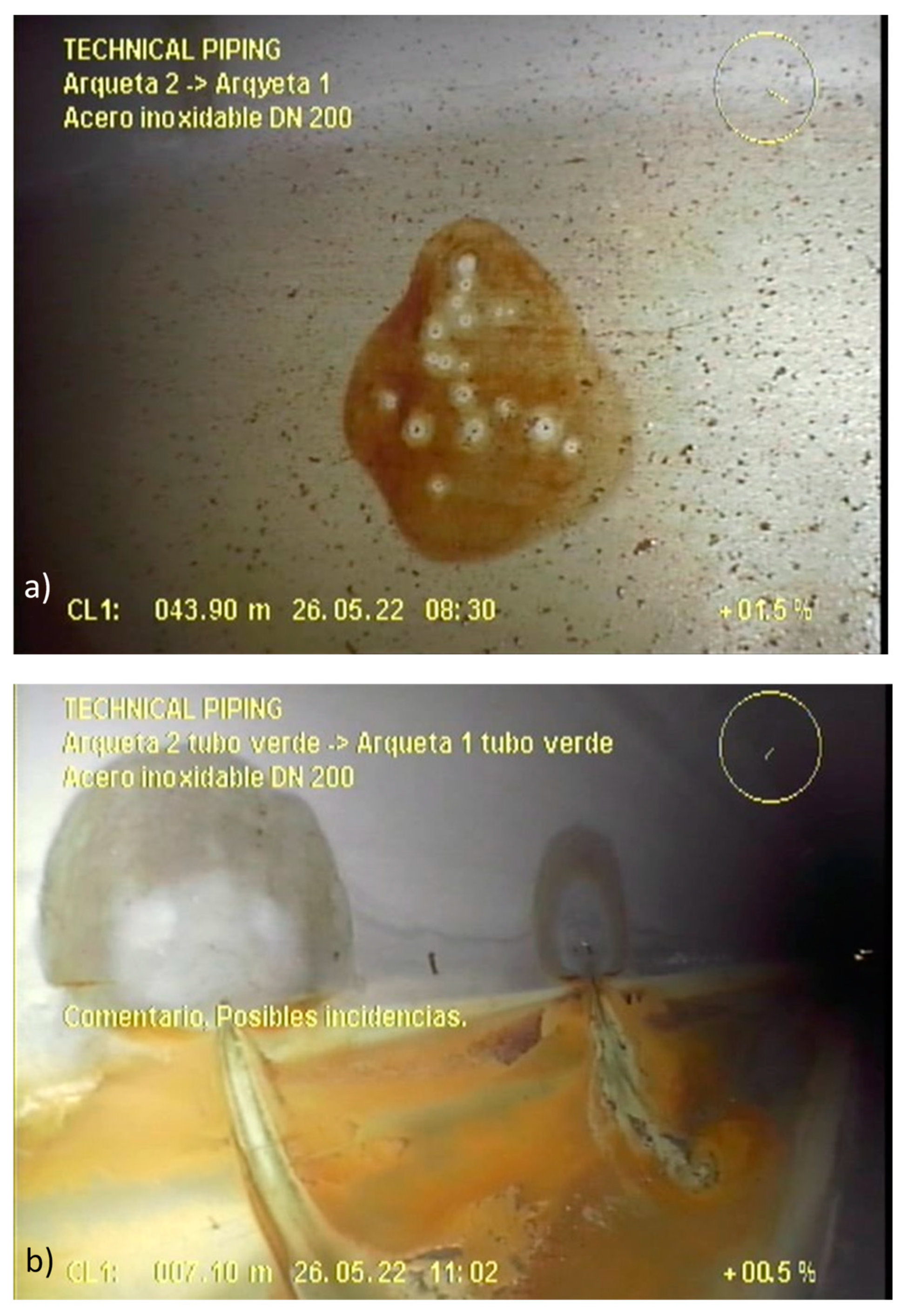
- Visual analysis of the video-inspection performed with a robotic submersible camera inside the pipes. There were more than two hours of records, and the most interesting and singular frames were extracted as images and will be shown here.
- -
- Analysis of the chemical composition of pipe material. This was carried out by spark emission spectroscopy, which allows for the determination of the content of alloying elements with high accuracy.
- -
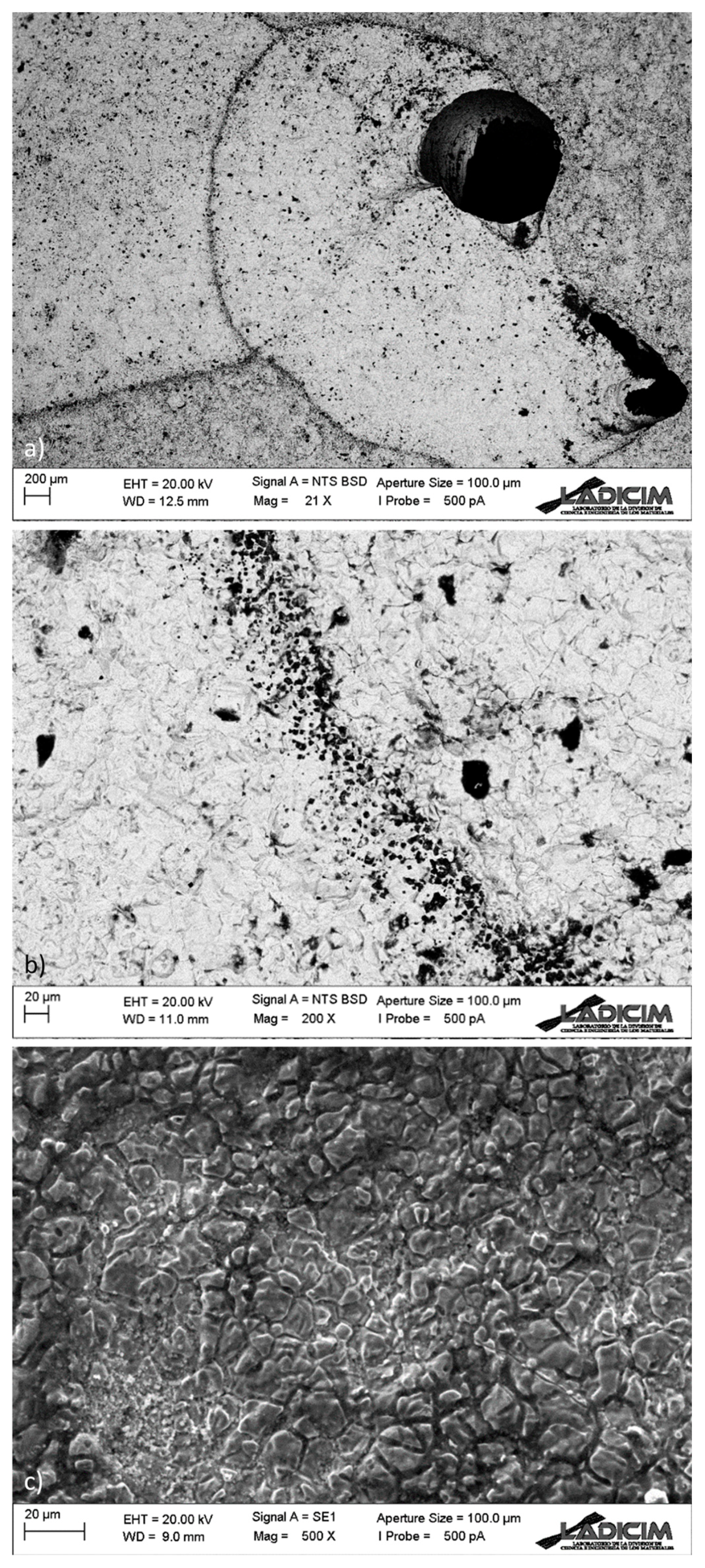
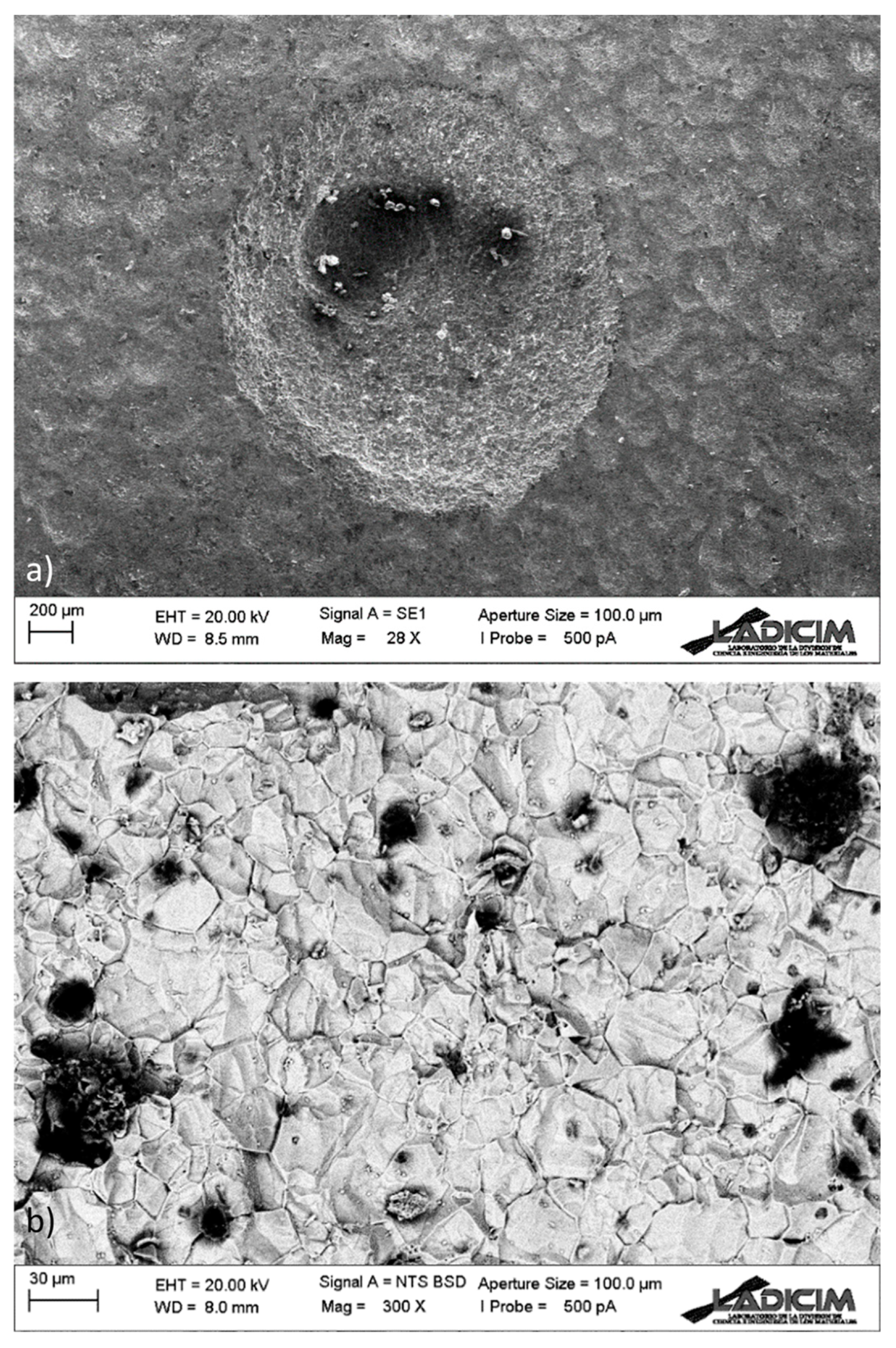
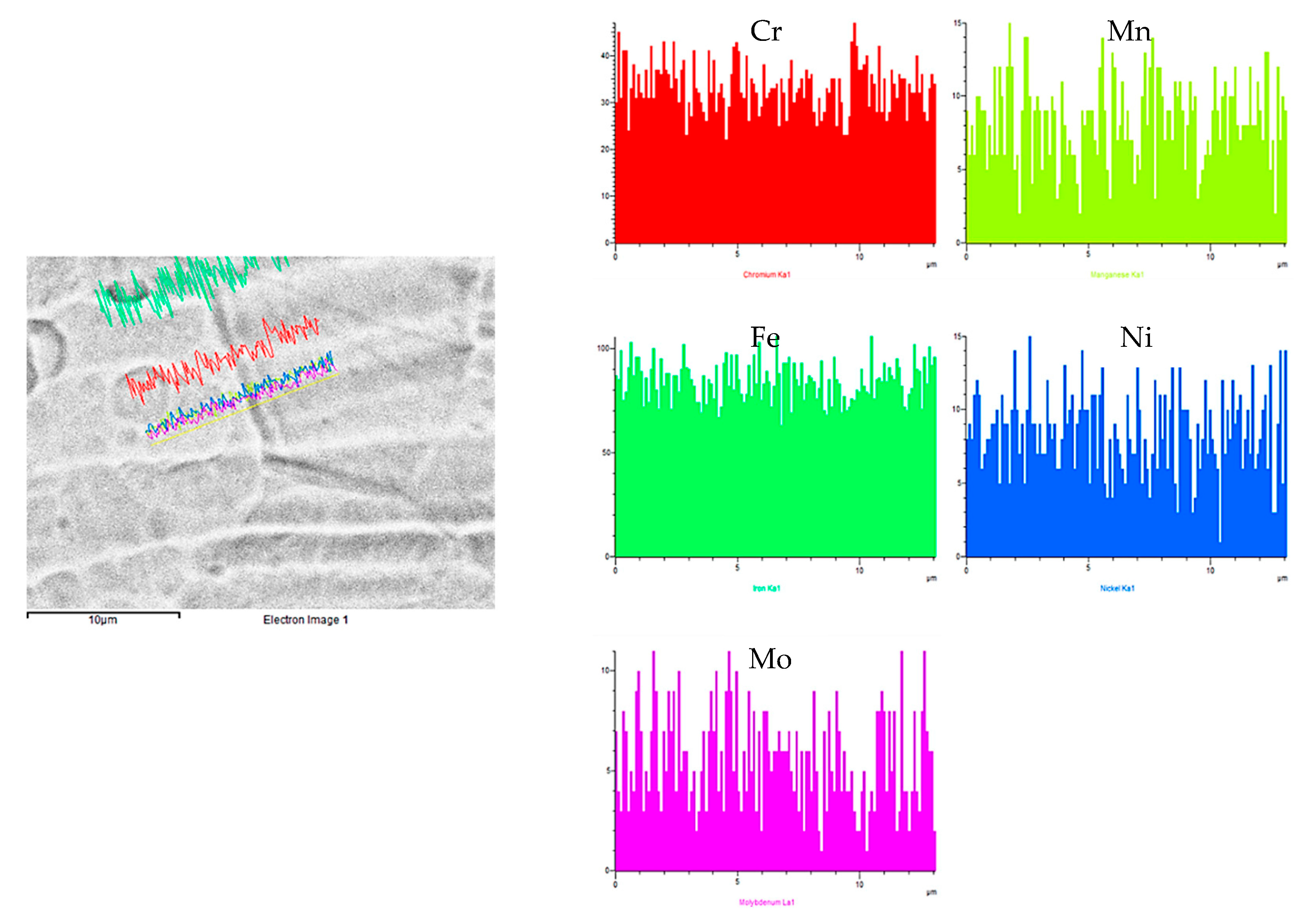
- Analysis of the pipe pitting defects by scanning electron microscopy (SEM). The micromechanisms of the pits were analyzed, as well as the semi-quantitative chemical composition of the material surrounding the pits and other singular points of interest, such as grain boundaries or corrosion products.
- -
- Bacterial culture of the water remaining inside the piping system in an area with an abundance of corrosion products. The sample was analyzed by culturing the mixture of microorganisms present in Kliguer medium and inoculating in a flute beak at 25 °C for one week.
3. Results and Discussion
3.1. Visual Inspection of the Sample
3.2. Video-Inspection from the Inside of the Piping System
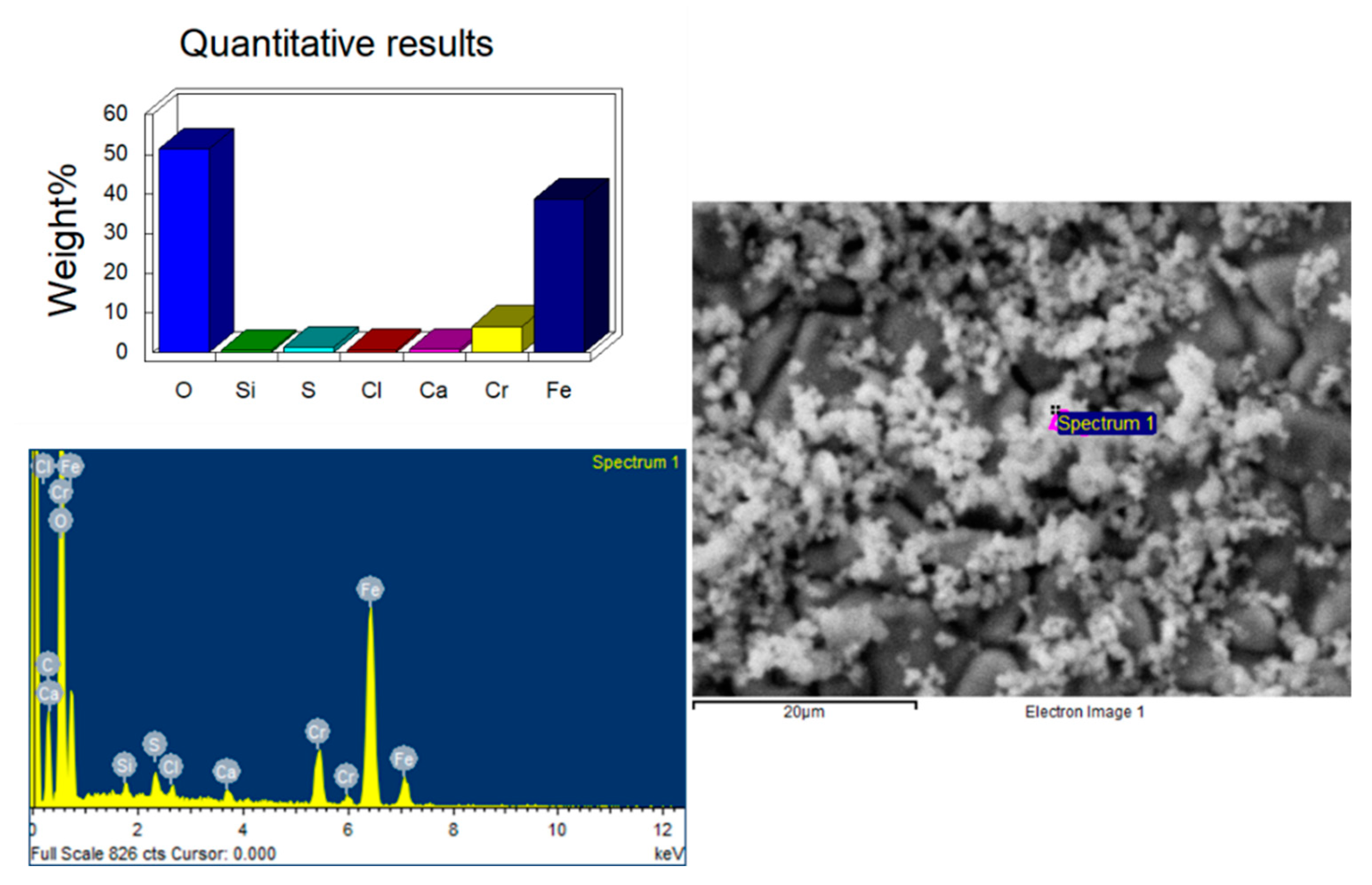
3.3. Chemical Composition of the Pipes
3.4. SEM Analysis of the Pitting Defects
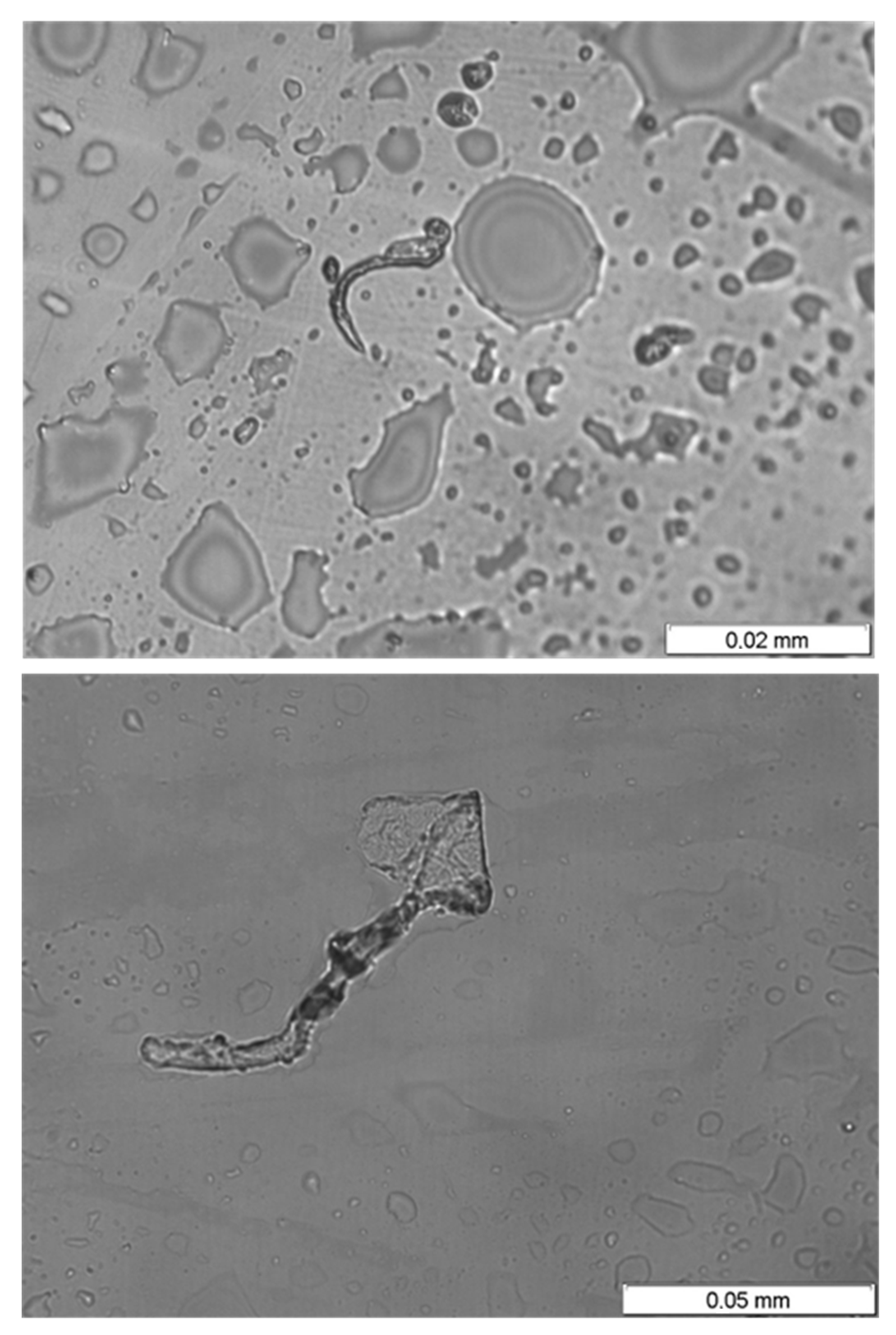
3.5. Bacterial Culture of the Water Found Inside the Piping System
3.6. Discussion
- -
- the type of corrosion defects observed, which are mainly pit-type defects,
- -
- the presence of gas pockets detected in some of the pits,
- -
- the development of the process in a short time, before the piping system was put into service, and about five months after its construction,
- -
- the lack of water circulation in this period, as well as the evidence of the existence of stagnant water,
- -
- the presence of sulfur in the corrosion deposits, as revealed by the SEM semi-quantitative analysis,
- -
- the presence of sulfate-reducing bacteria (SRB), as revealed by the bacterial culture, and,
- -
- the absence of other obvious signs of aggressiveness of the environment or defects in the material.
4. Conclusions
- The robotic video-inspection confirmed that generalized pitting corrosion with thorough-wall pitting is present in the whole facility to a higher or lower degree. Pockets of gas associated with some of the pits can be observed, while in others “mushroom-shaped” marks show the previous presence of gas bags.
- The chemical composition of the material fits the requirements of AISI 316L grade stainless steel, so material deficiencies are not the cause of the corrosion processes.
- There is also evidence (level marks of corrosion products) of the presence of stagnant water (corrosion products characterized by a reddish hue under the level mark). The culture of the remains on the stagnant water samples from the facility has been positive in terms of the presence of sulfate-reducing bacteria (SRB).
- During the SEM analysis, in the more developed pits, a preferentially radial direction was not observed, as would occur in the case of pitting by chlorides, discarding this type of process; the pits follow an intricate path from the inner face to the outer one. Additionally, it revealed no evidence of sensitization in the steel (roughly constant Cr content in grains and boundaries), but the presence of sulfur was detected in non-negligible quantities in the corrosion products.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, Y.T.; Dong, P.F.; Jiang, L.; Xiao, K.; Dong, C.F.; Wu, J.S. Corrosion behavior of riveted joints of TC4 Ti-Alloy and 316L Stainless Steel in simulated marine atmosphere. J. Chin. Soc. Corros. Prot. 2020, 40, 167–174. [Google Scholar]
- Bazgir, M.; Rahmani, K. Anodic protection of 316L stainless steel piping in sulfuric acid service: Failure causes and remedial actions. Corros. Rev. 2021, 39, 453–464. [Google Scholar] [CrossRef]
- Wigen, S.M.; Osvoll, H.; Gartland, P.O.; Huang, W. Efficient cathodic protection of stainless steel small bore tubing. In Proceedings of the CORROSION 2007, Nashville, TN, USA, 11–15 March 2007; pp. 070781–0707814. [Google Scholar]
- Kajiyama, F.; Okamura, K. Evaluating cathodic protection reliability on steel pipe in microbially active soils. Corrosion 1999, 55, 74–80. [Google Scholar] [CrossRef]
- Tseluikin, V.N. Electrodeposition and properties of composite coatings modified by fullerene C60. Prot. Met. Phys. Chem. Surf. 2017, 53, 433–436. [Google Scholar] [CrossRef]
- Tseluikin, V.N.; Koreshkova, A.A. Pulsed electrodeposition of composite coatings based on zinc–nickel alloy. Prot. Met. Phys. Chem. Surf. 2018, 54, 453–456. [Google Scholar] [CrossRef]
- Lanzutti, A.; Lekka, M.; de Leitenburg, C.; Fedrizzi, L. Effect of pulse current on wear behaviour of Ni matrix micro- and nano-SiC composite coatings at room and elevated temperature. Tribol. Int. 2019, 132, 50–61. [Google Scholar] [CrossRef]
- Stoltenhoff, T.; Kreye, H.; Richter, H.J. An analysis of the cold spray process and its coatings. J. Therm. Spray Technol. 2002, 11, 542–550. [Google Scholar] [CrossRef]
- Chiodo, M.S.G.; Ruggieri, C. Failure assessments of corroded pipelines with axial defects using stress-based criteria: Numerical studies and verification analyses. Int. J. Press. Vessel. Pip. 2009, 86, 164–176. [Google Scholar] [CrossRef]
- Greenshields, C.J.; Leevers, P.S.; Morris, J. Brittle fracture of plastic water pipes. Pipes Pipelines Int. 1997, 42, 11–16. [Google Scholar]
- Schmitt, C.; Pluvinage, G.; Hadj-Taieb, E.; Akid, R. Water pipeline failure due to water hammer effects. Fatigue Fract. Eng. Mater. Struct. 2006, 29, 1075–1082. [Google Scholar] [CrossRef]
- Gonzalez, M.; Machado, R.; Gonzalez, J. Fatigue analysis of PE-100 pipe under axial loading. Am. Soc. Mech. Eng. Press. Vessel. Pip. Div. (Publ.) PVP 2011, 6, 905–911. [Google Scholar]
- Guedes, R.M.; Sá, A.; Faria, H. On the prediction of long-term creep-failure of GRP pipes in aqueous environment. Polym. Compos. 2010, 31, 1047–1055. [Google Scholar] [CrossRef]
- Sadiq, R.; Rajani, B.; Kleiner, Y. Probabilistic risk analysis of corrosion associated failures in cast iron water mains. Reliab. Eng. Syst. Saf. 2004, 86, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Abouswa, K.; Elshawesh, F.; Elragei, O.; Elhood, A. Corrosion investigation of Cu-Ni tube desalination plant. Desalination 2007, 205, 140–146. [Google Scholar] [CrossRef]
- Xia, Y.; Shi, M.; Zhang, C.; Wang, C.; Sang, X.; Liu, R.; Zhao, P.; An, G.; Fang, H. Analysis of flexural failure mechanism of ultraviolet cured-in-place-pipe materials for buried pipelines rehabilitation based on curing temperature monitoring. Eng. Fail. Anal. 2022, 142, 106763. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.T.; Song, L.J.; Shi, Q.F.; Li, Y. Effects of pH on electrochemical properties of 316L Stainless Steel. Corros. Prot. 2013, 34, 593–596. [Google Scholar]
- Wang, J.; Shang, X.C.; Lu, M.X.; Zhang, L. Pitting nucleation of 316L Stainless Steel in different environments. J. Mater. Eng. 2015, 43, 12–18. [Google Scholar]
- Liu, B.; Duan, J.Z.; Hou, B.R. Study on corrosion behavior of 316L Stainless Steel by microbial film in seawater. J. Chin. Soc. Corros. Prot. 2012, 32, 48–53. [Google Scholar]
- Khandouzi, M.K.; Bahrami, A.; Hosseini-Abari, A.; Khandouzi, M.; Taheri, P. Microbiologically Influenced Corrosion of a pipeline in a petrochemical plant. Metals 2019, 9, 459. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Sun, M.H.; Lu, F.Y.; Du, C.W.; Li, X.G. Study of biofilm-influenced corrosion on X80 pipeline steel by a nitrate-reducing bacterium, bacillus cereus, in artificial Beijing soil. Colloids Surf. B Biointerfaces 2021, 197, 111356. [Google Scholar] [CrossRef]
- A276/A276M-17; Standard Specification for Stainless Steel Bars and Shapes. ASTM International: West Conshohocken, PA, USA, 2021.
- Procópio, L. The era of ‘omics’ technologies in the study of microbiologically influenced corrosion. Biotechnol Lett. 2020, 42, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Ilhan-Sungur, E.; Cansever, N. Case History 02.15.25.001. In Corrosion Atlas; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Hussain, M.; Zhang, T. Case History 02.21.25.002. In Corrosion Atlas; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Van der Kolk, F. Micro-Organisms Destroyed Stainless Steel Installation, Maintworld, Vols. 3–4. Available online: https://www.maintworld.com/Applications/Micro-organisms-Destroyed-Stainless-Steel-Installation (accessed on 20 January 2023).
- Ress, J.; Monrrabal, G.; Díaz, A.; Pérez-Pérez, J.; Bastidas, J.M.; Bastidas, D.M. Microbiologically influenced corrosion of welded AISI 304 stainless steel pipe in well water. Eng. Fail. Anal. 2020, 116, 104734. [Google Scholar] [CrossRef]
- Miller, B.A.; Shipley, R.J.; Parrington, R.J.; Dennies, D.P. (Eds.) ASM Handbook, Volume 11: Failure Analysis and Prevention; ASM International: Materials Park, OH, USA, 2002. [Google Scholar]
- Stoecker, J.G. A Practical Manual on Microbiologically Influenced Corrosion; NACE International, The Corrosion Society: Houston, TX, USA, 2001. [Google Scholar]
- Otero, E.; Bastidas, J.M.; Lopez, V. Analysis of a premature failure of welded AlSl316L stainless steel pipes originated by microbial induced corrosion. Mater. Corros. 1997, 48, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Wang, Z.; Zhang, Z.; Zhang, B.; Ma, G.; Yao, Q.; Gan, Z.; Wu, J.; Li, X. Influence of crevice width on sulfate-reducing bacteria (SRB)-induced corrosion of stainless steel 316L. Corros. Commun. 2021, 4, 33–44. [Google Scholar] [CrossRef]
- Liu, H.; Sharma, M.; Wang, J.; Cheng, Y.F.; Liu, H. Microbiologically influenced corrosion of 316L stainless steel in the presence of Chlorella vulgaris. Int. Biodeterior. Biodegrad. 2018, 129, 209–216. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Cheng, G.; Zhu, W. Localized corrosion behavior of 316L stainless steel in the presence of sulfate-reducing and iron-oxidizing bacteria. Mater. Sci. Eng. A 2007, 443, 235–241. [Google Scholar] [CrossRef]
- Dao, V.H.; Ryu, H.K.; Yoon, K.B. Leak failure at the TP316L welds of a water pipe caused by microbiologically influenced corrosion. Eng. Fail. Anal. 2021, 122, 105244. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, B.; Zhao, J.; Yang, B.; Zheng, X. Benzothiazole derivatives-based supramolecular assemblies as efficient corrosion inhibitors for copper in artificial seawater: Formation, interfacial release and protective mechanisms. Corros. Sci. 2023, 212, 110957. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, B.; Xu, B.; Yang, B. Ambient-stable polyethyleneimine functionalized Ti3C2Tx nanohybrid corrosion inhibitor for copper in alkaline electrolyte. Mater. Lett. 2023, 337, 133979. [Google Scholar] [CrossRef]
- Praveen, B.M.; Alhadhrami, A.; Prasanna, B.M.; Hebbar, N.; Prabhu, R. Anti-corrosion behavior of olmesartan for soft-cast steel in 1 mol dm−3 HCl. Coatings 2021, 11, 965. [Google Scholar] [CrossRef]
- Hebbar, N.; Praveen, B.M.; Prasanna, B.M.; Vishwanath, P. Electrochemical and adsorption studies of 4-Chloro, 8-(Trifluoromethyl) Quinoline (CTQ) for mild steel in acidic medium. J. Fail. Anal. Prev. 2020, 20, 1516–1523. [Google Scholar] [CrossRef]






| Test | Required [22] | |
|---|---|---|
| C | 0.022 ± 0.005 | <0.03 |
| Si | 0.450 ± 0.072 | <1.00 |
| Mn | 1.507 ± 0.080 | <2.00 |
| P | 0.035 ± 0.005 | <0.045 |
| S | <0.008 | <0.03 |
| Cr | 16.22 ± 0.48 | 16.0–18.0 |
| Ni | 9.86 ± 0.37 | 10.0–14.0 |
| N | 0.037 ± 0.009 | -- |
| Mo | 1.944 ± 0.091 | 2.0–3.0 |
| Cu | 0.319 ± 0.028 | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arroyo, B.; Lacalle, R.; Álvarez, J.A.; Cicero, S.; Moreno-Ventas, X. Analysis of Unexpected Leaks in AISI 316L Stainless Steel Pipes Used for Water Conduction in a Port Area. Appl. Sci. 2023, 13, 2598. https://doi.org/10.3390/app13042598
Arroyo B, Lacalle R, Álvarez JA, Cicero S, Moreno-Ventas X. Analysis of Unexpected Leaks in AISI 316L Stainless Steel Pipes Used for Water Conduction in a Port Area. Applied Sciences. 2023; 13(4):2598. https://doi.org/10.3390/app13042598
Chicago/Turabian StyleArroyo, Borja, Roberto Lacalle, José A. Álvarez, Sergio Cicero, and Xabier Moreno-Ventas. 2023. "Analysis of Unexpected Leaks in AISI 316L Stainless Steel Pipes Used for Water Conduction in a Port Area" Applied Sciences 13, no. 4: 2598. https://doi.org/10.3390/app13042598





