Experimental Investigation on Single-Phase Immersion Cooling of a Lithium-Ion Pouch-Type Battery under Various Operating Conditions
Abstract
:1. Introduction
2. Experimental Setup and Procedure
- Before each experiment, the battery is charged using an electronic power supply at 3.65 V under constant current and constant voltage (CC/CV) mode.
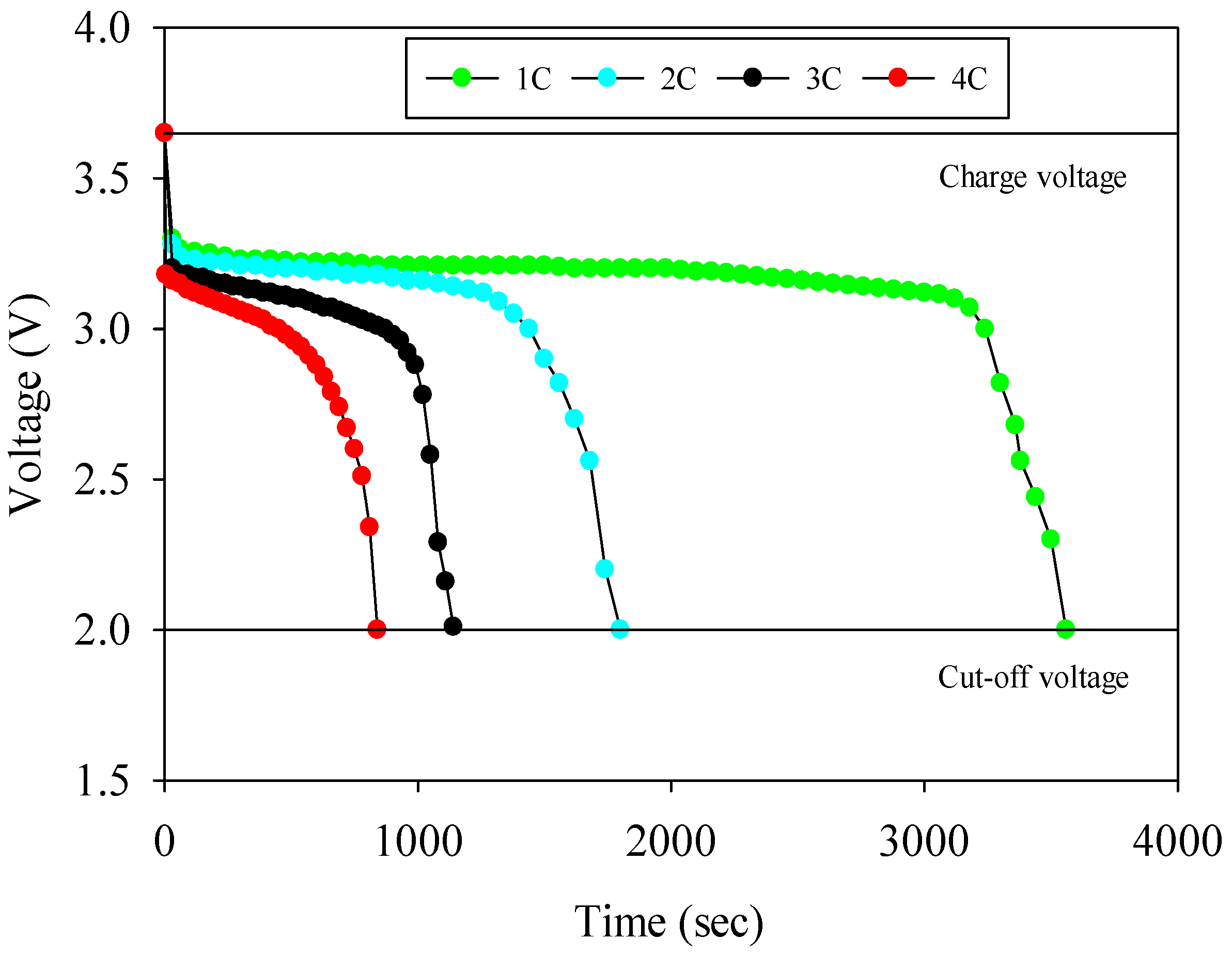
- The experiments are started by discharging the battery at a desired charge (1C, 2C, 3C and 4C) rate by using an electronic load; the voltage and current values of the battery are recorded during the experiment.
- Then, the cooling water is circulated by means of a frequency-controlled water circulation pump, and its temperature is measured with a data acquisition system at the inlet of the test section.
- After the test section, its temperature is measured again with the data acquisition system, and it is directed to the water bath. The cooling water, which is conditioned by using a water bath, is directed to a flowmeter for flow rate measurement.
- During the experiments, the temperature measurements are recorded by means of a data acquisition system every 10 s.
- The arithmetic mean and the highest and the lowest values of the thermocouple measurements are determined as the average, maximum and minimum temperatures, respectively.
- Each experiment ends when the voltage is 2 V which is called cut-off voltage. It is known that the lower values of this voltage lead to capacity reduction problems.
3. Results and Discussion
4. Conclusions
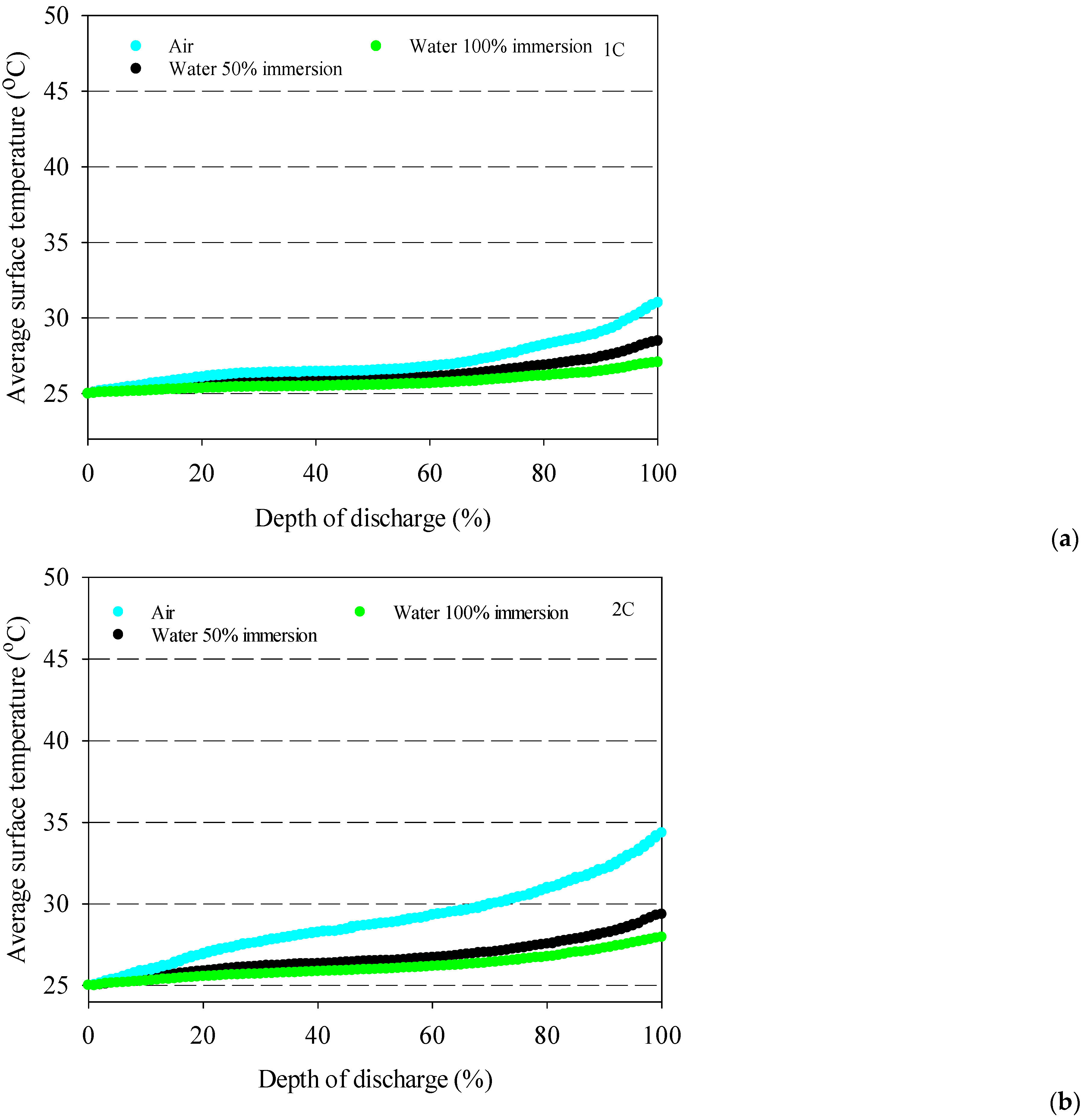
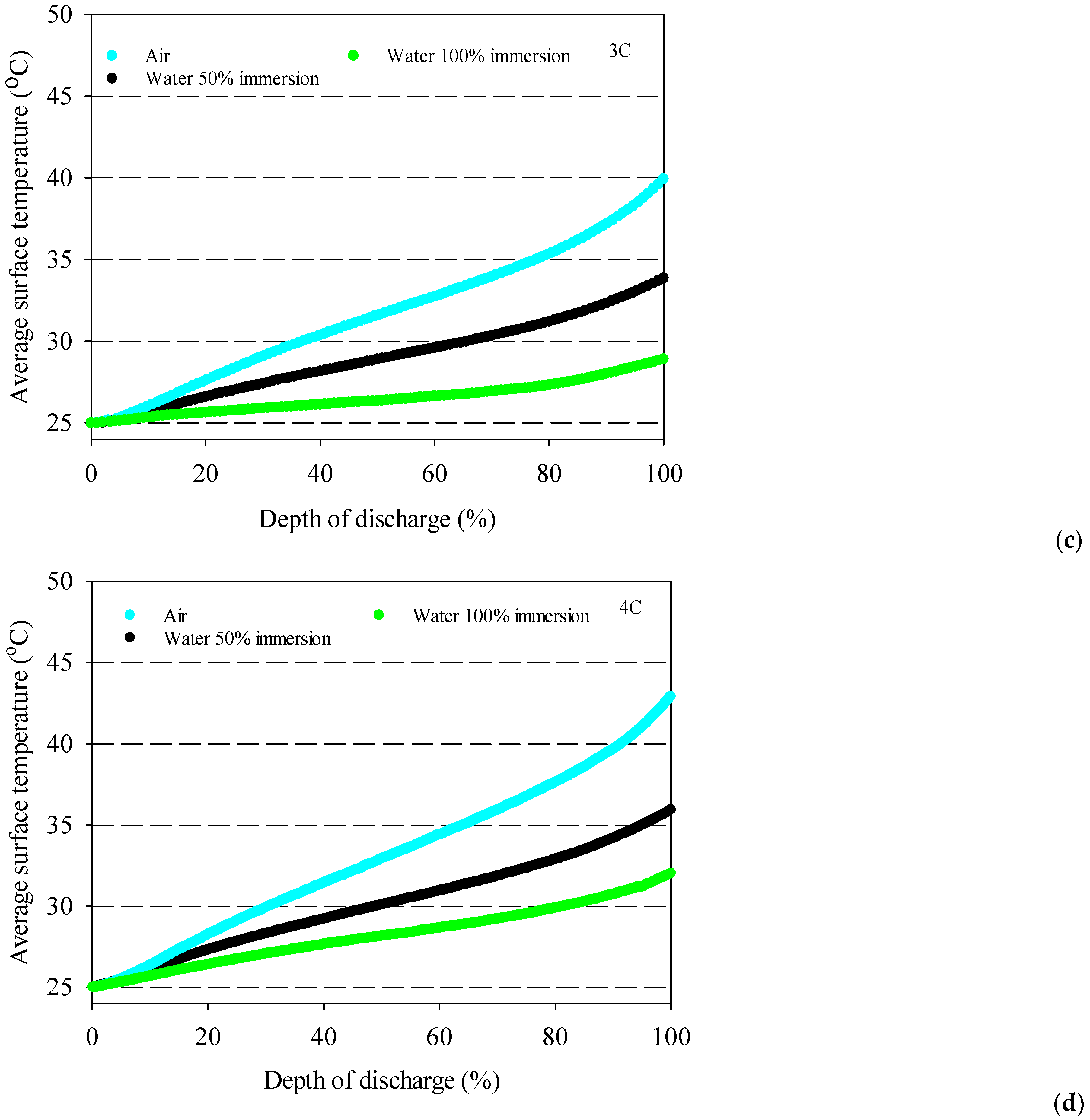
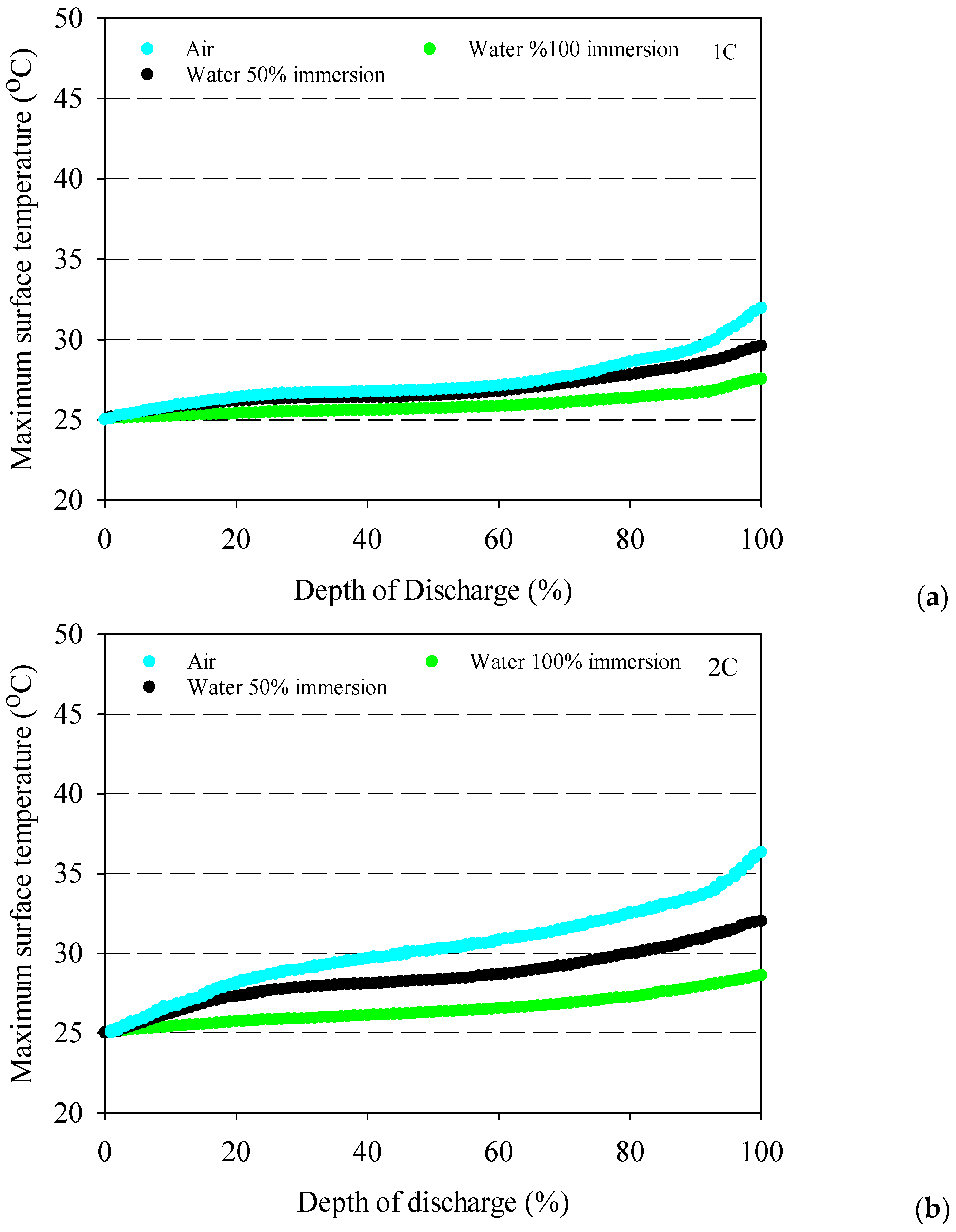
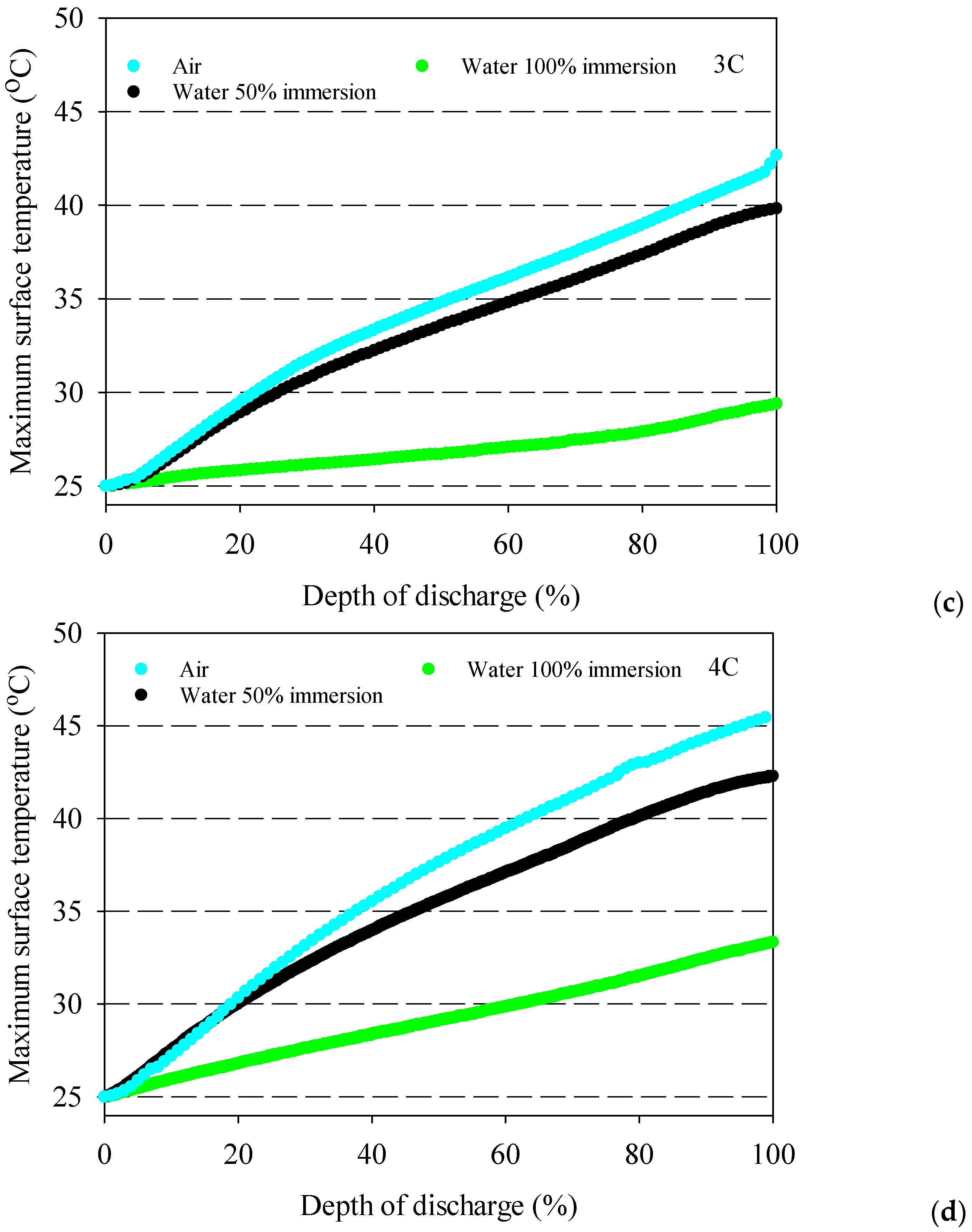
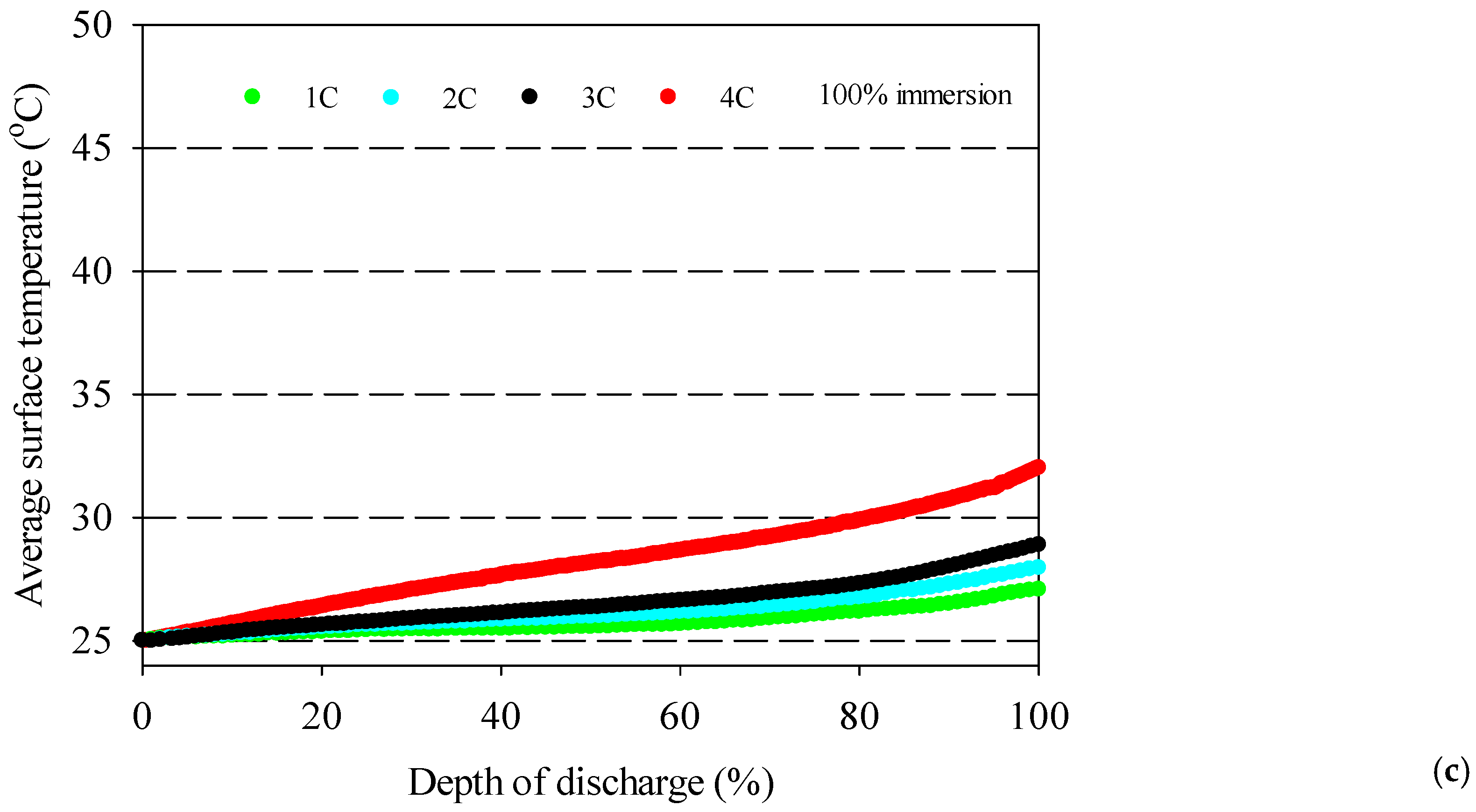
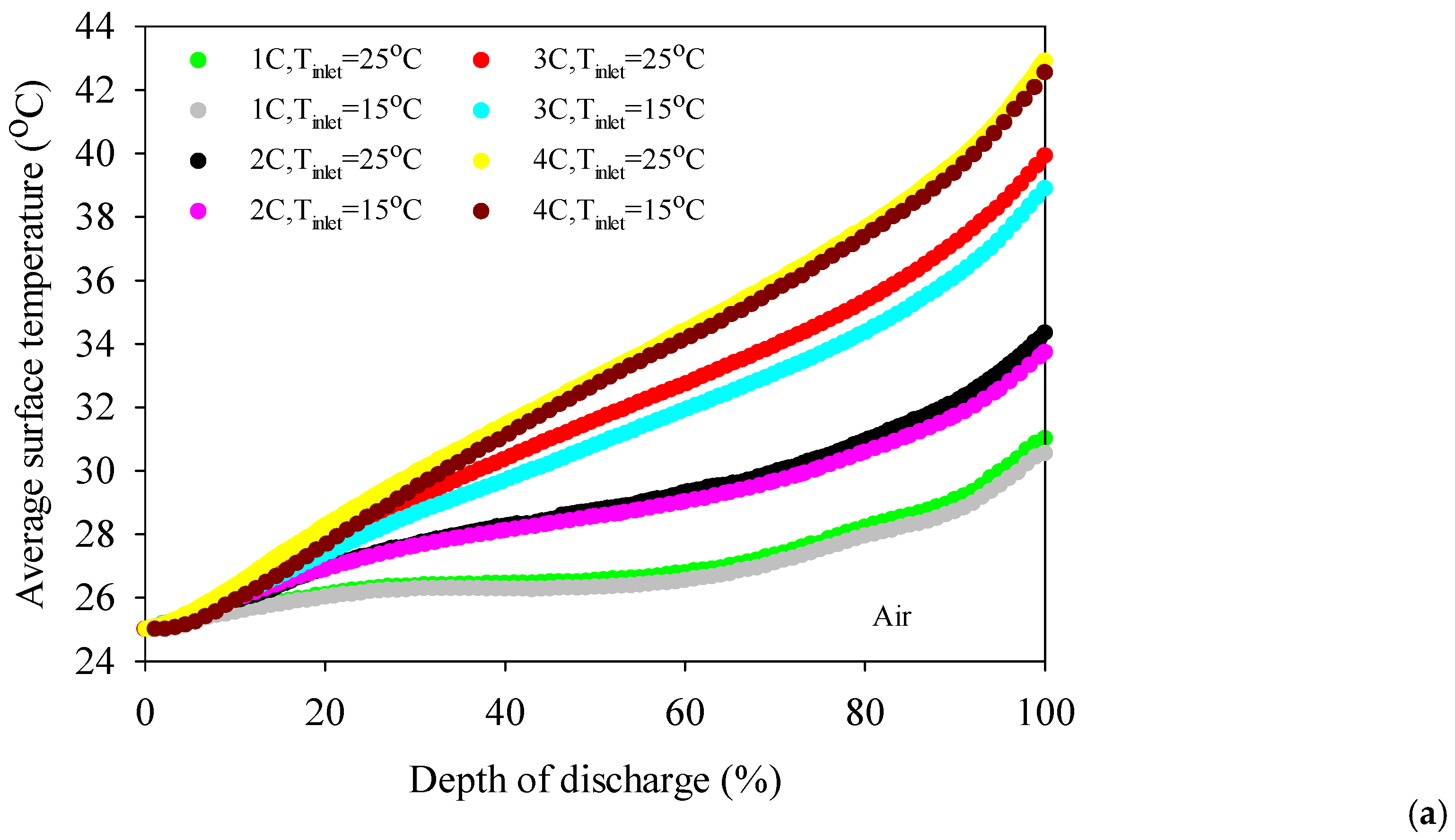
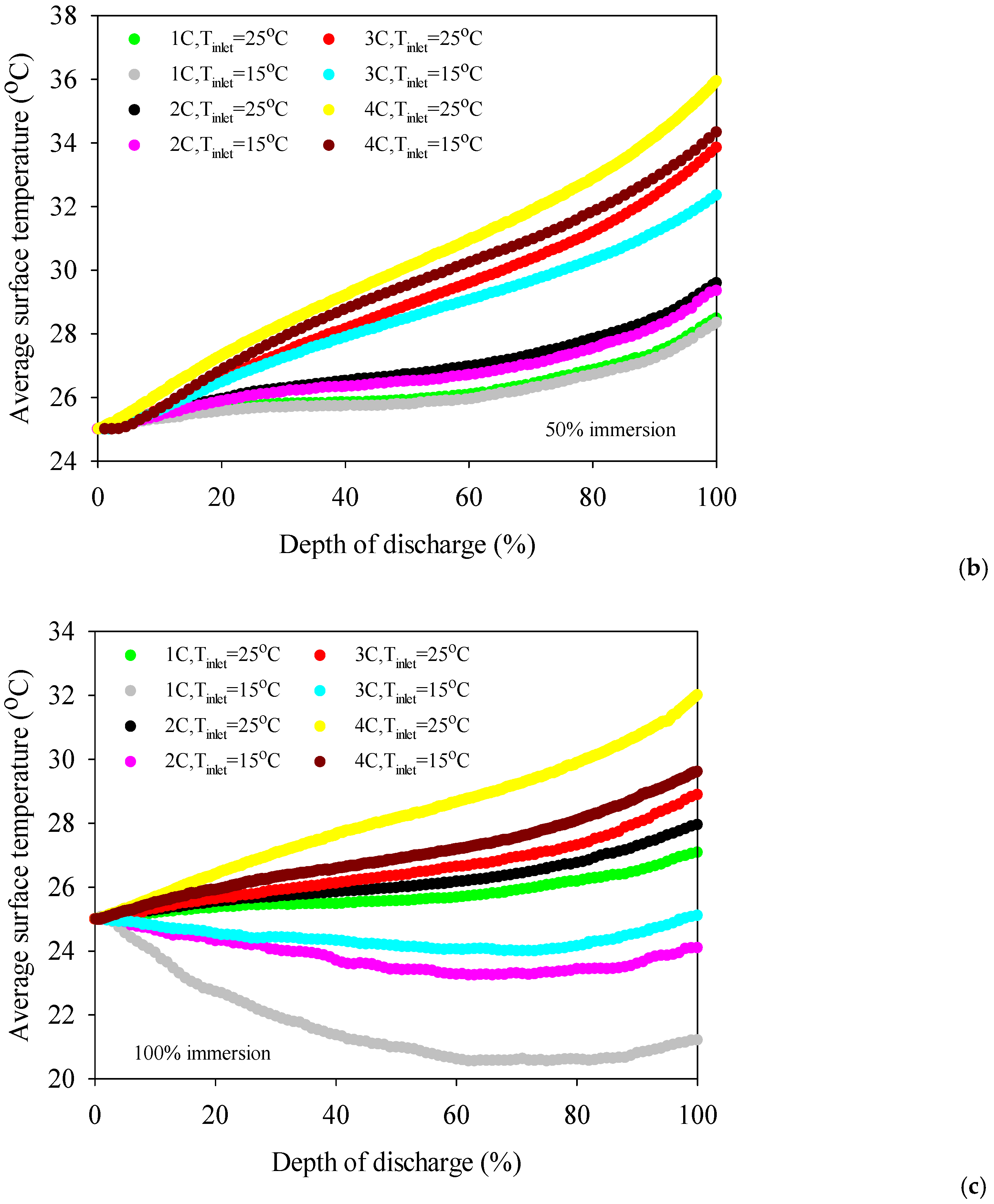
- The battery surface temperatures increase with an increment of discharge rates for both air and distilled water cooling conditions because of the exothermic chemical reaction occurring in the battery.
- The highest average battery surface temperature is observed for air cooling experiments due to the low specific heat capacity of air. This situation is valid for all discharge rates, and the highest increment average battery surface temperature is obtained at 10.9 °C for air at the 4C discharge rate compared to 100% water immersion.
- 100% immersion cooling has a significant impact on battery surface temperature since it can reduce average battery temperatures by up to 28% for tested working conditions.
- The lowest battery average surface temperature is recorded with the application of 100% distilled water immersion because of direct contact with fluid, and it increases by approximately 5 °C for different discharge rates in the range between 1–4C.
- The 50% immersion ratio has no significant effect on minimum battery temperature compared to 100%one for all discharge rates.
- The coolant inlet temperature has no significant impact on battery surface temperature except 100% immersion.
- The maximum temperature differences can be significantly reduced with the application of immersion cooling.
- The experimental results revealed that immersion cooling could be a good solution for battery thermal management systems, and their performance can be improved by using dielectric fluid having higher specific heat capacity and thermal conductivity.
Funding
Data Availability Statement
Conflicts of Interest
References
- International Energy Agency. Electric Cars Fend Off Supply Challenges to More Than Double Global Sales. Available online: https://www.iea.org/commentaries/electric-cars-fend-off-supply-challenges-to-more-than-double-global-sales (accessed on 28 December 2022).
- Dinçer, I.; Hamut, H.S.; Javani, N. Thermal Management of Electric Vehicle Battery Systems, 1st ed.; John Wiley & Sons: West Sussex, UK, 2016; pp. 6–7. [Google Scholar]
- Kim, J.; Oh, J.; Lee, H. Review on battery thermal management system for electric vehicles. Appl. Therm. Eng. 2019, 149, 192–212. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, X.; Negnevitsky, M.; Zhang, H. A Review of air-cooling battery thermal management systems for electric and hybrid electric vehicles. J. Power Sources 2021, 501, 230001. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, B.; Li, B.; Yan, Y. A critical review of thermal management models and solutions of lithium-ion batteries for the development of pure electric vehicles. Renew. Sustain. Energy Rev. 2016, 64, 106–128. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, X.; Ji, J.; Xu, X.; Zhang, Y. Research progress on power battery cooling technology for electric vehicles. J. Energy Storage 2020, 27, 101155. [Google Scholar] [CrossRef]
- Ouyang, D.; Weng, J.; Chen, M.; Wang, J. Impact of high-temperature environment on the optimal cycle rate of lithium-ion battery. J. Energy Storage 2020, 28, 101242. [Google Scholar] [CrossRef]
- Xu, B.; Lee, J.; Kwon, D.; Kong, L.; Pecht, M. Mitigation strategies for li-ion battery thermal runaway: A review. Renew. Sustain. Energy Rev. 2021, 150, 111437. [Google Scholar] [CrossRef]
- Situ, W.; Yang, X.; Li, X.; Zhang, G.; Rao, M.; Wei, C.; Huang, Z. Effect of high temperature environment on the performance of Lini0.5Co0.2Mn0.3O2 Battery. Int. J. Heat Mass Transf. 2017, 104, 743–748. [Google Scholar] [CrossRef]
- Widyantara, R.D.; Zulaikah, S.; Juangsa, F.B.; Budiman, B.A.; Aziz, M. Review on battery packing design strategies for superior thermal management in electric vehicles. Batteries 2022, 8, 287. [Google Scholar] [CrossRef]
- Hu, Y.; Choe, S.Y.; Garrick, T.R. Measurement of heat generation rate and heat sources of pouch type Li-ion cells. Appl. Therm. Eng. 2021, 189, 116709. [Google Scholar] [CrossRef]
- Hu, Y.; Choe, S.Y.; Garrick, T.R. Measurement of two-dimensional heat generation rate of pouch type lithium-ion battery using a multifunctional calorimeter. J. Power Sources 2022, 532, 231350. [Google Scholar] [CrossRef]
- Lin, C.; Xu, S.; Liu, J. Measurement of heat generation in a 40 Ah LiFePO4 prismatic battery using accelerating rate calorimetry. Int. J. Hydrogen Energy 2018, 43, 8375–8384. [Google Scholar] [CrossRef]
- Sheng, L.; Su, L.; Zhang, H.; Fang, Y.; Xu, H.; Ye, W. An improved calorimetric method for characterizations of the specific heat and the heat generation rate in a prismatic lithium ion battery cell. Energy Convers. Manag. 2019, 180, 724–732. [Google Scholar] [CrossRef]
- Yin, Y.; Zheng, Z.; Choe, S.Y. Design of a calorimeter for measurement of heat generation rate of lithium ion battery using thermoelectric device. SAE Int. J. Altern. Powertrains 2017, 6, 252–260. [Google Scholar] [CrossRef]
- Hu, X.; Liu, W.; Lin, X.; Xie, Y.; Foley, A.M.; Hu, L. A control-oriented electrothermal model for pouch-type electric vehicle batteries. IEEE Trans. Power Electron. 2021, 36, 5530–5544. [Google Scholar] [CrossRef]
- Yazdanpour, M.; Taheri, P.; Mansouri, A.; Bahrami, M. A distributed analytical electro-thermal model for pouch-type lithium-ion batteries. J. Electrochem. Soc. 2014, 161, A1953. [Google Scholar] [CrossRef]
- Rao, Z.; Wang, S. A Review of power battery thermal energy management. Renew. Sustain. Energy Rev. 2011, 15, 4554–4571. [Google Scholar] [CrossRef]
- Akinlabi, A.H.; Solyali, D. Configuration, design, and optimization of air-cooled battery thermal management system for electric vehicles: A review. Renew. Sustain. Energy Rev. 2020, 125, 109815. [Google Scholar] [CrossRef]
- Wu, S.; Lao, L.; Wu, L.; Liu, L.; Lin, C.; Zhang, Q. Effect analysis on integration efficiency and safety performance of a battery thermal management system based on direct contact liquid cooling. Appl. Therm. Eng. 2022, 201, 117788. [Google Scholar] [CrossRef]
- Roe, C.; Feng, X.; White, G.; Li, R.; Wang, H.; Rui, X.; Li, C.; Zhang, F.; Null, V.; Parkes, M.; et al. Immersion cooling for lithium-ion batteries—A review. J. Power Sources 2022, 525, 231094. [Google Scholar] [CrossRef]
- Pambudi, N.A.; Sarifudin, A.; Firdaus, R.A.; Ulfa, D.K.; Gandidi, I.M.; Romadhon, R. The immersion cooling technology: Current and future development in energy saving. Alex. Eng. J. 2022, 61, 9509–9527. [Google Scholar] [CrossRef]
- Zhou, G.; Zhou, J.; Huai, X.; Zhou, F.; Jiang, Y. A two-phase liquid immersion cooling strategy utilizing vapor chamber heat spreader for data center servers. Appl. Therm. Eng. 2022, 210, 118289. [Google Scholar] [CrossRef]
- Kanbur, B.B.; Wu, C.; Fan, S.; Duan, F. System-level experimental investigations of the direct immersion cooling data center units with thermodynamic and thermoeconomic assessments. Energy 2021, 217, 119373. [Google Scholar] [CrossRef]
- Liu, C.; Yu, H. Evaluation and optimization of a two-phase liquid-immersion cooling system for data centers. Energies 2021, 14, 1395. [Google Scholar] [CrossRef]
- Yuan, X.; Zhou, X.; Pan, Y.; Kosonen, R.; Cai, H.; Gao, Y.; Wang, Y. Phase change cooling in data centers: A review. Energy Build. 2021, 236, 110764. [Google Scholar] [CrossRef]
- Barnes, C.M.; Tum, P.E. Practical considerations relating to immersion cooling of power electronics in traction systems. IEEE Trans. Power Electron. 2010, 25, 2478–2485. [Google Scholar] [CrossRef]
- Birbarah, P.; Gebrael, T.; Foulkes, T.; Stillwell, A.; Moore, A.; Pilawa-Podgurski, R.; Miljkovic, N. Water immersion cooling of high power density electronics. Int. J. Heat Mass Transf. 2020, 147, 118918. [Google Scholar] [CrossRef]
- Tuma, P.E. Design considerations relating to non-thermal aspects of passive 2-phase immersion cooling. In Proceedings of the 27th Annual IEEE Semiconductor Thermal Measurement and Management Symposium, San Jose, CA, USA, 20–24 March 2011; pp. 1–9. [Google Scholar] [CrossRef]
- An, X.; Arora, M.; Huang, W.; Brantley, W.C.; Greathous, J.L. 3D Numerical Analysis of Two-Phase Immersion Cooling for Electronic Components. In Proceedings of the 17th IEEE Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems (ITherm), San Diego, CA, USA, 29 May–1 June 2018; pp. 609–614. [Google Scholar] [CrossRef]
- Van Gils, R.W.; Danilov, D.; Notten, P.H.L.; Speetjens, M.F.M.; Nijmeijer, H. Battery thermal management by boiling heat-transfer. Energy Convers. Manag. 2014, 79, 9–17. [Google Scholar] [CrossRef]
- Wang, Y.F.; Wu, J.T. Thermal performance predictions for an HFE-7000 direct flow boiling cooled battery thermal man-agement system for electric vehicles. Energy Convers. Manag. 2020, 207, 112569. [Google Scholar] [CrossRef]
- Dubey, P.; Pulugundla, G.; Srouji, A.K. Direct comparison of immersion and cold-plate based cooling for automotive Li-ion battery modules. Energies 2021, 14, 1259. [Google Scholar] [CrossRef]
- Luo, M.; Cao, J.; Liu, N.; Zhang, Z.; Fang, X. Experimental and simulative investigations on a water immersion cooling system for cylindrical battery cells. Front. Energy Res. 2022, 10, 803882. [Google Scholar] [CrossRef]
- Jithin, K.V.; Rajesh, P.K. Numerical analysis of single-phase liquid immersion cooling for lithium-ion battery thermal management using different dielectric fluids. Int. J. Heat Mass Transf. 2022, 188, 122608. [Google Scholar] [CrossRef]
- Trimbake, A.; Singh, C.P.; Krishnan, S. Mineral oil immersion cooling of lithium-ion batteries: An experimental investigation. J. Electrochem. Energy Convers. Storage 2022, 19, 021007. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Hu, L.; Bai, M.; Gao, L.; Li, Y.; Liu, X.; Li, Y.; Song, Y. Experimental studies of liquid immersion cooling for 18650 lithium-ion battery under different discharging conditions. Case Stud. Therm. Eng. 2022, 34, 102034. [Google Scholar] [CrossRef]
- Chen, D.; Jiang, J.; Kim, G.H.; Yang, C.; Pesaran, A. Comparison of different cooling methods for lithium ion battery cells. Appl. Therm. Eng. 2016, 94, 846–854. [Google Scholar] [CrossRef] [Green Version]
- Hirano, H.; Tajima, T.; Hasegawa, T.; Sekiguchi, T.; Uchino, M. Boiling liquid battery cooling for electric vehicle. In Proceedings of the 2014 IEEE Conference and Expo Transportation Electrification Asia-Pacific (ITEC Asia-Pacific), Beijing, China, 31 August–3 September 2014; pp. 1–4. [Google Scholar] [CrossRef]
- Chen, K.; Li, X. Accurate determination of battery discharge characteristics–A comparison between two battery temperature control methods. J. Power Sources 2014, 247, 961–966. [Google Scholar] [CrossRef]
- Sundin, D.W.; Sponholtz, S. Thermal management of li-ion batteries with single-phase liquid immersion cooling. IEEE Open J. Veh. Technol. 2020, 1, 82–92. [Google Scholar] [CrossRef]
- Larrañaga-Ezeiza, M.; Vertiz, G.; Arroiabe, P.F.; Martinez-Agirre, M.; Berasategi, J. A novel direct liquid cooling strategy for electric vehicles focused on pouch type battery cells. Appl. Therm. Eng. 2022, 216, 11886. [Google Scholar] [CrossRef]
- Patil, M.S.; Seo, H.J.; Lee, M.Y. A Novel dielectric fluid immersion cooling technology for li-ion battery thermal management. Energy Convers. Manag. 2021, 229, 113715. [Google Scholar] [CrossRef]
- Zhou, H.; Dai, C.; Liu, Y.; Fu, X.; Du, Y. Experimental investigation of battery thermal management and safety with heat pipe and immersion phase change liquid. J. Power Sources 2020, 473, 228545. [Google Scholar] [CrossRef]
- Wang, H.; Tao, T.; Xu, J.; Shi, H.; Mei, X.; Gou, P. Thermal performance of a liquid-immersed battery thermal management system for lithium-ion pouch batteries. J. Energy Storage 2022, 46, 103835. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Mohanty, R.K.; Ghosh, A. Design of an optimized thermal management system for li-ion batteries under different discharging conditions. Energies 2020, 13, 5695. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Xie, Z.; Wang, Y. Parametric investigation on the performance of a battery thermal management system with immersion cooling. Energies 2022, 15, 2554. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Su, L.; Bai, M.; Gao, L.; Li, Y.; Liu, X.; Li, Y.; Song, Y. Numerical simulations for indirect and direct cooling of 54 V LiFePO4 battery pack. Energies 2022, 15, 4581. [Google Scholar] [CrossRef]





| Parameter | Value |
|---|---|
| Nominal capacity | 20 Ah |
| Nominal voltage | 3.3 V |
| Charge cut-off voltage | 3.65 V |
| Discharge cut-off voltage | 2 V |
| Cathode material | LiFePO4 |
| Anode material | Graphite |
| Weight | 496 g |
| Dimensions | 7.25 × 160 × 227 mm |
| Measured Parameter | Uncertainty |
|---|---|
| Pt1oo | ±0.1 °C |
| Thermocouple | ±1.0 °C |
| Data acquisition system | ±0.6 °C |
| Flow meter | ±1% |
| Power supply | ±0.1% |
| Discharge Rate | Air | 50% Immersion | 100% Immersion |
|---|---|---|---|
| 1C | 29.5 | 27.6 | 26.6 |
| 2C | 32.5 | 27.7 | 27.3 |
| 3C | 35.8 | 29.7 | 28.1 |
| 4C | 41 | 30.8 | 31.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celen, A. Experimental Investigation on Single-Phase Immersion Cooling of a Lithium-Ion Pouch-Type Battery under Various Operating Conditions. Appl. Sci. 2023, 13, 2775. https://doi.org/10.3390/app13052775
Celen A. Experimental Investigation on Single-Phase Immersion Cooling of a Lithium-Ion Pouch-Type Battery under Various Operating Conditions. Applied Sciences. 2023; 13(5):2775. https://doi.org/10.3390/app13052775
Chicago/Turabian StyleCelen, Ali. 2023. "Experimental Investigation on Single-Phase Immersion Cooling of a Lithium-Ion Pouch-Type Battery under Various Operating Conditions" Applied Sciences 13, no. 5: 2775. https://doi.org/10.3390/app13052775
APA StyleCelen, A. (2023). Experimental Investigation on Single-Phase Immersion Cooling of a Lithium-Ion Pouch-Type Battery under Various Operating Conditions. Applied Sciences, 13(5), 2775. https://doi.org/10.3390/app13052775







