A Comparative Study on the Development of Bioactive Films Based on β-glucan from Spent Brewer’s Yeast and Pomegranate, Bilberry, or Cranberry Juices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
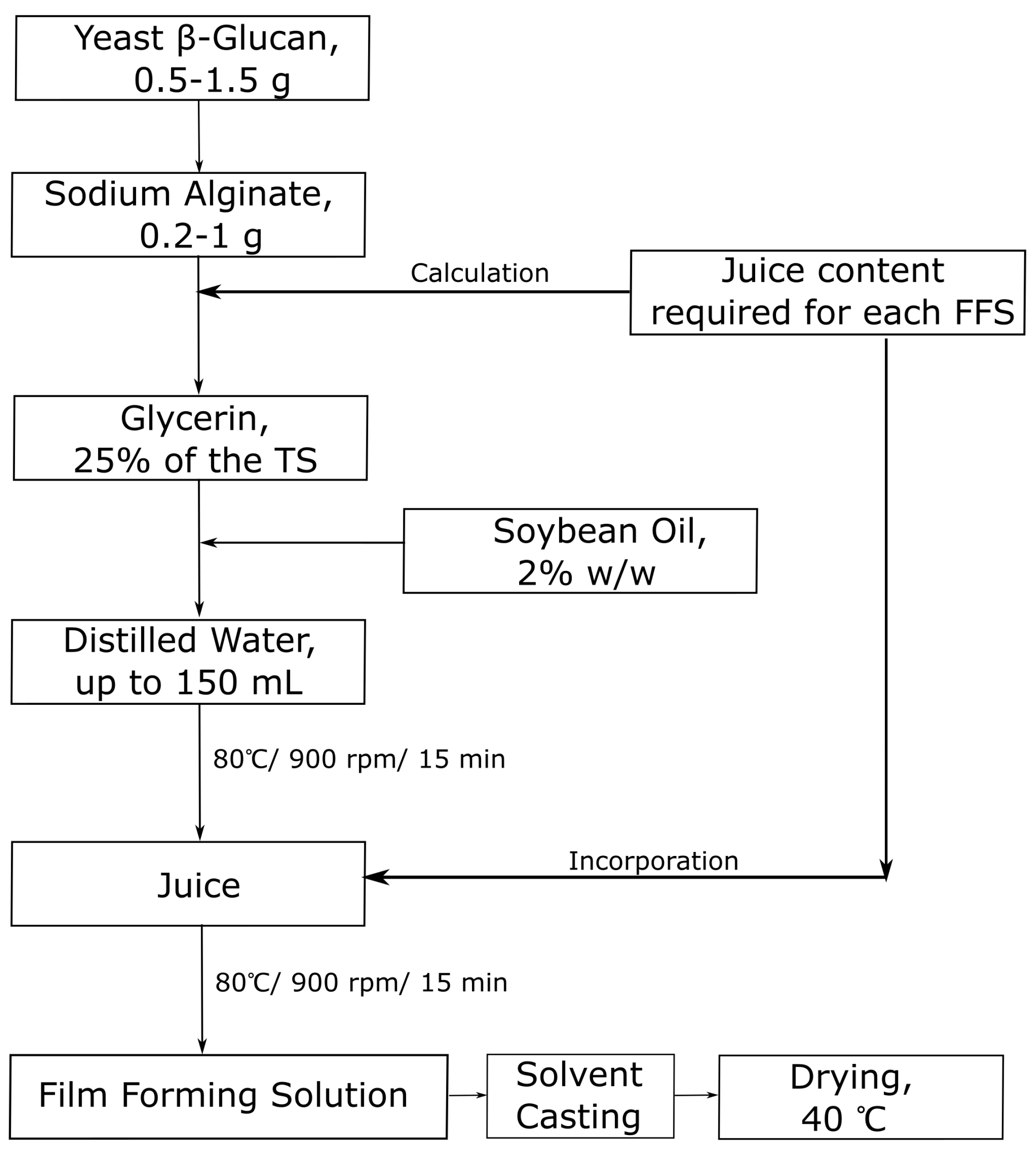
2.2. Film Preparation and Casting
2.3. Methods
2.3.1. Thickness
2.3.2. Water Vapor Transmission Rate (WVTR)
2.3.3. Water Vapor Permeability (WVP)
2.3.4. Dry Weight Determination
2.3.5. Dissolution Time
2.3.6. Scanning Electron Microscopy (SEM)
2.3.7. Optical Properties of the Film Samples
2.3.8. Statistical Analysis of the Results
3. Results
3.1. The Gelling Agent Characterization in Order to Obtain the Film-Forming Solution
3.2. Film Thickness
3.3. Water Vapor Transmission Rate (WVTR)
3.4. Water Vapor Permeability (WVP)
3.5. Dissolution Time
3.6. Color and Optical Properties of the Film Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, C.; Ji, N.; Wang, Y.; Xiong, L.; Sun, Q. Bioactive and intelligent starch-based films: A review. Trends Food Sci. Technol. 2021, 116, 854–869. [Google Scholar] [CrossRef]
- Moeini, A.; Pedram, P.; Fattahi, E.; Cerruti, P.; Santagata, G. Edible Polymers and Secondary Bioactive Compounds for Food Packaging Applications: Antimicrobial, Mechanical, and Gas Barrier Properties. Polymers 2022, 14, 2395. [Google Scholar] [CrossRef]
- Rezzani, G.D.; Choque, E.; Salvay, A.G.; Mathieu, F.; Peltzer, M.A. New Antioxidant Active Packaging Films Based on Yeast Cell Wall and Naphtho-γ-Pyrone Extract. Polymers 2022, 14, 2066. [Google Scholar] [CrossRef]
- Chaudhary, V.; Thakur, N.; Kajla, P.; Thakur, S.; Punia, S. Application of encapsulation technology in edible films: Carrier of bioactive compounds. Front. Sustain. Food Syst. 2021, 5, 734921. [Google Scholar] [CrossRef]
- La Storia, A.; Di Giuseppe, F.A.; Volpe, S.; Oliviero, V.; Villani, F.; Torrieri, E. Physical properties and antimicrobial activity of bioactive film based on whey protein and Lactobacillus curvatus 54M16 producer of bacteriocins. Food Hydrocoll. 2020, 108, 105959. [Google Scholar] [CrossRef]
- Puscaselu, R.; Gutt, G.; Amariei, S. Rethinking the future of food packaging: Biobased edible films for powdered food and drinks. Molecules 2019, 24, 3136. [Google Scholar] [CrossRef] [Green Version]
- Puscaselu, R.; Gutt, G.; Amariei, S. Biopolymer-Based Films Enriched with Stevia rebaudiana Used for the Development of Edible and Soluble Packaging. Coatings 2019, 9, 360. [Google Scholar] [CrossRef] [Green Version]
- Hulda, N.M.C.; Bianca, S.d.C.; Wiliene, C.d.L.; Daniel, C.K.; Flvio, L.S. Fruit juices in polysaccharides edible films. Afr. J. Food Sci. 2020, 14, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Odjo, K.; Al-Maqtari, Q.A.; Yu, H.; Xie, Y.; Guo, Y.; Li, M.; Du, Y.; Liu, K.; Chen, Y.; Yao, W. Preparation and characterization of chitosan-based antimicrobial films containing encapsulated lemon essential oil by ionic gelation and cranberry juice. Food Chem. 2022, 397, 133781. [Google Scholar] [CrossRef]
- Peltzer, M.A.; Salvay, A.G.; Delgado, J.F.; de la Osa, O.; Wagner, J.R. Use of Residual Yeast Cell Wall for New Biobased Materials Production: Effect of Plasticization on Film Properties. Food Bioprocess Technol. 2018, 11, 1995–2007. [Google Scholar] [CrossRef]
- Topalović, A.; Knežević, M.; Ivanović, L.; Gačnik, S.; Mikulic-Petkovsek, M. Phytochemical screening of wild pomegranate (Punica granatum L.) juices from the market. J. Food Compos. Anal. 2021, 100, 103933. [Google Scholar] [CrossRef]
- Kamau, C.M. Fruit Juices: Ellagic Acid Concentration and Sensory Appeal. Ph.D. Thesis, Bowling Green State University, Bowling Green, OH, USA, 2007. [Google Scholar]
- Poyrazoğlu, E.; Gökmen, V.; Artιk, N. Organic acids and phenolic compounds in pomegranates (Punica granatum L.) grown in Turkey. J. Food Compos. Anal. 2002, 15, 567–575. [Google Scholar] [CrossRef]
- Adami, R.; Salvo, G.; Meneses, M.; Järvenpää, E.; Huopalahti, R.; Sesti Osséo, L.; Reverchon, E. Innovative treatment of bilberry by-products for a selective recovery of anthocyanin compounds. In Proceedings of the 10th Conference on Supercritical Fluids and Their Applications, Naples, Italy, 29 April–6 May 2013. [Google Scholar]
- Laaksonen, O.; Sandell, M.; Kallio, H. Chemical factors contributing to orosensory profiles of bilberry (Vaccinium myrtillus) fractions. Eur. Food Res. Technol. 2010, 231, 271–285. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Petrikaite, V.; Pukalskas, A.; Sipailiene, A.; Raudone, L. Exploring Vaccinium vitis-idaea L. as a potential source of therapeutic agents: Antimicrobial, antioxidant, and anti-inflammatory activities of extracts and fractions. J. Ethnopharmacol. 2022, 292, 115207. [Google Scholar] [CrossRef]
- Kowalska, K. Lingonberry (Vaccinium vitis-idaea L.) Fruit as a Source of Bioactive Compounds with Health-Promoting Effects—A Review. Int. J. Mol. Sci. 2021, 22, 5126. [Google Scholar] [CrossRef]
- Fernandes, L.; Pereira, J.A.; Lopéz-Cortés, I.; Salazar, D.M.; González-Álvarez, J.; Ramalhosa, E. Physicochemical composition and antioxidant activity of several pomegranate (Punica granatum L.) cultivars grown in Spain. Eur. Food Res. Technol. 2017, 243, 1799–1814. [Google Scholar] [CrossRef]
- Papanov, S.I.; Petkova, E.G.; Ivanov, I.G. Polyphenols Content and Antioxidant Activity of Bilberry Juice Obtained from Different Altitude Samples. J. Pharm. Res. Int 2021, 33, 218–223. [Google Scholar] [CrossRef]
- Güder, A.; Gür, M.; Engin, M.S. Antidiabetic and antioxidant properties of bilberry (Vaccinium myrtillus Linn.) fruit and their chemical composition. J. Agric. Sci. Technol. 2015, 17, 401–414. [Google Scholar]
- Rocha, D.M.U.P.; Caldas, A.P.S.; da Silva, B.P.; Hermsdorff, H.H.M.; Alfenas, R. de C.G. Effects of blueberry and cranberry consumption on type 2 diabetes glycemic control: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1816–1828. [Google Scholar] [CrossRef]
- Vetvicka, V. Glucan-immunostimulant, adjuvant, potential drug. World J. Clin. Oncol. 2011, 2, 115. [Google Scholar] [CrossRef]
- Dey, L.; Attele, A.S.; Yuan, C.-S. Alternative therapies for type 2 diabetes. Altern. Med. Rev. 2002, 7, 45–58. [Google Scholar]
- Heiss, C.; Istas, G.; Feliciano, R.P.; Weber, T.; Wang, B.; Favari, C.; Mena, P.; Del Rio, D.; Rodriguez-Mateos, A. Daily consumption of cranberry improves endothelial function in healthy adults: A double blind randomized controlled trial. Food Funct. 2022, 13, 3812–3824. [Google Scholar] [CrossRef]
- Carlos, O.S.; Enrique, F.I.H.; Eduardo, F.M.; Araceli, O.R.M.; Raquel, C.C.; Alberto, A.O.J.; Carlos, H.G.J.; Diana, O.; Carmen, V.V.; Helen, B.M.; et al. Potential Mechanisms of the Improvement of Glucose Homeostasis in Type 2 Diabetes by Pomegranate Juice. Antioxidants 2022, 11, 553. [Google Scholar] [CrossRef]
- Kandylis, P.; Kokkinomagoulos, E. Food applications and potential health benefits of pomegranate and its derivatives. Foods 2020, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Han, X.; Huang, X.; Xie, W.; Zhang, X.; Zhang, Z.; Yu, Q.; Tao, L.; Li, T.; Li, S. Effects of different sources of β-glucan on pasting, gelation, and digestive properties of pea starch. Food Hydrocoll. 2023, 135, 108172. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- Thomas, S.; Rezoagli, E.; Abidin, I.Z.; Major, I.; Murray, P.; Murphy, E.J. β-Glucans from Yeast—Immunomodulators from Novel Waste Resources. Appl. Sci. 2022, 12, 5208. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Q.; Wu, X.; Algharib, S.A.; Gong, F.; Hu, J.; Luo, W.; Zhou, M.; Pan, Y.; Yan, Y. Structure, preparation, modification, and bioactivities of β-glucan and mannan from yeast cell wall: A review. Int. J. Biol. Macromol. 2021, 173, 445–456. [Google Scholar] [CrossRef]
- Caruso, A.; Piermaria, J.A.; Abraham, A.G.; Micaela, M. β-glucans obtained from beer spent yeasts as functional food grade additive: Focus on biological activity. Food Hydrocoll. 2022, 133, 107963. [Google Scholar] [CrossRef]
- Jaeger, A.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Brewer’s Spent Yeast (BSY), an Underutilized Brewing By-Product. Fermentation 2020, 6, 123. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, D.; Regenstein, J.M.; Xia, W.; Dong, J. A comprehensive review on natural bioactive films with controlled release characteristics and their applications in foods and pharmaceuticals. Trends Food Sci. Technol. 2021, 112, 690–707. [Google Scholar] [CrossRef]
- Volman, J.J.; Ramakers, J.D.; Plat, J. Dietary modulation of immune function by β-glucans. Physiol. Behav. 2008, 94, 276–284. [Google Scholar] [CrossRef]
- Baeghbali, V.; Niakousari, M.; Farahnaky, A. Refractance Window drying of pomegranate juice: Quality retention and energy efficiency. LWT—Food Sci. Technol. 2016, 66, 34–40. [Google Scholar] [CrossRef]
- Bernaert, N.; Van Droogenbroeck, B.; Van Pamel, E.; De Ruyck, H. Innovative refractance window drying technology to keep nutrient value during processing. Trends Food Sci. Technol. 2019, 84, 22–24. [Google Scholar] [CrossRef]
- Avramia, I.; Amariei, S. Formulation, Characterization and Optimization of β–Glucan and Pomegranate Juice Based Films for Its Potential in Diabetes. Nutrients 2022, 14, 2142. [Google Scholar] [CrossRef]
- Avramia, I.; Amariei, S. Formulation of Fast Dissolving β-Glucan/Bilberry Juice Films for Packaging Dry Powdered Pharmaceuticals for Diabetes. Plants 2022, 11, 2040. [Google Scholar] [CrossRef]
- ASTM E96-00; Standard Test Methods for Water Vapor Transmission of Materials 1. ASTM International: West Conshohocken, PA, USA, 2018; pp. 1–14.
- Yıldırım-Yalçın, M.; Şeker, M.; Sadıkoğlu, H. Development and characterization of edible films based on modified corn starch and grape juice. Food Chem. 2019, 292, 6–13. [Google Scholar] [CrossRef]
- Siddiqui, M.H.B.; Islam, M.S.; Razu, M.R.; Zaman, A.N.; Jadi, B.; Saha, T.; Pathan, M.S.I. Preparation and evaluation of sublingual film of ketorolac tromethamine. Drug Dev. Ind. Pharm. 2022, 48, 438–445. [Google Scholar] [CrossRef]
- Kwon, S.; Orsuwan, A.; Bumbudsanpharoke, N.; Yoon, C.; Choi, J.; Ko, S. A short review of light barrier materials for food and beverage packaging. Korean J. Packag. Sci. Technol. 2018, 24, 141–148. [Google Scholar] [CrossRef]
- Mancini, M.; Moresi, M.; Sappino, F. Rheological behaviour of aqueous dispersions of algal sodium alginates. J. Food Eng. 1996, 28, 283–295. [Google Scholar] [CrossRef]
- Roland, C.M. Chapter 6—Rheological Behavior and Processing of Unvulcanized Rubber. In The Science and Technology of Rubber, 4th ed.; Mark, J., Erman, B., Roland, M., Eds.; Academic Press: Boston, MA, USA, 2013; pp. 285–336. ISBN 978-0-12-394584-6. [Google Scholar]
- Xiao, Q.; Tong, Q.; Lim, L.T. Pullulan-sodium alginate based edible films: Rheological properties of film forming solutions. Carbohydr. Polym. 2012, 87, 1689–1695. [Google Scholar] [CrossRef]
- Ma, J.; Lin, Y.; Chen, X.; Zhao, B.; Zhang, J. Flow behavior, thixotropy and dynamical viscoelasticity of sodium alginate aqueous solutions. Food Hydrocoll. 2014, 38, 119–128. [Google Scholar] [CrossRef]
- Arham, R.; Mulyati, M.T.; Metusalach, M.; Salengke, S. Physical and mechanical properties of agar based edible film with glycerol plasticizer. Int. Food Res. J. 2016, 23, 1669–1675. [Google Scholar] [CrossRef]
- Brasil, I.M.; Gomes, C.; Puerta-Gomez, A.; Castell-Perez, M.E.; Moreira, R.G. Polysaccharide-based multilayered antimicrobial edible coating enhances quality of fresh-cut papaya. LWT 2012, 47, 39–45. [Google Scholar] [CrossRef]
- Nagendra, B.; Vignola, E.; Daniel, C.; Rizzo, P.; Guerra, G. Dependence on film thickness of guest-induced c perpendicular orientation in PPO films. Polymers 2021, 13, 4384. [Google Scholar] [CrossRef]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef]
- Severo, C.; Anjos, I.; Souza, V.G.L.; Canejo, J.P.; Bronze, M.R.; Fernando, A.L.; Coelhoso, I.; Bettencourt, A.F.; Ribeiro, I.A.C. Development of cranberry extract films for the enhancement of food packaging antimicrobial properties. Food Packag. Shelf Life 2021, 28, 100646. [Google Scholar] [CrossRef]
- Daskalova, A.; Trifonov, A.; Bliznakova, I.; Nathala, C.; Ajami, A.; Husinsky, W.; Declercq, H.; Buchvarov, I. Selective cell response on natural polymer bio-interfaces textured by femtosecond laser. Appl. Phys. A 2018, 124, 207. [Google Scholar] [CrossRef]
- An, J.; Im, S.; Kang, H.; Kim, H.; Yoo, Y.; Noh, J.; Huh, S. A Study on the Wear Characteristics of Al7075 with Changes in Surface Roughness and Ti Thin Film Deposition Time. Adv. Mater. Sci. Eng. 2020, 2020, 7934842. [Google Scholar] [CrossRef]
- Valovirta, I. Water Vapor Permeability and Thermal Conductivity as a Function of Temperature and Relative Humidity. 2004. Available online: https://trepo.tuni.fi/bitstream/handle/10024/128675/Valovirta_Water_Vapor_Permeability_and_Thermal_Conductivity_as_a_Function_of_Temperature_and_Relative_Humidity.pdf?sequence=1 (accessed on 16 February 2023).
- Azeredo, H.M.C.; Morrugares-Carmona, R.; Wellner, N.; Cross, K.; Bajka, B.; Waldron, K.W. Development of pectin films with pomegranate juice and citric acid. Food Chem. 2016, 198, 101–106. [Google Scholar] [CrossRef]
- Chang, J.; Li, W.; Liu, Q.; Zhou, Y.; Chen, X.; Lyu, Q.; Liu, G. Preparation, properties, and structural characterization of β-glucan/pullulan blend films. Int. J. Biol. Macromol. 2019, 140, 1269–1276. [Google Scholar] [CrossRef]
- Park, S.I.; Zhao, Y. Development and characterization of edible films from cranberry pomace extracts. J. Food Sci. 2006, 71, E95–E101. [Google Scholar] [CrossRef]
- Crank, J.; Park, G.S. Diffusion in high polymers: Some anomalies and their significance. Trans. Faraday Soc. 1951, 47, 1072–1084. [Google Scholar] [CrossRef]
- Duan, Z. Water Vapour Permeability of Bio-Based Polymers. Ph.D. Thesis, Loughborough University, Loughborough, UK, 2013. [Google Scholar]
- Ramakrishnan, R.K.; Miroslav, Č.; Padil, V.V.T. Biomacromolecule assembly based on gum kondagogu-sodium alginate composites and their expediency in flexible packaging films. Int. J. Biol. Macromol. 2021, 177, 526–534. [Google Scholar] [CrossRef]
- Beigomi, M.; Mohsenzadeh, M.; Salari, A. Characterization of a novel biodegradable edible film obtained from Dracocephalum moldavica seed mucilage. Int. J. Biol. Macromol. 2018, 108, 874–883. [Google Scholar] [CrossRef]
- Naushad Emmambux, M.; Stading, M. In situ tensile deformation of zein films with plasticizers and filler materials. Food Hydrocoll. 2007, 21, 1245–1255. [Google Scholar] [CrossRef]
- Pacheco, M.S.; Barbieri, D.; da Silva, C.F.; de Moraes, M.A. A review on orally disintegrating films (ODFs) made from natural polymers such as pullulan, maltodextrin, starch, and others. Int. J. Biol. Macromol. 2021, 178, 504–513. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M.; Durairaj, R.; Mageshwaran, G.; Joseph, G.B. A brief review on edible food packing materials. J. Glob. Eng. Probl. Solut. 2017, 1, 9–19. [Google Scholar]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef]
- Luchese, C.L.; Abdalla, V.F.; Spada, J.C.; Tessaro, I.C. Evaluation of blueberry residue incorporated cassava starch film as pH indicator in different simulants and foodstuffs. Food Hydrocoll. 2018, 82, 209–218. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, J.; Zhou, H.; Zhou, S.; Lv, Y.; Cheng, Y.; Tao, Y.; Lu, J.; Wang, H. Biodegradable intelligent film for food preservation and real-time visual detection of food freshness. Food Hydrocoll. 2022, 129, 107665. [Google Scholar] [CrossRef]
- Neves, D.; Andrade, P.B.; Videira, R.A.; de Freitas, V.; Cruz, L. Berry anthocyanin-based films in smart food packaging: A mini-review. Food Hydrocoll. 2022, 133, 107885. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avramia, I.; Amariei, S. A Comparative Study on the Development of Bioactive Films Based on β-glucan from Spent Brewer’s Yeast and Pomegranate, Bilberry, or Cranberry Juices. Appl. Sci. 2023, 13, 2807. https://doi.org/10.3390/app13052807
Avramia I, Amariei S. A Comparative Study on the Development of Bioactive Films Based on β-glucan from Spent Brewer’s Yeast and Pomegranate, Bilberry, or Cranberry Juices. Applied Sciences. 2023; 13(5):2807. https://doi.org/10.3390/app13052807
Chicago/Turabian StyleAvramia, Ionut, and Sonia Amariei. 2023. "A Comparative Study on the Development of Bioactive Films Based on β-glucan from Spent Brewer’s Yeast and Pomegranate, Bilberry, or Cranberry Juices" Applied Sciences 13, no. 5: 2807. https://doi.org/10.3390/app13052807






