Low-Dose Non-Thermal Atmospheric Plasma Promotes the Proliferation and Migration of Human Normal Skin Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. NTP Device
2.3. Cell Viability
2.4. Extracellular Reactive Species Detection
2.5. Intracellular Reactive Species Detection
2.6. Mitochondrial Membrane Potential Detection
2.7. Scratch Wound Assay
2.8. Cell Migration Assay
2.9. Cell Cycle Analysis
2.10. Western Blot
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effect of NTP on Viability of HaCaT Cells
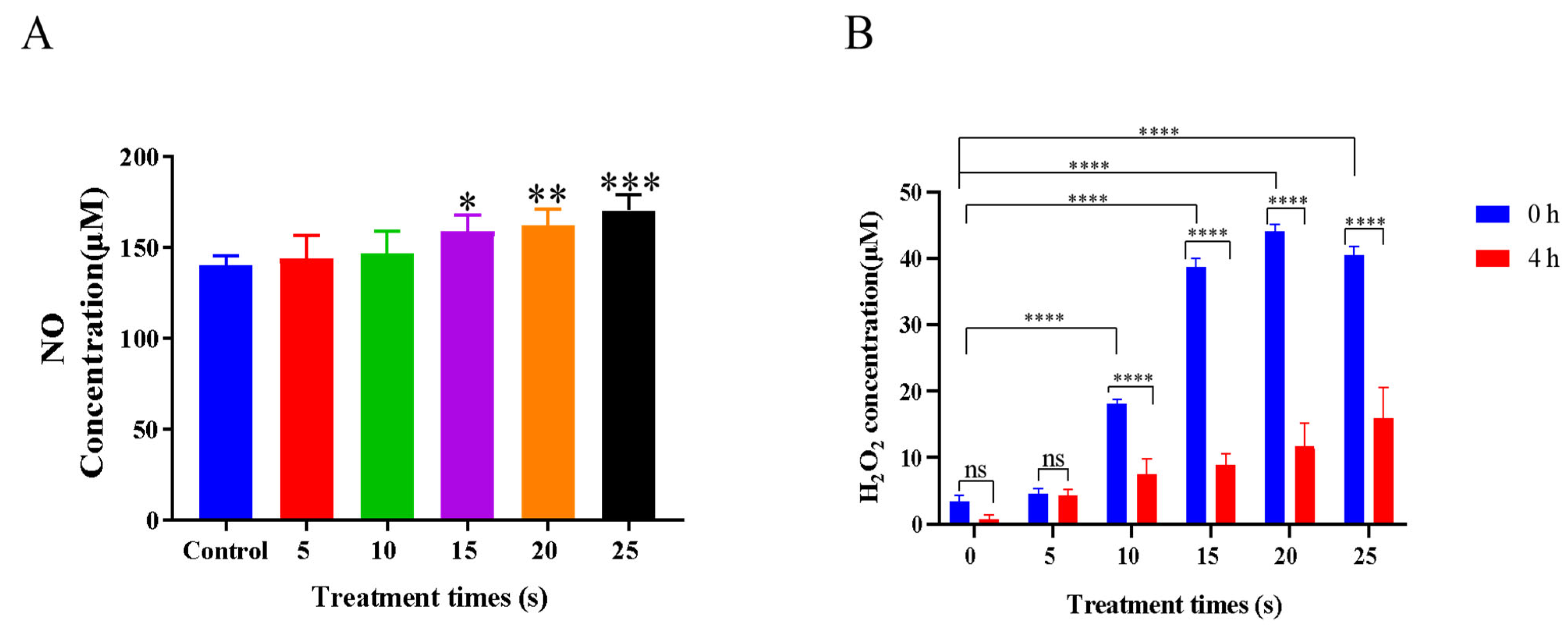
3.2. Effects of NTP Treatment on Extracellular Reactive Species Generation
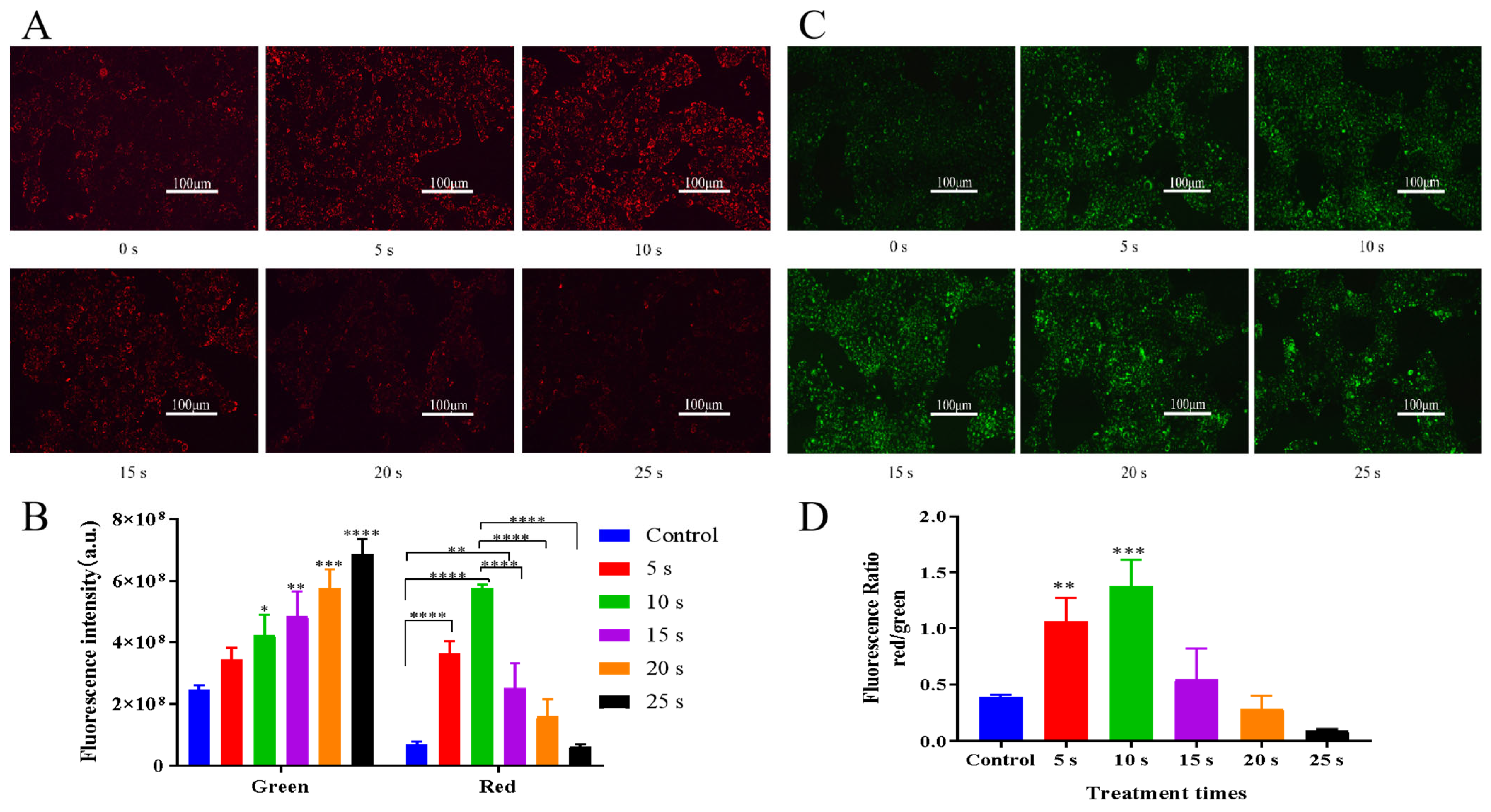
3.3. Effects of NTP Treatment on Intracellular Reactive Species Generation
3.4. Effects of NTP Treatment on Mitochondrial Membrane Potential
3.5. Effects of NTP Treatment on Cell Migration
3.6. Effects of NTP Treatment on Cell Cycle
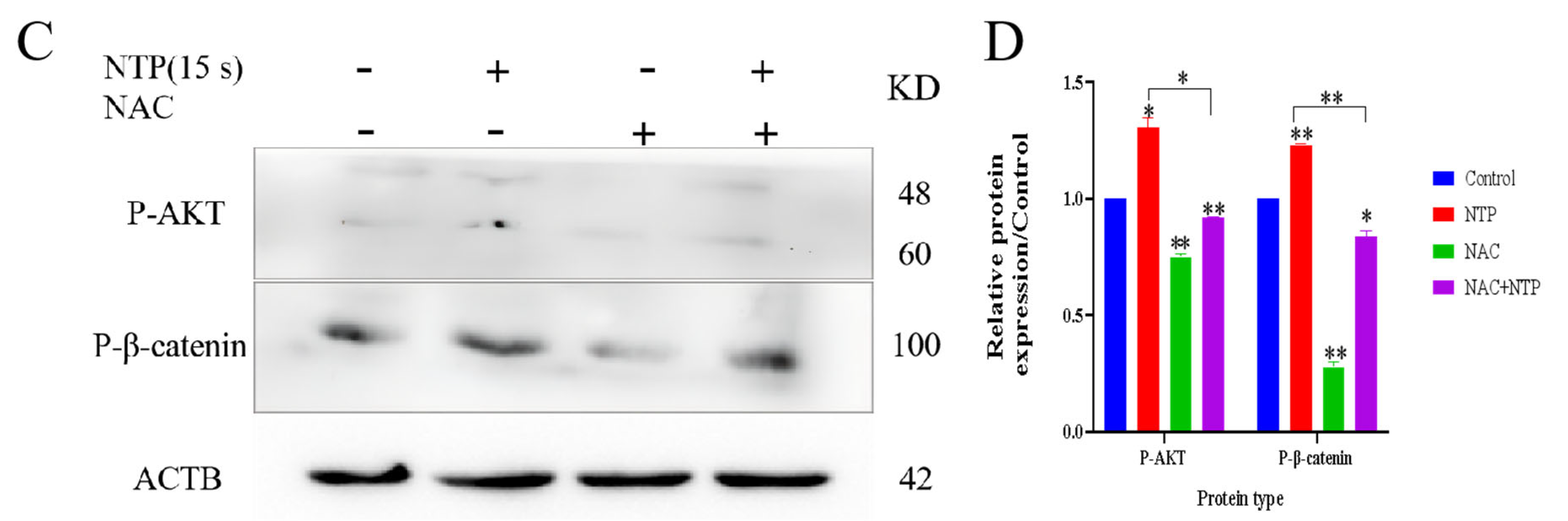
3.7. NTP Activated the PI3K/AKT and STAT3 Pathways in HaCaT Cells
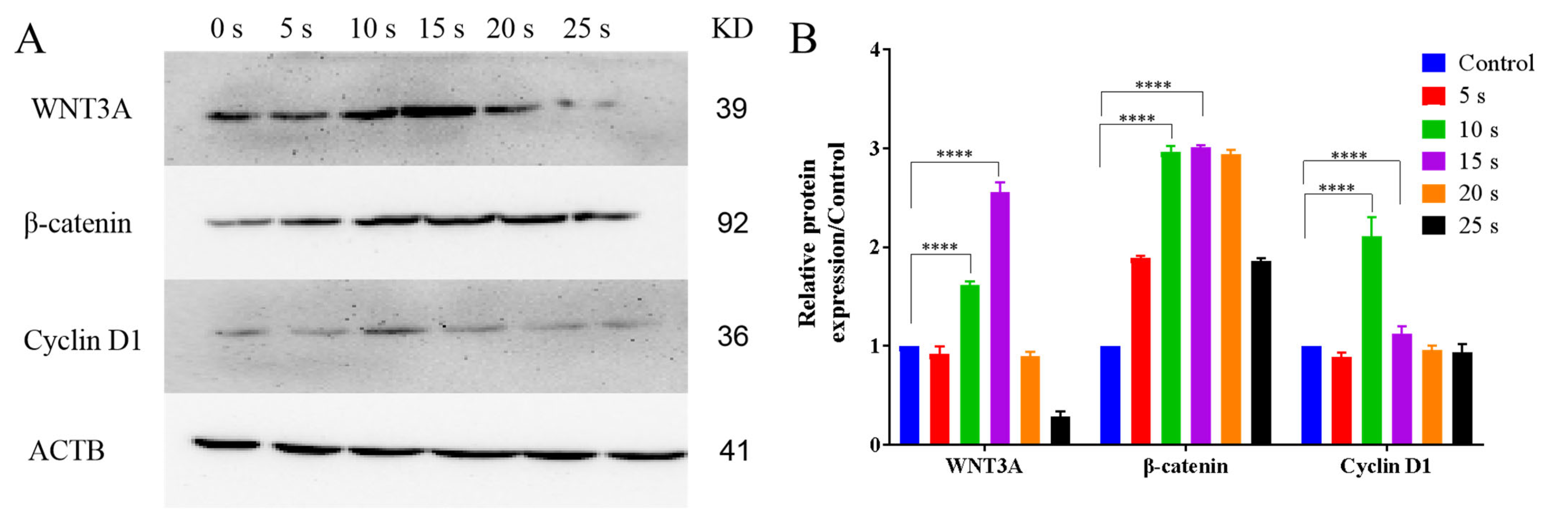
3.8. NTP Activated the WNT3A/β-Catenin Pathways in HaCaT Cells
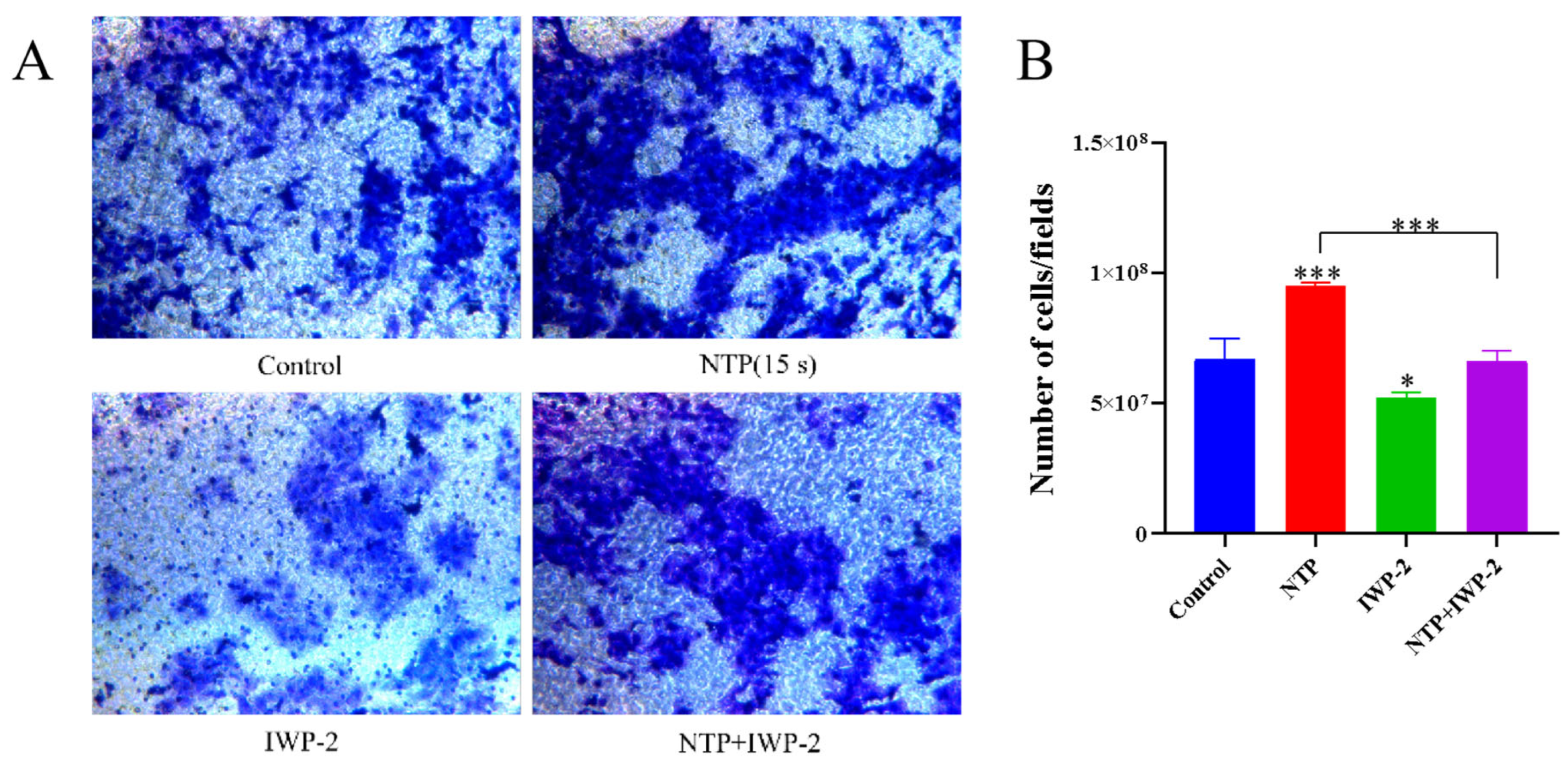
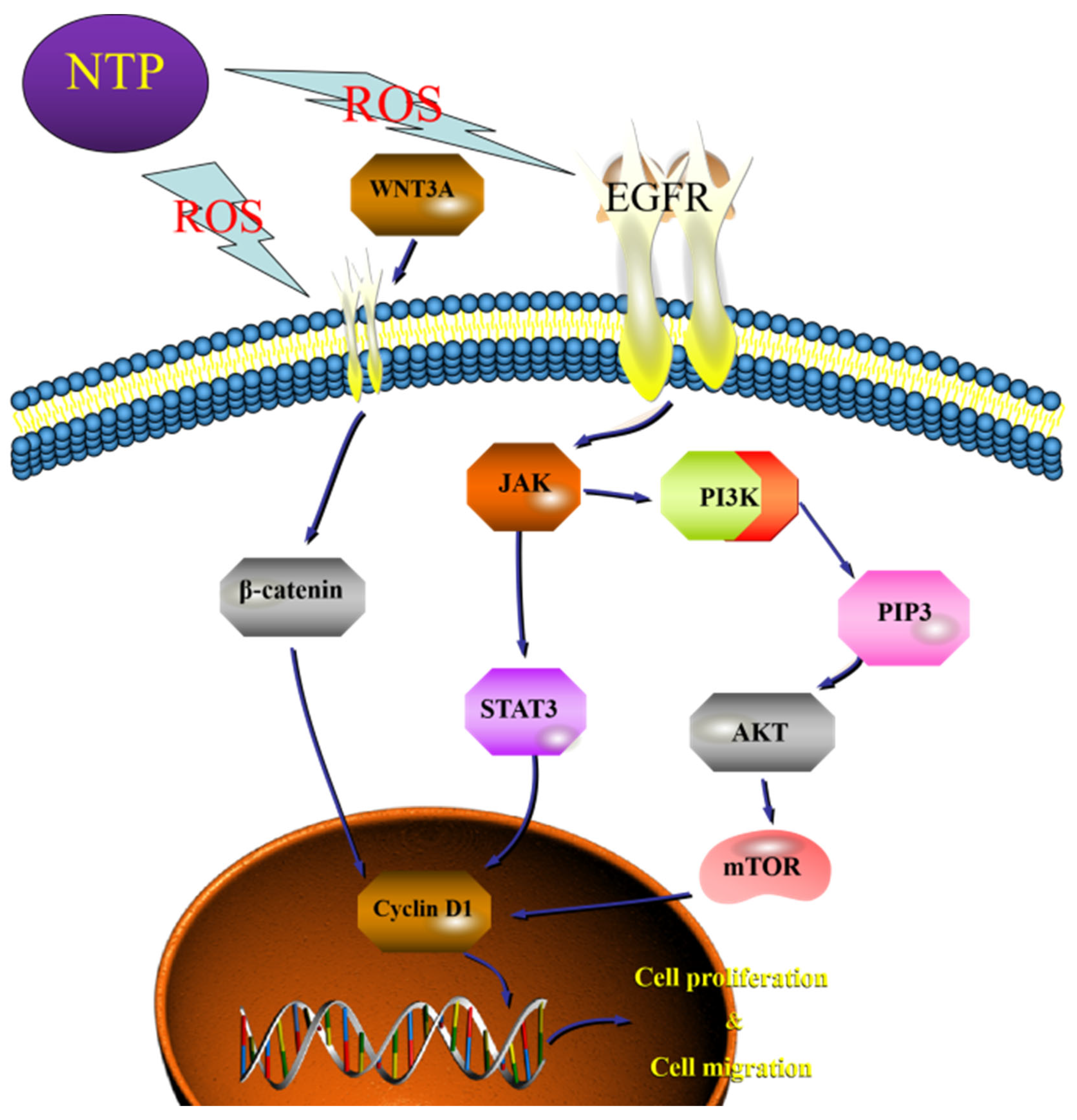
3.9. Low-Dose ROS Promotes HaCaT Cell Proliferation and Migration by Activating the PI3K/AKT and Wnt/β-Catenin Pathways
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adhikari, B.R.; Khanal, R. Introduction to the Plasma State of Matter. Himal. Phys. 2013, 4, 60. [Google Scholar] [CrossRef] [Green Version]
- Weltmann, K.D.; Kindel, E.; Brandenburg, R.; Meyer, C.; Bussiahn, R.; Wilke, C.; von Woedtke, T. Atmospheric Pressure Plasma Jet for Medical Therapy: Plasma Parameters and Risk Estimation. Contrib. Plasma Phys. 2009, 49, 631–640. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Nontarget 2017, 8, 15977–15995. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.; Pomicter, A.D.; Li, F.; Bhatt, S.; Chen, C.; Li, W.; Qi, M.; Huang, C.; Deininger, M.W.; Kong, M.G.; et al. Trident cold atmospheric plasma blocks three cancer survival pathways to overcome therapy resistance. Proc. Natl. Acad. Sci. USA 2021, 118, e2107220118. [Google Scholar] [CrossRef]
- Shahbazi Rad, Z.; Abbasi Davani, F.; Etaati, G. Determination of proper treatment time for in vivo blood coagulation and wound healing application by non-thermal helium plasma jet. Australas. Phys. Eng. Sci. Med. 2018, 41, 905–917. [Google Scholar] [CrossRef]
- Eggers, B.; Stope, M.B.; Marciniak, J.; Marciniak, J.; Mustea, A.; Deschner, J.; Nokhbehsaim, M.; Kramer, F.J. Modulation of Inflammatory Responses by a Non-Invasive Physical Plasma Jet during Gingival Wound Healing. Cells 2022, 11, 2740. [Google Scholar] [CrossRef]
- Sarthak, D.; Veda, P.G.; Sarita, M.; Gagandeep, S.; Satyananda, K. Role of cold atmospheric plasma in microbial inactivation and the factors affecting its efficacy. Health Sci. Rev. 2022, 4, 100037. [Google Scholar] [CrossRef]
- Zhai, S.; Xu, M.; Li, Q.; Guo, K.; Chen, H.; Kong, M.G.; Xia, Y. Successful Treatment of Vitiligo with Cold Atmospheric Plasma—Activated Hydrogel. J. Invest. Dermatol. 2021, 141, 2710–2719.e6. [Google Scholar] [CrossRef]
- Kang, S.U.; Choi, J.W.; Chang, J.W.; Kim, K.; Kim, Y.S.; Park, J.K.; Kim, Y.E.; Lee, Y.S.; Yang, S.S.; Kim, C.H. N2 non-thermal atmospheric pressure plasma promotes wound healing in vitro and in vivo: Potential modulation of adhesion molecules and matrix metalloproteinase-9. Exp. Dermatol. 2017, 26, 163–170. [Google Scholar] [CrossRef]
- Stratmann, B.; Costea, T.C.; Nolte, C.; Nolte, C.; Hiller, J.; Schmidt, J.; Reindel, J.; Masur, K.; Motz, W.; Timm, J.; et al. Effect of Cold Atmospheric Plasma Therapy vs Standard Therapy Placebo on Wound Healing in Patients with Diabetic Foot Ulcers: A Randomized Clinical Trial. JAMA Netw. Open. 2020, 3, e2010411. [Google Scholar] [CrossRef]
- Brun, P.; Bernabè, G.; Marchiori, C.; Scarpa, M.; Zuin, M.; Cavazzana, R.; Zaniol, B.; Martines, E. Antibacterial efficacy and mechanisms of action of low power atmospheric pressure cold plasma: Membrane permeability, biofilm penetration and antimicrobial sensitization. J. Appl. Microbiol. 2018, 125, 398–408. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Fouché, M.; Willers, C.; Hamman, S.; Malherbe, C.; Steenekamp, J. Wound Healing Effects of Aloe muth-muth: In Vitro Investigations Using Immortalized Human Keratinocytes (HaCaT). Biology 2020, 9, 350. [Google Scholar] [CrossRef]
- Catanzano, O.; Quaglia, F.; Boateng, J.S. Wound dressings as growth factor delivery platforms for chronic wound healing. Expert Opin. Drug Deliv. 2021, 18, 737–759. [Google Scholar] [CrossRef]
- Koo, M.A.; Hee Hong, S.; Hee Lee, M.; Kwon, B.J.; Mi Seon, G.; Sung Kim, M.; Kim, D.; Chang Nam, K.; Park, J.C. Effective stacking and transplantation of stem cell sheets using exogenous ROS-producing film for accelerated wound healing. Acta Biomater. 2019, 95, 418–426. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.A.; Afzali, H.; Raoofi, A.; Nouri, M.; Naser, R.; Gholami, O.; Nasiry, D.; Mohammadnia, A.; Razzaghi, Z.; Alimohammadi, A.; et al. Topical spray of Wharton’s jelly mesenchymal stem cells derived from umbilical cord accelerates diabetic wound healing. J Cosmet Dermatol. 2022, 21, 5156–5167. [Google Scholar] [CrossRef]
- Shi, X.M.; Xu, G.M.; Zhang, G.J.; Liu, J.R.; Wu, Y.M.; Gao, L.G.; Yang, Y.; Chang, Z.S.; Yao, C.W. Low-temperature Plasma Promotes Fibroblast Proliferation in Wound Healing by ROS-activated NF-κB Signaling Pathway. Curr. Med. Sci. 2018, 38, 107–114. [Google Scholar] [CrossRef]
- Arndt, S.; Unger, P.; Berneburg, M.; Bosserhoff, A.K.; Karrer, S. Cold atmospheric plasma (CAP) activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis in an autocrine and paracrine mode. J. Dermatol. Sci. 2018, 89, 181–190. [Google Scholar] [CrossRef]
- Frescaline, N.; Duchesne, C.; Favier, M.; Onifarasoaniaina, R.; Guilbert, T.; Uzan, G.; Banzet, S.; Rousseau, A.; Lataillade, J.J. Physical plasma therapy accelerates wound re-epithelialisation and enhances extracellular matrix formation in cutaneous skin grafts. J. Pathol. 2020, 252, 451–464. [Google Scholar] [CrossRef]
- Yu, Y.; Tan, M.; Chen, H.; Wu, Z.; Xu, L.; Li, J.; Cao, J.; Yang, Y.; Xiao, X.; Lian, X.; et al. Non-thermal plasma suppresses bacterial colonization on skin wound and promotes wound healing in mice. J. Huazhong Univ Sci. Technol. Med. Sci. 2011, 31, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Kalghatgi, S.; Friedman, G.; Fridman, A.; Clyne, A.M. Endothelial cell proliferation is enhanced by low dose non-thermal plasma through fibroblast growth factor-2 release. Ann. Biomed. Eng. 2010, 38, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Unger, P.; Wacker, E.; Shimizu, T.; Heinlin, J.; Li, Y.F.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L.; Bosserhoff, A.K.; et al. Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PLoS ONE 2013, 8, e79325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arjunan, K.P.; Clyne, A.M. Non-thermal dielectric barrier discharge plasma induces angiogenesis through reactive oxygen species. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2011, 2011, 2447–2450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Shaw, T.J.; Martin, P. Wound repair: A showcase for cell plasticity and migration. Curr. Opin. Cell Biol. 2016, 42, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef]
- Abba, M.; Patil, N.; Leupold, J.; Allgayer, H. MicroRNA Regulation of Epithelial to Mesenchymal Transition. J. Clin. Med. 2016, 5, 8. [Google Scholar] [CrossRef]
- Thomason, H.A.; Cooper, N.H.; Ansell, D.M.; Chiu, M.; Merrit, A.J.; Hardman, M.J.; Garrod, D.R. Direct evidence that PKCα positively regulates wound re-epithelialization: Correlation with changes in desmosomal adhesiveness. J. Pathol. 2012, 227, 346–356. [Google Scholar] [CrossRef]
- Nunan, R.; Campbell, J.; Mori, R.; Pitulescu, M.E.; Jiang, W.G.; Harding, K.G.; Adams, R.H.; Nobes, C.D.; Martin, P. Ephrin-Bs Drive Junctional Downregulation and Actin Stress Fiber Disassembly to Enable Wound Re-epithelialization. Cell Rep. 2015, 13, 1380–1395. [Google Scholar] [CrossRef] [Green Version]
- Rousselle, P.; Braye, F.; Dayan, G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 344–365. [Google Scholar] [CrossRef]
- Santoro, M.M.; Gaudino, G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp. Cell Res. 2005, 304, 274–286. [Google Scholar] [CrossRef]
- Baum, C.L.; Arpey, C.J. Normal cutaneous wound healing: Clinical correlation with cellular and molecular events. Dermatol. Surg. 2005, 31, 674–686. [Google Scholar] [CrossRef]
- Hasse, S.; Duong Tran, T.; Hahn, O.; Kindler, S.; Metelmann, H.R.; von Woedtke, T.; Masur, K. Induction of proliferation of basal epidermal keratinocytes by cold atmospheric-pressure plasma. Clin. Exp. Dermatol. 2016, 41, 202–209. [Google Scholar] [CrossRef]
- Ren, S.; Chen, J.; Duscher, D.; Liu, Y.; Guo, G.; Kang, Y.; Xiong, H.; Zhan, P.; Wang, Y.; Wang, C.; et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem. Cell Res. Ther. 2019, 10, 47. [Google Scholar] [CrossRef]
- Lu, C.C.; Yang, J.S.; Chiu, Y.J.; Tsai, F.J.; Hsu, Y.M.; Yin, M.C.; Juan, Y.N.; Ho, T.J.; Chen, H.P. Dracorhodin perchlorate enhances wound healing via β-catenin, ERK/p38, and AKT signaling in human HaCaT keratinocytes. Exp. Ther. Med. 2021, 22, 822. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, Y.; Song, W.; Wang, H. Cold Atmospheric Plasma Inhibits the Proliferation of CAL-62 Cells through the ROS-Mediated PI3K/Akt/mTOR Signaling Pathway. Sci. Technol. Nucl. Install. 2022, 2022, 3884695. [Google Scholar] [CrossRef]
- Liu, J.R.; Xu, G.M.; Shi, X.M.; Zhang, G.J. Low temperature plasma promoting fibroblast proliferation by activating the NF-κB pathway and increasing cyclinD1 expression. Sci. Rep. 2017, 7, 11698. [Google Scholar] [CrossRef] [Green Version]
- Veeraperumal, S.; Qiu, H.M.; Zeng, S.S.; Yao, W.Z.; Wang, B.P.; Liu, Y.; Cheong, K.L. Polysaccharides from Gracilaria lemaneiformis promote the HaCaT keratinocytes wound healing by polarised and directional cell migration. Carbohydr. Polym. 2020, 241, 116310. [Google Scholar] [CrossRef]
- Leng, X.; Shang, J.; Gao, D.; Wu, J. Low-intensity pulsed ultrasound promotes proliferation and migration of HaCaT keratinocytes through the PI3K/AKT and JNK pathways. Braz. J. Med. Biol. Res. 2018, 51, e7862. [Google Scholar] [CrossRef]
- Duchesne, C.; Banzet, S.; Lataillade, J.J.; Rousseau, A.; Frescaline, N. Cold atmospheric plasma modulates endothelial nitric oxide synthase signalling and enhances burn wound neovascularisation. J. Pathol. 2019, 249, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Pekshev, A.V.; Shekhter, A.B.; Vagapov, A.B.; Sharapov, N.A.; Vanin, A.F. Study of plasma-chemical NO-containing gas flow for treatment of wounds and inflammatory processes. Nitric. Oxide. 2018, 73, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Chung, T.H. Cold atmospheric plasma jet-generated RONS and their selective effects on normal and carcinoma cells. Sci. Rep. 2016, 6, 20332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, C.; Shetti, D.; Wei, K. Erianin Inhibits Proliferation and Induces Apoptosis of HaCaT Cells via ROS-Mediated JNK/c-Jun and AKT/mTOR Signaling Pathways. Molecules 2019, 24, 2727. [Google Scholar] [CrossRef] [Green Version]
- Martinotti, S.; Laforenza, U.; Patrone, M.; Moccia, F.; Ranzato, E. Honey-Mediated Wound Healing: H₂O₂ Entry through AQP3 Determines Extracellular Ca2+ Influx. Int. J. Mol. Sci. 2019, 20, 764. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Chen, G.; Yu, K.N.; Yang, M.; Peng, S.; Ma, J.; Qin, F.; Cao, W.; Cui, S.; Nie, L.; et al. Correction: Cold atmospheric plasma induces GSDME-dependent pyroptotic signaling pathway via ROS generation in tumor cells. Cell Death Dis. 2022, 13, 690. [Google Scholar] [CrossRef]
- Cheraghi, G.; Hajiabedi, E.; Niaghi, B.; Nazari, F.; Naserzadeh, P.; Hosseini, M.J. High doses of sodium tungstate can promote mitochondrial dysfunction and oxidative stress in isolated mitochondria. J. Biochem. Mol. Toxicol. 2019, 33, e22266. [Google Scholar] [CrossRef]
- Cao, X.; Fu, M.; Bi, R.; Zheng, X.; Fu, B.; Tian, S.; Liu, C.; Li, Q.; Liu, J. Cadmium induced BEAS-2B cells apoptosis and mitochondria damage via MAPK signaling pathway. Chemosphere 2021, 263, 128346. [Google Scholar] [CrossRef]
- Azmi, L.; Shukla, I.; Goutam, A.; Allauddin, R.C.V.; Jawaid, T.; Kamal, M.; Awaad, A.S.; Alqasoumi, S.I.; AlKhamees, O.A. In vitro wound healing activity of 1-hydroxy-5,7-dimethoxy-2-naphthalene-carboxaldehyde (HDNC) and other isolates of Aegle marmelos L: Enhances keratinocytes motility via Wnt/β-catenin and RAS-ERK pathways. Saudi Pharm. J. 2019, 27, 532–539. [Google Scholar] [CrossRef]
- Misiura, M.; Baszanowska, W.; Ościłowska, I.; Pałka, J.; Miltyk, W. Prolidase Stimulates Proliferation and Migration through Activation of the PI3K/Akt/mTOR Signaling Pathway in Human Keratinocytes. Int. J. Mol. Sci. 2020, 21, 9243. [Google Scholar] [CrossRef]
- Kim, D.; Ku, B.; Choi, E.M. Se-methylselenocysteine stimulates migration and antioxidant response in HaCaT keratinocytes: Implications for wound healing. J. Trace Elem. Med. Biol. 2020, 58, 126426. [Google Scholar] [CrossRef]
- Li, Y.; Lv, Y.; Zhu, Y.; Yang, X.; Lin, B.; Li, M.; Zhou, Y.; Tan, Z.; Choi, E.H.; Wang, J.; et al. Low-Temperature Plasma-Activated Medium Inhibited Proliferation and Progression of Lung Cancer by Targeting the PI3K/Akt and MAPK Pathways. Oxid. Med. Cell Longev. 2022, 2022, 9014501. [Google Scholar] [CrossRef]
- Liu, Y.; Piao, X.J.; Xu, W.T.; Zhang, Y.; Zhang, T.; Xue, H.; Li, Y.N.; Zuo, W.B.; Sun, G.; Fu, Z.R.; et al. Calycosin induces mitochondrial-dependent apoptosis and cell cycle arrest, and inhibits cell migration through a ROS-mediated signaling pathway in HepG2 hepatocellular carcinoma cells. Toxicol. In Vitro 2021, 70, 105052. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, X.; Song, D.; Liu, Y.; Kong, W. Anticancer activity of Eremanthin against the human cervical cancer cells is due to G2/M phase cell cycle arrest, ROS-mediated necrosis-like cell death and inhibition of PI3K/AKT signalling pathway. J. BUON 2020, 25, 1547–1553. [Google Scholar]
- Karki, S.B.; Gupta, T.T.; Yildirim-Ayan, E.; Eisenmann, K.M.; Ayan, H. Miniature Non-thermal Plasma Induced Cell Cycle Arrest and Apoptosis in Lung Carcinoma Cells. Plasma Chem. Plasma Process. 2019, 40, 99–117. [Google Scholar] [CrossRef]
- Cui, H.S.; Cho, Y.S.; Joo, S.Y.; Mun, C.H.; Seo, C.H.; Kim, J.B. Wound Healing Potential of Low Temperature Plasma in Human Primary Epidermal Keratinocytes. Tissue Eng. Regen. Med. 2019, 16, 585–593. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Wang, H.; Liu, S.; Hu, N.; Li, X. AKT Regulated Phosphorylation of GSK-3β/Cyclin D1, p21 and p27 Contributes to Cell Proliferation Through Cell Cycle Progression From G1 to S/G2M Phase in Low-Dose Arsenite Exposed HaCat Cells. Front. Pharmacol. 2019, 10, 1176. [Google Scholar] [CrossRef] [Green Version]
- Miao, X.; Xiang, Y.; Mao, W.; Chen, Y.; Li, Q.; Fan, B. TRIM27 promotes IL-6-induced proliferation and inflammation factor production by activating STAT3 signaling in HaCaT cells. Am. J. Physiol. Cell Physiol. 2020, 318, C272–C281. [Google Scholar] [CrossRef]
- He, L.; Zhu, C.; Jia, J.; Hao, X.Y.; Yu, X.Y.; Liu, X.Y.; Shu, M.G. ADSC-Exos containing MALAT1 promotes wound healing by targeting miR-124 through activating Wnt/β-catenin pathway. Biosci. Rep. 2020, 40, BSR20192549. [Google Scholar] [CrossRef]
- Mendoza-Reinoso, V.; Beverdam, A. Epidermal YAP activity drives canonical WNT16/β-catenin signaling to promote keratinocyte proliferation in vitro and in the murine skin. Stem. Cell Res. 2018, 29, 15–23. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y. Crosstalk Between Peroxisome Proliferator-Activated Receptor Gamma and the Canonical WNT/β-Catenin Pathway in Chronic Inflammation and Oxidative Stress During Carcinogenesis. Front. Immunol. 2018, 9, 745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, L.; Duan, J.; Zhang, S.; Liu, X.; Poorun, D.; Liu, X.; Lu, X.; Duan, X.; Liu, D.; Chen, H. Cold atmospheric plasma ameliorates imiquimod-induced psoriasiform dermatitis in mice by mediating antiproliferative effects. Free Radic. Res. 2019, 53, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.K.; Adhikari, M.; Kumar, S.; Ghimire, B.; Han, I.; Kim, M.H.; Choi, E.H. Cold atmospheric plasma generated reactive species aided inhibitory effects on human melanoma cells: An in vitro and in silico study. Sci. Rep. 2020, 10, 3396. [Google Scholar] [CrossRef] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Zhang, Y.; Zhou, Y.; Yan, Z.; Chen, J.; Lu, T.; Song, W. Low-Dose Non-Thermal Atmospheric Plasma Promotes the Proliferation and Migration of Human Normal Skin Cells. Appl. Sci. 2023, 13, 2866. https://doi.org/10.3390/app13052866
Wu H, Zhang Y, Zhou Y, Yan Z, Chen J, Lu T, Song W. Low-Dose Non-Thermal Atmospheric Plasma Promotes the Proliferation and Migration of Human Normal Skin Cells. Applied Sciences. 2023; 13(5):2866. https://doi.org/10.3390/app13052866
Chicago/Turabian StyleWu, Hui, Yan Zhang, Yuanyuan Zhou, Zhuna Yan, Jinwu Chen, Tingting Lu, and Wencheng Song. 2023. "Low-Dose Non-Thermal Atmospheric Plasma Promotes the Proliferation and Migration of Human Normal Skin Cells" Applied Sciences 13, no. 5: 2866. https://doi.org/10.3390/app13052866
APA StyleWu, H., Zhang, Y., Zhou, Y., Yan, Z., Chen, J., Lu, T., & Song, W. (2023). Low-Dose Non-Thermal Atmospheric Plasma Promotes the Proliferation and Migration of Human Normal Skin Cells. Applied Sciences, 13(5), 2866. https://doi.org/10.3390/app13052866




