Does the Nature of Added Bioactive Lipids Affect the Biological Properties of Yogurts?—Case Study Coconut and Avocado Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Bigel Production
2.3. Yogurt Production
2.4. Fatty Acids Profile
2.5. In Vitro Digestion
2.6. Biological Properties
2.6.1. Cell Lines
2.6.2. Metabolic Activity
2.6.3. Hepatic Lipid Accumulation
2.6.4. Adipolysis
2.6.5. Adipokines Secretion
2.6.6. Immunomodulation in Raw 264.7
2.7. Statistical Analysis
3. Results and Discussion
3.1. Fatty Acids Profile
3.2. Impact of In Vitro Digestion on Fatty Acids Profile
3.3. Biological Properties
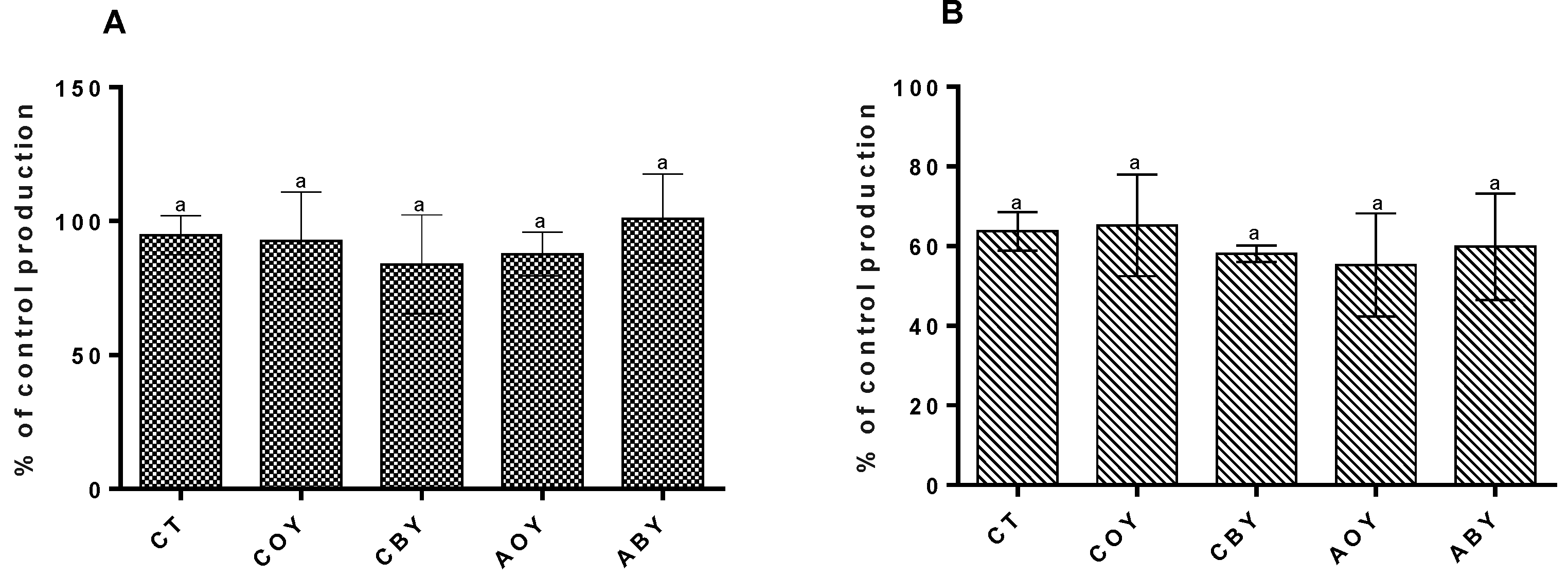
3.3.1. Metabolic Activity
3.3.2. Impact on Hepatic Lipid Accumulation
3.3.3. Impact on Adipolysis and Adipokines Secretion
3.3.4. Immunomodulation Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vázquez, L.; Corzo-martínez, M.; Arranz-martínez, P.; Barroso, E.; Reglero, G.; Torres, C. Bioactive Lipids. In Bioactive Molecules in Food; Mérillon, J.M., Ramawat, K.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–61. ISBN 9783319545288. [Google Scholar]
- De Carvalho, C.C.; Caramujo, M.J. The various roles of fatty acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef] [Green Version]
- Aluko, R. Bioactive Lipids 2 2.1. In Functional Foods and Nutraceuticals; Springer: Berlin/Heidelberg, Germany, 2012; pp. 23–35. ISBN 9781461434801. [Google Scholar]
- Chen, B.; McClements, D.J.; Decker, E.A. Design of Foods with Bioactive Lipids for Improved Health. Annu. Rev. Food Sci. Technol. 2012, 4, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Zabielski, P.; Ksi, M. The Impact of OMEGA-3 Fatty Acids Supplementation on Insulin Resistance and Content of Adipocytokines and Biologically Active Lipids in Adipose Tissue of High-Fat Diet Fed Rats. Nutrients 2019, 11, 835. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot. Essent. Fat. Acids 2008, 79, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S. Omega-3 fatty acids and cardiovascular disease: A case for omega-3 index as a new risk factor. Pharmacol. Res. 2007, 55, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Kidd, P.M. Omega-3 DHA and EPA for Cognition, Behavior, and Mood: Clinical Findings and Structural-Functional Synergies with Cell Membrane Phospholipids. Altern. Med. Rev. 2007, 12, 207–227. [Google Scholar] [CrossRef] [Green Version]
- Gladine, C.; Newman, J.W.; Durand, T.; Pedersen, T.L.; Galano, J.M.; Demougeot, C.; Berdeaux, O.; Pujos-Guillot, E.; Mazur, A.; Comte, B. Lipid profiling following intake of the omega 3 fatty acid DHA identifies the peroxidized metabolites F4-neuroprostanes as the best predictors of atherosclerosis prevention. PLoS ONE 2014, 9, e89393. [Google Scholar] [CrossRef]
- Phillips, M.C. Molecular mechanisms of cellular cholesterol efflux. J. Biol. Chem. 2014, 289, 24020–24029. [Google Scholar] [CrossRef] [Green Version]
- Gumus, C.E.; Gharibzahedi, S.M.T. Yogurts supplemented with lipid emulsions rich in omega-3 fatty acids: New insights into the fortification, microencapsulation, quality properties, and health-promoting effects. Trends Food Sci. Technol. 2021, 110, 267–279. [Google Scholar] [CrossRef]
- Jamshidi, A.; Cao, H.; Xiao, J.; Simal-Gandara, J. Advantages of techniques to fortify food products with the benefits of fish oil. Food Res. Int. 2020, 137, 109353. [Google Scholar] [CrossRef]
- Donovan, S.M.; Rao, G. Health benefits of yogurt among infants and toddlers aged 4 to 24 months: A systematic review. Nutr. Rev. 2019, 77, 478–486. [Google Scholar] [CrossRef]
- Kok, C.R.; Hutkins, R. Yogurt and other fermented foods as sources of health-promoting bacteria. Nutr. Rev. 2018, 76, 4–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.X.; Chong, G.H.; Hamzah, H.; Ghazali, H.M. Hypocholesterolaemic and hepatoprotective effects of virgin avocado oil in diet-induced hypercholesterolaemia rats. Int. J. Food Sci. Technol. 2018, 53, 2706–2713. [Google Scholar] [CrossRef] [Green Version]
- Cervantes-Paz, B.; Yahia, E.M. Avocado oil: Production and market demand, bioactive components, implications in health, and tendencies and potential uses. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4120–4158. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Sousa, S.; Morais, P.; Miranda, A.; Rodriguez-Alcalá, L.M.; Gomes, A.M.; Pintado, M. Novel avocado oil-functionalized yogurt with anti-obesity potential: Technological and nutraceutical perspectives. Food Biosci. 2022, 50, 101983. [Google Scholar] [CrossRef]
- Machado, M.; Sousa, S.; Morais, P.; Miranda, A.; Rodríguez-Alcalá, L.M.; Gomes, A.M.; Pintado, M.M. Medium-chain triglycerides and conjugated linolenic acids in functional yogurts: Impact of GIT and potential biological activities. Food Funct. 2022, 13, 10937–10946. [Google Scholar] [CrossRef] [PubMed]
- Rial, S.A.; Jutras-Carignan, A.; Bergeron, K.F.; Mounier, C. A high-fat diet enriched in medium chain triglycerides triggers hepatic thermogenesis and improves metabolic health in lean and obese mice. Biochim. Biophys. Acta–Mol. Cell Biol. Lipids 2020, 1865, 158582. [Google Scholar] [CrossRef]
- Thomas, D.D.; Stockman, M.C.; Yu, L.; Meshulam, T.; McCarthy, A.C.; Ionson, A.; Burritt, N.; Deeney, J.; Cabral, H.; Corkey, B.; et al. Effects of medium chain triglycerides supplementation on insulin sensitivity and beta cell function: A feasibility study. PLoS ONE 2019, 14, e0226200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djurasevic, S.; Bojic, S.; Nikolic, B.; Dimkic, I.; Todorovic, Z.; Djordjevic, J.; Mitic-Culafic, D. Beneficial Effect of Virgin Coconut Oil on Alloxan-Induced Diabetes and Microbiota Composition in Rats. Plant Foods Hum. Nutr. 2018, 73, 295–301. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of omega-3 fatty acids on the gut microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef] [Green Version]
- Shakeel, A.; Farooq, U.; Gabriele, D.; Marangoni, A.G.; Lupi, F.R. Bigels and multi-component organogels: An overview from rheological perspective. Food Hydrocoll. 2021, 111, 106190. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional indices for assessing fatty acids: A mini-review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Costa, E.M.; Silva, S.; Rodriguez-Alcalá, L.M.; Gomes, A.M.; Pintado, M. Pomegranate Oil’s Potential as an Anti-Obesity Ingredient. Molecules 2022, 27, 4958. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.; Machado, M.; Pintado, M.; Silva, S. Biological macromolecules as immunomodulators. In Biological Macromolecules; Elsevier: Amsterdam, The Netherlands, 2022; pp. 273–287. [Google Scholar]
- Ribeiro, T.B.; Bonifácio-Lopes, T.; Morais, P.; Miranda, A.; Nunes, J.; Vicente, A.A.; Pintado, M. Incorporation of olive pomace ingredients into yoghurts as a source of fibre and hydroxytyrosol: Antioxidant activity and stability throughout gastrointestinal digestion. J. Food Eng. 2021, 297, 110476. [Google Scholar] [CrossRef]
- Ahmad, N.; Manzoor, M.F.; Shabbir, U.; Ahmed, S.; Ismail, T.; Saeed, F.; Nisa, M.U.; Anjum, F.M.; Hussain, S. Health lipid indices and physicochemical properties of dual fortified yogurt with extruded flaxseed omega fatty acids and fibers for hypercholesterolemic subjects. Food Sci. Nutr. 2020, 8, 273–280. [Google Scholar] [CrossRef] [Green Version]
- ISO-10993-5; Biological Evaluation of Medical Devices–Tests for In Vitro Cytotoxicity. International Organization Standardization: Geneva, Switzerland, 2009; Volume 2007, pp. 1–11.
- DeVito, L.M.; Dennis, E.A.; Kahn, B.B.; Shulman, G.I.; Witztum, J.L.; Sadhu, S.; Nickels, J.; Spite, M.; Smyth, S.; Spiegel, S. Bioactive lipids and metabolic syndrome—A symposium report. Ann. N. Y. Acad. Sci. 2022, 1511, 87–106. [Google Scholar] [CrossRef]
- Wang, B.; Fu, J.; Li, L.; Gong, D.; Wen, X.; Yu, P.; Zeng, Z. Medium-chain fatty acid reduces lipid accumulation by regulating expression of lipid-sensing genes in human liver cells with steatosis. Int. J. Food Sci. Nutr. 2016, 67, 288–297. [Google Scholar] [CrossRef]
- Giulitti, F.; Petrungaro, S.; Mandatori, S.; Tomaipitinca, L.; de Franchis, V.; D’Amore, A.; Filippini, A.; Gaudio, E.; Ziparo, E. Anti-tumor Effect of Oleic Acid in Hepatocellular Carcinoma Cell Lines via Autophagy Reduction. Front. Cell Dev. Biol. 2021, 9, 629182. [Google Scholar] [CrossRef]
- Zeng, X.; Zhu, M.; Liu, X.; Chen, X.; Yuan, Y.; Li, L.; Liu, J.; Lu, Y.; Cheng, J.; Chen, Y. Erratum: Oleic acid ameliorates palmitic acid induced hepatocellular lipotoxicity by inhibition of ER stress and pyroptosis. Nutr. Metab. 2020, 17, 11. [Google Scholar] [CrossRef] [Green Version]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef] [PubMed]
- Granados, N.; Amengual, J.; Ribot, J.; Palou, A.; Luisa Bonet, M. Distinct effects of oleic acid and its trans-isomer elaidic acid on the expression of myokines and adipokines in cell models. Br. J. Nutr. 2011, 105, 1226–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bueno, A.A.; Oyama, L.M.; De Oliveira, C.; Pisani, L.P.; Ribeiro, E.B.; Silveira, V.L.F.; Oller Do Nascimento, C.M. Effects of different fatty acids and dietary lipids on adiponectin gene expression in 3T3-L1 cells and C57BL/6J mice adipose tissue. Pflugers Arch. Eur. J. Physiol. 2008, 455, 701–709. [Google Scholar] [CrossRef] [PubMed]
- de Vasconcelos, M.H.A.; Tavares, R.L.; Torres Junior, E.U.; Dorand, V.A.M.; Batista, K.S.; Toscano, L.T.; Silva, A.S.; de Magalhães Cordeiro, A.M.T.; de Albuquerque Meireles, B.R.L.; da Silva Araujo, R.; et al. Extra virgin coconut oil (Cocos nucifera L.) exerts anti-obesity effect by modulating adiposity and improves hepatic lipid metabolism, leptin and insulin resistance in diet-induced obese rats. J. Funct. Foods 2022, 94, 105122. [Google Scholar] [CrossRef]
- Han, J.; Hamilton, J.A.; Kirkland, J.L.; Corkey, B.E.; Guo, W. Medium-chain oil reduces fat mass and down-regulates expression of adipogenic genes in rats. Obes. Res. 2003, 11, 734–744. [Google Scholar] [CrossRef]
- Scoditti, E.; Massaro, M.; Carluccio, M.A.; Pellegrino, M.; Wabitsch, M.; Calabriso, N.; Storelli, C.; De Caterina, R. Additive regulation of adiponectin expression by the mediterranean diet olive oil components oleic acid and hydroxytyrosol in human adipocytes. PLoS ONE 2015, 10, e0128218. [Google Scholar] [CrossRef]
- Ravaut, G.; Légiot, A.; Bergeron, K.F.; Mounier, C. Monounsaturated fatty acids in obesity-related inflammation. Int. J. Mol. Sci. 2021, 22, 330. [Google Scholar] [CrossRef]
- Rohman, A.; Irnawati; Erwanto, Y.; Lukitaningsih, E.; Rafi, M.; Fadzilah, N.A.; Windarsih, A.; Sulaiman, A.; Zakaria, Z. Virgin Coconut Oil: Extraction, Physicochemical Properties, Biological Activities and Its Authentication Analysis. Food Rev. Int. 2021, 37, 46–66. [Google Scholar] [CrossRef]
- Deen, A.; Visvanathan, R.; Wickramarachchi, D.; Marikkar, N.; Nammi, S.; Jayawardana, B.C.; Liyanage, R. Chemical composition and health benefits of coconut oil: An overview. J. Sci. Food Agric. 2021, 101, 2182–2193. [Google Scholar] [CrossRef]
- Vysakh, A.; Ratheesh, M.; Rajmohanan, T.P.; Pramod, C.; Premlal, S.; Girish Kumar, B.; Sibi, P.I. Polyphenolics isolated from virgin coconut oil inhibits adjuvant induced arthritis in rats through antioxidant and anti-inflammatory action. Int. Immunopharmacol. 2014, 20, 124–130. [Google Scholar] [CrossRef]
- Zicker, M.C.; Silveira, A.L.M.; Lacerda, D.R.; Rodrigues, D.F.; Oliveira, C.T.; de Souza Cordeiro, L.M.; Lima, L.C.F.; Santos, S.H.S.; Teixeira, M.M.; Ferreira, A.V.M. Virgin Coconut Oil Is Effective to Treat Metabolic and Inflammatory Dysfunction Induced by High Refined Carbohydrate-Containing Diet in Mice; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 63, ISBN 3134099853. [Google Scholar]
- Jose, S.P.; kumar, K.; Sandya, S.S.; Rajmohan, R.V. Polyphenolic fraction of virgin coconut oil inhibits the inflammatory response in oxidized LDL activated human peripheral blood mononuclear cells by modulating TLR/NF-κB signaling pathways. Eur. J. Integr. Med. 2017, 10, 59–65. [Google Scholar] [CrossRef]




| CT | COY | CBY | AOY | ABY | |
|---|---|---|---|---|---|
| C6 | n.d. | 0.08 ± 0.04 a | 0.01 ± 0.01 b | n.d. | n.d. |
| C8 | n.d. | 1.36 ± 0.61 a | 0.20 ± 0.02 b | n.d. | n.d. |
| C10 | 0.02 ± 0.01 b | 1.27 ± 0.54 a | 0.20 ± 0.02 b | n.d. | n.d. |
| C12 | 0.03 ± 0.02 b | 1.47 ± 0.20 a | 2.16 ± 0.60 a | n.d. | n.d. |
| C14 | 0.11 ± 0.07 b | 5.51 ± 2.05 a | 0.92 ± 0.08 b | 0.04 ± 0.001 b | 0.04 ± 0.001 b |
| C16 | 0.36 ± 0.22 b | 2.50 ± 0.85 a | 0.84 ± 0.08 b | 0.68 ± 0.36 b | 1.38 ± 0.56 ab |
| C16:1 | n.d. | 0.01 ± 0.001 b | n.d. | 0.15 ± 0.09 ab | 0.29 ± 0.13 a |
| C18 | 0.12 ± 0.07 c | 1.01 ± 0.33 a | 0.60 ± 0.06 ab | 0.11 ± 0.04 c | 0.32 ± 0.11 bc |
| C18:1 c9 | 0.24 ± 0.14 b | 1.64 ± 0.58 ab | 0.65 ± 0.05 b | 2.08 ± 1.29 ab | 4.10 ± 1.78 a |
| C18:1 c11 | n.d. | n.d. | n.d. | 0.16 ± 0.10 a | 0.30 ± 0.14 a |
| C18:2 | 0.03 ± 0.01 b | 0.22 ± 0.19 b | 0.06 ± 0.01 b | 0.44 ± 0.28 ab | 0.85 ± 0.38 a |
| C18:3 c6c9c12 | n.d. | 0.09 ± 0.10 a | n.d. | 0.02 ± 0.01 a | 0.03 ± 0.01 a |
| C20:1 | n.d. | n.d. | n.d. | n.d. | 0.02 ± 0.01 |
| C18:3 c9c13c15 | n.d. | n.d. | n.d. | n.d. | 0.02 ± 0.01 |
| ∑Fatty acids | 0.91 ± 0.53 b | 15.16 ± 4.99 a | 5.62 ± 0.88 b | 3.69 ± 2.16 b | 7.35 ± 3.13 b |
| Nutritional Quality Indexes | |||||
| AI | 3.52 ± 0.07 c | 13.23 ± 0.11 a | 9.43 ± 0.76 b | 0.31 ± 0.05 d | 0.28 ± 0.02 d |
| TI | 4.37 ± 0.12 c | 9.15 ± 0.07 a | 7.30 ± 0.20 b | 0.61 ± 0.08 d | 0.60 ± 0.03 d |
| HH | 2.82 ± 0.13 b | 0.23 ± 0.02 c | 0.40 ± 0.01 c | 3.60 ± 0.42 a | 3.67 ± 0.16 a |
| HPI | 0.33 ± 0.01 b | 0.08 ± 0.001 b | 0.11 ± 0.01 b | 3.28 ± 0.58 a | 3.56 ± 0.26 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, M.; Rodríguez-Alcalá, L.M.; Maria Gomes, A.; Pintado, M. Does the Nature of Added Bioactive Lipids Affect the Biological Properties of Yogurts?—Case Study Coconut and Avocado Oils. Appl. Sci. 2023, 13, 3101. https://doi.org/10.3390/app13053101
Machado M, Rodríguez-Alcalá LM, Maria Gomes A, Pintado M. Does the Nature of Added Bioactive Lipids Affect the Biological Properties of Yogurts?—Case Study Coconut and Avocado Oils. Applied Sciences. 2023; 13(5):3101. https://doi.org/10.3390/app13053101
Chicago/Turabian StyleMachado, Manuela, Luís M. Rodríguez-Alcalá, Ana Maria Gomes, and Manuela Pintado. 2023. "Does the Nature of Added Bioactive Lipids Affect the Biological Properties of Yogurts?—Case Study Coconut and Avocado Oils" Applied Sciences 13, no. 5: 3101. https://doi.org/10.3390/app13053101
APA StyleMachado, M., Rodríguez-Alcalá, L. M., Maria Gomes, A., & Pintado, M. (2023). Does the Nature of Added Bioactive Lipids Affect the Biological Properties of Yogurts?—Case Study Coconut and Avocado Oils. Applied Sciences, 13(5), 3101. https://doi.org/10.3390/app13053101










