Recent Advances in H2S Removal from Gas Streams
Abstract
:1. Introduction
2. The Technologies
2.1. Absorption Processes to Remove Hazardous H2S from Gas Streams
2.2. Adsorption Processes to Remove Hazardous H2S from Gas Streams
2.3. Membranes and Membrane Contactors to Remove Hazardous H2S from Gas Streams
3. Simultaneous and Selective H2S–CO2 Removal: A Case Study
| Technology | Characteristics | Industrial Use | Reference |
|---|---|---|---|
| Ionic liquid absorption | Azole-based protic ionic liquids | No | [66] |
| Inorganic membranes | Ceramic-based | No | [73] |
| Carbon molecular sieve | No | ||
| Hybrid membrane | Metal organic framework-polyimide mixed | No | [67] |
| Adsorption on pristine materials | Molecular-sieve bases materials | Yes | [26,68] |
| Adsorption on composite materials | Metal oxide/silica | No | [26,68] |
| Metal oxide/activated carbon | No |
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, G.; Bhat, S.A.; Li, W.; Ishiguro, Y.; Wei, Y.; Li, F. H2S, MeSH, and NH3 emissions from activated sludge: An insight towards sludge characteristics and microbial mechanisms. Int. Biodeterior. Biodegrad. 2022, 166, 105331. [Google Scholar]
- Han, Z.; Qi, F.; Li, R.; Wang, H.; Sun, D. Health impact of ordor from on-situ sewage sludge aerobic composting throughout different seasons and during anaerobic digestion with hydrolysis pretreatment. Chemosphere 2020, 249, 126077. [Google Scholar]
- Wang, Y.-C.; Han, M.-F.; Jia, T.-P.; Hu, X.-R.; Zhu, H.-Q.; Tong, Z.; Lin, Y.-T.; Wang, C.; Liu, D.-Z.; Peng, Y.-Z.; et al. Emissions, measurement, and control of odor in livestock farms: A review. Sci. Total Environ. 2021, 776, 145735. [Google Scholar]
- Konkol, D.; Popiela, E.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Moustakas, K.; Opaliński, S.; Korczyński, M.; Witek-Krowiak, A.; Chojnacka, K. Recent innovations in various methods of harmful gases conversion and its mechanism in poultry farms. Environ. Res. 2022, 21, 113825. [Google Scholar]
- de Oliveira Carneiro, L.; de Vasconcelos, S.F.; de Farias Neto, G.W.; Brito, R.P.; Brito, K.D. Improving H2S removal in the coke oven gas purification process. Sep. Purif. Technol. 2021, 257, 117862. [Google Scholar]
- Lee, W.Y.; Park, S.Y.; Lee, K.B.; Nam, S.C. Simultaneous removal of CO2 and H2S from biogas by blending amine adsorbents: A performance comparison study. Energy Fuel. 2020, 34, 1992–2000. [Google Scholar]
- Chan, Y.H.; Loh, S.K.; Chin, B.L.F.; Yiin, C.L.; How, B.S.; Cheah, K.W.; Wong, M.K.; Loy, A.C.M.; Gwee, Y.L.; Lo, S.L.Y.; et al. Fractionation and extraction of bio-oil for production of greener fuel and value-added chemicals: Recent advances and future prospects. Chem. Eng. J. 2020, 397, 125406. [Google Scholar]
- Tian, X.F.; Wang, L.M.; Zhang, P.; Fu, D.; Wang, Z.Y. A high efficient absorbent for the separation of H2S from low partial pressure coke oven gas. Environ. Sci. Pollut. Control Ser. 2021, 28, 5822–5832. [Google Scholar]
- Wibowo, H.; Susanto, H.; Grisdanurak, N.; Hantoko, D.; Yoshikawa, K.; Qun, H.; Yan, M. Recent development of deep eutectic solvent as absorbent for CO2 removal from syngas produced from gasification: Current status, challenges, and further research. J. Environ. Chem. Eng. 2021, 9, 105439. [Google Scholar]
- Wang, B.; Cheng, J.; Wang, D.; Li, X.; Meng, Q.; Zhang, Z.; An, J.; Liu, X.; Li, M. Study on the desulfurization and regeneration performance of functional deep eutectic solvents. ACS Omega 2020, 5, 15353–15361. [Google Scholar]
- Liu, X.; Wang, B.; Dong, X.; Qiu, Y.; Meng, Q. Enhancement effect of nanofluids on the desulfurization and regeneration performance of ionic liquid-based system. J. Hazard Mater. 2021, 419, 126394. [Google Scholar]
- Liu, X.; Wang, B.; Lv, X.; Meng, Q.; Li, M. Enhanced removal of hydrogen sulfide using novel nanofluid system composed of deep eutectic solvent and Cu nanoparticles. J. Hazard Mater. 2021, 405, 124271. [Google Scholar]
- Kang, J.-H.; Yoon, Y.; Song, J. Effects of pH on the simultaneous removal of hydrogen sulfide and ammonia in a combined absorption and electro-oxidation system. J. Hazard Mater. 2020, 382, 121011. [Google Scholar]
- Marín, D.; Vega, M.; Lebrero, R.; Muñoz, R. Optimization of a chemical scrubbing process based on a Fe-EDTA-carbonate based solvent for the simultaneous removal of CO2 and H2S from biogas. J. Water Proc. Eng. 2020, 37, 101476. [Google Scholar]
- Zhan, J.; Wang, B.; Zhang, L.; Sun, B.-C.; Fu, J.; Chu, G.-w.; Zou, H. Simultaneous absorption of H2S and CO2 into the MDEA + PZ aqueous solution in a rotating packed bed. Ind. Eng. Chem. Res. 2020, 59, 8295–8303. [Google Scholar]
- Zhang, C.; Wang, X.; Liu, H.; Liu, C.; Li, S.; Xue, J.; Zeng, X. Development and application of modified lye for treating hydrogen sulphide in coal mine. Fuel 2020, 269, 117233. [Google Scholar]
- Mei, Y.; Dai, J.; Wang, X.; Nie, Y.; He, D. Novel low-temperature H2S removal technology by developing yellow phosphorus and phosphate rock slurry as absorbent. J. Hazard Mater. 2021, 413, 125386. [Google Scholar]
- Alkhatib, I.I.I.; Ferreira, M.L.; Alba, C.G.; Bahamon, D.; Llovell, F.; Pereiro, A.B.; Araújo, J.M.M.; Abu-Zahra, M.R.M.; Vega, L.F. Screening of ionic liquids and deep eutectic solvents for physical CO2 absorption by Soft-SAFT using key performance indicators. J. Chem. Eng. Data 2020, 65, 5844–5861. [Google Scholar]
- Liu, X.; Wang, B.; Qiu, Y.; Dong, X.; Song, Y.; Meng, Q.; Li, M. Study on the desulfurization performance of iron/ethanolamine/deep eutectic solvent system. Environ. Sci. Pollut. Control Ser. 2021, 28, 38026–38033. [Google Scholar]
- Zhang, M.; Dong, B.; Wu, Y.; Hu, H.; Huang, H. Mechanism study on H2S capture of ionic liquids based on triethylenetetramine blended with ethylene glycol. J. Molec. Liq. 2022, 368, 120704. [Google Scholar]
- Jafari, F.D.; Ameri, E. Effect of sodium dodecyl sulfate on CO2 and H2S absorption enhancement of functionalized multiwall carbon nanotubes in water: Experimental study and empirical model. Arabian J. Chem. 2022, 15, 104314. [Google Scholar]
- Askari, M.; Salehi, E.; Baluchi, A. Intensification of hydrogen sulfide absorption by diethanolamine in industrial scale via combined simulation and data-based optimization strategies. Chem. Eng. Res. Design 2022, 188, 545–554. [Google Scholar]
- Abdi, J.; Hadipoor, M.; Esmaeili-Faraj, S.H.; Vaferi, B. A modeling approach for estimating hydrogen sulfide solubility in fifteen different imidazole-based ionic liquids. Sci. Rep. 2022, 12, 4415. [Google Scholar]
- Abotaleb, A.; Gladich, I.; Alkhateeb, A.; Mardini, N.; Bicer, Y.; Sinopoli, A. Chemical and physical systems for sour gas removal: An overview from reaction mechanisms to industrial implications. J. Nat. Gas Sci. Eng. 2022, 10, 104755. [Google Scholar]
- Qiu, K.; Liu, Z.; Dong, Y.; Liu, L.; Li, W.; Niu, S.; Jin, Z. [Bmim]FeCl4 efficient catalytic oxidative removal of H2S by Cu2+ synergistic reinforcement. Chem. Eng. Technol. 2022, 45, 1867–1875. [Google Scholar]
- Georgiadis, A.G.; Charisiou, N.D.; Goula, M.A. Removal of hydrogen sulfide from various industrial gases: A review of the most promising adsorbing materials. Catalysts 2020, 10, 521. [Google Scholar]
- Florent, M.; Bandosz, T.J. Surfactant-modified biosolid-derived materials as efficient H2S removal media: Synergistic effects of carbon phase properties and inorganic phase chemistry on reactive adsorption. Chem. Eng. J. 2020, 401, 125986. [Google Scholar]
- Hassankiadeh, M.N.; Hallajisani, A. Application of molybdenum oxide nanoparticles in H2S removal from natural gas under different operational and geometrical conditions. J. Petrol. Sci. Eng. 2020, 190, 107131. [Google Scholar]
- Jafari, M.J.; Zendehdel, R.; Rafieepour, A.; Pour, M.N.; Irvani, H.; Khodakarim, S. Comparison of Y and ZSM-5 zeolite modified with magnetite nanoparticles in removal of hydrogen sulfide from air. Int. J. Environ. Sci. Technol. 2020, 17, 187–194. [Google Scholar]
- Mao, J.; Ma, Y.; Zang, L.; Xue, R.; Xiao, C.; Ji, D. Efficient adsorption of hydrogen sulfide at room temperature using fumed silica-supported deep eutectic solvents. Aerosol Air Qual. Res. 2020, 20, 203–215. [Google Scholar]
- Nicolae, S.A.; Szilágyi, P.Á.; Titirici, M.M. Soft templating production of porous carbon adsorbents for CO2 and H2S capture. Carbon 2020, 169, 193–204. [Google Scholar]
- Ahmadi, R.; Alivand, M.S.; Tehrani, N.H.M.H.; Ardjmand, M.; Rashidi, A.; Rafizadeh, M.; Seif, A.; Mollakazemi, F.; Noorpoor, Z.; Rudd, J. Preparation of fiber-like nanoporous carbon from jute thread waste for superior CO2 and H2S removal from natural gas: Experimental and DFT stusdy. Chem. Eng. J. 2021, 415, 129076. [Google Scholar]
- Wang, Y.; Ning, P.; Zhao, R.; Li, K.; Wang, C.; Sun, X.; Song, X.; Lin, Q. A Cu modified active carbon fiber significantly promoted H2S and PH3 simultaneous removal at a low reaction temperature. Front. Environ. Sci. Eng. 2021, 15, 132. [Google Scholar]
- Wang, S.; Nam, H.; Nam, H. Preparation of activated carbon from peanut shell with KOH activation and its application for H2S adsorption in confined space. J. Environ. Chem. Eng. 2020, 8, 103683. [Google Scholar]
- Gasquet, V.; Kim, B.; Sigot, L.; Benbelkacem, H. H2S adsorption from biogas with thermal treatment residues. Waste Biomass Valorization. 2020, 11, 5363–5373. [Google Scholar]
- Zhao, C.; Wang, B.; Theng, B.K.G.; Wu, P.; Liu, F.; Wang, S.; Lee, X.; Chen, M.; Li, L.; Zhang, X. Formation and mechanisms of nano-metal oxide-biochar composites for pollutants removal: A review. Sci. Total Environ. 2021, 767, 145305. [Google Scholar]
- Kozyatnyk, I.; Yacout, D.M.M.; Caneghem, J.V.; Jansson, S. Comparative environmental assessment of end-of-life carbonaceous water treatment adsorbents. Bioresour. Technol. 2020, 302, 122866. [Google Scholar]
- Nowrouzi, M.; Abyar, H.; Younesi, H.; Khaki, E. Life cycle environmental and economic assessment of highly efficient carbon-based CO2 adsorbents: A comparative study. J. CO2 Util. 2021, 47, 101491. [Google Scholar]
- Izhar, T.N.T.; Kee, G.Z.; Saad, F.N.M.; Rahim, S.Z.A.; Zakarya, I.A.; Besom, M.R.C.; Ib, M.; Syafiuddin, A. Adsorption of hydrogen sulfide (H2S) from municipal solid waste by using biochars. Bioint. Res. Appl. Chem. 2022, 12, 8057–8069. [Google Scholar]
- Alguacil, F.J.; Alonso, M.A.; Lopez, F.A.; Robla, J.I. Dynamic adsorption of H2S onto a goethite-based material. Molecules 2022, 27, 7983. [Google Scholar]
- Cai, X.; Yang, Q.; Tong, Y.; Wang, M.; Zhang, S. Metal-modified s-C3N6 as a potential superior sensing medium for effective capture of toxic waste gases CO, H2S and SO2 in the iron and steel industry based on first-principles investigations. Appl. Surf. Sc. 2022, 606, 154947. [Google Scholar]
- Cheng, W.-Y.; Chang, C.-R.; Fuh, H.-R. First principles study of NH3, H2S, Cl2, and C2H2 gases adsorption on defective GaSe monolayer. Appl. Surf. Sci. 2022, 606, 154722. [Google Scholar]
- Xu, Y.; Chen, Y.; Ma, C.; Qiao, W.; Wang, J.; Ling, L. Functionalization of activated carbon fiber mat with bimetallic active sites for NH3 and H2S adsorption at room temperature. Sep. Purif. Technol. 2022, 303, 122335. [Google Scholar]
- Juntarachat, N.; Onthong, U. Removal of hydrogen sulfide from biogas using banana peel and banana empty fruit bunch biochars as alternative adsorbents. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y. Preparation of hydrogen sulfide adsorbent derived from spent Fenton-like reagent modified biochar and its removal characteristics for hydrogen sulfide. Fuel Proc. Technol. 2022, 238, 107495. [Google Scholar]
- Cui, S.; Zhao, Y.; Liu, Y.; Pan, J. Preparation of copper-based biochar adsorbent with outstanding H2S adsorption capacity and study on H2S removal. J. Energy Inst. 2022, 105, 481–490. [Google Scholar]
- He, X.; Kumakiri, I.; Hillestad, M. Conceptual process design and simulation of membrane systems for integrated natural gas dehydration and sweetening. Sep. Purif. Technol. 2020, 247, 116993. [Google Scholar]
- Shi, Y.; Liang, B.; Lin, R.-B.; Zhang, C.; Chen, B. Gas separation via hybrid metalorganic framework/polymer membranes. Trends Chem. 2020, 2, 254–269. [Google Scholar]
- Han, Y.; Ho, W.S.W. Polymeric membranes for CO2 separation and capture. J. Membr. Sci. 2021, 628, 119244. [Google Scholar]
- Valappil, R.S.K.; Ghasem, N.; Al-Marzouqi, M. Current and future trends in polymer membrane-based gas separation technology: A comprehensive review. J. Ind. Eng. Chem. 2021, 98, 103–129. [Google Scholar]
- Yong, W.F.; Zhang, H. Recent advances in polymer blend membranes for gas separation and pervaporation. Prog. Mater. Sci. 2021, 116, 100713. [Google Scholar]
- Liu, Y.; Liu, Z.; Morisato, A.; Bhuwania, N.; Chinn, D.; Koros, W.J. Natural gas sweetening using a cellulose triacetate hollow fiber membrane illustrating controlled plasticization benefits. J. Membr. Sci. 2020, 601, 117910. [Google Scholar]
- Liu, Y.; Liu, Z.; Liu, G.; Qiu, W.; Bhuwania, N.; Chinn, D.; Koros, W.J. Surprising plasticization benefits in natural gas upgrading using polyimide membranes. J. Membr. Sci. 2020, 593, 117430. [Google Scholar]
- Harrigan, D.J.; Lawrence, J.A.; Reid, H.W.; Rivers, J.B.; O’Brien, J.T.; Sharber, S.A.; Sundell, B.J. Tunable sour gas separations: Simultaneous H2S and CO2 removal from natural gas via crosslinked telechelic poly(ethylene glycol) membranes. J. Membr. Sci. 2020, 602, 117947. [Google Scholar]
- Lawrence, J.A.; Harrigan, D.J.; Maroon, C.R.; Sharber, S.A.; Long, B.K.; Sundell, B.J. Promoting acid gas separations via strategic alkoxysilyl substitution of vinyladded poly(norbornene)s. J. Membr. Sci. 2020, 616, 118569. [Google Scholar]
- Yahaya, G.O.; Hayek, A.; Alsamah, A.; Shalabi, Y.A.; Sultan, M.M.B.; Alhajry, R.H. Copolyimide membranes with improved H2S/CH4 selectivity for high-pressure sour mixed-gas separation. Sep. Purif. Technol. 2021, 272, 118897. [Google Scholar]
- Shamsabadi, A.A.; Rezakazemi, M.; Seidi, F.; Riazi, H.; Aminabhavi, T.; Soroush, M. Next generation polymers of intrinsic microporosity with tunable moieties for ultrahigh permeation and precise molecular CO2 separation. Prog. Energy Combust. Sci. 2021, 84, 100903. [Google Scholar]
- Chuah, C.Y.; Kim, K.; Lee, J.; Koh, D.-Y.; Bae, T.-H. CO2 absorption using membrane contactors: Recent progress and future perspective. Ind. Eng. Chem. Res. 2020, 59, 6773–6794. [Google Scholar]
- Pang, H.; Chen, Z.; Gong, H.; Du, M. Fabrication of a super hydrophobic polyvinylidene fluoride-hexadecyltrimethoxysilane hybrid membrane for carbon dioxide absorption in a membrane contactor. J. Membr. Sci. 2020, 595, 117536. [Google Scholar]
- Zhang, H.; Xue, K.; Cheng, C.; Gao, D.; Chen, H. Study on the performance of CO2 capture from flue gas with ceramic membrane contactor. Sep. Purif. Technol. 2021, 265, 118521. [Google Scholar]
- Signorini, V.; Giacinti Baschetti, M.; Pizzi, D.; Merlo, L. Permeation of ternary mixture containing H2S, CO2 and CH4 in Aquivion® perfluorosulfonic acid (PFSA) ionomer membranes. Membranes 2022, 12, 1034. [Google Scholar]
- Das, J.; Lens, P.N.L. Resilience of hollow fibre membrane bioreactors for treating H2S under steady state and transient conditions. Chemosphere 2022, 307, 136142. [Google Scholar]
- Das, J.; Nolan, S.; Lens, P.N.L. Simultaneous removal of H2 and NH3 from raw biogas in hollow fibre membrane bioreactors. Environ. Technol. Innov. 2022, 28, 102777. [Google Scholar]
- Li, Y.; Li, F.; Zheng, M.; Yan, H.; Liu, Q. H2S adsorption performance of alkali lignocarbon/PVA composite membrane. Korean J. Chem. Eng. 2022, 39, 2368–2378. [Google Scholar]
- Ma, Y.; Liao, Y.; Su, Y.; Wang, B.; Yang, Y.; Ji, D.; Li, H.; Zhou, H.; Wang, D. Comparative investigation of different CO2 capture technologies for coal to ethylene glycol process. Processes 2021, 9, 207. [Google Scholar]
- Zhang, X.; Xiong, W.; Peng, L.; Wu, Y.; Hu, X. Highly selective absorption separation of H2S and CO2 from CH4 by novel azole-based protic ionic liquids. AIChE J. 2020, 66, 16936. [Google Scholar]
- Ahmad, M.Z.; Peters, T.A.; Konnertz, N.M.; Visser, T.; T’ellez, C.; Coronas, J.; Fila, V.; de Vos, W.M.; Benes, N.E. High-pressure CO2/CH4 separation of Zr-MOFs based mixed matrix membranes. Sep. Purif. Technol. 2020, 230, 115858. [Google Scholar]
- Liu, D.; Li, B.; Wu, J.; Liu, Y. Sorbents for hydrogen sulfide capture from biogas at low temperature: A review. Environ. Chem. Lett. 2020, 18, 113–128. [Google Scholar]
- Huertas, J.K.; Quipuzco, L.; Hassanein, A.; Lansing, S. Comparing hydrogen sulfide removal efficiency in a field-scale digester using microaeration and iron filters. Energies 2020, 13, 4793. [Google Scholar]
- Shaikh, A.R.; Posada-Pérez, S.; Brotons-Rufes, A.; Pajski, J.J.; Vajiha Kumar, G.; Mateen, A.; Poater, A.; Solà, M.; Chawla, M.; Cavallo, L. Selective absorption of H2S and CO2 by azole based protic ionic liquids: A combined density functional theory and molecular dynamics study. J. Molec. Liq. 2022, 367, 120558. [Google Scholar]
- Raksajati, A.; Purwanto, H.K.; Baskoro, A.N.; Indarto, A.; Ariono, D. Selective H2S absorption using the mixture of NaOH-NaHCO3-Na2CO3 buffer solvent solution. J. Eng. Technol. Sci. 2022, 54, 220513. [Google Scholar]
- Xue, J.; Yang, C.; Fu, J.; He, J.; Li, J. Research and performance evaluation on selective absorption of H2S from gas mixtures by using secondary alkanolamines. Processes 2022, 10, 1795. [Google Scholar]
- Makertihartha, I.G.B.N.; Kencana, K.S.; Dwiputra, T.R.; Khoiruddin, K.; Lugito, G.; Mukti, R.R.; Wenten, I.G. SAPO-34 zeotype membrane for gas sweetening. Rev. Chem. Eng. 2022, 38, 431–450. [Google Scholar]
- Rocher-Rivas, R.; González-Sánchez, A.; Ulloa-Mercado, G.; Muñoz, R.; Quijano, G. Biogas desulfurization and calorific value enhancement in compact H2S/CO2 absorption units coupled to a photobioreactor. J. Environ. Chem. Eng. 2022, 10, 108336. [Google Scholar]
- Agarwal, N.; Nhien, L.C.; Lee, M. Rate-based modeling and assessment of an amine-based acid gas removal process through a comprehensive solvent selection procedure. Energies 2022, 15, 6817. [Google Scholar]
- Moreira, L.C.; Borges, P.O.; Cavalcante, R.M.; Young, A.F. Simulation and economic evaluation of process alternatives for biogas production and purification from sugarcane vinasse. Renew. Sust. Energ. Rev. 2022, 163, 112532. [Google Scholar]
- Peng, L.; Shi, M.; Zhang, X.; Xiong, W.; Hu, X.; Tu, Z.; Wu, Y. Facilitated transport separation of CO2 and H2S by supported liquid membrane based on task-specific protic ionic liquids. Green Chem. Eng. 2022, 3, 259–266. [Google Scholar]
- Elfving, J.; Kauppinen, J.; Jegoroff, M.; Ruuskanen, V.; J¨arvinen, L.; Sainio, T. Experimental comparison of regeneration methods for CO2 concentration from air using amine-based adsorbent. Chem. Eng. J. 2021, 404, 126337. [Google Scholar]
- Hafeez, S.; Safdar, T.; Pallari, E.; Manos, G.; Aristodemou, E.; Zhang, Z.; Al-Salem, S.M.; Constantinou, A. CO2 capture using membrane contactors: A systematic literature review. Front. Chem. Sci. Eng. 2021, 15, 720–754. [Google Scholar]
- Nemestóthy, N.; Bakonyi, P.; Lajtai-Szabó, P.; Bélafi-Bakó, K. The impact of various natural gas contaminant exposures on CO2/CH4 separation by a polyimide membrane. Membranes 2020, 10, 324. [Google Scholar]
- Sun, M.-H.; Wang, X.-Z.; Zhao, Z.-B.; Qiu, J.-S. Review of H2S selective oxidation over carbon-based materials at low temperature: From pollutant to energy storage materials. N. Carbon Mater. 2022, 37, 675–694. [Google Scholar]
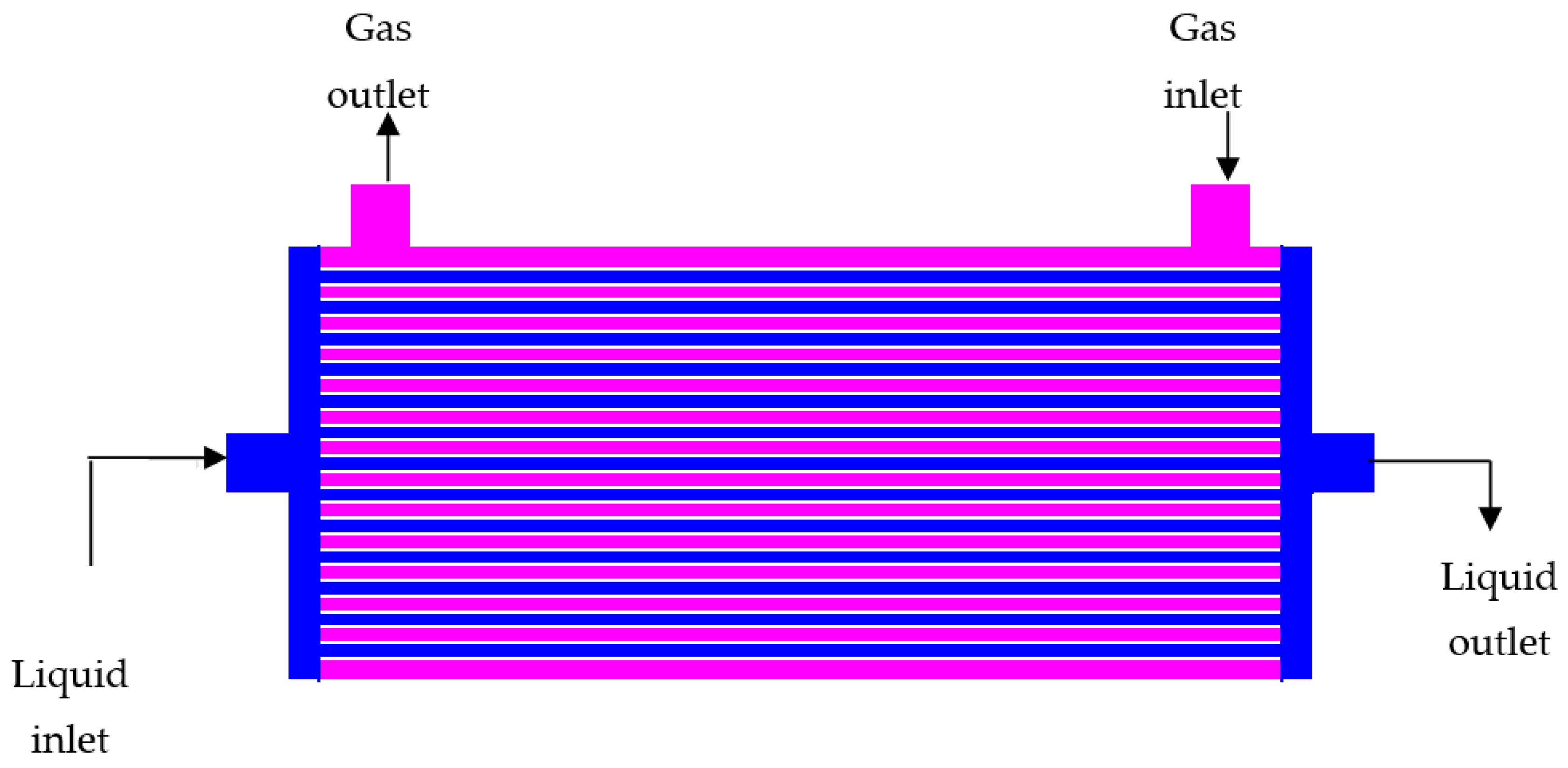
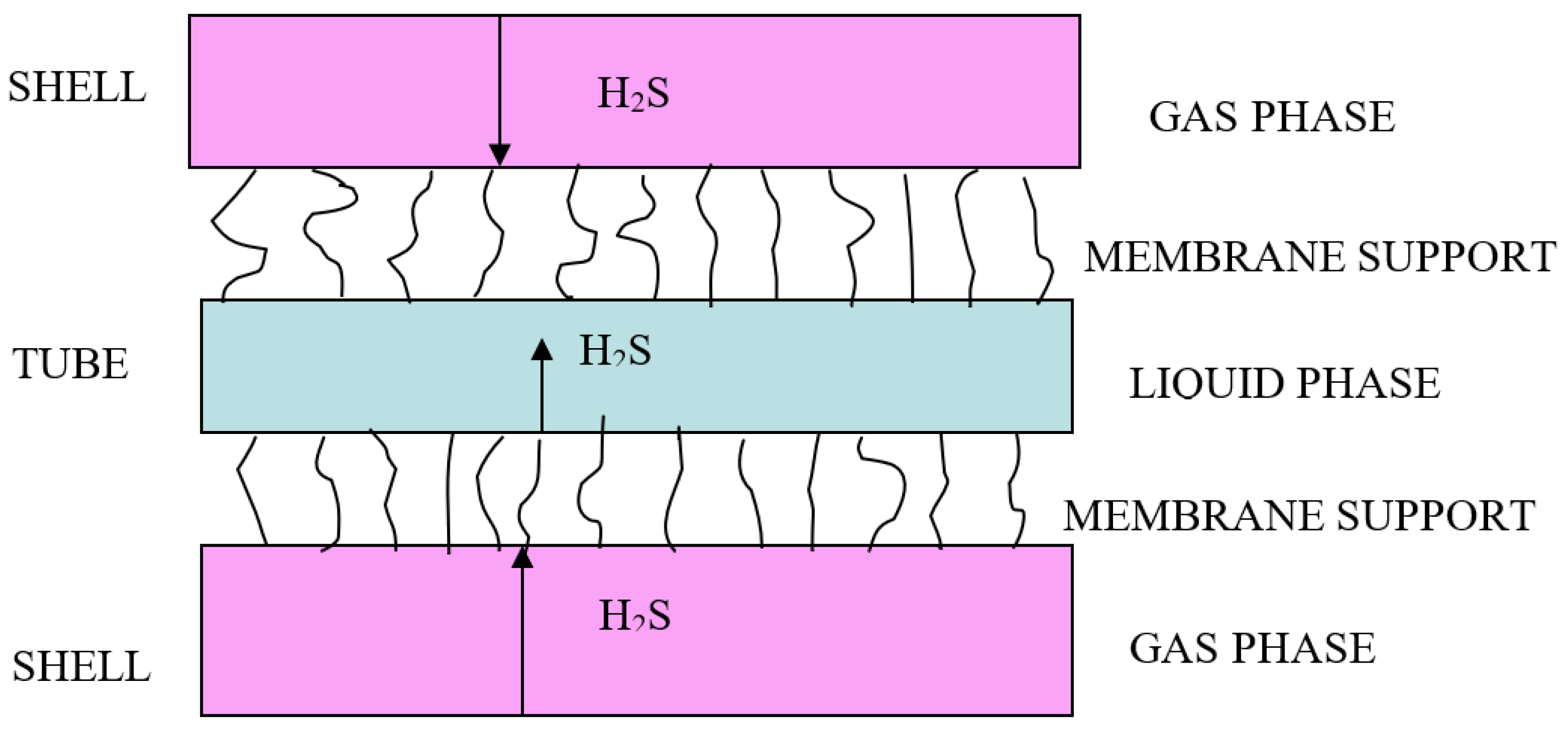
| Solvent | Gas Stream | Contactor | Temperature | % Capture | Reference |
|---|---|---|---|---|---|
| NaCl | H2S and NH3 in N2 | Column | 25 °C | Near 99 | [13] |
| Fe-EDTA and others | H2S and CO2 in CH4 | Column | Ambient | 100 | [14] |
| ChCl and others | H2S in N2 | Bubbled stirrer reactor | 30–70 °C | 100 | [10] |
| MDEA | H2S and CO2 in N2 | Rotating packed bed | 30–45 °C | Near 100 | [15] |
| Modified lye | H2S in N2 | reactor | No data | 98 | [16] |
| Yellow phosphorous and phosphate rock | H2S in N2 | Bubble reactor | 55–80 °C | 88 | [17] |
| Adsorbent | SBET, m2/g | Conditions | H2S Capacity | Reference |
|---|---|---|---|---|
| Modified biosolid adsorbent | 110–180 | [H2S] = 1000 ppm | 89–221 mg/g | [27] |
| MoO2 nanoparticle | 48–65 | [H2S] = 38–73 ppm T = 65–89 °C | 0.033–0.081 g/g | [28] |
| Modified zeolite | 333–550 | [H2S] = 30–120 ppm T = 100–300 °C | 14.7–70 mg/g | [29] |
| DES supported on fumed silica | 124–133 | [H2S] = 800 ppm T = 20–60 °C | 3.9–14 mg/g | [30] |
| Carbon adsorbents | 229–3217 | [H2S] = 20 ppm | 6.3–25.7 mmol/g | [31] |
| Jute-derived nanoporous carbons | 1065–2580 | T = 25 °CT | 6.5–50 mmol/g | [32] |
| Cu-modified activated carbon | 981–1769 | [H2S] = 0.46 mg/L T = 25–110 °C | 22.4–76 mg/g | [33] |
| Membrane | Feed Gas | Working Conditions | H2S Capture | Reference |
|---|---|---|---|---|
| Crosslinked poly(ethylene glycol membrane | 5% H2S | T = 25 °C P = 800 psi | 0.08–25 barrier | [54] |
| Vinyl-poly(norborene) membrane | 5–20% H2S | T = 25 °C P = 800 psi | Depending on feed gas composition | [55] |
| Cellulose triacetate HFM | 20 mol% H2S | T = 35–50 °C P = 6.9–31.9 bar | 140 GPU | [52] |
| Dense polymer membrane | 0.5–20% H2S | T = 35 °C P = 7–46 bar | Depending on membrane type | [53] |
| Copolymide membranes | 20% H2S | T = 22 °C P = 24–46 bar | Depending on membrane type | [56] |
| Raw Material | Plasticizers | Dopants | Acronym |
|---|---|---|---|
| Alkali lignocarbon and polivynil alcohol | Glycerol and water | nano-CuO and Cu2+ | CLA/PVA CuO-CLA/PVA-1 Cu-CLA/PVA-2 |
| Technology | Characteristics | Industrial Use | Reference |
|---|---|---|---|
| Physical absorption | Dimethyl ether of polyethylene glycol | Yes | [65] |
| N-methyl-2-pyrrolidene | Yes | ||
| Ionic liquid absorption | Ionic liquids | No | [66] |
| Hybrid membrane | Supported liquid membranes | No | [67] |
| Adsorption on pristine materials | Iron-oxide-based materials | Yes | [26,68] |
| Molecular sieves-based materials | Yes | ||
| Adsorption on composite materials | Metal oxide/silica | No | [26,68] |
| Metal oxide/activated carbon | No |
| Technology | Advantages | Disadvantages |
|---|---|---|
| Absorption | Established technology, possibility to treat tail gas | Chemistry of alkanolamines, solvent regeneration seems difficult |
| Adsorption | Established technology, high removal capacity | Generation of toxic wastes, difficult to operate offshore, stability of the adsorbent |
| Membranes and membrane contactors | Modular configuration, large surface area per unit volume | Possible limitations due to permeability, resistance due to membrane, degradation of the membrane |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alguacil, F.J. Recent Advances in H2S Removal from Gas Streams. Appl. Sci. 2023, 13, 3217. https://doi.org/10.3390/app13053217
Alguacil FJ. Recent Advances in H2S Removal from Gas Streams. Applied Sciences. 2023; 13(5):3217. https://doi.org/10.3390/app13053217
Chicago/Turabian StyleAlguacil, Francisco Jose. 2023. "Recent Advances in H2S Removal from Gas Streams" Applied Sciences 13, no. 5: 3217. https://doi.org/10.3390/app13053217
APA StyleAlguacil, F. J. (2023). Recent Advances in H2S Removal from Gas Streams. Applied Sciences, 13(5), 3217. https://doi.org/10.3390/app13053217






