Bond Characterization in Cementitious Material Binders Using Fourier-Transform Infrared Spectroscopy
Abstract
1. Introduction
1.1. A Brief Background on the Use of FTIR in Material Characterization
1.2. Mechanism of Fourier Infrared Spectroscopy
1.3. Molecular Bond Vibrations
1.4. Sampling Methods in FTIR
1.4.1. Transmission Method
1.4.2. Reflection Methods
- a.
- The Attenuate transmission reflection (ATR) technique:
- b.
- Specular reflectance technique
- c.
- Diffuse reflectance IR transmittance spectroscopy (DRIFTS) method
1.5. Factors Affecting Bond Vibrations
2. Historical Background of the Utilization of FTIR for Bond Characterization in Cementitious Binders
2.1. FTIR Spectra of Si-O-, T-O, Si-O-T and the Degree of Crystallization and Polymerization
2.2. The Effect of Silica Modulus, Alkali, and Aluminium on Si-O-T and T-O Vibrations of FTIR
2.3. Analyses of CO32− Vibration in FTIR Spectra
2.4. FTIR Spectra Vibrations for Ca(OH)2, -OH, H-OH, and Si-OH
3. The Use of FTIR Spectra for Binder Durability
3.1. The Effect of an Acidic Attack on the H-O-H and S-O-T Bond Vibrations in FTIR Spectra
3.2. The Effect of a Sulfate Attack on the H-O-H Bond and Si-O-T Vibrations in FTIR Spectra
4. Conclusions
- (a)
- FTIR is a simple technique for bond characterization and its sampling methods include transmission, attenuated total reflectance (ATR), diffusive reflectance infrared transmittance spectroscopy technique (DRIFTS), and specular aperture grazing angle (SAGA).
- (b)
- FTIR can be used to study the aluminosilicate and calcium silicate hydrate bond characteristics in the cementitious binders which include traditional cement (OPC), geopolymers (GPs), and alkaline-activated slag or binders (AASs). The attachment of cations and their atomic weight have effects on the skeletal framework and the frequency of vibration of Si-O-T bonds.
- (c)
- FTIR could give an indication of the crystalline nature of the binder, thereby giving hints on the silicate organization by observing Si-O-T within 900–1100 cm−1. The more the frequency of vibration of the Si-O-Si bond, the more the silicate re-organizes or polymerizes the binder.
- (d)
- FTIR spectra bands could also be used to study the durability of a binder qualitatively by observing the Si-O-T and -OH bands upon being exposed to aggressive environments, such as sulfates and acids.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kristó, J.; Frost, R.; Felinger, A.; Mink, J. FTIR spectroscopic study of intercalated kaolinite. J. Mol. Struct. 1997, 410–411, 119–122. [Google Scholar] [CrossRef]
- Ketov, A.; Rudakova, L.; Vaisman, I.; Ketov, I.; Haritonovs, V.; Sahmenko, G. Recycling of rice husks ash for the preparation of resistant, lightweight and environment-friendly fired bricks. Constr. Build. Mater. 2021, 302, 124385. [Google Scholar] [CrossRef]
- Lee, W.K.W.; van Deventer, J.S.J. The Effect of Inorganic Salt contamination on the strength and durability of geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2002, 211, 115–126. [Google Scholar] [CrossRef]
- van Jaarsveld, J.; van Deventer, J. The effect of metal contaminants on the formation and properties of waste-based geopolymers. Cem. Concr. Res. 1999, 29, 1189–1200. [Google Scholar] [CrossRef]
- Davidovits, J. Application of Ca-based geopolymer with blast furnace slag, a review. In Proceedings of the Second International Slag Valorisation Symposium, Leuven, Belgium, 18–20 April 2011; pp. 33–49. [Google Scholar]
- Davidovits, J. Geopolymer Chemistry and Applications; Geopolymer Institute: Saint-Quentin, France, 2008. [Google Scholar]
- Van Jaarsveld, J.; van Deventer, J.; Lukey, G. The characterization of source materials in fly ash-based geopolymers. Mater. Lett. 2003, 57, 1272–1280. [Google Scholar] [CrossRef]
- King, P.L.; Mcmillan, P.F.; Moore, G.M. Infrared Spectroscopy of Silicate Glasses with Application to Natural Systems; Mineralogical Association of Canada: Ottawa, ON, Canada, 2004. [Google Scholar]
- Fadlelmoula, A.; Pinho, D.; Carvalho, V.H.; Catarino, S.O.; Minas, G. Fourier Transform Infrared (FTIR) Spectroscopy to Analyse Human Blood over the Last 20 Years: A Review towards Lab-on-a-Chip Devices. Micromachines 2022, 13, 187. [Google Scholar] [CrossRef]
- Coates, J. Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Dumas, P.; Sockalingum, G.D.; Sulé-Suso, J. Adding synchrotron radiation to infrared microspectroscopy: What’s new in biomedical applications? Trends Biotechnol. 2007, 25, 40–44. [Google Scholar] [CrossRef]
- Kylili, A.; Fokaides, P.A.; Christou, P.; Kalogirou, S.A. Infrared thermography (IRT) applications for building diagnostics: A review. Appl. Energy 2014, 134, 531–549. [Google Scholar] [CrossRef]
- Hermann, P.; Kästner, B.; Hoehl, A.; Kashcheyevs, V.; Patoka, P.; Ulrich, G.; Feikes, J.; Ries, M.; Tydecks, T.; Beckhoff, B.; et al. Enhancing the sensitivity of nano-FTIR spectroscopy. Opt. Express 2017, 25, 16574–16588. [Google Scholar] [CrossRef]
- Kiefer, J.; Stärk, A.; Kiefer, A.L.; Glade, H. Infrared Spectroscopic Analysis of the Inorganic Deposits from Water in Domestic and Technical Heat Exchangers. Energies 2018, 11, 798. [Google Scholar] [CrossRef]
- Keller, W.D.; Spotts, J.H.; Biggs, D.L. Infrared Spectra of some rock forming materials. Am. J. Sci. 1952, 250, 438–471. [Google Scholar] [CrossRef]
- De Lloyd, D. Instrumental Infrared and Fourier Transform Spectroscopy. Delloyd’s Lab Tech Resources, Reagents and Instrumentation. 2000. Available online: http://delloyd.50megs.com/MOBILE/infrared_spectroscopy.html (accessed on 18 February 2023).
- Filip, Z.; Hermann, S.; Demnerová, K. FT-IR spectroscopic characteristics of differently cultivated Escherichia coli. Czech J. Food Sci. 2008, 26, 458–463. [Google Scholar] [CrossRef]
- Mylläri, V.; Ruoko, T.-P.; Syrjälä, S. A comparison of rheology and FTIR in the study of polypropylene and polystyrene photodegradation. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Sagar, A. Infrared (IR) Spectroscopy-Definition, Principle, Parts, Uses. 2022. Available online: https://microbenotes.com/infrared-ir-spectroscopy/ (accessed on 25 December 2022).
- Cooley, J.W.; Tukey, J.W. An Algorithm for the Machine Calculation of Complex Fourier Series. Math. Comput. 1965, 19, 297–301. [Google Scholar] [CrossRef]
- Daudon, M.; Bazin, D. Vibrational spectroscopies to investigate concretions and ectopic calcifications for medical diagnosis. Comptes Rendus Chim. 2016, 19, 1416–1423. [Google Scholar] [CrossRef]
- Afremow, L.C.; Vanderberg, J.T. High resolution spectra of inorganic pigments and extenders in the mid-infrared region from 1500 cm−1 to 200 cm−1. J. Paint Technol. 1966, 38, 169–202. [Google Scholar]
- Fernández-Carrasco, L.; Torrens-Martín, D.; Morales, L.M.; Martínez-Ramírez, S. Infrared Spectroscopy in the Analysis of Building and Construction Materials. In Infrared Spectroscopy—Materials Science, Engineering and Technology; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Beasley, M.M.; Bartelink, E.J.; Taylor, L.; Miller, R.M. Comparison of transmission FTIR, ATR, and DRIFT spectra: Implications for assessment of bone bioapatite diagenesis. J. Archaeol. Sci. 2014, 46, 16–22. [Google Scholar] [CrossRef]
- Davidovits, J. Properties of Geopolymer Cement; Geopolymer Institute: Saint-Quentin, France, 1994; pp. 1–19. [Google Scholar]
- Brown, T.L. Infrared Intensities And Molecular Structure. Chem. Rev. 1958, 58, 581–608. [Google Scholar] [CrossRef]
- Yu, P.; Kirkpatrick, R.J.; Poe, B.; McMillan, P.F.; Cong, X. Structure of Calcium Silicate Hydrate (C-S-H): Near-, Mid-, and Far-Infrared Spectroscopy. J. Am. Ceram. Soc. 2004, 82, 742–748. [Google Scholar] [CrossRef]
- Yusuf, M.O. Performance of slag blended alkaline activated palm oil fuel ash mortar in sulfate environments. Constr. Build. Mater. 2015, 98, 417–424. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Impacts of silica modulus on the early strength of alkaline activated ground slag/ultrafine palm oil fuel ash based concrete. Mater. Struct. 2014, 48, 733–741. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Effects of addition of Al(OH)3 on the strength of alkaline activated ground blast furnace slag-ultrafine palm oil fuel ash (AAGU) based binder. Constr. Build. Mater. 2014, 50, 361–367. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Evolution of alkaline activated ground blast furnace slag–ultrafine palm oil fuel ash based concrete. Mater. Des. 2014, 55, 387–393. [Google Scholar] [CrossRef]
- Atta, A.; Jibril, B.; Aderemi, B.; Adefila, S. Preparation of analcime from local kaolin and rice husk ash. Appl. Clay Sci. 2012, 61, 8–13. [Google Scholar] [CrossRef]
- Uchino, T.; Sakka, T.; Hotta, K.; Iwasaki, M.; Uchino, T.; Sakka, T.; Hotta, K.; Iwasaki, M. Attenuated Toatal Reflectance Fourier-Transform Infrared Spectra of a Hydrated Sodium Soilicate Glass. J. Am. Ceram. Soc. 1989, 72, 2173–2175. [Google Scholar] [CrossRef]
- Setnička, V. FT-IR Reflection Techniques. 2021. Available online: https://old.vscht.cz/anl/vibspec/FTIR%20Reflection%20Techniques.pdf (accessed on 25 December 2022).
- ASTM C494/C494M-08. Standard Specification for Chemical Admixtures for Concrete. 2008. Available online: https://webstore.ansi.org/standards/astm/astmc494c494m08a (accessed on 31 December 2022).
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. Attenuated Total Reflectance Fourier Transform Infrared Analysis of Fly Ash Geopolymer Gel Aging. Langmuir 2007, 23, 8170–8179. [Google Scholar] [CrossRef]
- Nedeljković, M.; Šavija, B.; Zuo, Y.; Luković, M.; Ye, G. Effect of natural carbonation on the pore structure and elastic modulus of the alkali-activated fly ash and slag pastes. Constr. Build. Mater. 2018, 161, 687–704. [Google Scholar] [CrossRef]
- Michael, R.B. Infrared Spectra of Adsorbed Molecules. Appl. Spectrosc. Rev. 1968, 1, 289–378. [Google Scholar] [CrossRef]
- Casale, A.J.; Doukakis, J.; Najm, H.; Davis, K. Quantitative Assessment of Infrared Analysis of Concrete Admixtures. Int. J. Concr. Struct. Mater. 2013, 7, 203–214. [Google Scholar] [CrossRef]
- Fuller, M.P.; Griffiths, P.R. Diffuse reflectance measurements by infrared Fourier transform spectrometry. Anal. Chem. 1978, 50, 1906–1910. [Google Scholar] [CrossRef]
- ThermoFisher Scientific. Effect of Water on Infrared Signal. 2023. Available online: https://www.thermofisher.com/blog/identifying-threats/how-water-affects-raman-and-ftir-identification-of-unknown-substances/#:~:text=FTIR%20is%20often%20blinded%20or,overwhelming%20signal%20produced%20by%20water (accessed on 25 December 2022).
- Zhang, X.; He, A.; Guo, R.; Zhao, Y.; Yang, L.; Morita, S.; Xu, Y.; Noda, I.; Ozaki, Y. A new approach to removing interference of moisture from FTIR spectrum. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2021, 265, 120373. [Google Scholar] [CrossRef]
- Giordanengo, T.; Charpentier, J.-P.; Roger, J.-M.; Roussel, S.; Brancheriau, L.; Chaix, G.; Baillères, H. Correction of moisture effects on near infrared calibration for the analysis of phenol content in eucalyptus wood extracts. Ann. For. Sci. 2008, 65, 803. [Google Scholar] [CrossRef]
- Povnnennykh, A.S. The use of infrared spectra for the determination of minerals. Am. Mineral. 1978, 63, 956–959. [Google Scholar]
- Lodeiro, I.G.; Fernández-Jimenez, A.; Palomo, A.; Macphee, D. Effect on fresh C-S-H gels of the simultaneous addition of alkali and aluminium. Cem. Concr. Res. 2010, 40, 27–32. [Google Scholar] [CrossRef]
- Provis, J.L. Geopolymers and other alkali activated materials: Why, how, and what? Mater. Struct. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Composition and microstructure of alkali activated fly ash binder: Effect of the activator. Cem. Concr. Res. 2005, 35, 1984–1992. [Google Scholar] [CrossRef]
- Hunt, J.M.; Wisherd, M.P.; Bonham, L.C. Infrared Absorption Spectra of Minerals and Other Inorganic Compounds. Anal. Chem. 1950, 22, 1478–1497. [Google Scholar] [CrossRef]
- Farmer, V. The Infrared Spectra of Minerals; Mineralogical Society of Great Britain and Ireland: Twickenham, UK, 1974. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared Spectra of Inorganic and Coordination Compounds. In Handbook of Vibrational Spectroscopy; Griffiths, P.R., Ed.; Wiley: New York, NY, USA, 1970. [Google Scholar] [CrossRef]
- Bensted, J. Uses of Raman Spectroscopy in Cement Chemistry. J. Am. Ceram. Soc. 1976, 59, 140–143. [Google Scholar] [CrossRef]
- Bensted, J.; Varma, S.P. Some Applications in Infrared and Raman Spectral Studies in Cement Industry (Part 2: Portland Cement and Its Constituents); Cement Technology: Covington, GA, USA, 1974; pp. 378–382. [Google Scholar]
- Ghosh, S.N.; Handoo, S.K. Infrared and Raman spectral studies in cement and concrete (review). Cem. Concr. Res. 1980, 10, 771–782. [Google Scholar] [CrossRef]
- EStolper, E. Contributions to Mineralogy and Petrology Water in Silicate Glasses: An Infrared Spectroscopic Study. Contrib. Mineral. Petrol. 1982, 81, 1–17. [Google Scholar] [CrossRef]
- Wallah, S.E.; Rangan, B.V. Low-Calcium Fly Ash-Based Geopolymer Concrete: Long-Term Properties; Curtin University of Technology: Perth, Australia, 2006; p. 107. [Google Scholar] [CrossRef]
- Lloyd, N.A.; Rangan, B.V. Geopolymer concrete with fly ash. In Proceedings of the Second International Conference on Sustainable Construction Materials and Technologies, Ancona, Italy, 28–30 June 2010; Volume 3, pp. 1493–1504. [Google Scholar]
- Bartholomew, R.F.; Butler, B.L.; Hoover, H.L.; Wu, C.K. Infrared Spectra of a Water-Containing Glass. J. Am. Ceram. Soc. 1980, 63, 481–485. [Google Scholar] [CrossRef]
- Williams, G.P. The national synchrotron light source in the infrared region. Nucl. Instruments Methods Phys. Res. 1982, 195, 383–387. [Google Scholar] [CrossRef]
- Mawhinney, D.B.; Glass, J.J.A.; Yates, J.J.T. FTIR Study of the Oxidation of Porous Silicon. J. Phys. Chem. B 1997, 101, 1202–1206. [Google Scholar] [CrossRef]
- Karakassides, M.A. An Infrared Reflectance Study of Si-O Vibrations in Thermally Treated Alkali-Saturated Montmorillonites. Clay Miner. 1999, 34, 429–438. [Google Scholar] [CrossRef]
- Tararushkin, E.V.; Shchelokova, T.N.; Kudryavtseva, V.D. A study of strength fluctuations of Portland cement by FTIR spectroscopy. IOP Conf. Series: Mater. Sci. Eng. 2020, 919. [Google Scholar] [CrossRef]
- Criado, M.; Palomo, A.; Fernandezjimenez, A. Alkali activation of fly ashes. Part 1: Effect of curing conditions on the carbonation of the reaction products. Fuel 2005, 84, 2048–2054. [Google Scholar] [CrossRef]
- Lecomte, I.; Henrist, C.; Liégeois, M.; Maseri, F.; Rulmont, A.; Cloots, R. (Micro)-structural comparison between geopolymers, alkali-activated slag cement and Portland cement. J. Eur. Ceram. Soc. 2006, 26, 3789–3797. [Google Scholar] [CrossRef]
- Provis, J.; van Deventer, J. What controls the durability of geopolymer binders and concretes. In Concrete under Severe Conditions, Two Volume Set; CRC Press: Boca Raton, FL, USA, 2010; pp. 1535–1542. [Google Scholar] [CrossRef]
- Lodeiro, I.G.; Macphee, D.; Palomo, A.; Fernández-Jiménez, A. Effect of alkalis on fresh C–S–H gels. FTIR analysis. Cem. Concr. Res. 2009, 39, 147–153. [Google Scholar] [CrossRef]
- Ismail, I.; A Bernal, S.; Provis, J.L.; Hamdan, S.; Van Deventer, J.S.J. Microstructural changes in alkali activated fly ash/slag geopolymers with sulfate exposure. Mater. Struct. 2012, 46, 361–373. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Jimenez, A.M.F.; Blanco-Varela, M.T.; Palomo, A. FTIR study of the sol–gel synthesis of cementitious gels: C–S–H and N–A–S–H. J. Sol-Gel Sci. Technol. 2007, 45, 63–72. [Google Scholar] [CrossRef]
- Hubicki, Z.; Zieba, E.; Wójcik, G.; Ryczkowski, J. FT-IR/PAS and SEM EDX Studies on Aluminosilicates Modified by Cs(I), Th(IV) and U(VI). Acta Phys. Pol. A 2009, 116, 312–314. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Maltseva, O.; Fernández-Jiménez, A.; Torroja, I.E. Hybrid alkaline cement: Part I Fundamentals. Rev. Română Mater./Rom. J. Mater. 2012, 42, 330–335. [Google Scholar]
- Peyvandi, A.; Holmes, D.; Soroushian, P.; Balachandra, A.M. Monitoring of Sulfate Attack in Concrete by Al27 and Si29 MAS NMR Spectroscopy. J. Mater. Civ. Eng. 2015, 27. [Google Scholar] [CrossRef]
- Kramar, S.; Ducman, V. Mechanical and microstructural characterization of geopolymer synthesized from low calcium fly ash. Chem. Ind. Chem. Eng. Q. 2015, 21, 13–22. [Google Scholar] [CrossRef]
- Xu, Z.; Cornilsen, B.C.; Popko, D.C.; Wood, J.R.; Hwang, J.-Y. Quantitative Mineral Analysis by FTIR Spectroscopy. Internet J. Vib. Spectrosc. 2001, 5, 4. [Google Scholar]
- Chen, J.J.; Thomas, J.J.; Taylor, H.F.; Jennings, H.M. Solubility and structure of calcium silicate hydrate. Cem. Concr. Res. 2004, 34, 1499–1519. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Jimenez, A.M.F.; Palomo, A.; Macphee, D. Effect of Calcium Additions on N-A-S-H Cementitious Gels. J. Am. Ceram. Soc. 2010, 93, 1934–1940. [Google Scholar] [CrossRef]
- Rovnaník, P. Influence of C12A7 admixture on setting properties of fly ash geopolymer. Ceramics—Silikaty 2010, 54, 362–367. [Google Scholar]
- Rovnaník, P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Gougazeh, M.; Buhl, J.-C. Synthesis and characterization of zeolite A by hydrothermal transformation of natural Jordanian kaolin. J. Assoc. Arab. Univ. Basic Appl. Sci. 2014, 15, 35–42. [Google Scholar] [CrossRef]
- Lloyd, N.A.; Rangan, B.V. Geopolymer Concrete: A Review of Development and Opportunities. In Proceedings of the 35th Conference on Our World in Concrete and Structure, Singapore, 25–27 August 2010. [Google Scholar]
- Criado, M.; Fernández-Jiménez, A.; de la Torre, A.G.; Aranda, M.A.G.; Palomo, A. An XRD study of the effect of the SiO2/Na2O ratio on the alkali activation of fly ash. Cem. Concr. Res. 2007, 37, 671–679. [Google Scholar] [CrossRef]
- El-Didamony, H.; Amer, A.A.; Ela-Ziz, H.A. Properties and durability of alkali-activated slag pastes immersed in sea water. Ceram. Int. 2012, 38, 3773–3780. [Google Scholar] [CrossRef]
- Criado, M.; Aperador, W.; Sobrados, I. Microstructural and Mechanical Properties of Alkali Activated Colombian Raw Materials. Materials 2016, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.O.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Influence of curing methods and concentration of NaOH on strength of the synthesized alkaline activated ground slag-ultrafine palm oil fuel ash mortar/concrete. Constr. Build. Mater. 2014, 66, 541–548. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Effects of H2O/Na2O molar ratio on the strength of alkaline activated ground blast furnace slag-ultrafine palm oil fuel ash based concrete. Mater. Des. 2014, 56, 158–164. [Google Scholar] [CrossRef]
- Palomo, A.; Fernández-Jiménez, A.; Criado, M. Activación alcalina de cenizas volantes. Estudio comparativo entre activadores sódicos y potásicos. 2006, 56. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Provis, J.L.; van Deventer, J.S.J. Effect of Alumina Release Rate on the Mechanism of Geopolymer Gel Formation. Chem. Mater. 2010, 22, 5199–5208. [Google Scholar] [CrossRef]
- Bakharev, T. Durability of geopolymer materials in sodium and magnesium sulfate solutions. Cem. Concr. Res. 2005, 35, 1233–1246. [Google Scholar] [CrossRef]
- Clayden, N.; Esposito, S.; Aronne, A.; Pernice, P. Solid state 27Al NMR and FTIR study of lanthanum aluminosilicate glasses. J. Non-Crystalline Solids 1999, 258, 11–19. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Reedwood Books: Trowbridge, UK, 1990. [Google Scholar]
- Brindley, G.W.; McKinstry, H.A. The Kaolinite-Mullite Reaction Series: IV, The Coordination of Aluminum. J. Am. Ceram. Soc. 1961, 44, 506–507. [Google Scholar] [CrossRef]
- Bernal, S.A.; de Gutierrez, R.M.; Provis, J.L.; Rose, V. Effect of silicate modulus and metakaolin incorporation on the carbonation of alkali silicate-activated slags. Cem. Concr. Res. 2010, 40, 898–907. [Google Scholar] [CrossRef]
- Herting, G.; Odnevall, I. Corrosion of Aluminium and Zinc in Concrete at Simulated Conditions of the Repository of Low Active Waste in Sweden. Corros. Mater. Degrad. 2021, 2, 150–162. [Google Scholar] [CrossRef]
- Isokoski, K.; Poteet, C.A.; Linnartz, H. Highly resolved infrared spectra of pure CO2ice (15–75 K). Astron. Astrophys. 2013, 555, A85. [Google Scholar] [CrossRef]
- Jang, J.; Lee, N.; Lee, H. Fresh and hardened properties of alkali-activated fly ash/slag pastes with superplasticizers. Constr. Build. Mater. 2014, 50, 169–176. [Google Scholar] [CrossRef]
- Bekiaris, G.; Bruun, S.; Peltre, C.; Houot, S.; Jensen, L.S. FTIR–PAS: A powerful tool for characterising the chemical composition and predicting the labile C fraction of various organic waste products. Waste Manag. 2015, 39, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.A.; Wilkins, C.H. Infrared Spectra and Characteristic Frequencies of Inorganic Ions. Anal. Chem. 1952, 24, 1253–1294. [Google Scholar] [CrossRef]
- Elyamany, H.E.; Elmoaty, A.E.M.A.; Elshaboury, A.M. Magnesium sulfate resistance of geopolymer mortar. Constr. Build. Mater. 2018, 184, 111–127. [Google Scholar] [CrossRef]
- Al-Akhras, N.M. Durability of metakaolin concrete to sulfate attack. Cem. Concr. Res. 2006, 36, 1727–1734. [Google Scholar] [CrossRef]
- Karakoç, M.B.; Türkmen, I.; Maraş, M.M.; Kantarci, F.; Demirboğa, R. Sulfate resistance of ferrochrome slag based geopolymer concrete. Ceram. Int. 2016, 42, 1254–1260. [Google Scholar] [CrossRef]
- Puertas, F.; de Gutierrez, R.; Fernández-Jiménez, A.; Delvasto, S.; Maldonado, J. Alkaline cement mortars. Chemical resistance to sulfate and seawater attack. Mater. Constr. 2002, 2002, 55–71. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Evaluation of Slag-Blended Alkaline-Activated Palm Oil Fuel Ash Mortar Exposed to the Sulfuric Acid Environment. J. Mater. Civ. Eng. 2015, 27. [Google Scholar] [CrossRef]
- Ariffin, M.; Bhutta, M.; Hussin, M.; Tahir, M.M.; Aziah, N. Sulfuric acid resistance of blended ash geopolymer concrete. Constr. Build. Mater. 2013, 43, 80–86. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.; Cheng, Y.-B. Resistance of alkali-activated slag concrete to acid attack. Cem. Concr. Res. 2003, 33, 1607–1611. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Resistance of alkali-activated slag to carbonation. Cem. Concr. Res. 2001, 31, 1277–1283. [Google Scholar] [CrossRef]
- Nasrazadani, S.; Eghtesad, R.; Sudoi, E.; Vupputuri, S.; Ramsey, J.D.; Ley, M.T. Application of Fourier transform infrared spectroscopy to study concrete degradation induced by biogenic sulfuric acid. Mater. Struct. 2015, 49, 2025–2034. [Google Scholar] [CrossRef]
- Satya, Y.S.D.; Saputra, E.; Olivia, M. Performance of Blended Fly Ash (FA) and Palm Oil Fuel Ash (POFA) Geopolymer Mortar in Acidic Peat Environment. Mater. Sci. Forum 2016, 841, 83–89. [Google Scholar] [CrossRef]
- Izzat, A.M.; Mohd, A.; Al Bakri Abdullah, M.M.; Kamarudin, H. Sulfuric acid attack on ordinary Portland cement and geopolymer material. Rev. Chim. 2013, 64, 1011–1014. [Google Scholar]
- Bhutta, M.A.R.; Ariffin, N.F.; Hussin, M.W.; Lim, N.H.A.S. Sulfate and Sulfuric Acid Resistance of Geopolymer Mortars Using Waste Blended Ash. J. Teknol. 2013, 61. [Google Scholar] [CrossRef]
- Korolevich, M.; Zhbankov, R.; Sivchik, V. Calculation of absorption band frequencies and intensities in the IR spectrum of α-d-glucose in a cluster. J. Mol. Struct. 1990, 220, 301–313. [Google Scholar] [CrossRef]
- Geng, G.; Taylor, R.; Bae, S.; Hernández-Cruz, D.; Kilcoyne, D.A.; Emwas, A.-H.; Monteiro, P.J. Atomic and nano-scale characterization of a 50-year-old hydrated C3S paste. Cem. Concr. Res. 2015, 77, 36–46. [Google Scholar] [CrossRef]
- Lee, W.; van Deventer, J. The effect of ionic contaminants on the early-age properties of alkali-activated fly ash-based cements. Cem. Concr. Res. 2002, 32, 577–584. [Google Scholar] [CrossRef]
- Panias, D.; Giannopoulou, I.P.; Perraki, T. Effect of synthesis parameters on the mechanical properties of fly ash-based geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 246–254. [Google Scholar] [CrossRef]
- Sandoval, M.; Henao, J.; Ríos, C.; Williams, C.; Apperley, D. Synthesis and characterization of zeotype ANA framework by hydrothermal reaction of natural clinker. Fuel 2009, 88, 272–281. [Google Scholar] [CrossRef]
- Barbosa, V.F.; MacKenzie, K.J.; Thaumaturgo, C. Synthesis and characterisation of materials based on inorganic polymers of alumina and silica: Sodium polysialate polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar] [CrossRef]
- Mollah, M.Y.; Kesmez, M.; Cocke, D.L. An X-ray diffraction (XRD) and Fourier transform infrared spectroscopic (FT-IR) investigation of the long-term effect on the solidification/stabilization (S/S) of arsenic(V) in Portland cement type-V. Sci. Total. Environ. 2004, 325, 255–262. [Google Scholar] [CrossRef]
- Uchino, T.; Sakka, T.; Iwasaki, M. Interpretation of Hydrated States of Sodium Silicate Glasses by Infrared and Raman Analysis. J. Am. Ceram. Soc. 1991, 74, 306–313. [Google Scholar] [CrossRef]
- Handke, M.; Mozgawa, W. Vibrational spectroscopy of the amorphous silicates. Vib. Spectrosc. 1993, 5, 75–84. [Google Scholar] [CrossRef]
- Stolper, E. The speciation of water in silicate melts. Geochim. Cosmochim. Acta 1982, 46, 2609–2620. [Google Scholar] [CrossRef]
- Taylor, W.R. Application of infrared spectroscopy to studies of silicate glass structure: Examples from the melilite glasses and the systems Na2O-SiO2 and Na2O-Al2O3-SiO2. J. Earth Syst. Sci. 1990, 99, 99–117. [Google Scholar] [CrossRef]
- Swapna, K.; Mahamuda, S.; Rao, A.S.; Shakya, S.; Sasikala, T.; Haranath, D.; Prakash, G.V. Optical studies of Sm3+ ions doped zinc alumino bismuth borate glasses. Spectrochim. Acta A 2014, 125, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Nahin, P.G. Infrared Analysis of Clays and Related Minerals. Clays Clay Miner. 1952, 1, 112–118. [Google Scholar] [CrossRef]


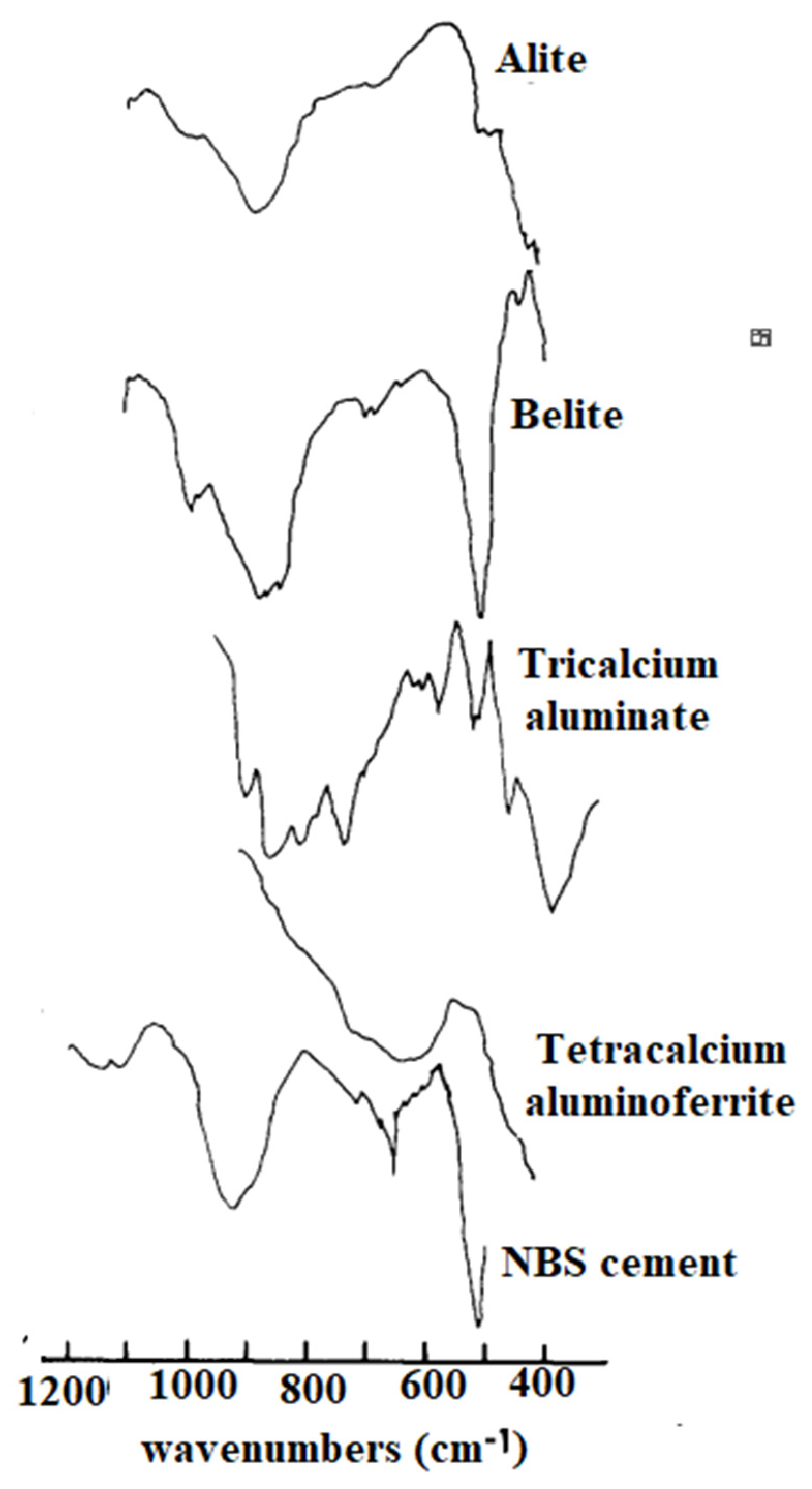

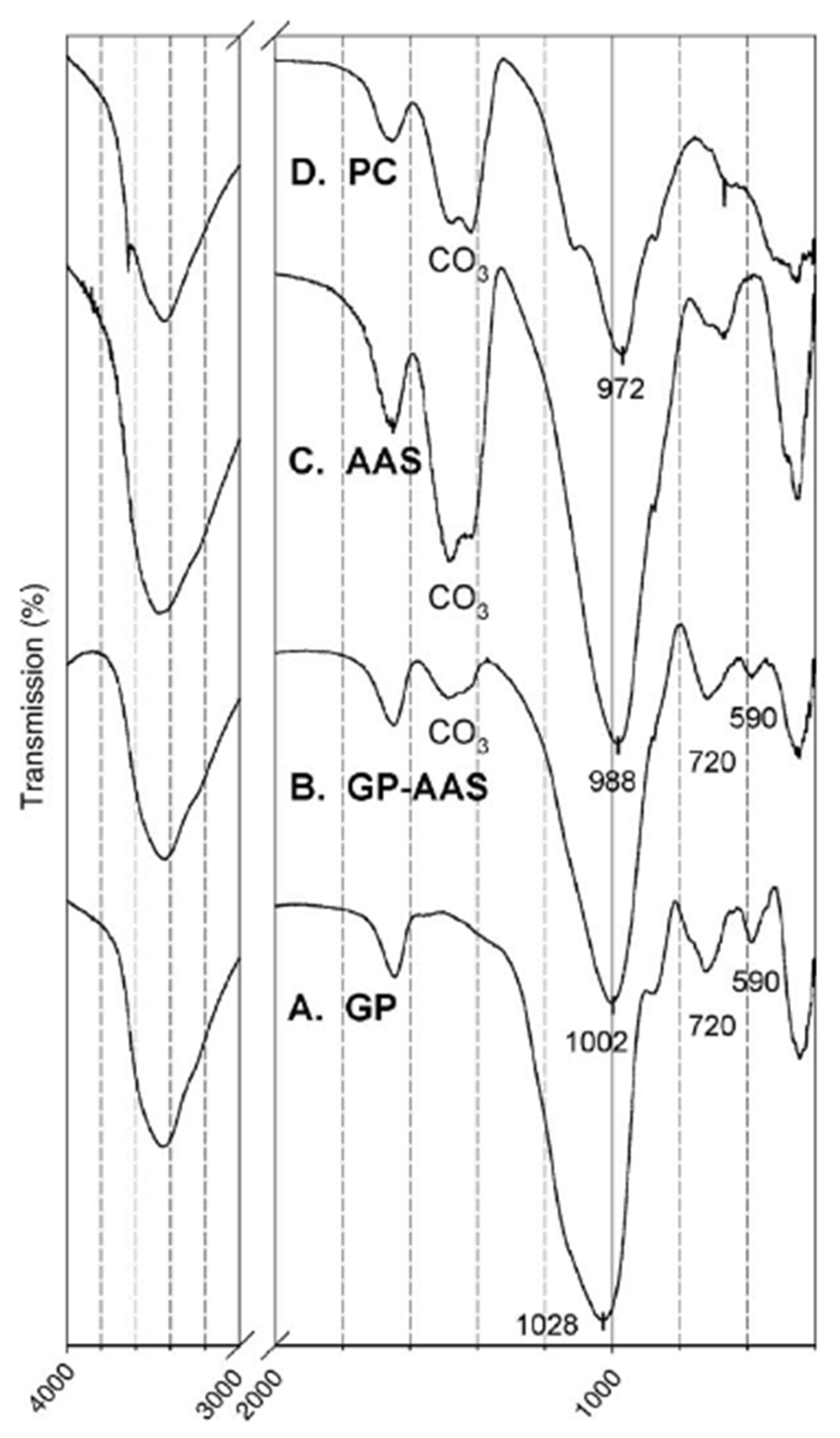

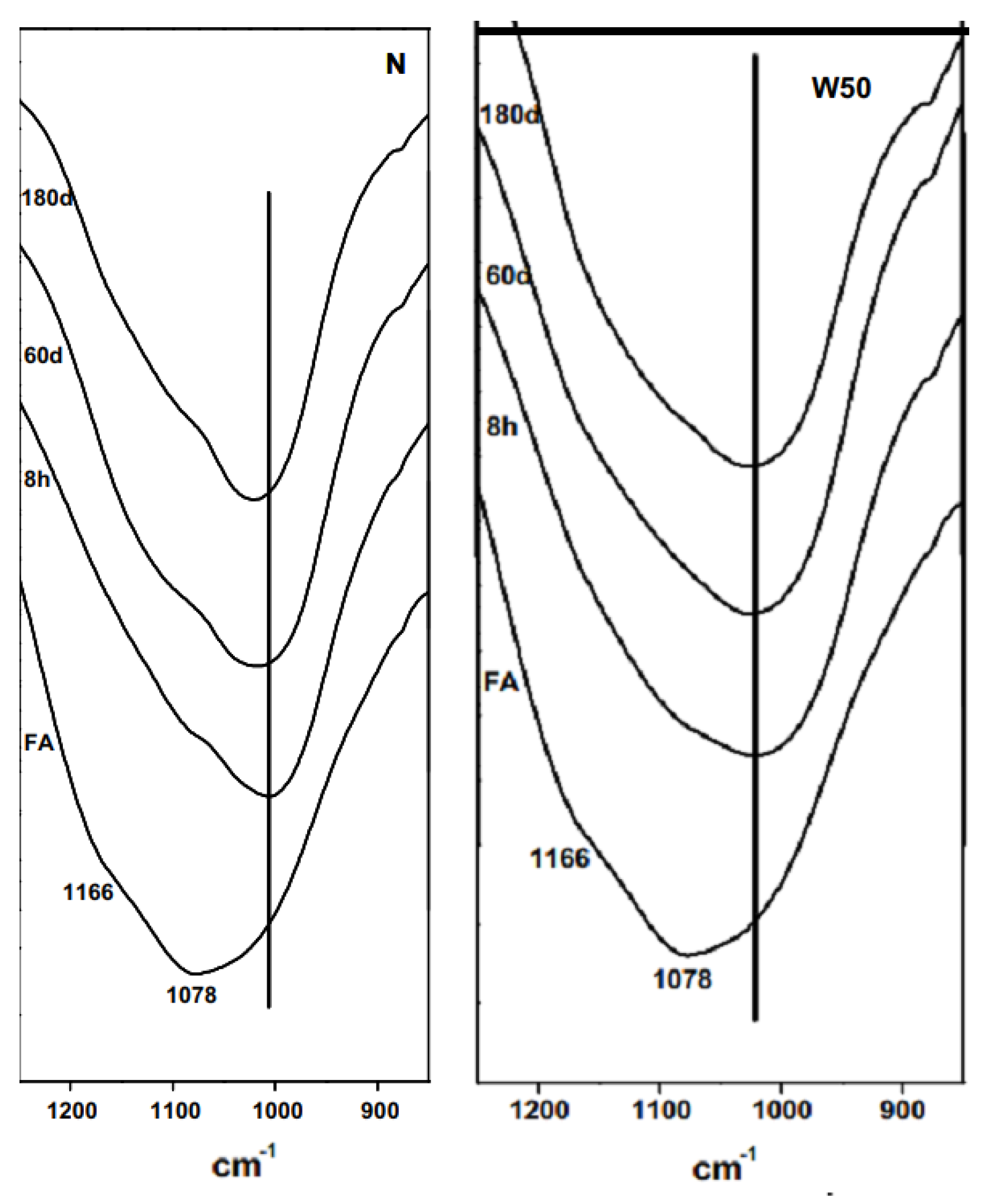



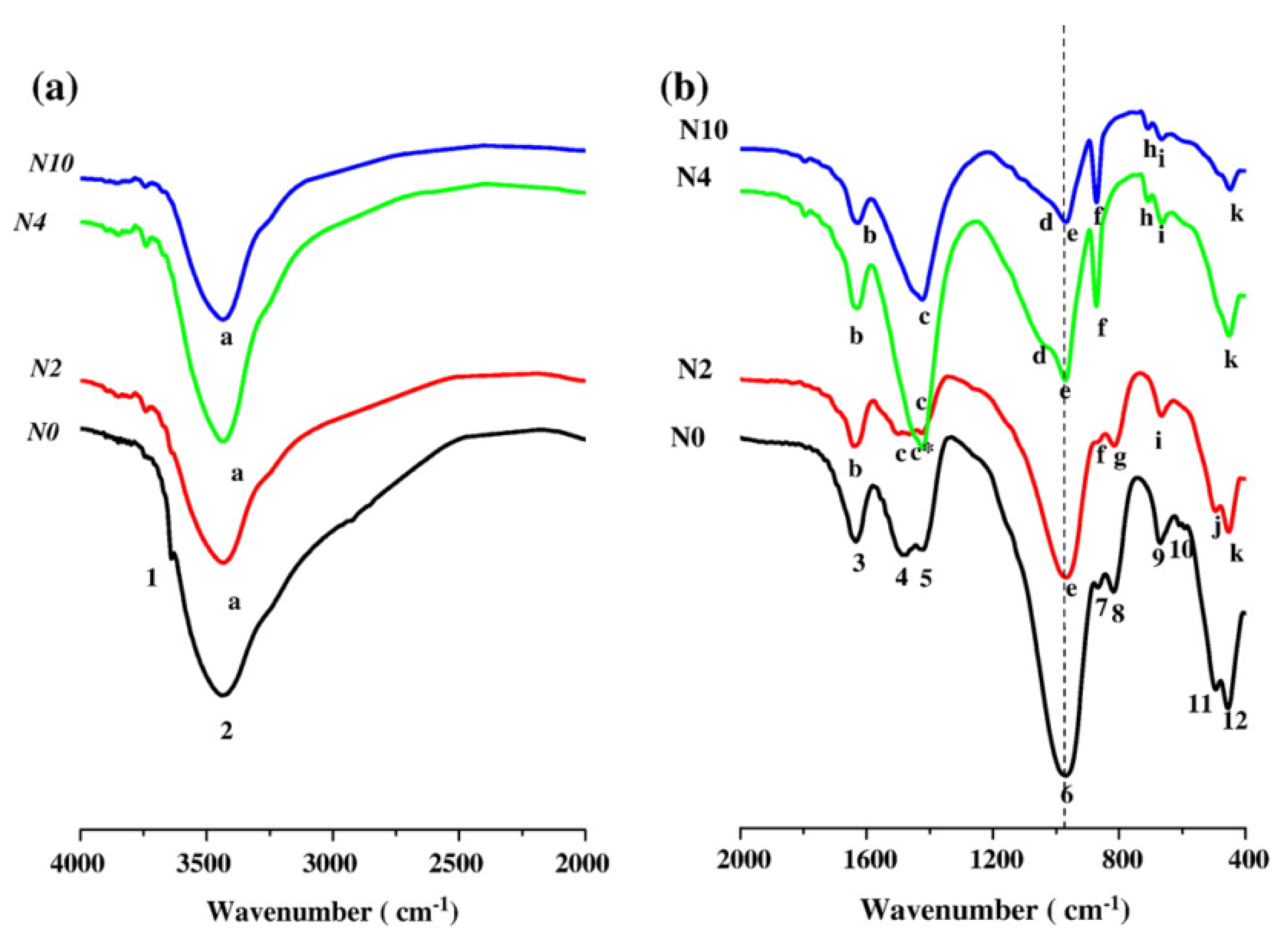


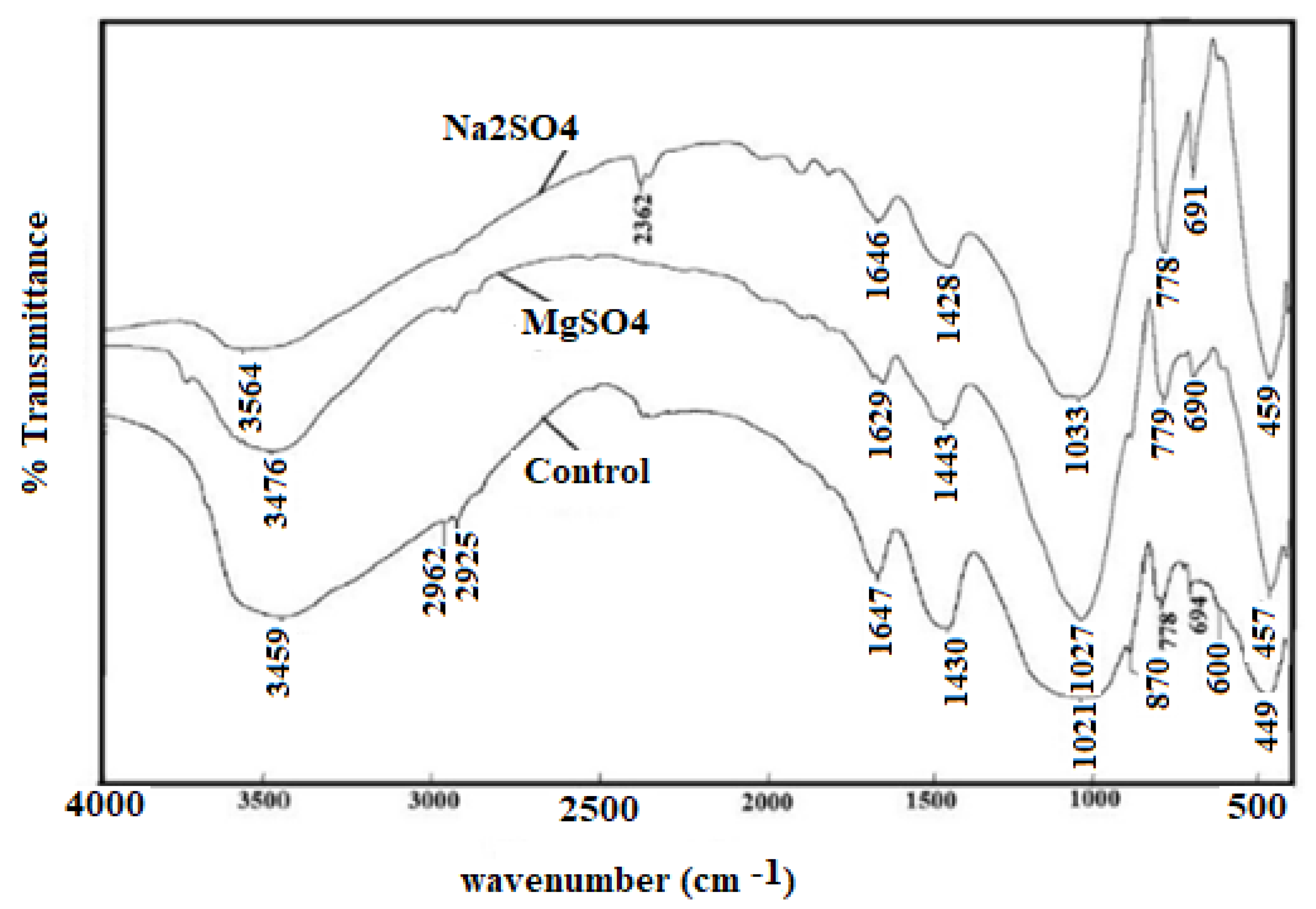
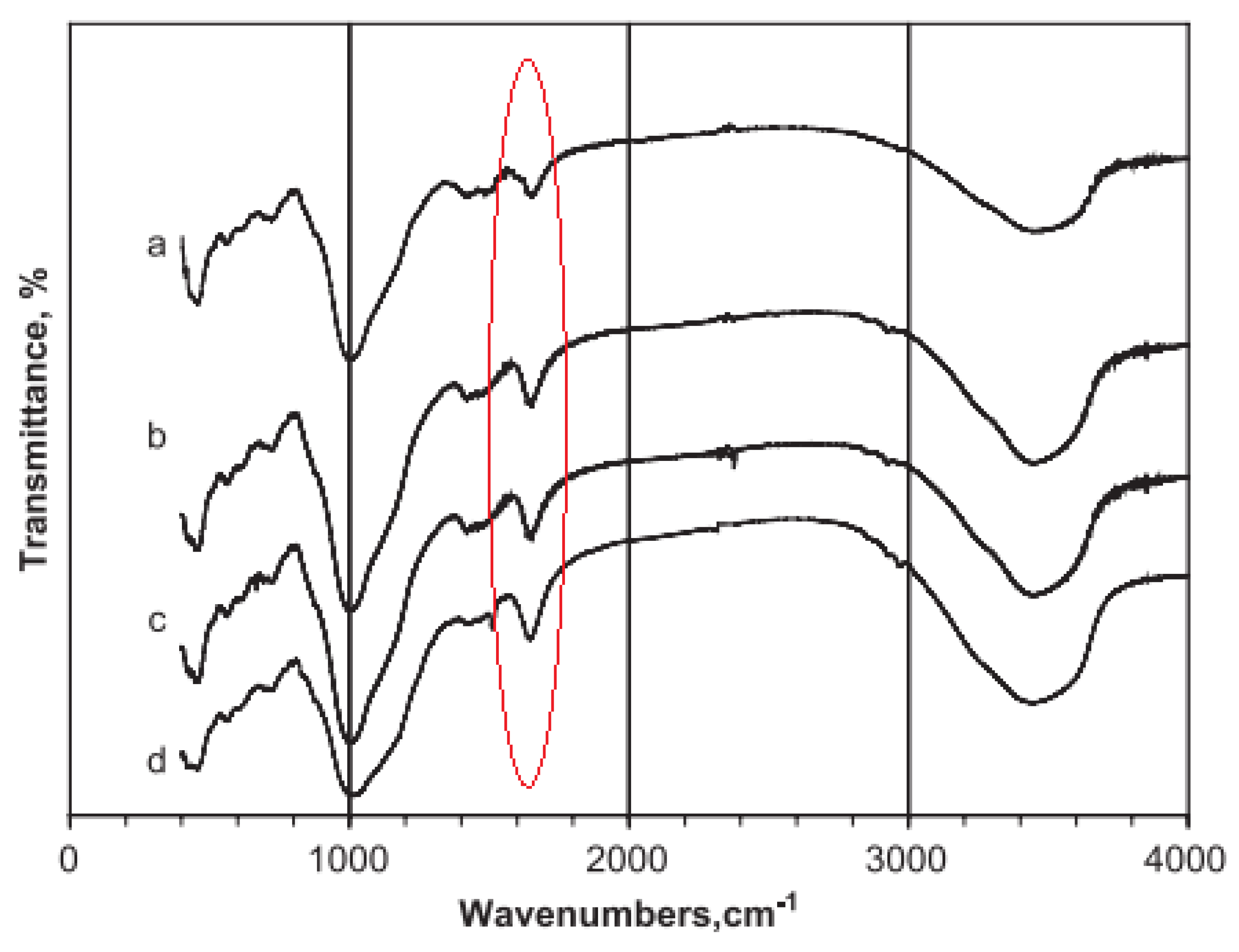
| Bonds | Wavenumber | Assignment | References |
|---|---|---|---|
| Si-O-T | 950–1200 cm−1 | Very strong band of asymmetric stretching | [22,23,63,65,112,113] |
| Si-O-T | 1116–1148 cm−1 | Unreacted sample of metakaolin and rice husk ash | [32,113,114] |
| Si-O-T | 671–717 cm−1 | symmetric stretching of Si-O-T | [60,63,80] |
| Si-O-T | 435–540 cm−1 | Bending | [22,23,63,65,77] |
| -Si-OH | 815–988 cm−1 (Q2Al) | Symmetric stretching | [63,69] |
| -C-O | 1421–1458 cm−1 | Very strong stretching vibration (CO32-) | [14,24,27,44,69,113,115,116] |
| -C-O | 2350-2355 cm−1 | Asymmetric stretching (CO2) | [94,95,96] |
| -C-O | 875–877 cm−1 | Strong vibration (CO2) | [10,95,113] |
| S-O (SO4) | 1115–1200 cm−1 | Very strong sulfate asymmetrical vibration | [10,14,23] |
| S-O (SO4) | 660–667 cm−1 | Bending vibration strong in CaSO4 and weak in Na2SO4 | [13,61,66,95] |
| -N-O (NO3) | 1385 cm−1 | Very strong diffuse asymmetric stretching | [3,10] |
| -N-O (NO3) | 815–840 cm−1 | Symmetric stretching of nitrate | [10,95] |
| -P-O (PO4) | 1000–1100 cm−1 | Asymmetric stretching | [10,95] |
| Si-O-Si | 795 cm−1 | Symmetric stretching | [3,116] |
| Si-O-Si, O-Si-O | 470 cm−1 | bending | [22,23,33,63,65,67,113,117] |
| Si-O | 1010–1104 cm−1 | Kaolinite in-plane vibration | [117] |
| T-O | 452 cm−1 | Associated with the T-O bending | [32] |
| kaolinite | 435 cm−1 | Strong kaolinite bending | [33,117] |
| Al-O | 600–800 cm−1 | Alumina in IV-fold coordination | [87,118] |
| -OH/HOH | 3500–3600 cm−1 | -asymmetric stretching | [33,117,119] |
| -OH | 875 cm−1 | bending | [33,117,119] |
| Al-O | 700 cm−1 | Tetrahedra alumina | [120] |
| Al-O | 620 cm−1 | Octahedral alumina | [120] |
| Si-OH | 875 cm-1 | Stretching bank | [117] |
| H-O-H | 2350–2800 cm−1 | -asymmetric stretching | [14,33,117,121] |
| H-O-H | 2810–2924.5 cm−1 | Hydrogen bonding | [10,121] |
| H-O-H | 1500–1700 cm−1 | Unbound water molecules | [122] |
| H-O-H | 1622–1652 cm−1 | Bending vibration | [75,76,113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusuf, M.O. Bond Characterization in Cementitious Material Binders Using Fourier-Transform Infrared Spectroscopy. Appl. Sci. 2023, 13, 3353. https://doi.org/10.3390/app13053353
Yusuf MO. Bond Characterization in Cementitious Material Binders Using Fourier-Transform Infrared Spectroscopy. Applied Sciences. 2023; 13(5):3353. https://doi.org/10.3390/app13053353
Chicago/Turabian StyleYusuf, Moruf Olalekan. 2023. "Bond Characterization in Cementitious Material Binders Using Fourier-Transform Infrared Spectroscopy" Applied Sciences 13, no. 5: 3353. https://doi.org/10.3390/app13053353
APA StyleYusuf, M. O. (2023). Bond Characterization in Cementitious Material Binders Using Fourier-Transform Infrared Spectroscopy. Applied Sciences, 13(5), 3353. https://doi.org/10.3390/app13053353






