Cyclodextrin Polymers as a Promising Drug Carriers for Stabilization of Meropenem Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
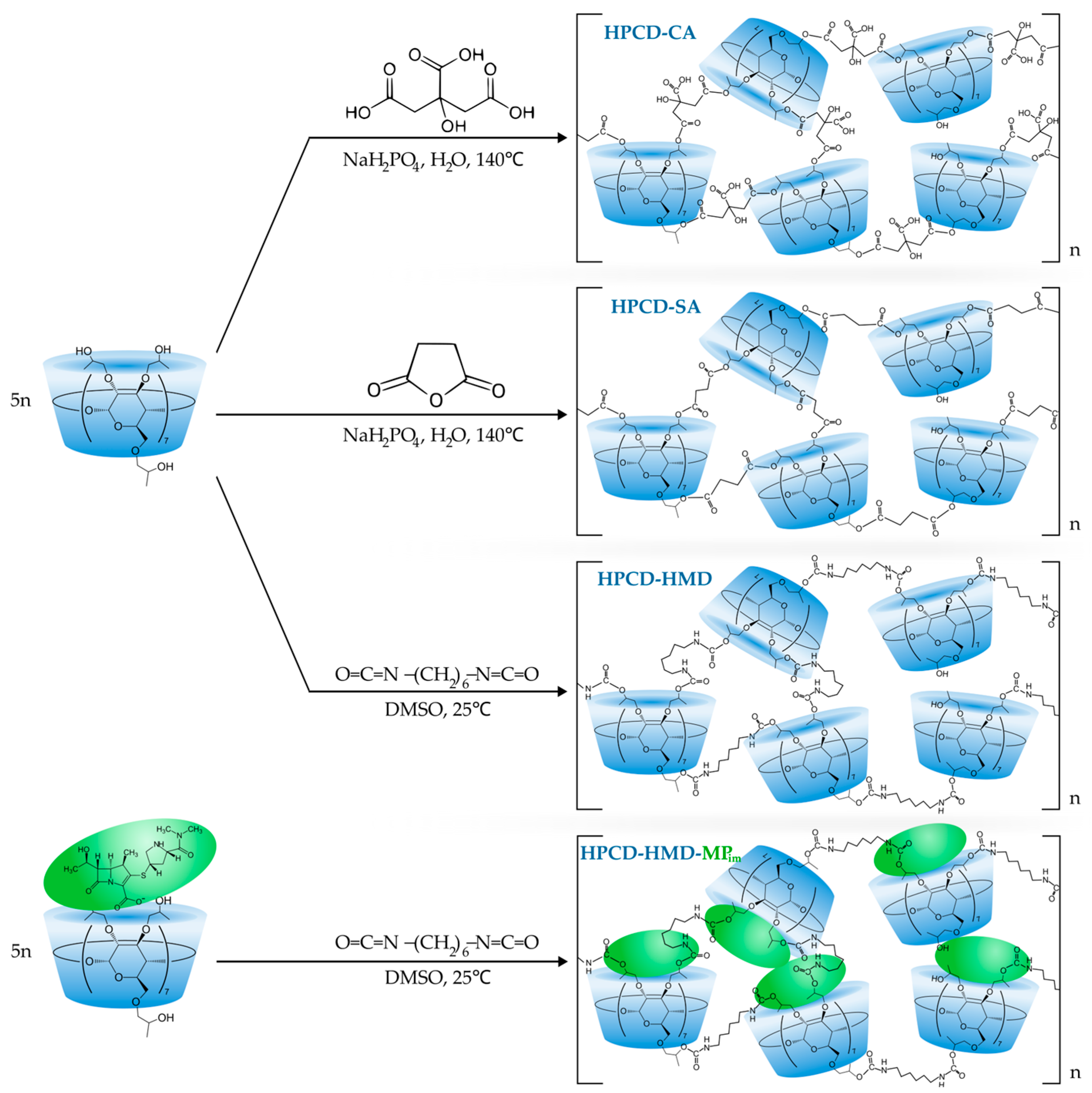
2.2.1. Synthesis of HPCD Polymers Linked by CA and SA
2.2.2. Synthesis of HPCD Polymer and HPCD Polymer with Encapsulated MP Linked by HMD
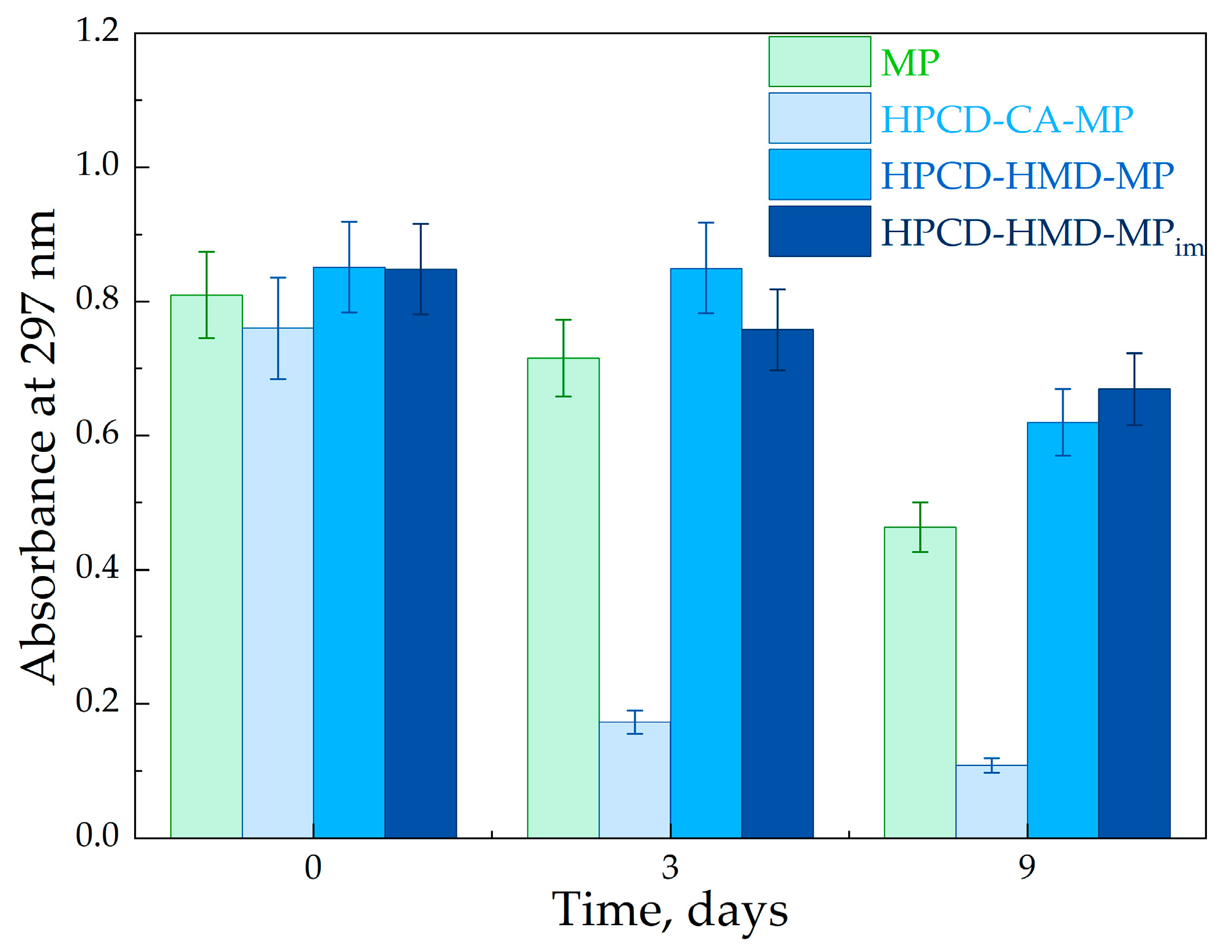
2.2.3. MP Stability Studies
2.2.4. MP Release Studies
2.2.5. Optical Microscopy
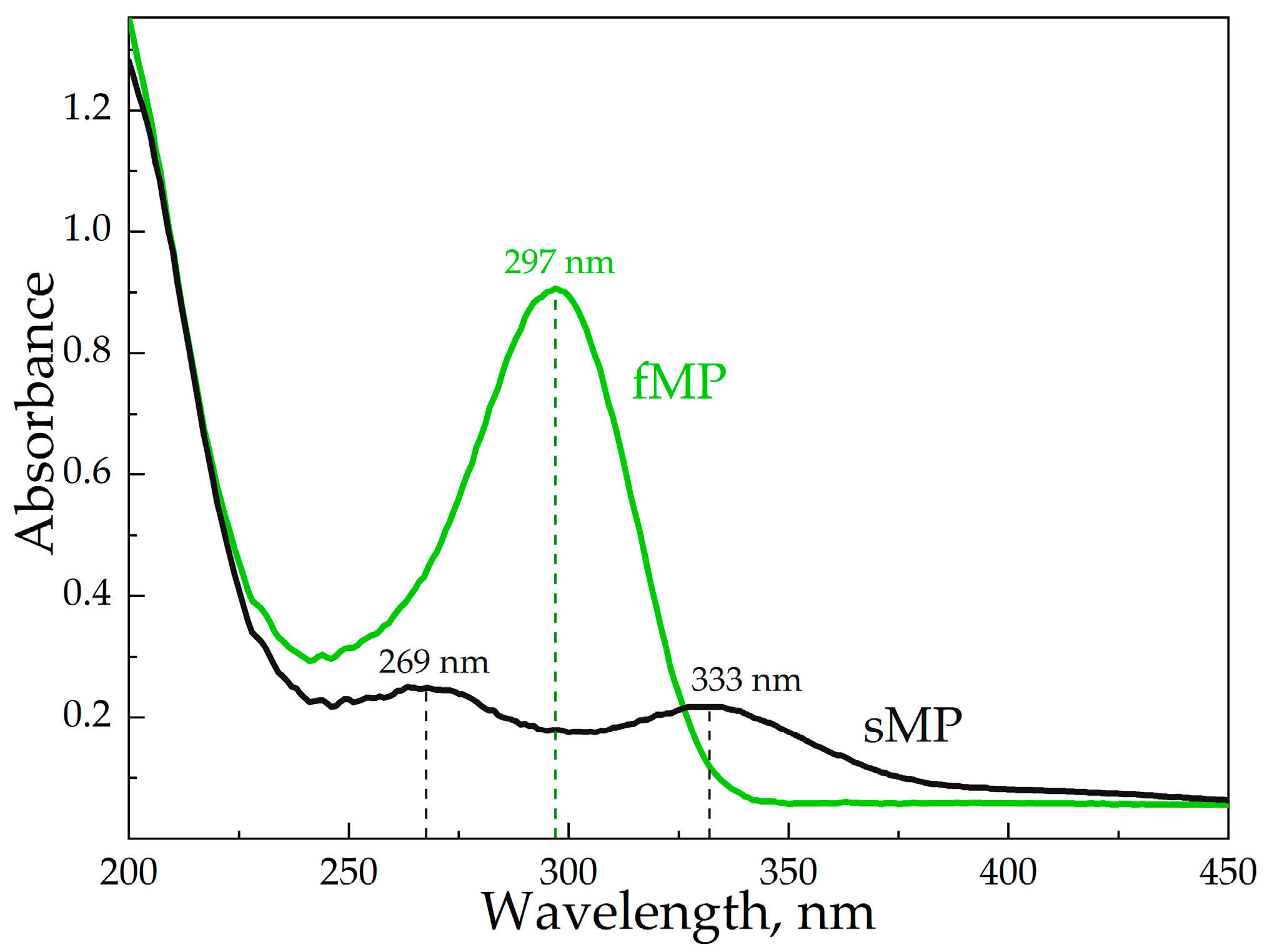
2.2.6. UV-Spectroscopy
2.2.7. NMR-Spectroscopy
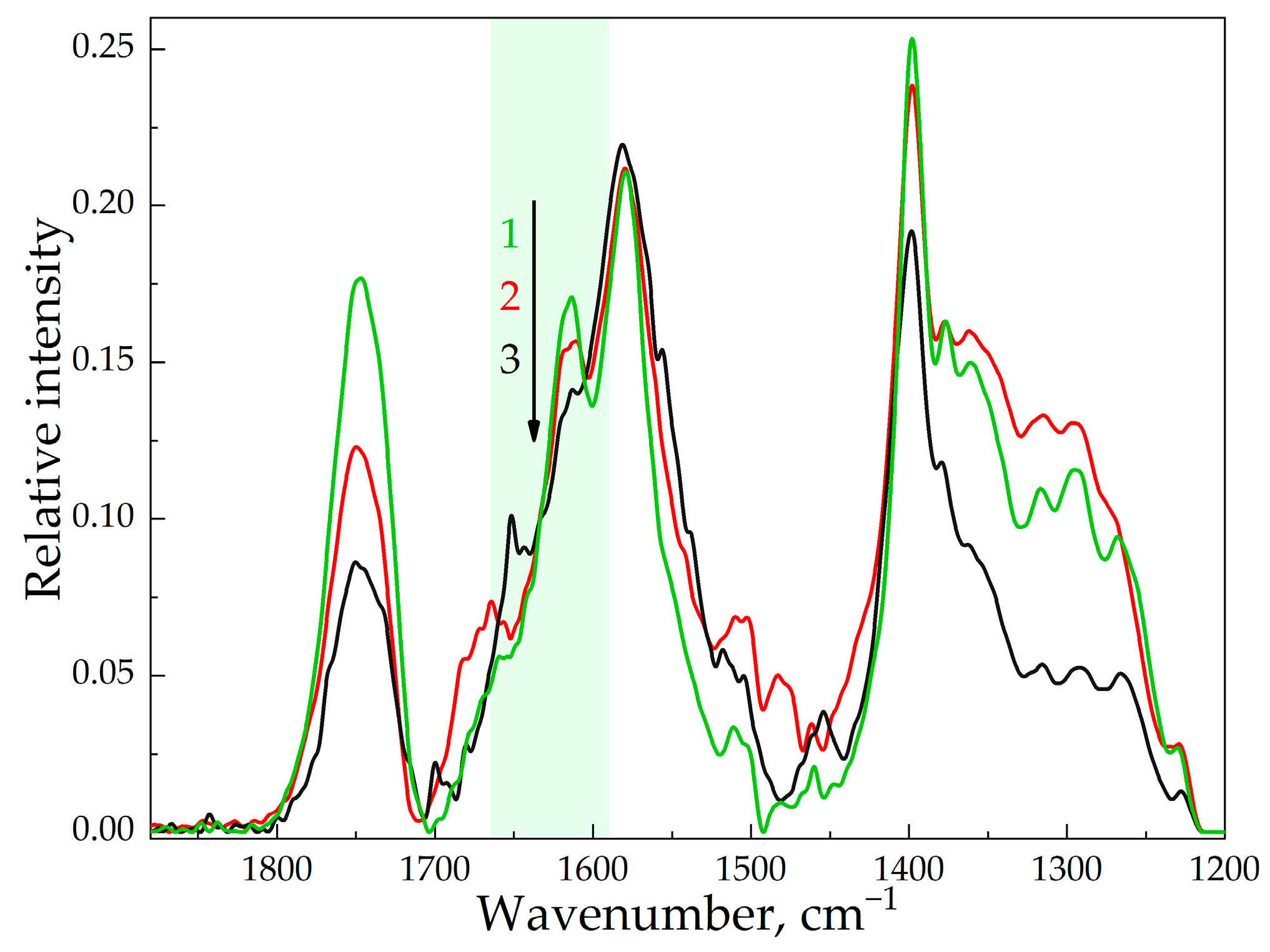
2.2.8. FTIR-Spectroscopy
2.2.9. Nanoparticle Tracking Analysis (NTA)
2.2.10. Molecular Weight of HPCD Polymers
2.2.11. Dynamic Light Scattering (DLS)
2.2.12. Powder X-ray Diffraction (PXRD) Analysis
3. Results and Discussion
3.1. DLS and NTA Analysis
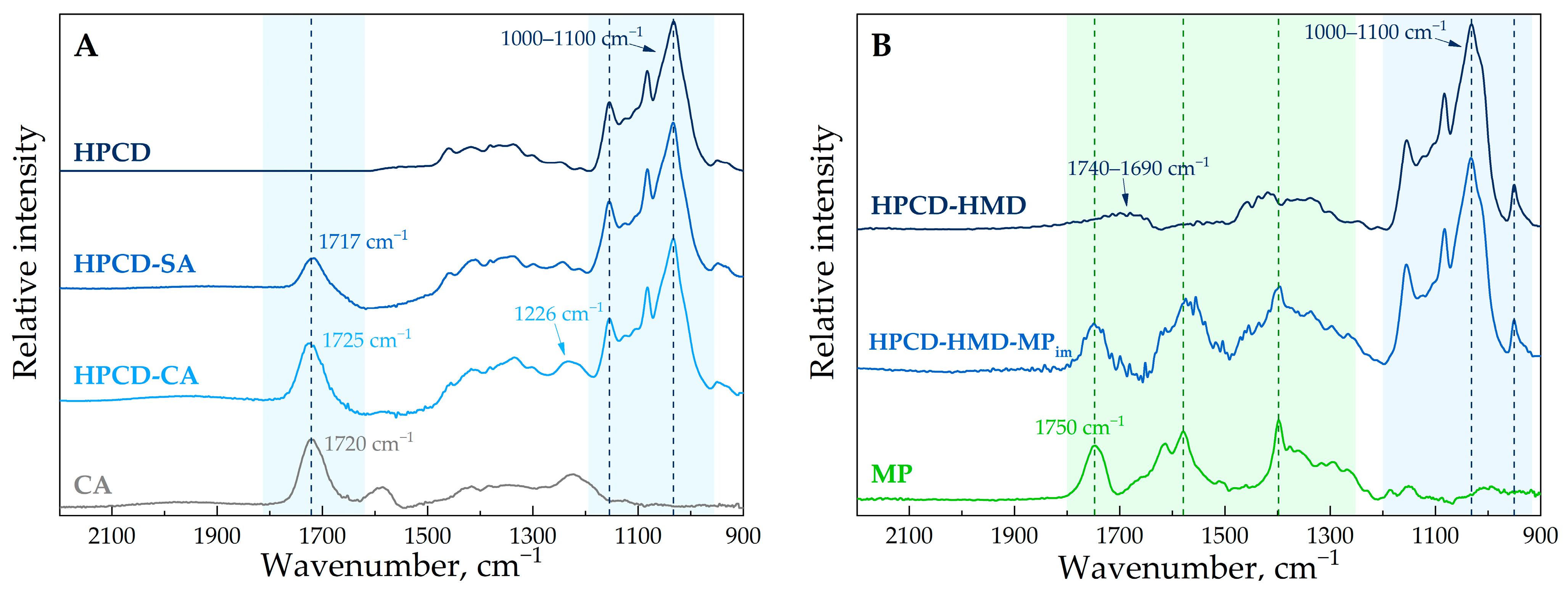
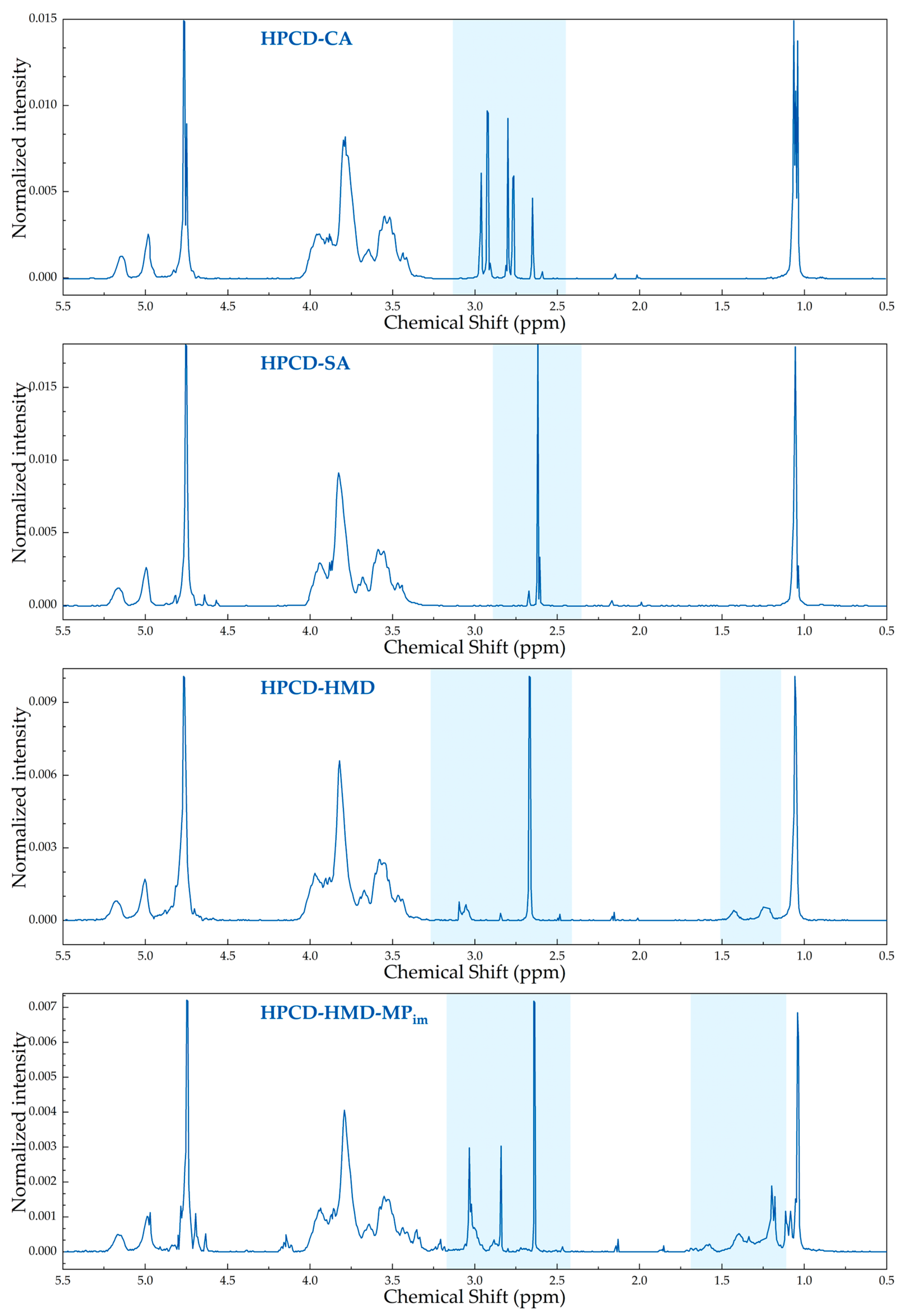
3.2. The Structure of HPCD-Polymers
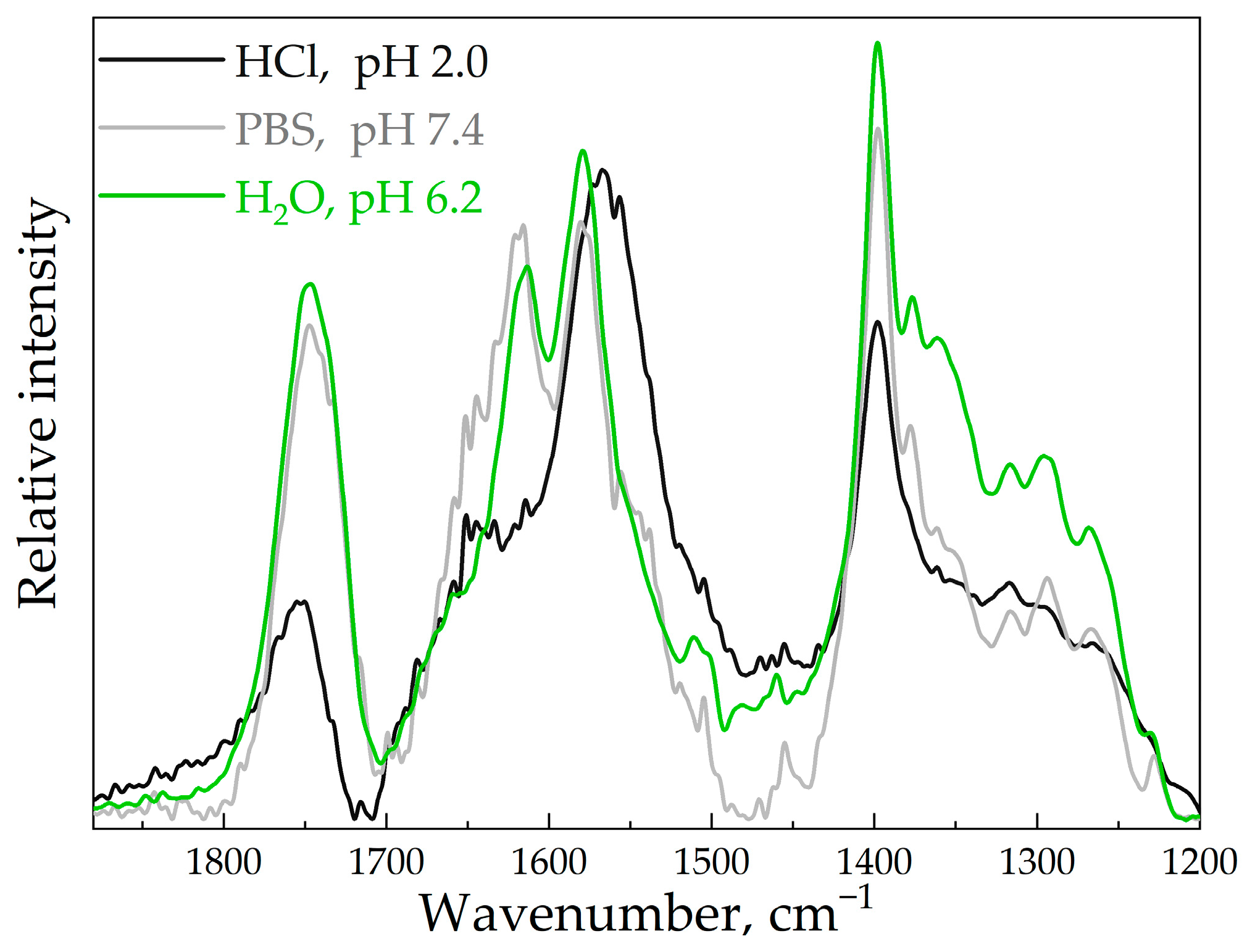
3.3. The Influence of HPCD Polymers on MP’s Stability in Aqueous Media
3.4. The Influence of HPCD Polymers on MP’s Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sulis, G.; Sayood, S.; Katukoori, S.; Bollam, N. Exposure to WHO AWaRe antibiotics and isolation of multi-drug resistant bacteria: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 1193–1202. [Google Scholar] [CrossRef]
- Paczkowska, M.; Mizera, M.; Szymanowska-Powałowska, D. β-Cyclodextrin complexation as an effective drug delivery system for meropenem. Eur. J. Pharm. Biopharm. 2016, 99, 24–34. [Google Scholar] [CrossRef]
- Raza, A.; Miles, J.; Sime, F.; Ross, B.; Roberts, J. PLGA encapsulated γ-cyclodextrin-meropenem inclusion complex formulation for oral delivery. Int. J. Pharm. 2021, 597, 120280. [Google Scholar] [CrossRef]
- Edwards, J.R. Meropenem: A microbiological overview. J. Antimicrob. Chemother. 1995, 36, 1–17. [Google Scholar] [CrossRef]
- Mendez, A.; Dalomo, J.; Steppe, M. Stability and degradation kinetics of meropenem in powder for injection and reconstituted sample. J. Pharm. Biomed. Anal. 2006, 41, 1363–1366. [Google Scholar] [CrossRef]
- Martin Del Valle, E.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Stella, V.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.Y.; Manne, A.S.N.; Kalthur, G.; Jain, S.; Mutalik, S. Cyclodextrins as carriers in targeted delivery of therapeutic agents: Focused review on traditional and inimitable applications. Curr. Pharm. Des. 2019, 25, 444–454. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Highly soluble cyclodextrin derivatives: Chemistry, properties, and trends in development. Adv. drug Deliv. 1999, 36, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ye, L.; Xi, J.; Wang, J.; Feng, Z.G. Cyclodextrin polymers: Structure, synthesis, and use as drug carriers. Prog. Polym. Sci. 2021, 118, 101408. [Google Scholar] [CrossRef]
- Barman, S.; Barman, B.K.; Roy, M.N. Preparation, characterization and binding behaviors of host-guest inclusion complexes of metoclopramide hydrochloride with α- and β-cyclodextrin molecules. J. Mol. Struct. 2018, 1155, 503–512. [Google Scholar] [CrossRef]
- Le-Deygen, I.M.; Skuredina, A.A.; Kudryashova, E.V. Drug delivery systems for fluoroquinolones: New prospects in tuberculosis treatment. Russ. J. Bioorganic Chem. 2017, 43, 487–501. [Google Scholar] [CrossRef]
- Biwer, A.; Antranikian, G.; Heinzle, E. Enzymatic production of cyclodextrins. Appl. Microbiol. Biotechnol. 2002, 59, 609–617. [Google Scholar] [CrossRef]
- Skuredina, A.; Le-Deygen, I.; Belogurova, N. Effect of cross-linking on the inclusion complex formation of derivatized β-cyclodextrins with small-molecule drug moxifloxacin. Carbohydr. Res. 2020, 498, 108183. [Google Scholar] [CrossRef]
- Deygen, I.M.; Kudryashova, E.V. New versatile approach for analysis of PEG content in conjugates and complexes with biomacromolecules based on FTIR spectroscopy. Colloids Surf. B Biointerfaces 2016, 141, 36–43. [Google Scholar] [CrossRef]
- Skuredina, A.A.; Yu Kopnova, T.; Tychinina, A.S.; Golyshev, S.A. The new strategy for studying drug-delivery systems with prolonged release: Seven-day in vitro antibacterial action. Molecules 2022, 27, 8026. [Google Scholar] [CrossRef] [PubMed]
- Trotta, F.; Caldera, F.; Cavalli, R.; Soster, M. Molecularly imprinted cyclodextrin nanosponges for the controlled delivery of L-DOPA: Perspectives for the treatment of Parkinson’s disease. Expert Opin. Drug Deliv. 2016, 13, 1671–1680. [Google Scholar] [CrossRef]
- Skuredina, A.; Tychinina, A.; Le-Deygen, I. Cyclodextrins and their polymers affect the lipid membrane permeability and increase levofloxacin’s antibacterial activity in vitro. Polymers 2022, 14, 4476. [Google Scholar] [CrossRef] [PubMed]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of nanoparticle tracking analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Sunagawa, M. Stability of a 1β-methylcarbapenem antibiotic, meropenem (SM-7338) in aqueous solution. Chem. Pharm. Bull. 1995, 43, 689–692. [Google Scholar] [CrossRef]
- Yang, S.K.; Yusoff, K.; Mai, C.W.; Lim, W.M.; Yap, W.S.; Lim, S.H.; Lai, K.S. Additivity vs. synergism: Investigation of the additive interaction of cinnamon bark oil and meropenem in combinatory therapy. Molecules 2017, 22, 1733. [Google Scholar] [CrossRef] [PubMed]
- Skuredina, A.A.; Tychinina, A.S.; Le-Deygen, I.M.; Golyshev, S.A.; Belogurova, N.G.; Kudryashova, E.V. The formation of quasi-regular polymeric network of cross-linked sulfobutyl ether derivative of β-cyclodextrin synthesized with moxifloxacin as a template. React. Funct. Polym. 2021, 159, 104811. [Google Scholar] [CrossRef]
- Aleem, O.; Kuchekar, B.; Pore, Y.; Late, S. Effect of β-cyclodextrin and hydroxypropyl β-cyclodextrin complexation on physicochemical properties and antimicrobial activity of cefdinir. J. Pharm. Biomed. Anal. 2008, 47, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhao, L.; Zhu, C.S.; Huang, W.Q.; Hu, J.L. Water-insoluble β-cyclodextrin polymer crosslinked by citric acid: Synthesis and adsorption properties toward phenol and methylene blue. J. Incl. Phenom. Macrocycl. Chem. 2009, 63, 195–201. [Google Scholar] [CrossRef]
- Wilpiszewska, K.; Antosik, A.K.; Zdanowicz, M. The effect of citric acid on physicochemical properties of hydrophilic carboxymethyl starch-based films. J. Polym. Environ. 2019, 27, 1379–1387. [Google Scholar] [CrossRef]
- Yilgor, I.; Yilgor, E.; Guler, I.G.; Ward, T.C.; Wilkes, G.L. FTIR investigation of the influence of diisocyanate symmetry on the morphology development in model segmented polyurethanes. Polymer 2006, 47, 4105–4114. [Google Scholar] [CrossRef]
- Baier, G.; Baumann, D.; Siebert, J.M.; Musyanovych, A.; Mailänder, V.; Landfester, K. Suppressing unspecific cell uptake for targeted delivery using hydroxyethyl starch nanocapsules. Biomacromolecules 2012, 13, 2704–2715. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J. Vibrational spectra of primary and secondary aliphatic amines. J. Chem. Phys. 1959, 30, 1259–1265. [Google Scholar] [CrossRef]
- Cielecka-Piontek, J.; Paczkowska, M.; Lewandowska, K.; Barszcz, B.; Zalewski, P.; Garbacki, P. Solid-state stability study of meropenem - solutions based on spectrophotometric analysis. Chem. Cent. J. 2013, 7, 2–9. [Google Scholar] [CrossRef]
- Berthoin, K.; Le, D.C.; Marchand-Brynaert, J. Transparency declarations. J. Antimicrob. Chemother. 2009, 64, 142–150. [Google Scholar]
- Muneer, S.; Wang, T.; Rintoul, L.; Ayoko, G.; Islam, N. Development and characterization of meropenem dry powder inhaler formulation for pulmonary drug delivery. Int. J. Pharm. 2020, 587, 119684. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, X.; Dang, L.; Wei, H. Solubility and pseudopolymorphic transitions in mixed solvent: Meropenem in methanol–water solution. Fluid Phase Equilib. 2013, 349, 25–30. [Google Scholar] [CrossRef]
- Kohut, A.; Demchuk, Z.; Kingsley, K.; Voronov, S.; Voronov, A. Dual role of methyl-β-cyclodextrin in the emulsion polymerization of highly hydrophobic plant oil-based monomers with various unsaturations. Eur. Polym. J. 2018, 108, 322–328. [Google Scholar] [CrossRef]
- Sala, A.; Hoossen, Z.; Bacchi, A. Two crystal forms of a hydrated 2: 1 β-cyclodextrin fluconazole complex: Single crystal X-ray structures, dehydration profiles, and conditions for their individual. Molecules 2021, 26, 4427. [Google Scholar] [CrossRef]
- Xu, Q.; Tang, Y.; Zhang, X.; Oshima, Y.; Chen, Q.; Jiang, D. Template Conversion of Covalent Organic Frameworks into 2D Conducting Nanocarbons for Catalyzing Oxygen Reduction Reaction. Adv. Mater. 2018, 30, 1706330. [Google Scholar] [CrossRef]
- Mendez, A.; Chagastelles, P.; Palma, E.; Nardi, N. Thermal and alkaline stability of meropenem: Degradation products and cytotoxicity. Int. J. Pharm. 2008, 350, 95–102. [Google Scholar] [CrossRef]
- Tomasello, C.; Leggieri, A.; Cavalli, R.; Di Perri, G.; D’avolio, A. In vitro stability evaluation of different pharmaceutical products containing meropenem. Hosp. Pharm. 2015, 50, 296–303. [Google Scholar] [CrossRef] [PubMed]


| Method | HPCD | HPCD-CA | HPCD-SA | HPCD-HMD | HPCD-HMD-MPim | |
|---|---|---|---|---|---|---|
| Hydrodynamic diameter, nm | NTA | ≈0.15 1 | 120.1 ± 3.2 | 199.2 ± 18.2 | 142.5 ± 13.5 | 178.8 ± 10.2 |
| DLS | ≈0.15 1 | 245 ± 15 | 283 ± 21 | 220 ± 17 | 289 ± 22 | |
| ζ-potential, mV | DLS | 0.5 ± 0.3 | – 2 | 5.6 ± 2.1 | 14.7 ± 0.4 | −2.9 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakupova, L.R.; Skuredina, A.A.; Markov, P.O.; Le-Deygen, I.M.; Kudryashova, E.V. Cyclodextrin Polymers as a Promising Drug Carriers for Stabilization of Meropenem Solutions. Appl. Sci. 2023, 13, 3608. https://doi.org/10.3390/app13063608
Yakupova LR, Skuredina AA, Markov PO, Le-Deygen IM, Kudryashova EV. Cyclodextrin Polymers as a Promising Drug Carriers for Stabilization of Meropenem Solutions. Applied Sciences. 2023; 13(6):3608. https://doi.org/10.3390/app13063608
Chicago/Turabian StyleYakupova, Linara R., Anna A. Skuredina, Pavel O. Markov, Irina M. Le-Deygen, and Elena V. Kudryashova. 2023. "Cyclodextrin Polymers as a Promising Drug Carriers for Stabilization of Meropenem Solutions" Applied Sciences 13, no. 6: 3608. https://doi.org/10.3390/app13063608
APA StyleYakupova, L. R., Skuredina, A. A., Markov, P. O., Le-Deygen, I. M., & Kudryashova, E. V. (2023). Cyclodextrin Polymers as a Promising Drug Carriers for Stabilization of Meropenem Solutions. Applied Sciences, 13(6), 3608. https://doi.org/10.3390/app13063608








