Physical and Chemical Features of Hydrogen Combustion and Their Influence on the Characteristics of Gas Turbine Combustion Chambers
Abstract
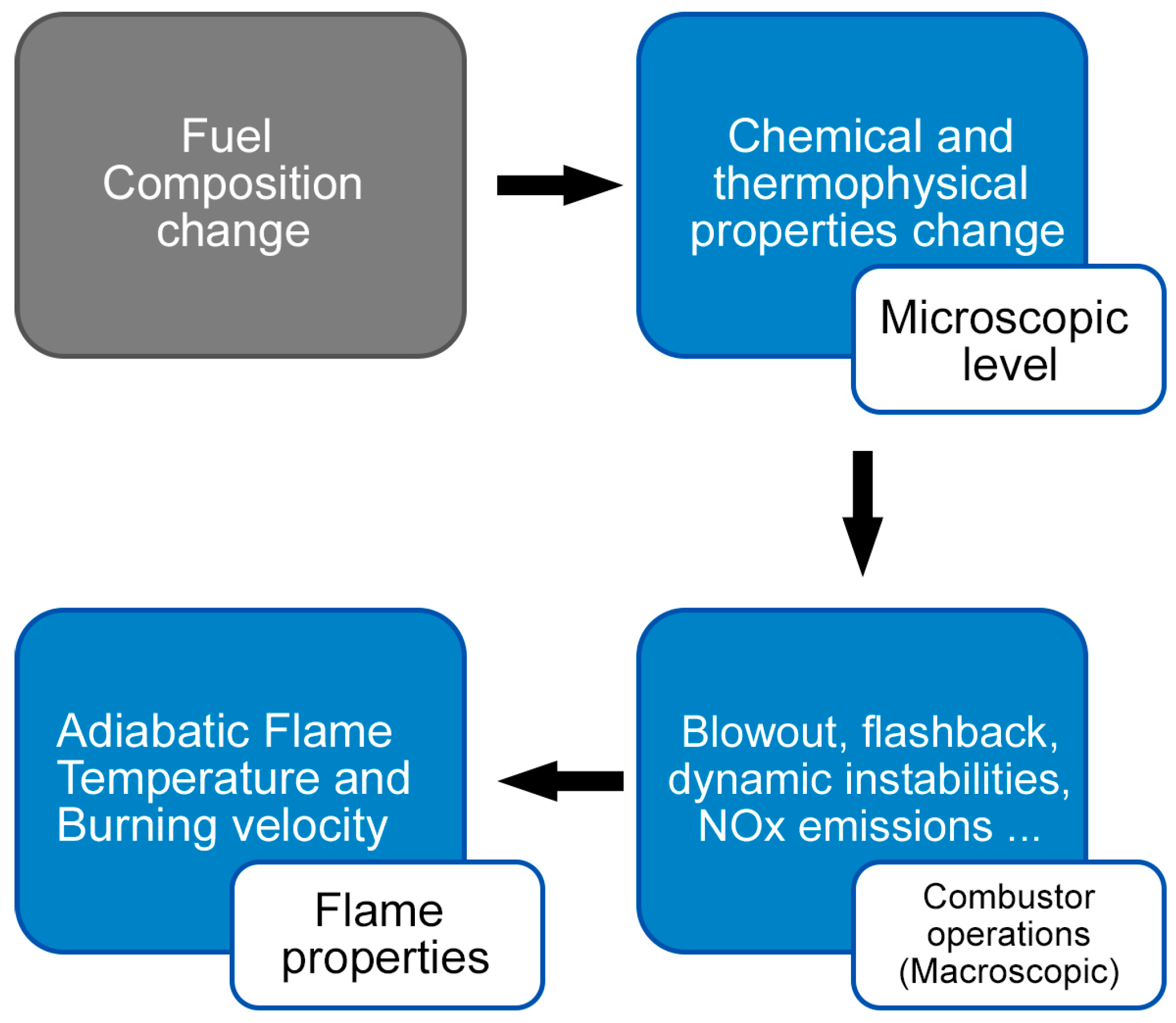
1. Introduction
2. Features of Hydrogen Combustion Processes
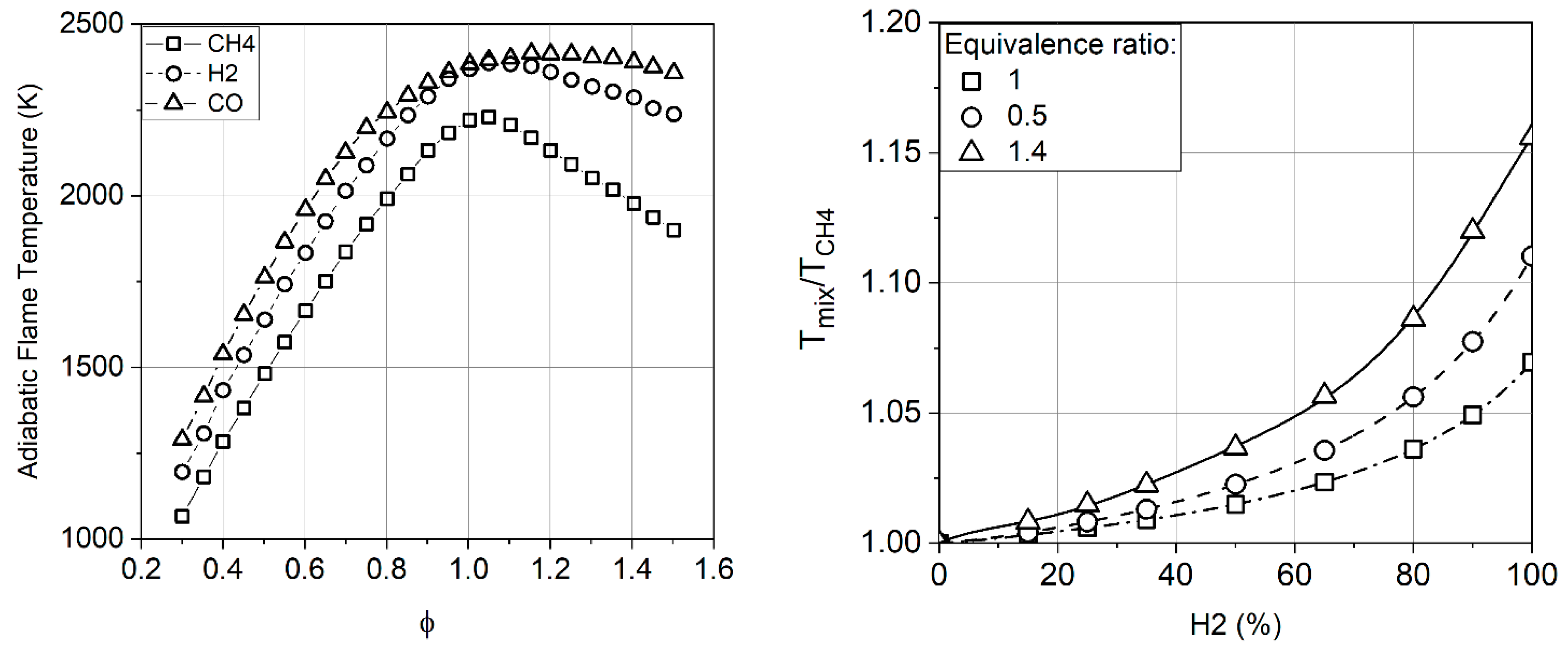
2.1. Properties of Hydrogen and Flame Temperature
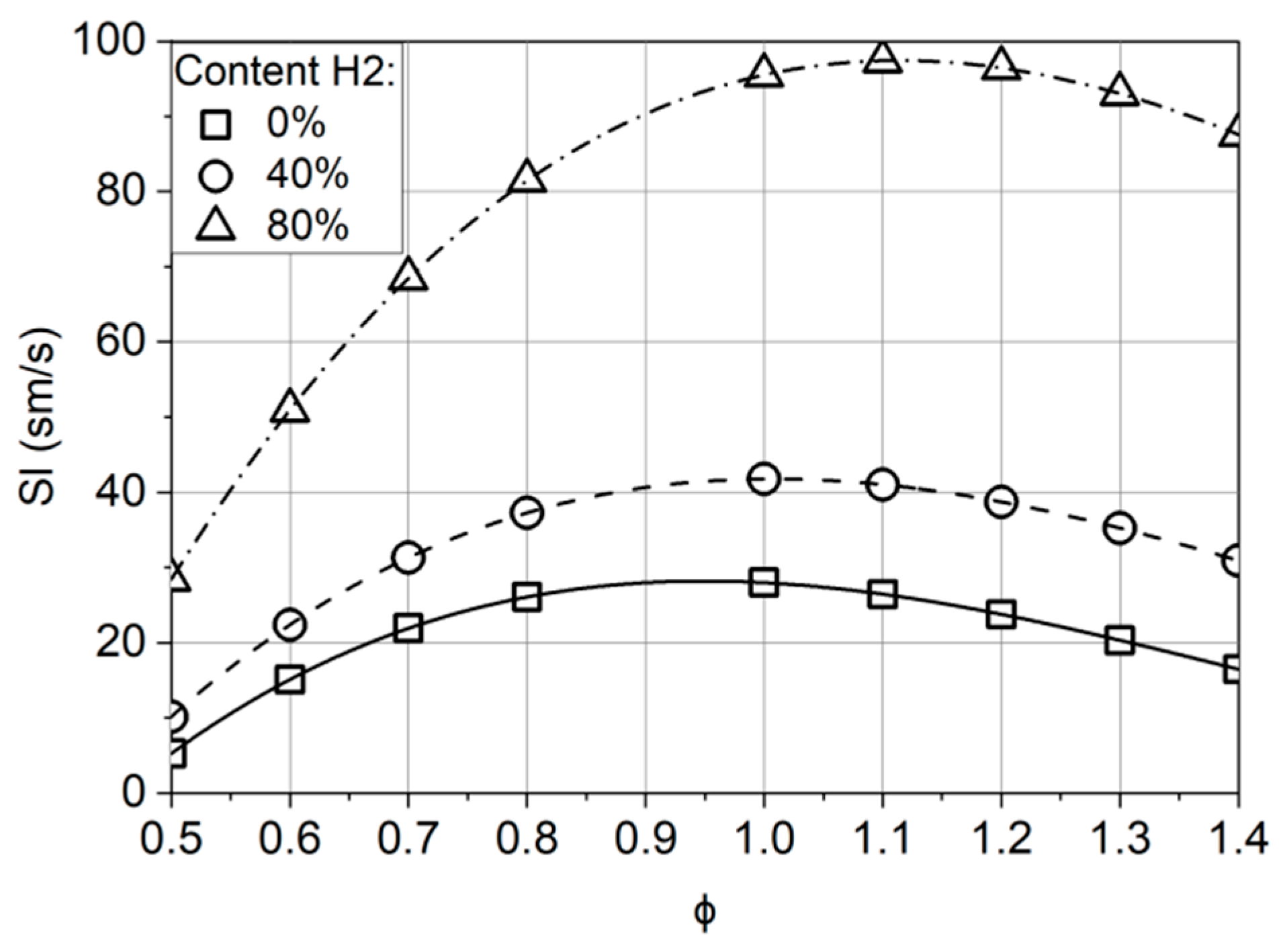
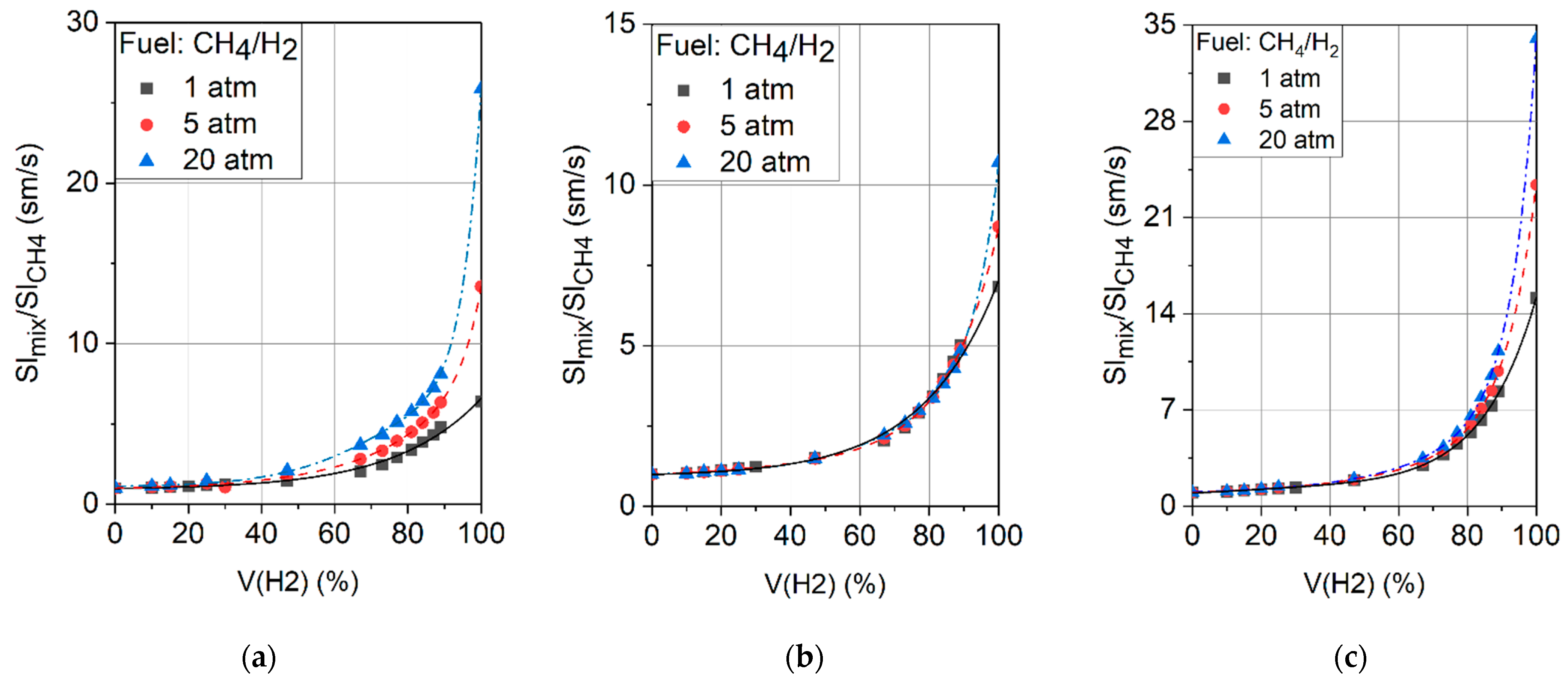
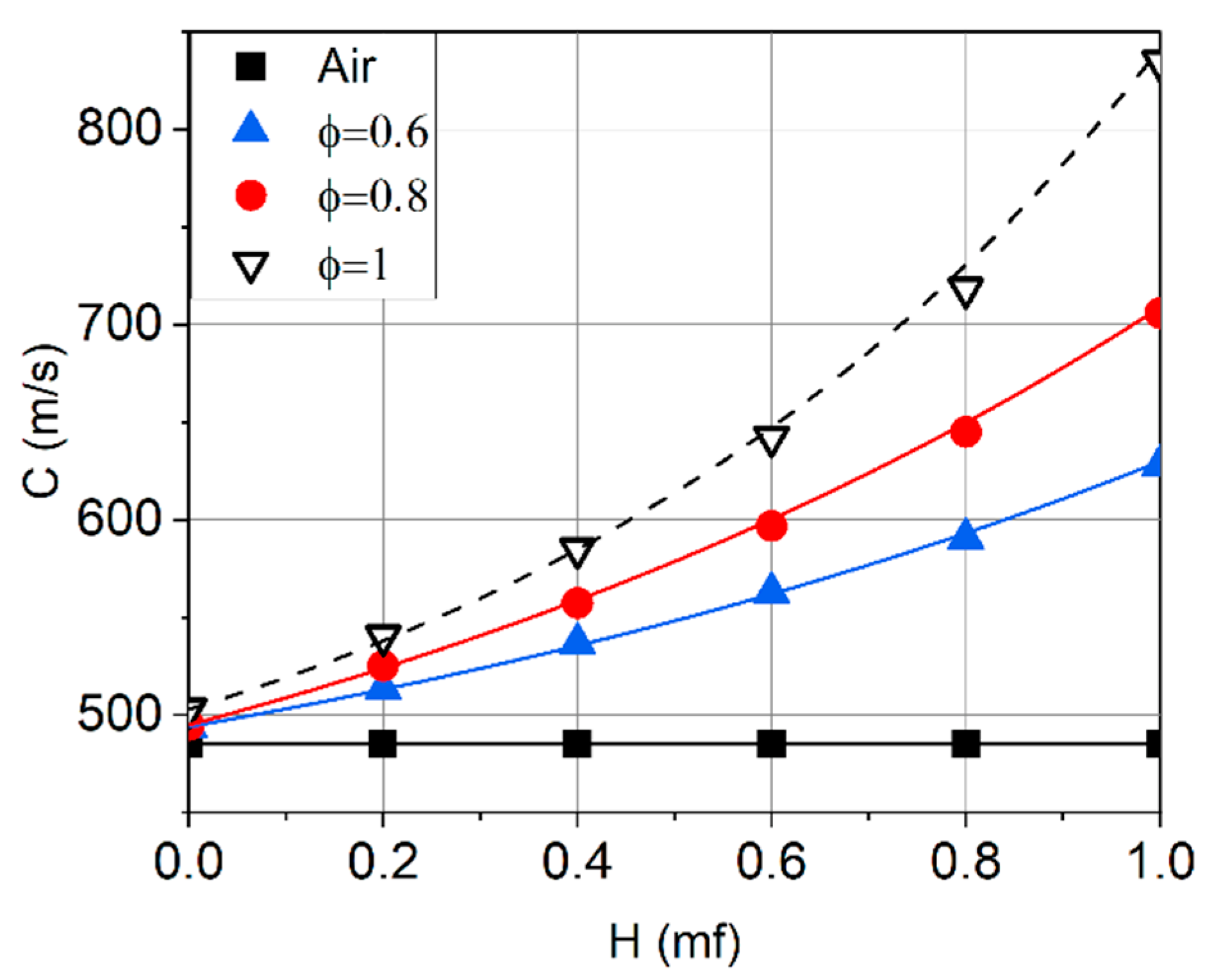
2.2. Laminar Flame Propagation Speed
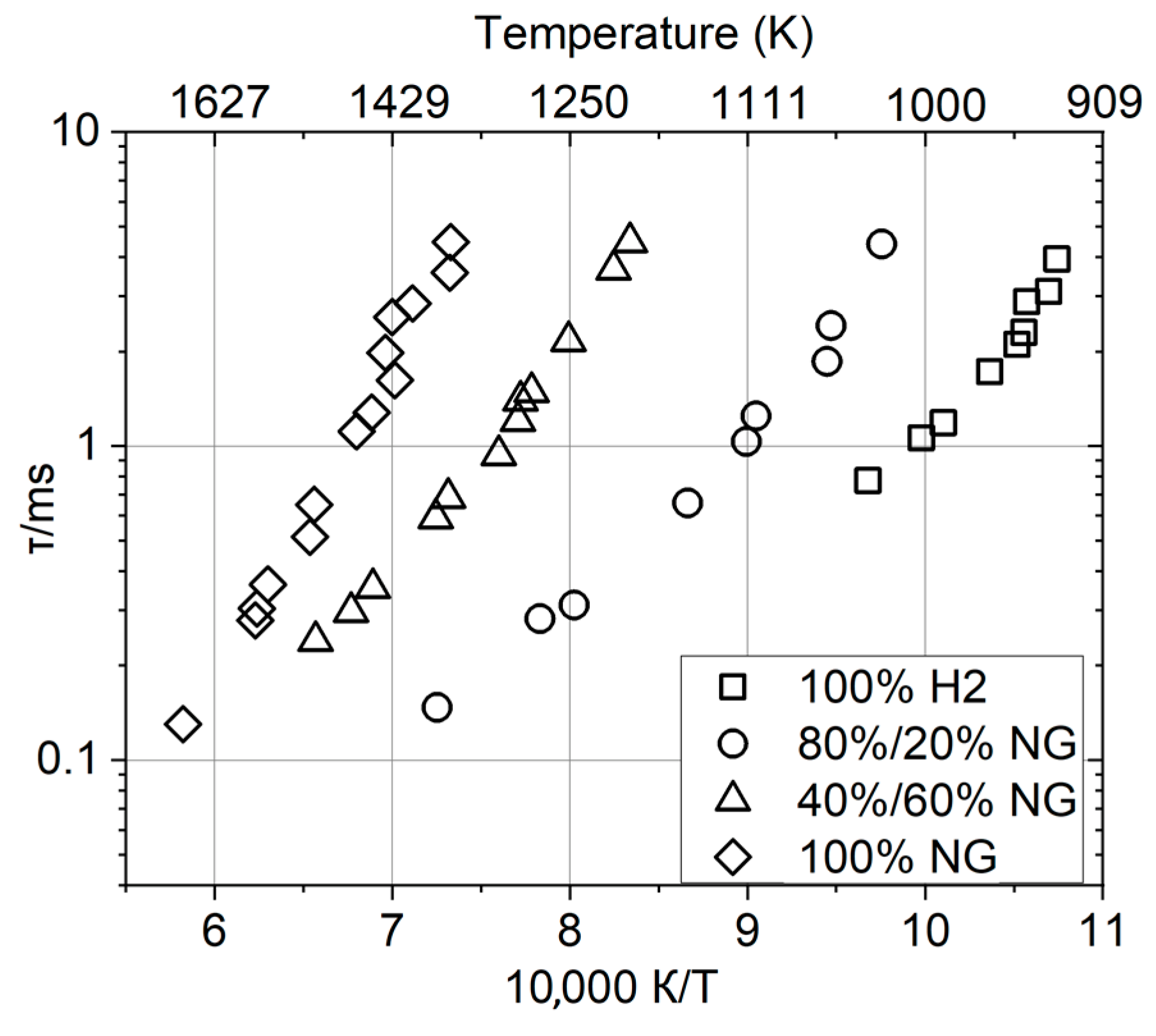
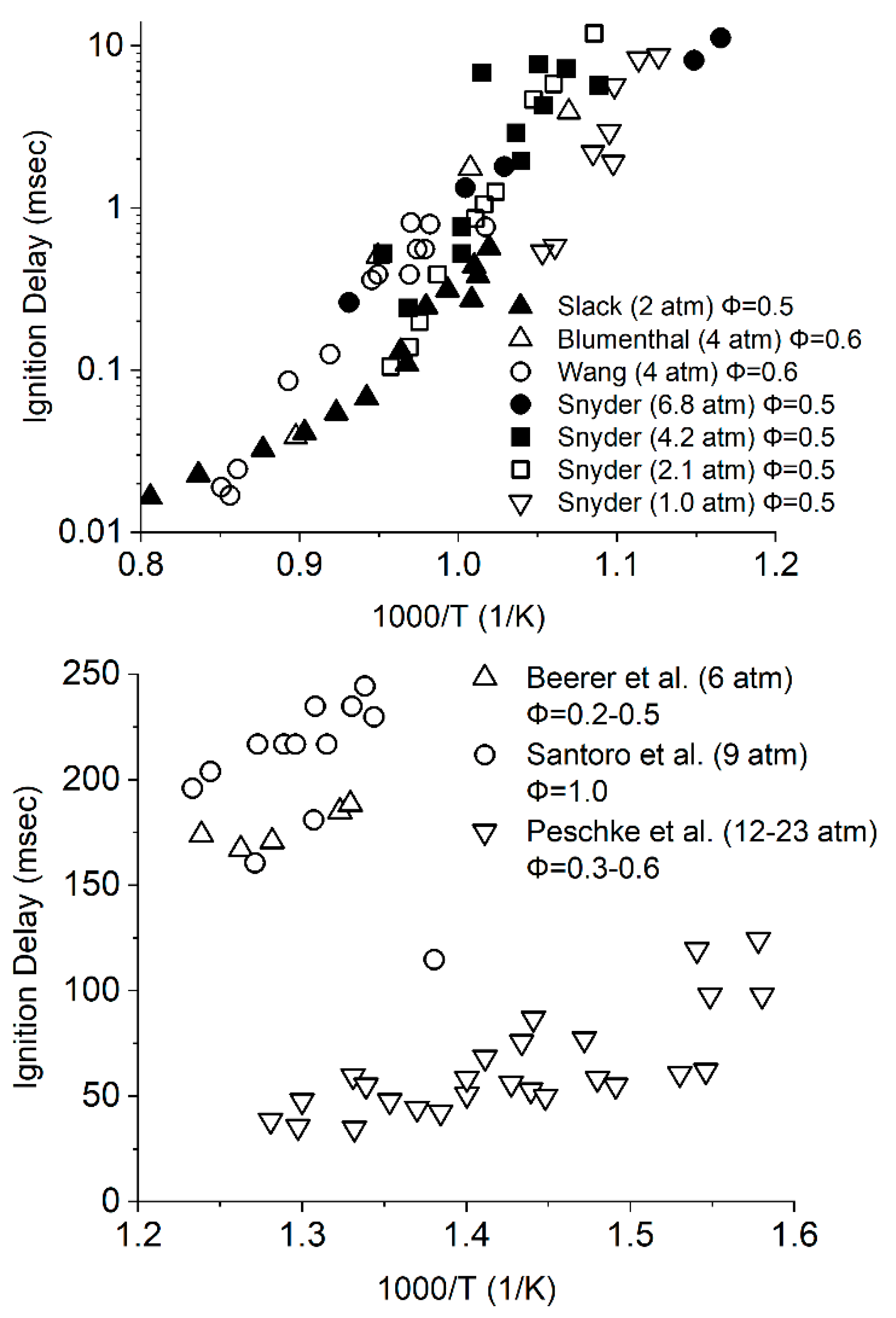
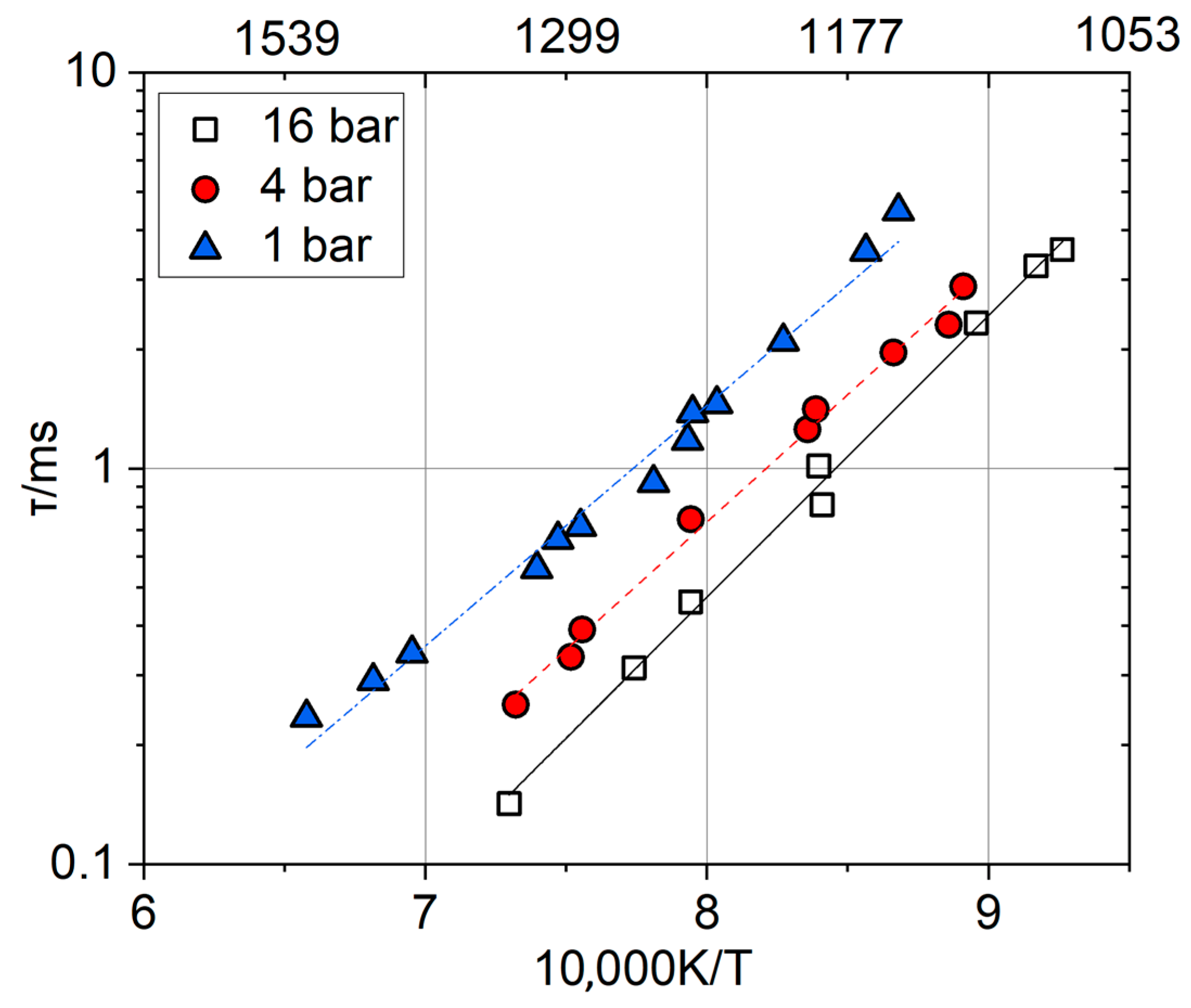
2.3. Ignition Delay Time and Autoignition Temperature
- (1)
- MCDI (methane chemistry dominating ignition) system—for methane–hydrogen fuel mixtures containing less than 40% hydrogen, a decrease in the ignition delay time typical of hydrocarbons with increasing pressure is observed.
- (2)
- CCMHDI (combined chemistry of methane and hydrogen dominating ignition) system—for a 40% CH4/60% H2 fuel mixture, there is no noticeable effect of pressure on the ignition time. In this case, the nature of ignition is determined neither by the kinetics of hydrogen nor by the kinetics of methane.
- (3)
- HCDI (hydrogen chemistry dominating ignition) system—the ignition typical of hydrogen and a complex pressure dependence that occurs when the mole hydrogen fraction of the mixture exceeds 80%.
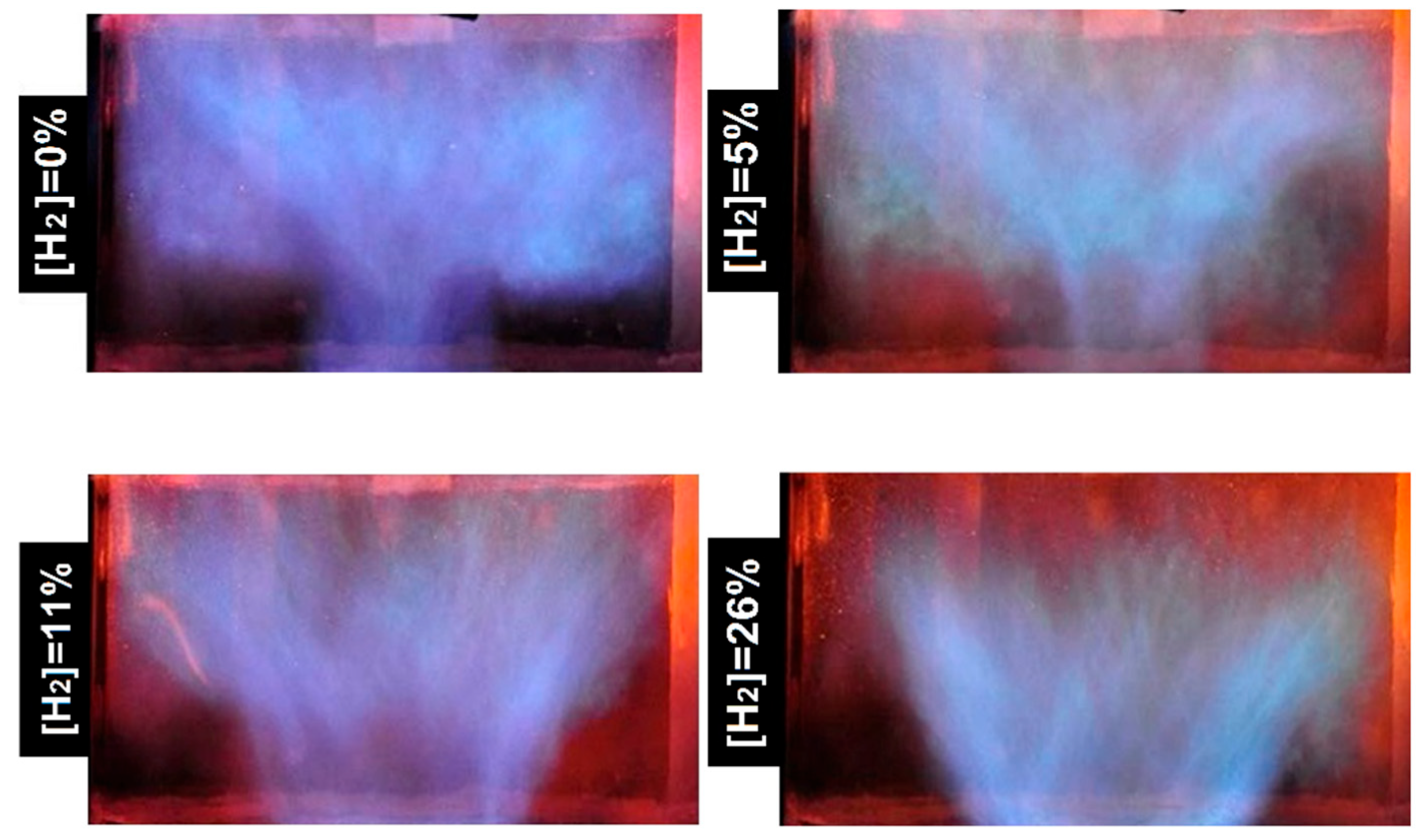
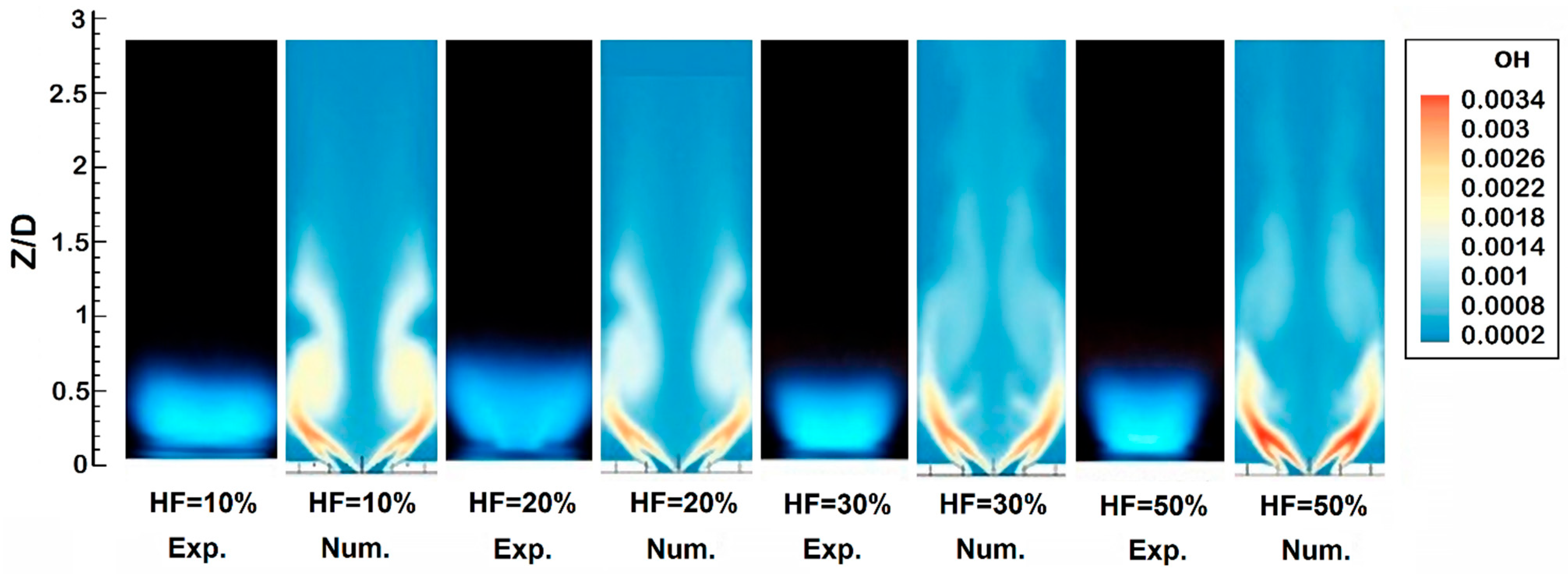
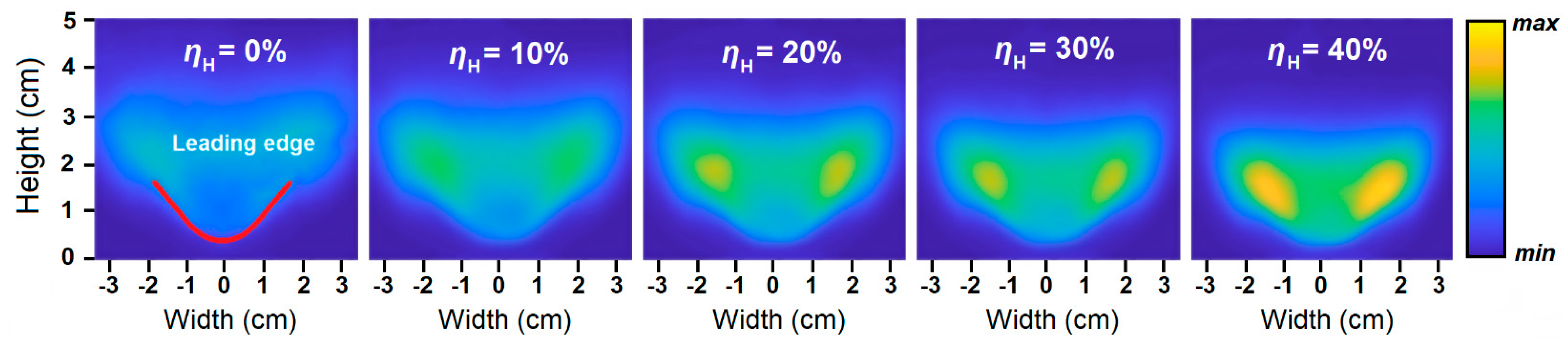
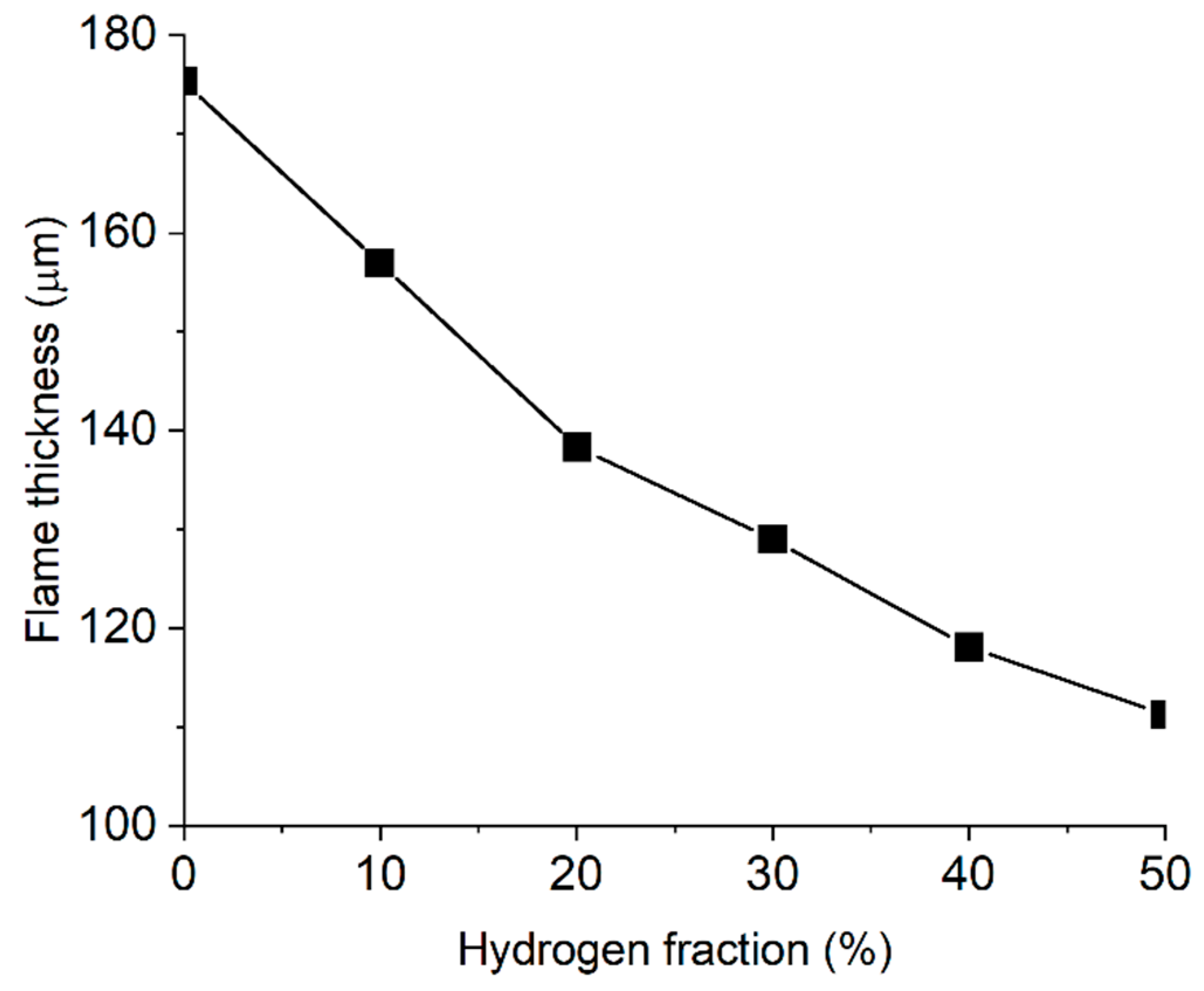
2.4. Flame Size and Shape
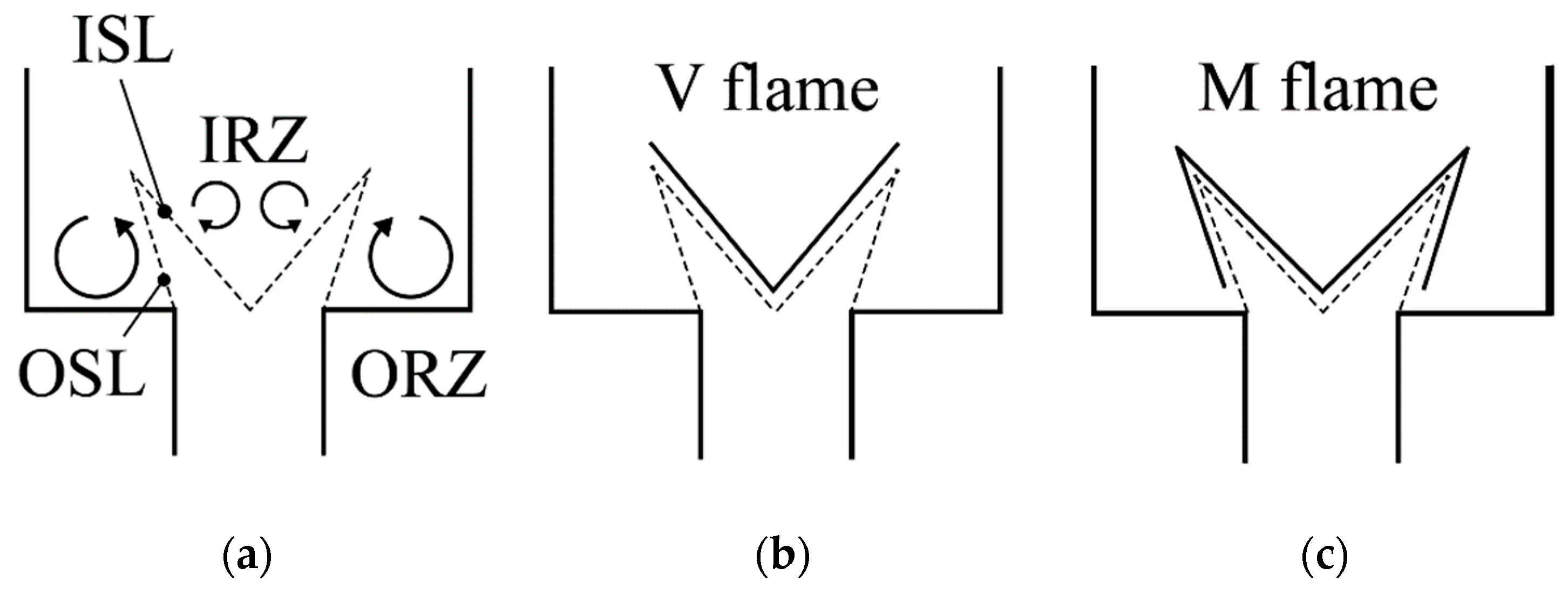
2.5. Flow Swirl
3. Influence of the Behavior Features of the Hydrogen Combustion Processes on the Characteristics of Combustion Chambers
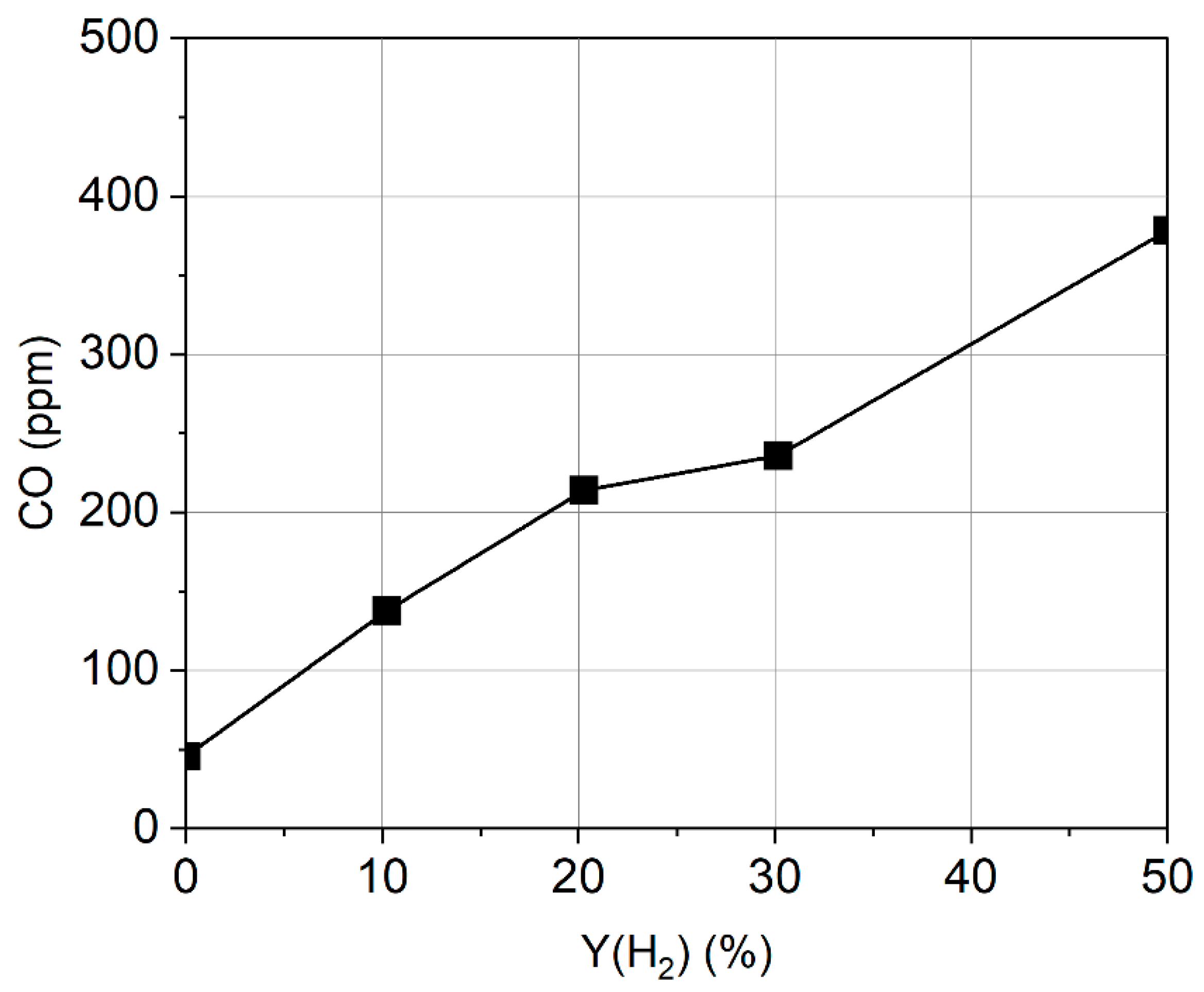
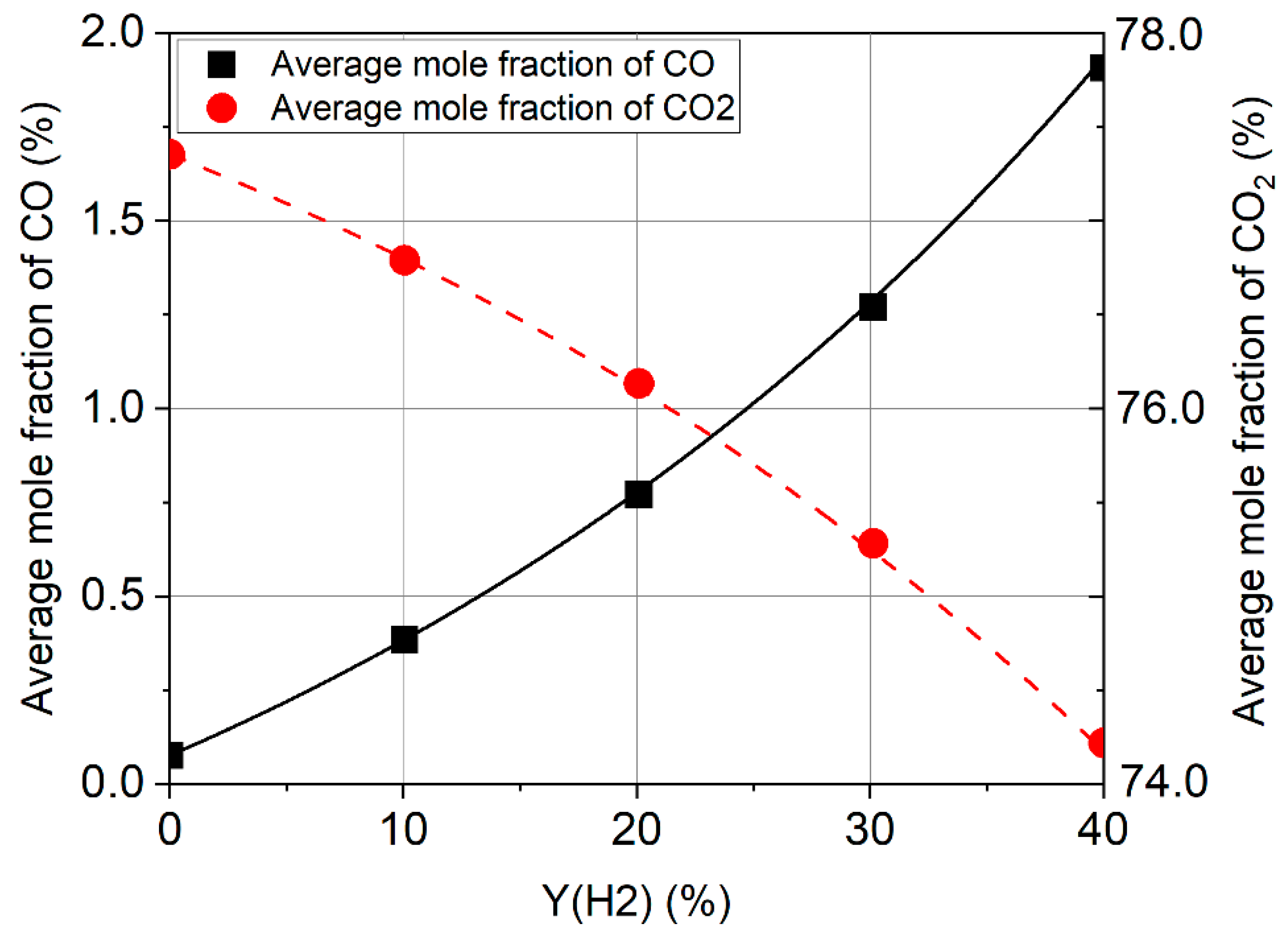
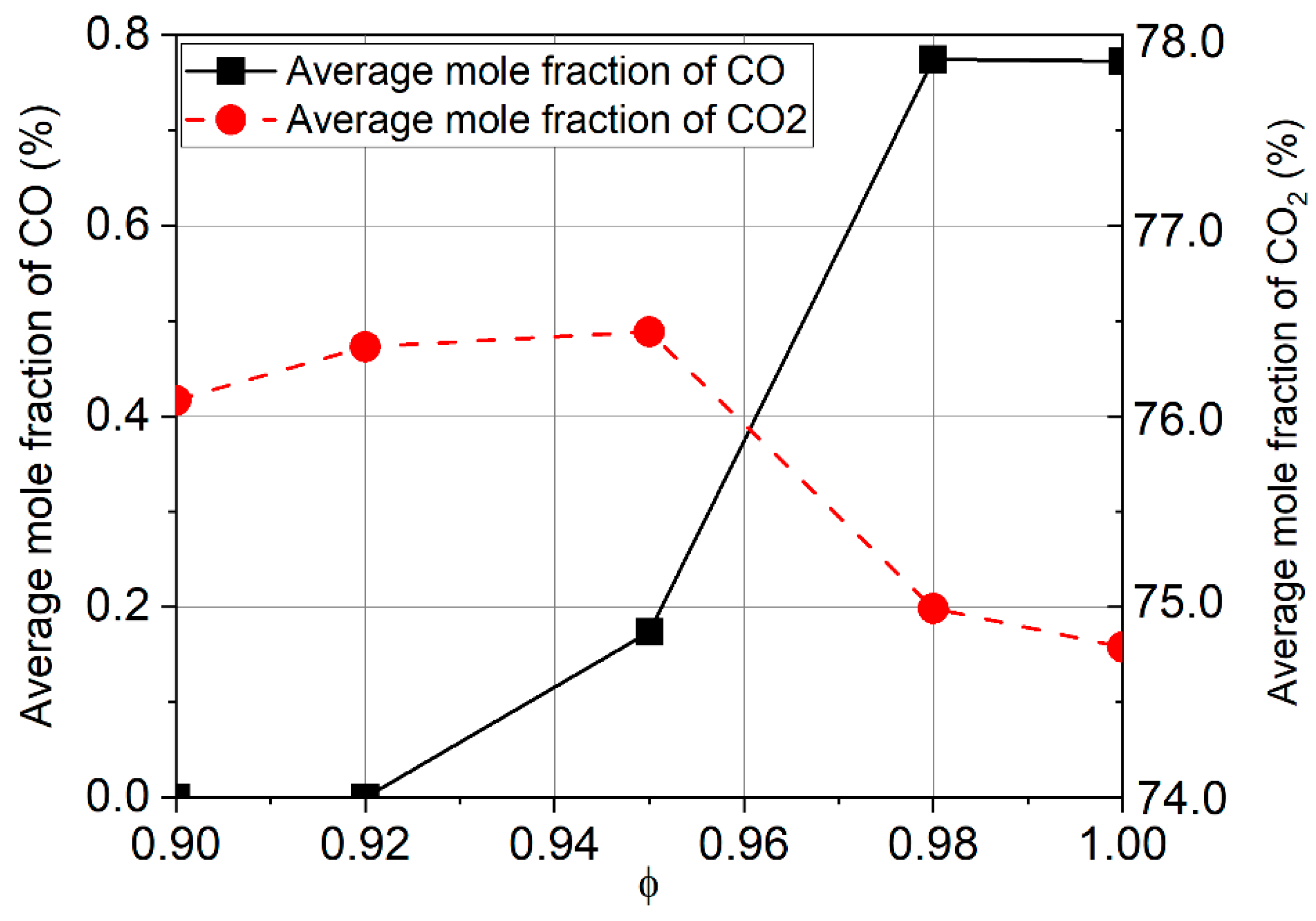
3.1. Formation of Pollutant Emissions: CO and CO2
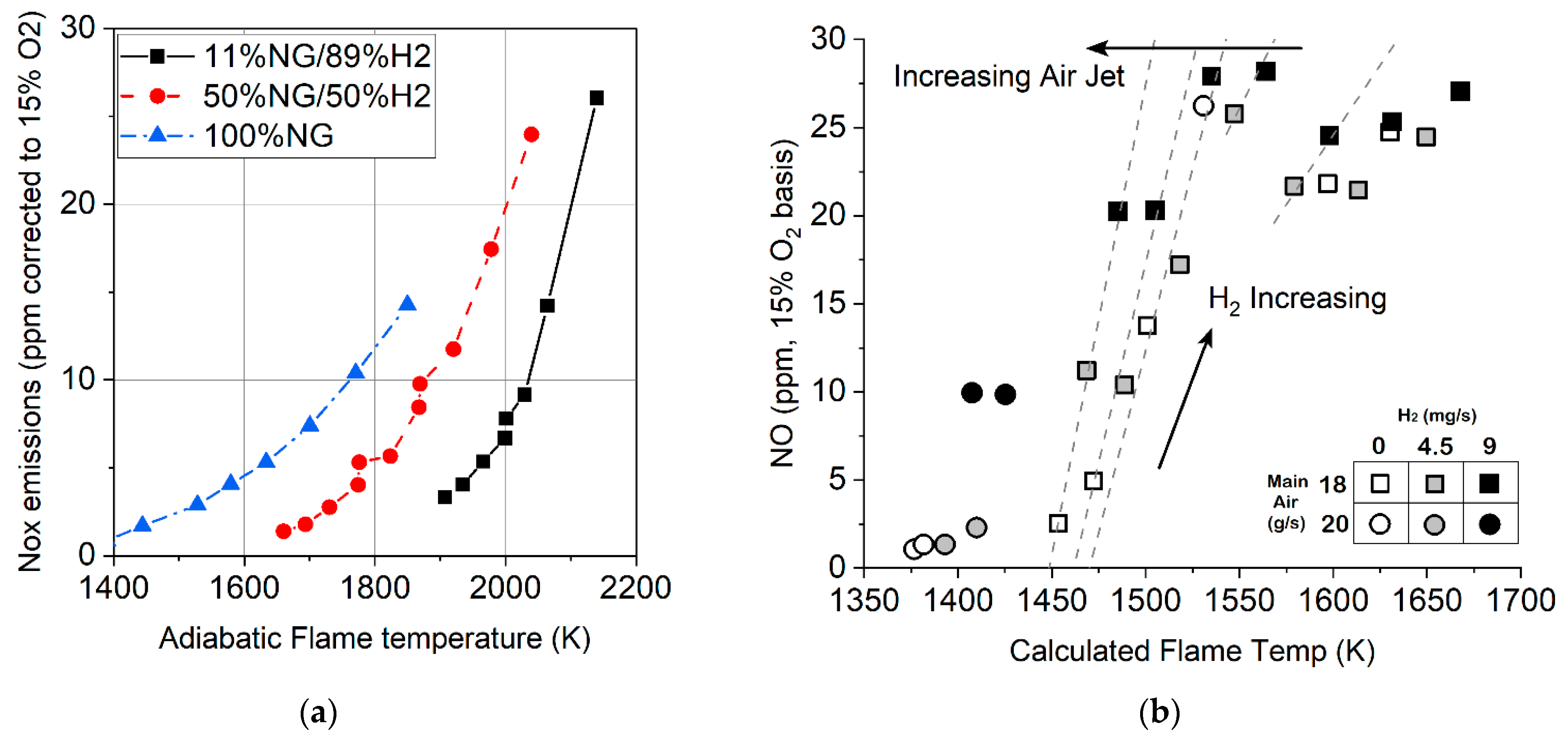
3.2. Formation of Pollutant Emissions: NOx
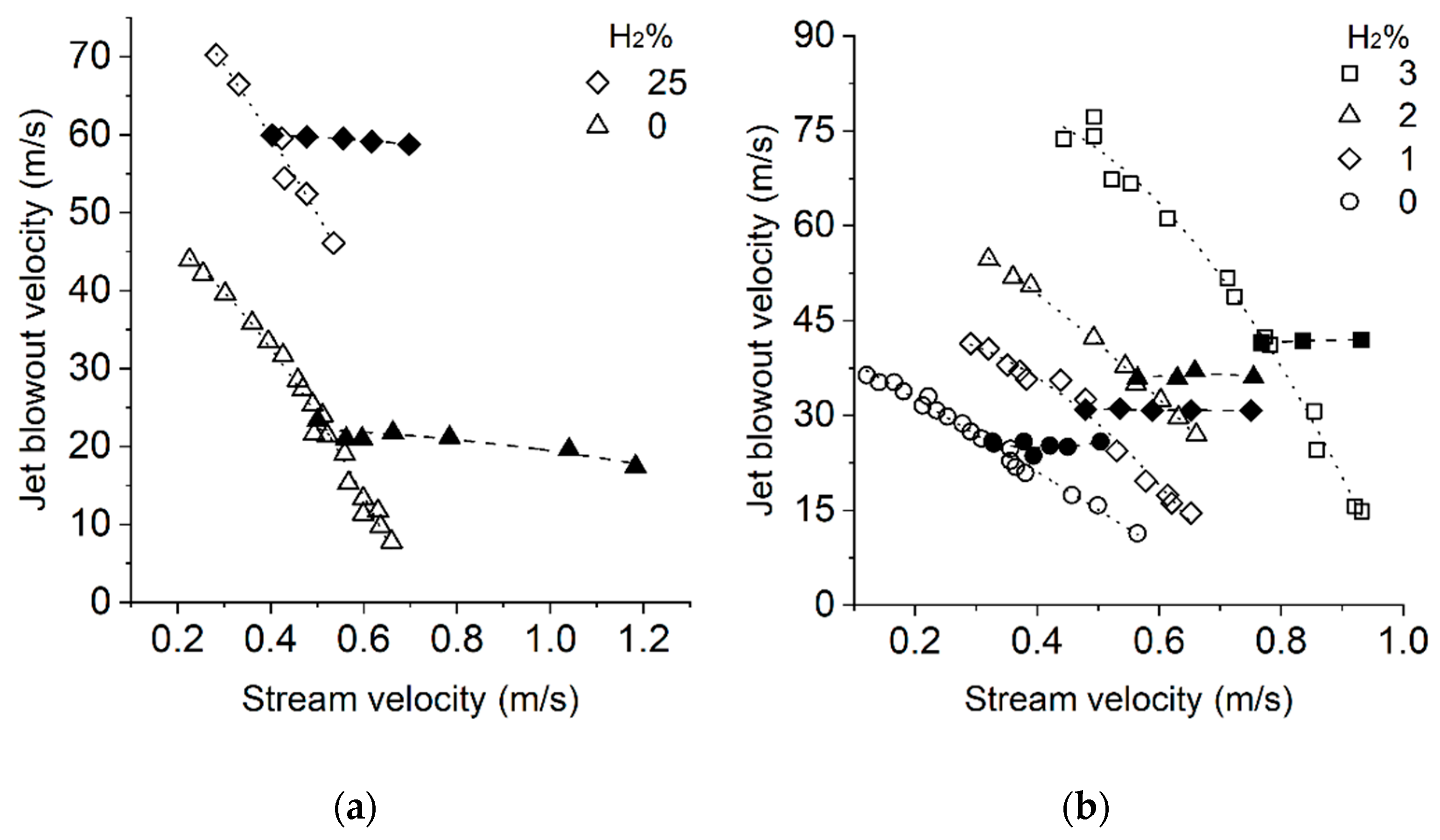
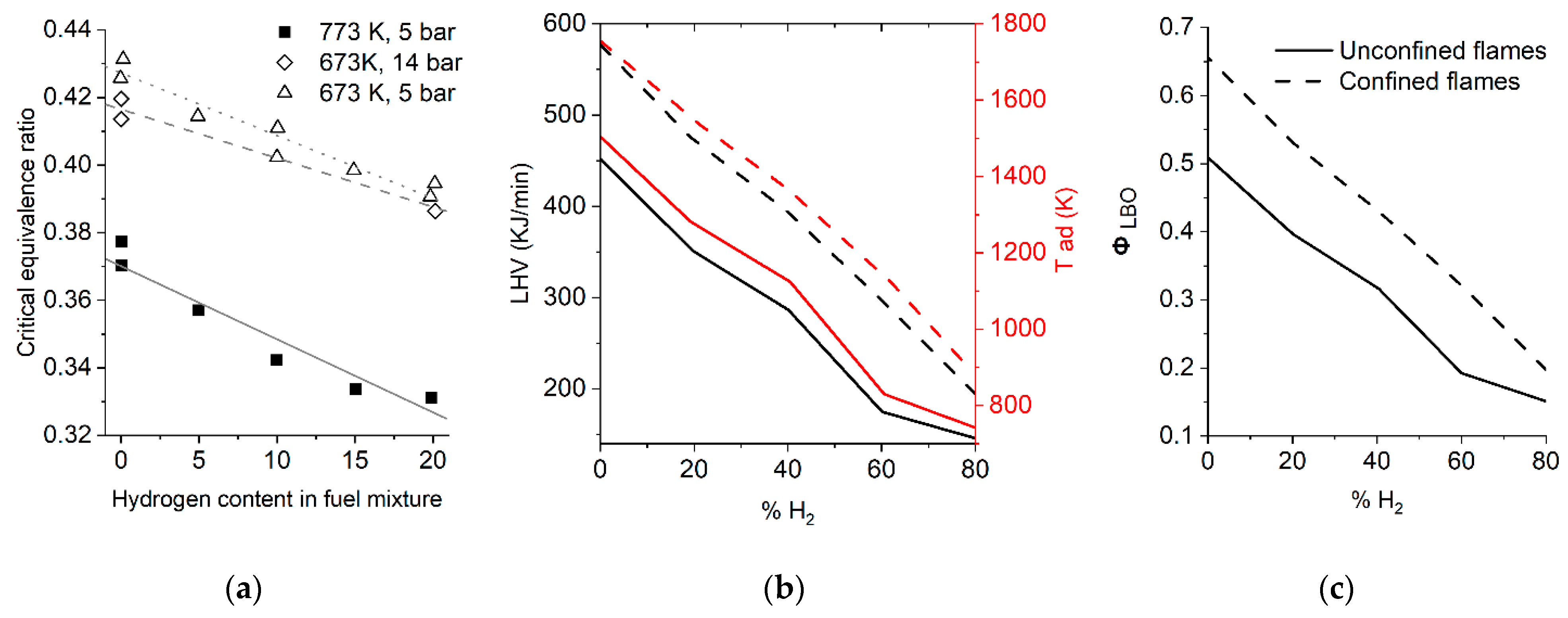
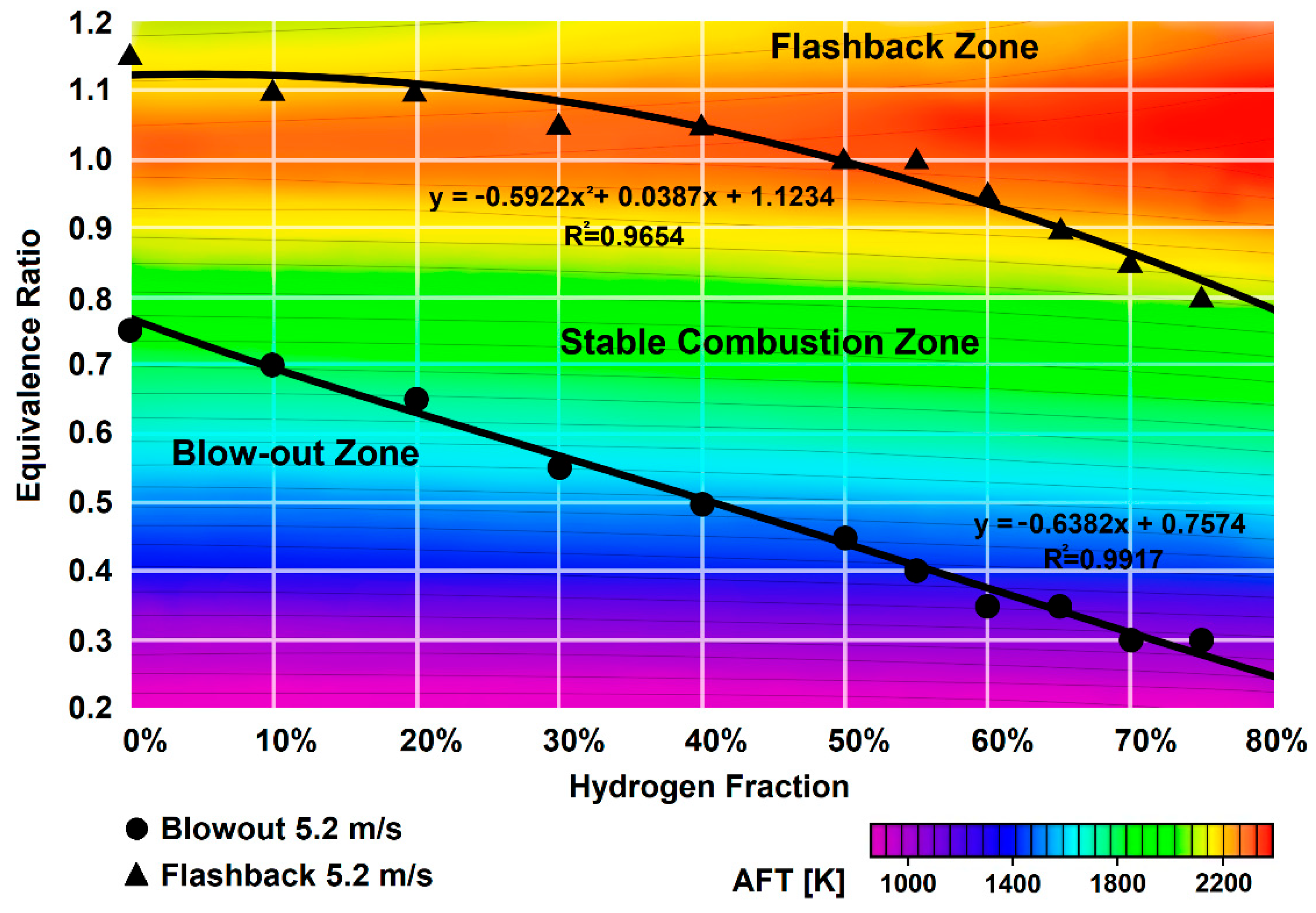
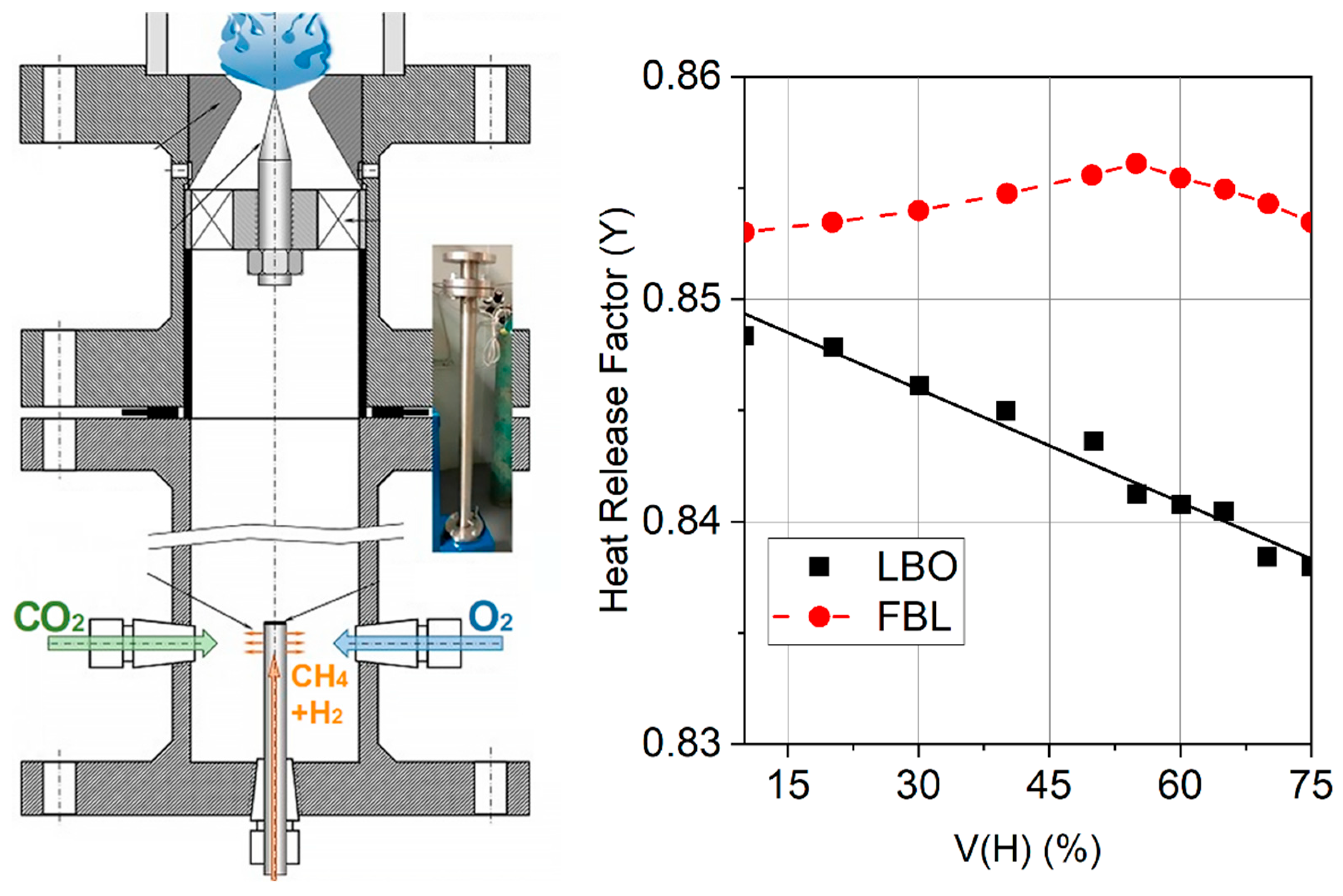
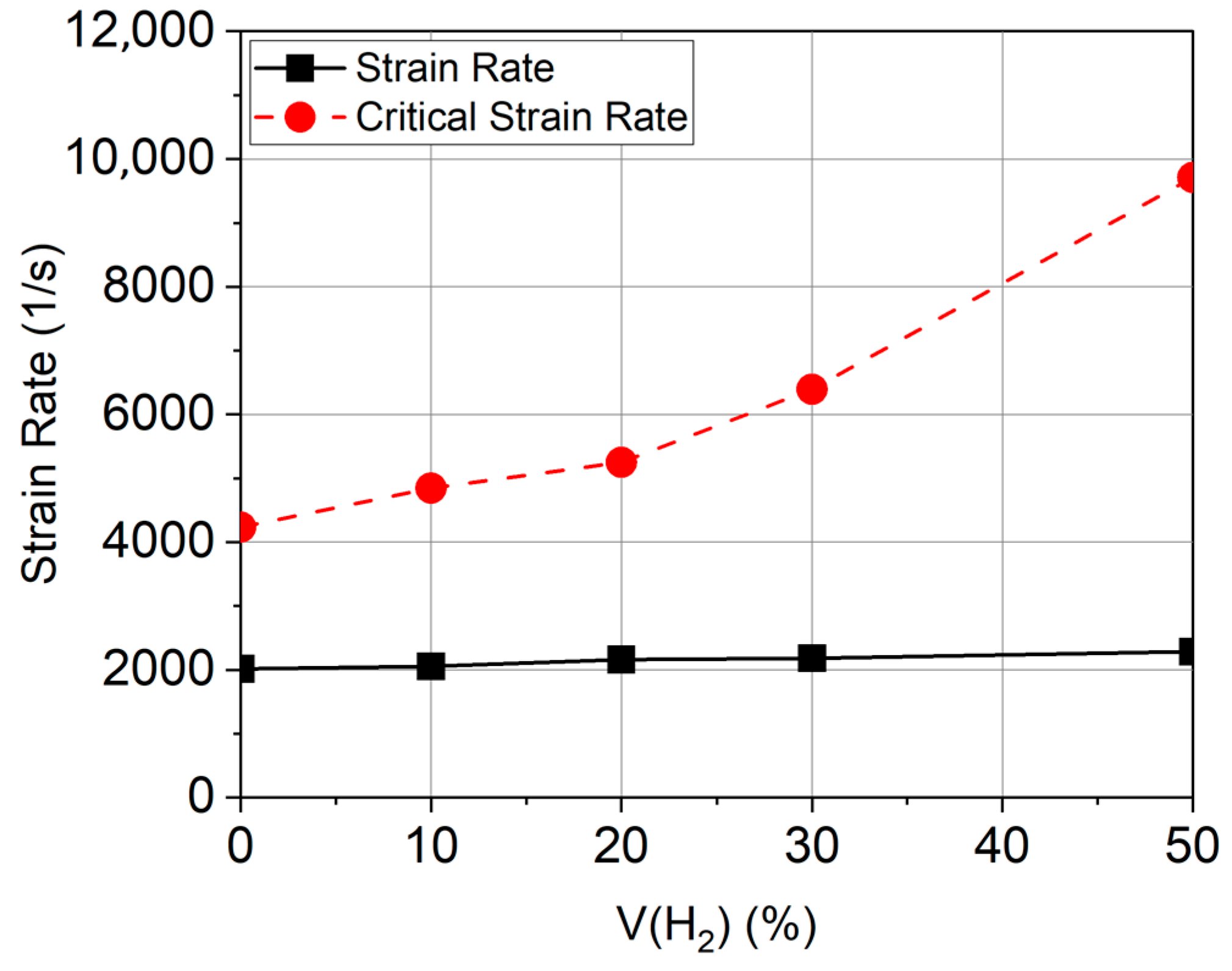
3.3. Flame Blowout and Flashback
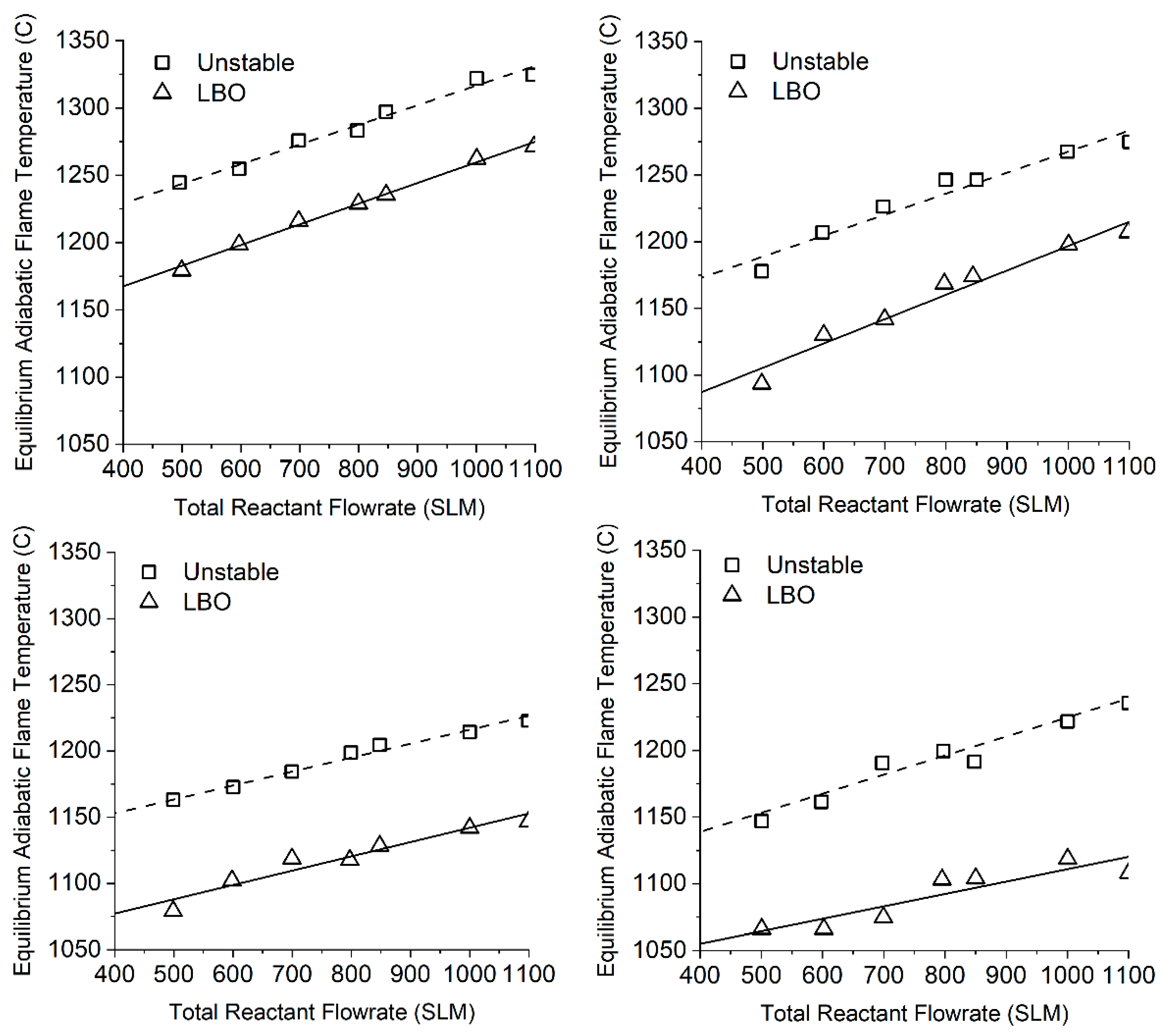
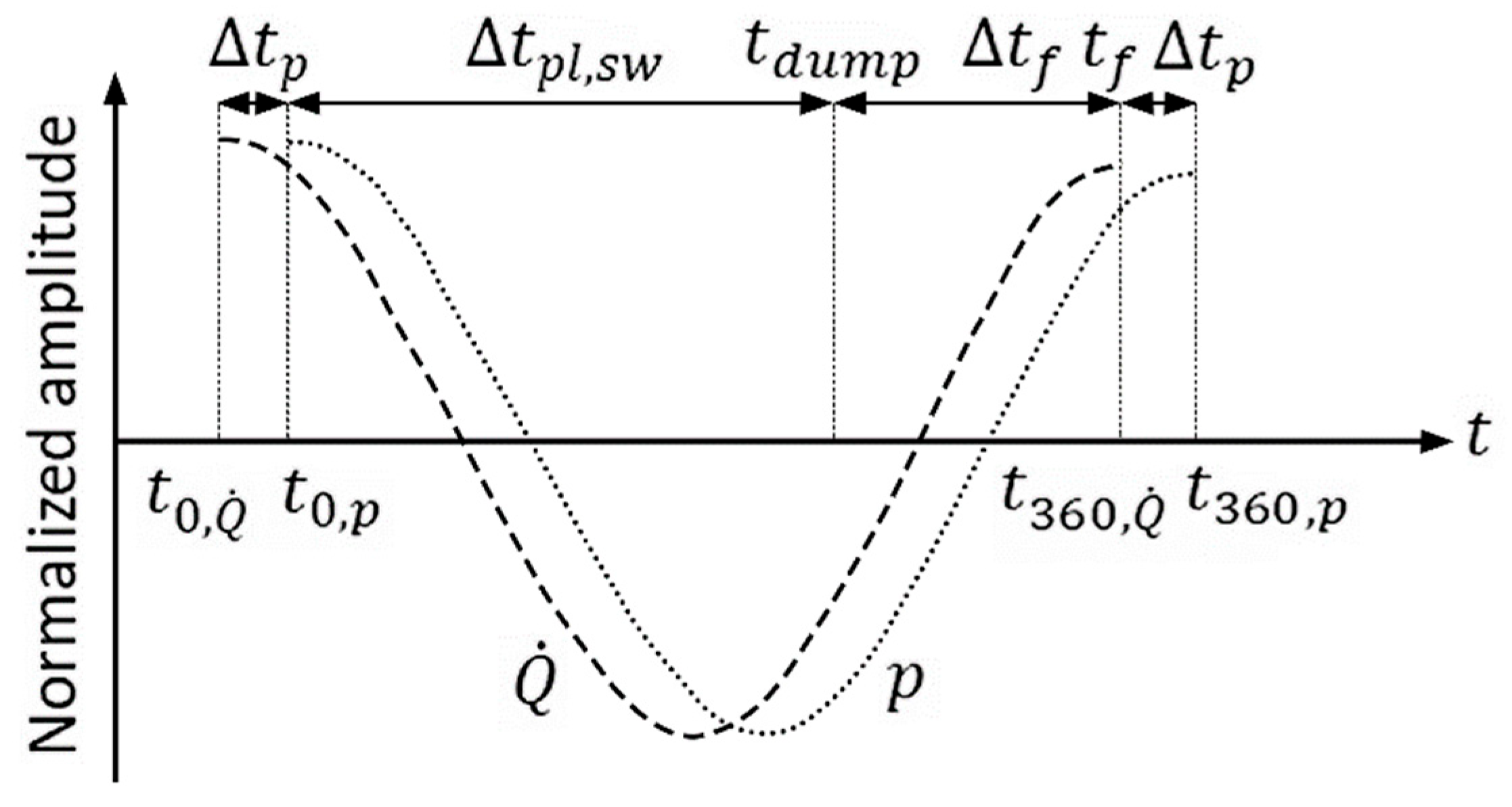
3.4. Unsteady Combustion
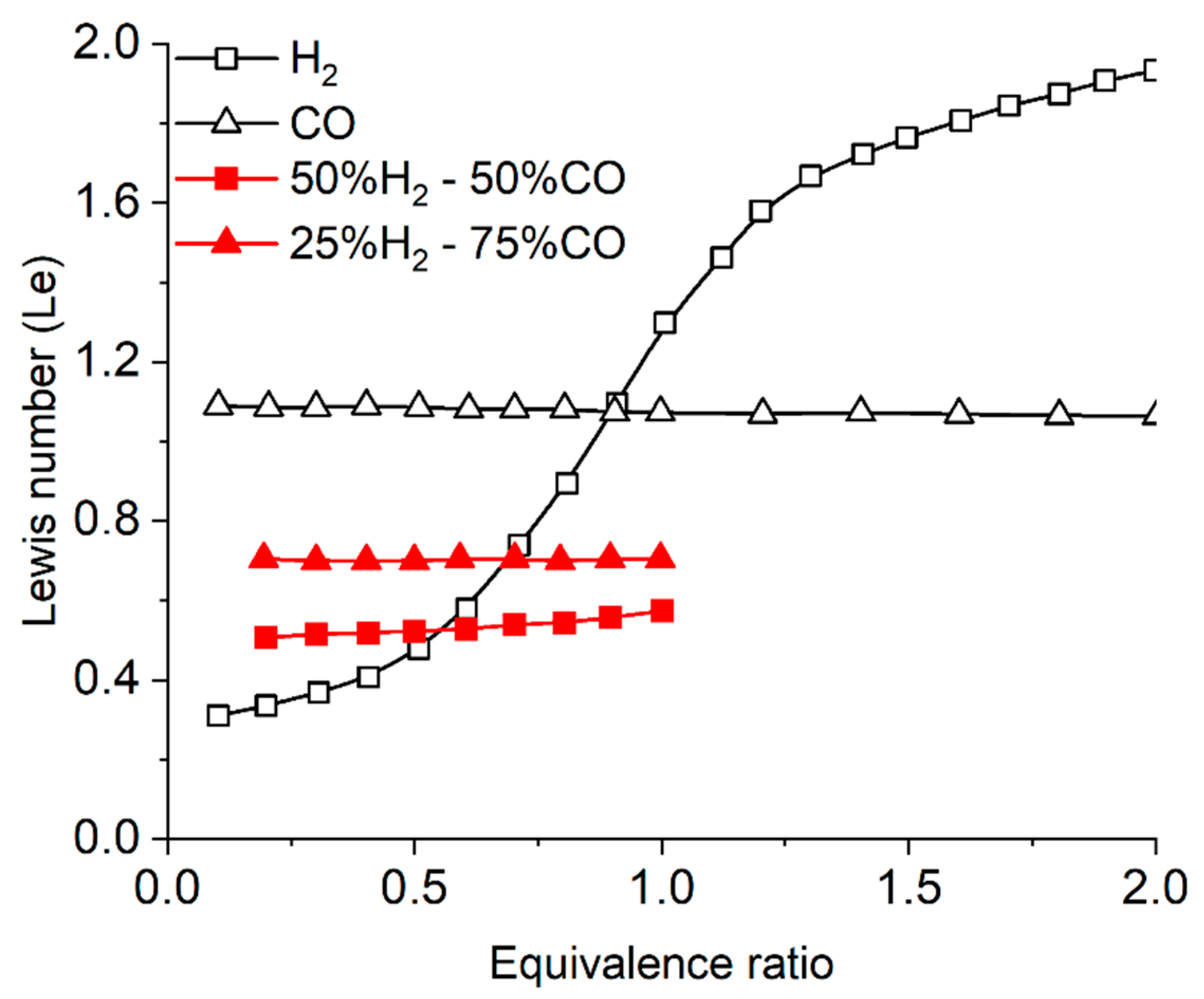
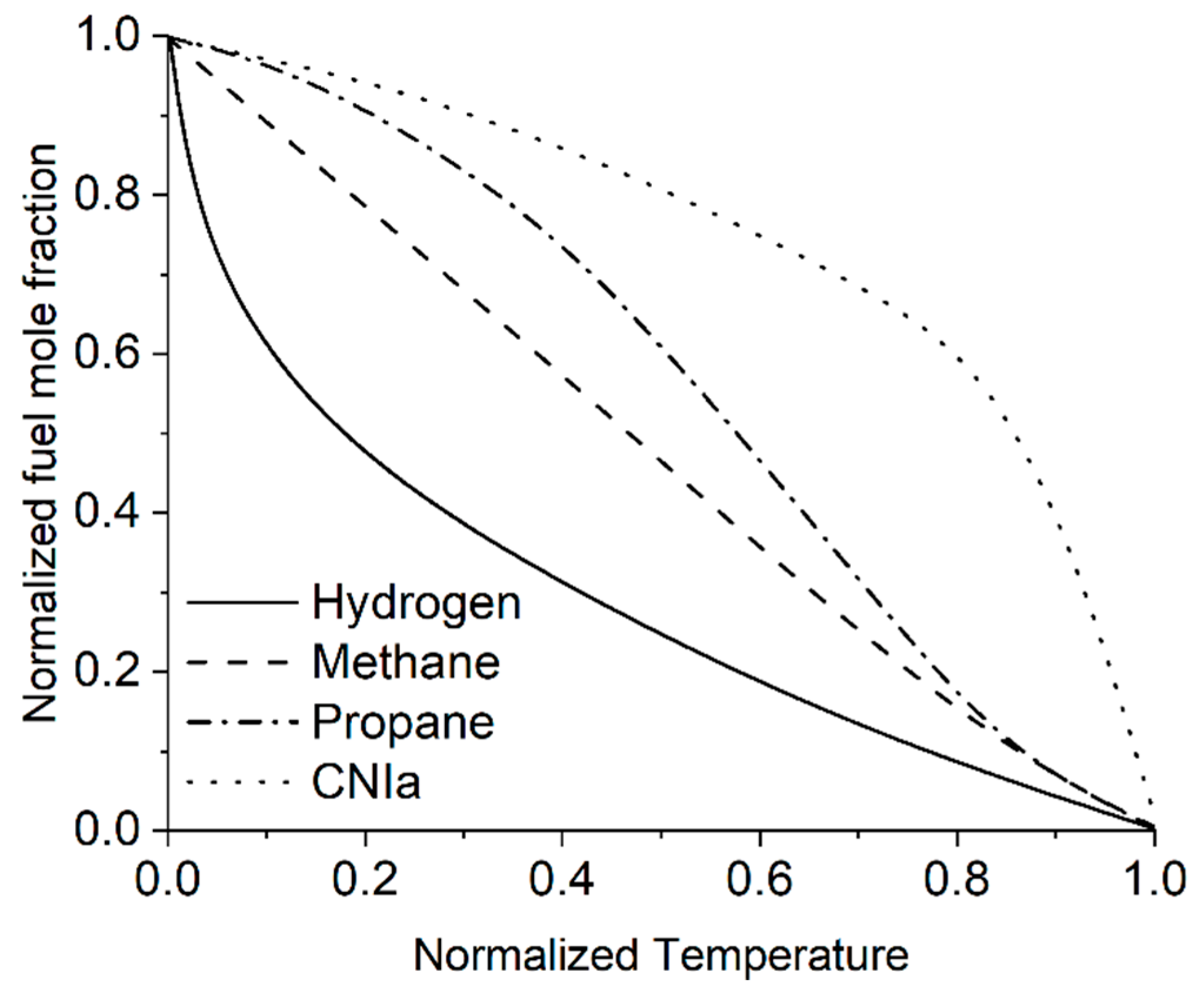
3.5. Lewis Number
4. Conclusions
- The density of hydrogen is almost an order of magnitude lower than the density of natural gas, whereas the net calorific value per cubic meter is much lower. Thus, while maintaining the parameters of the power plant when switching to hydrogen, it is necessary to pay attention to the flow sections of the fuel supply system, especially to the fuel injectors.
- The addition of hydrogen leads to an increased intensity of chemical processes per unit volume in the flame front. This results in the compactification of the flame, a change in its shape, and a decrease in its size.
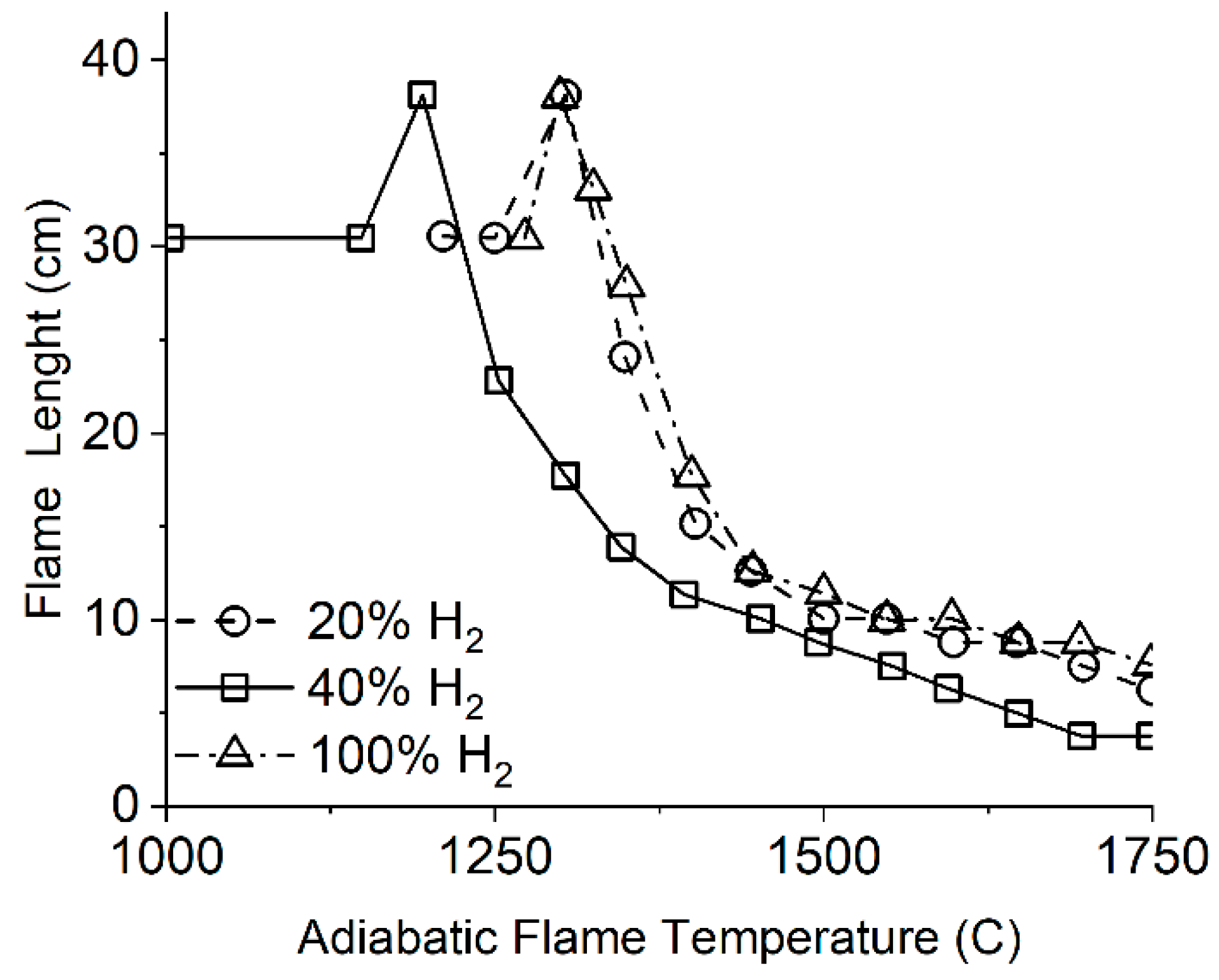
- The adiabatic combustion temperature of hydrogen flame is 5–15% higher than that of methane; however, the operating range of hydrogen covers some lower minimum temperatures of up to 1050 K, compared to ≈1400–1500 K for methane, due to the high concentration of active radicals (H, HO).
- The addition of hydrogen results in a significant increase in the flame propagation speed. The greatest effect is achieved at a hydrogen concentration in the fuel of 65% or more. In this case, the maximum velocity shifts to the region of rich mixtures. On a basis of high hydrogen velocity, the combustion chamber can theoretically be shortened, which will reduce both the engine weight and NOx emissions by cutting the residence time of the air–fuel mixture in the high-temperature zones.
- To ensure combustion with no flashbacks, it is necessary to increase the flow rate, which requires an increase in the pressure drop across the flame tube head and, in general, an increase in pressure losses in the combustion chamber.
- The ignition delay time of hydrogen is of several orders of magnitude shorter than that of methane. The minimum autoignition temperature shifts to the region of rich mixtures and is approximately 70–150 degrees lower than that of methane. Thus, for combustion chambers with the air temperature Tk > 700 K, it is advisable to use a combustion system without premixing, for example, LDI or a multicluster.
- The CO emission increases when hydrogen is added to the fuel, while CO2 decreases for stoichiometric and rich mixtures. Burning lean mixtures of hydrogen with air allows a reduction in the level of CO and CO2 emissions compared to the combustion of methane.
- The emission of NOx when hydrogen is added to the fuel increases at the same adiabatic temperature of the flame, which indicates different mechanisms for the formation of nitrogen oxides during the combustion of methane and methane–hydrogen mixtures. However, nitrogen oxide emissions can be achieved by lowering the combustion temperature.
- An increase in the concentration of hydrogen in the mixtures with methane and natural gas is accompanied by an increase in resistance to flame blowout but increases the tendency of the mixture to flashback [58].
- The change in the amplitude of pulsations with increased concentration of hydrogen in the fuel is determined by the initial mechanism of their formation. In this case, the pulsation frequency usually increases.
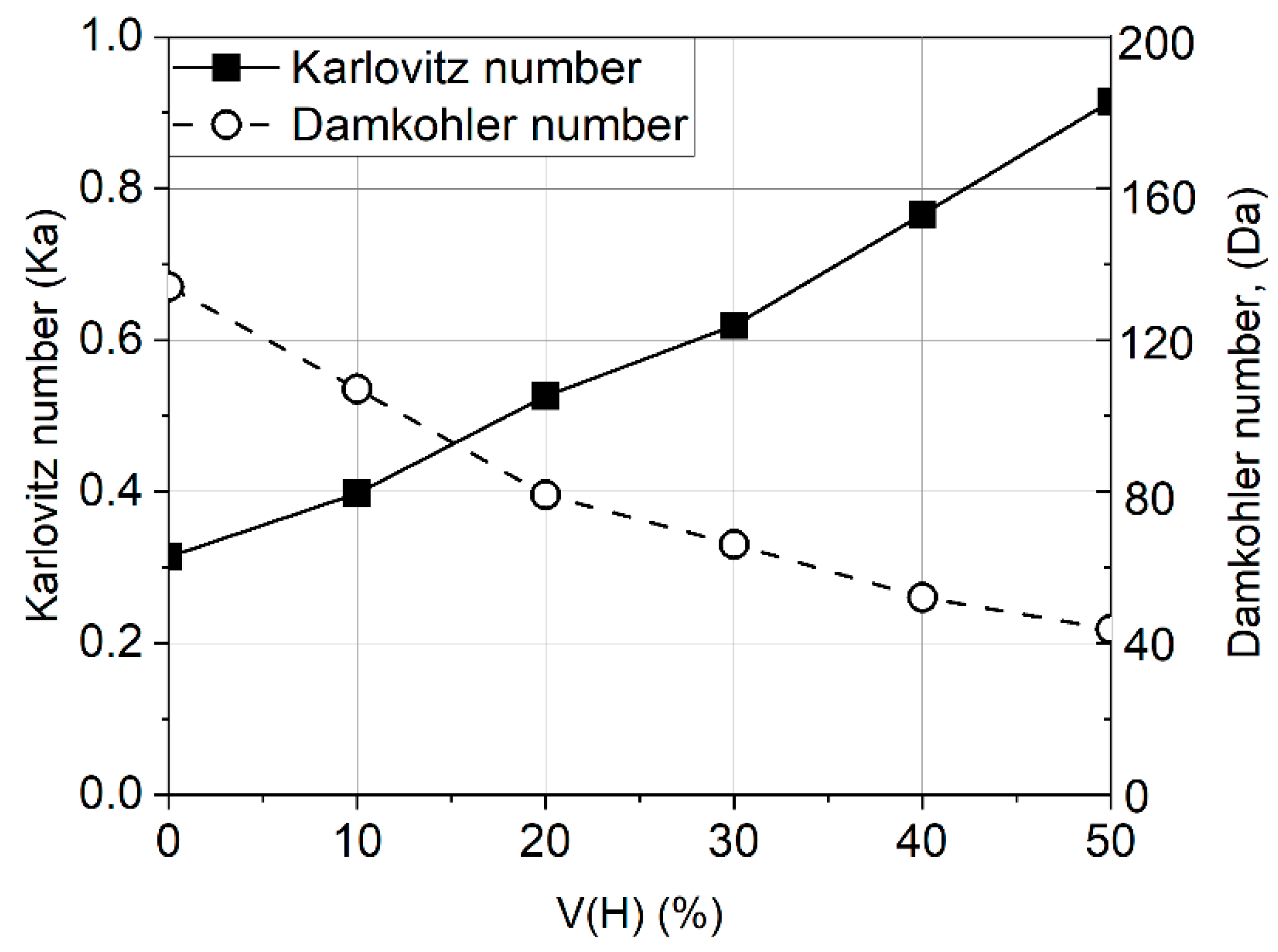
- As a rule, the effect of increased pressure is more pronounced for the hydrogen–air flame than for the methane–air flame, which complicates the process of interpolating the data obtained under atmospheric conditions for the engine parameters. Thus, further research into the influence of pressure on the combustion of hydrogen presents a crucial task.
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Russian Government. Available online: http://government.ru/docs/42971/ (accessed on 6 March 2023).
- Acar, C.; Dincer, I. The potential role of hydrogen as a sustainable transportation fuel to combat global warming. Int. J. Hydrogen Energy 2020, 45, 3396–3406. [Google Scholar] [CrossRef]
- Taamallah, S.; Vogiatzaki, K.; Alzahrani, F.M.; Mokheimer, E.M.A.; Habib, M.A.; Ghoniem, A.F. Fuel flexibility, stability and emissions in premixed hydrogen-rich gas turbine combustion: Technology, fundamentals, and numerical simulations. Appl. Energy 2015, 154, 1020–1047. [Google Scholar] [CrossRef]
- Griebel, P. Gas Turbines and Hydrogen. Hydrog. Sci. Eng. Mater. Process. Syst. Technol. 2016, 2, 1011–1032. [Google Scholar]
- Biryuk, V.; Lukachev, S.; Uglanov, D.; Tsybizov, Y. Gas in Engines, 1st ed.; Publishing House of Samara University: Samara, Russia, 2021; pp. 3–296. [Google Scholar]
- Wallner, T.; Ng, H.K.; Peters, R.W. The effects of blending hydrogen with methane on engine operation, efficiency, and emissions. SAE Tech. Pap. 2007, 2007, 0474. [Google Scholar]
- Wei, J. Boiling points and melting points of chlorofluprocarbons. Ind. Eng. Chem. Res. 2000, 39, 3116–3119. [Google Scholar] [CrossRef]
- GRI-Mech 3.0. Available online: http://combustion.berkeley.edu/gri-mech/version30/text30.html (accessed on 6 March 2023).
- Boushaki, T.; Dhué, Y.; Selle, L.; Ferret, B.; Poinsot, T. Effects of hydrogen and steam addition on laminar burning velocity of methane-air premixed flame: Experimental and numerical analysis. Int. J. Hydrogen Energy 2012, 37, 9412–9422. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Huang, M.; Li, Q.; Wang, W.; Zhao, D.; Cheng, S.; Deng, H.; Du, J.; Song, Y.; et al. Effect of hydrogen addition on the combustion and emission characteristics of methane under gas turbine relevant operating condition. Fuel 2022, 324, 124707. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, X.; Wei, W.; Qiu, L.; Wu, H. Effects of hydrogen addition on laminar flame speeds of methane, ethane and propane: Experimental and numerical analysis. Int. J. Hydrogen Energy 2017, 42, 24055–24066. [Google Scholar] [CrossRef]
- Ilbas, M.; Crayford, A.P.; Yilmaz, I.; Bowen, P.J.; Syred, N. Laminar-burning velocities of hydrogen-air and hydrogen-methane-air mixtures: An experimental study. Int. J. Hydrogen Energy 2006, 31, 1768–1779. [Google Scholar] [CrossRef]
- Herzler, J.; Naumann, C. Shock-tube study of the ignition of methane/ethane/hydrogen mixtures with hydrogen contents from 0% to 100% at different pressures. Proc. Combust. Inst. 2009, 32, 212–220. [Google Scholar] [CrossRef]
- Lieuwen, T.; McDonell, V.; Petersen, E.; Santavicca, D. Fuel flexibility influences on premixed combustor blowout, flashback, autoignition, and stability. J. Eng. Gas Turbines Power 2008, 130, 011506. [Google Scholar] [CrossRef]
- Beerer, D.J. Autoignition of Methane, Ethane, Propane and Hydrogen in Turbulent High Pressure and Temperature Flows. Master’s Thesis, The University of California, Irvine, CA, USA, 2009. [Google Scholar]
- Electronic Fond. Explosionprotected Electrical Apparatus. Part 4. Method of Test for Ignition Temperature. Available online: https://docs.cntd.ru/document/1200103115?ysclid=lbeo3uxkcl682084142 (accessed on 6 March 2023).
- Zhang, Y.; Huang, Z. Experimental and Modeling Study on Auto-Ignition of Methane/hydrogen Blends at Elevated Pressures; SAE Technical Paper: Warrendale, PA, USA, 2014; p. 1. [Google Scholar]
- Gupta, A.; Lilley, D.; Syred, N. Swirl Flows, 1st ed.; Abacus Press: Tunbridge Wells, UK, 1984; 488p. [Google Scholar]
- Zhang, Y.; Huang, Z.; Wei, L.; Zhang, J.; Law, C.K. Experimental and modeling study on ignition delays of lean mixtures of methane, hydrogen, oxygen, and argon at elevated pressures. Combust. Flame 2012, 159, 918–931. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, X.; Wei, L.; Zhang, J.; Tang, C.; Huang, Z. Experimental and modeling study on auto-ignition characteristics of methane/hydrogen blends under engine relevant pressure. Int. J. Hydrogen Energy 2012, 37, 19168–19176. [Google Scholar] [CrossRef]
- Ge, B.; Ji, Y.; Zhang, Z.; Zang, S.; Tian, Y.; Yu, H.; Chen, M.; Jiao, G.; Zhang, D. Experiment study on the combustion performance of hydrogen-enriched natural gas in a DLE burner. Int. J. Hydrogen Energy 2019, 44, 14023–14031. [Google Scholar] [CrossRef]
- Choi, J.; Lee, W.; Rajasegar, R.; Lee, T.; Yoo, J. Effects of hydrogen enhancement on mesoscale burner array flame stability under acoustic perturbations. Int. J. Hydrogen Energy 2021, 46, 37098–37107. [Google Scholar] [CrossRef]
- Chterev, I.; Boxx, I. Effect of hydrogen enrichment on the dynamics of a lean technically premixed elevated pressure flame. Combust. Flame 2021, 225, 149–159. [Google Scholar] [CrossRef]
- Figura, L.; Lee, J.G.; Quay, B.D.; Santavicca, D.A. The Effects of Fuel Composition on Flame Structure and Combustion Dynamics in a Lean Premixed Combustor. ASME 2007, 2, 181–187. [Google Scholar]
- Yilmaz, H.; Cam, O.; Yilmaz, I. Experimental investigation of flame instability in a premixed combustor. Fuel 2020, 262, 116594. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, X. Experimental study on flame stability of biogas / hydrogen combustion. Int. J. Hydrogen Energy 2019, 44, 5607–5614. [Google Scholar] [CrossRef]
- Indlekofer, T.; Ahn, B.; Kwah, Y.H.; Wiseman, S.; Mazur, M.; Dawson, J.R.; Worth, N.A. The effect of hydrogen addition on the amplitude and harmonic response of azimuthal instabilities in a pressurized annular combustor. Combust. Flame 2021, 228, 375–387. [Google Scholar] [CrossRef]
- Wicksall, D.M.; Agrawal, A.K. Acoustics measurements in a lean premixed combustor operated on hydrogen/hydrocarbon fuel mixtures. Int. J. Hydrogen Energy 2007, 32, 1103–1112. [Google Scholar] [CrossRef]
- Kashir, B.; Tabejamaat, S. A numerical study on the effects of H2 addition in non-premixed turbulent combustion of C3H8-H2-N2 mixture using a steady flamelet approach. Int. J. Hydrogen Energy 2013, 38, 9918–9927. [Google Scholar] [CrossRef]
- Araoye, A.A.; Abdelhafez, A.; Nemitallah, M.A.; Habib, M.A.; Ben-Mansour, R. Experimental and numerical investigation of stability and emissions of hydrogen-assisted oxy-methane flames in a multi-hole model gas-turbine burner. Int. J. Hydrogen Energy 2021, 46, 20093–20106. [Google Scholar] [CrossRef]
- Bongartz, D.; Ghoniem, A.F. Chemical kinetics mechanism for oxy-fuel combustion of mixtures of hydrogen sulfide and methane. Combust. Flame 2015, 162, 544–553. [Google Scholar] [CrossRef]
- Zhang, P.; Zsély, I.G.; Papp, M.; Nagy, T.; Turányi, T. Comparison of methane combustion mechanisms using laminar burning velocity measurements. Combust. Flame 2022, 238, 111867. [Google Scholar] [CrossRef]
- Verma, S.; Monnier, F.; Lipatnikov, A.N. Validation of leading point concept in RANS simulations of highly turbulent lean syngas-air flames with well-pronounced diffusional-thermal effects. Int. J. Hydrogen Energy 2021, 46, 9222–9233. [Google Scholar] [CrossRef]
- Nemitallah, M.A.; Imteyaz, B.; Abdelhafez, A.; Habib, M.A. Experimental and computational study on stability characteristics of hydrogen-enriched oxy-methane premixed flames. Appl. Energy 2019, 250, 433–443. [Google Scholar] [CrossRef]
- Bouras, F.; El Hadi Attia, M.; Khaldi, F.; Si-Ameur, M. Control of methane flame properties by hydrogen fuel addition: Application to power plant combustion chamber. Int. J. Hydrogen Energy 2017, 42, 8932–8939. [Google Scholar] [CrossRef]
- Kim, H.S.; Arghode, V.K.; Gupta, A.K. Flame characteristics of hydrogen-enriched methane–air premixed swirling flames. Int. J. Hydrogen Energy 2009, 34, 1063–1073. [Google Scholar] [CrossRef]
- Rahimi, S.; Mazaheri, K.; Alipoor, A.; Mohammadpour, A. The effect of hydrogen addition on methane-air flame in a stratified swirl burner. Energy 2023, 265, 126354. [Google Scholar] [CrossRef]
- Kim, H.S.; Arghode, V.K.; Linck, M.B.; Gupta, A.K. Hydrogen addition effects in a confined swirl-stabilized methane-air flame. Int. J. Hydrogen Energy 2009, 34, 1054–1062. [Google Scholar] [CrossRef]
- Leng, X.; Huang, H.; Ge, Q.; He, Z.; Zhang, Y.; Wang, Q.; He, D.; Long, W. Effects of hydrogen enrichment on the combustion and emission characteristics of a turbulent jet ignited medium speed natural gas engine: A numerical study. Fuel 2021, 290, 119966. [Google Scholar] [CrossRef]
- Abubakar, Z.; Shakeel, M.R.; Mokheimer, E.M.A. Experimental and numerical analysis of non-premixed oxy-combustion of hydrogen-enriched propane in a swirl stabilized combustor. Energy 2018, 165, 1401–1414. [Google Scholar] [CrossRef]
- Therkelsen, P.; Werts, T.; McDonell, V.; Samuelsen, S. Analysis of NOx Formation in a Hydrogen-Fueled Gas Turbine Engine. ASME 2009, 131, 031507. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Hajialigol, N.; Mazaheri, K.; Fattahi, A. Investigation of the effect of the flame holder geometry on the flame structure in non-premixed hydrogen-hydrocarbon composite fuel combustion. Combust. Explos. Shock. Waves 2014, 50, 32–41. [Google Scholar] [CrossRef]
- Ghoniem, A.F.; Annaswamy, A.; Park, S.; Sobhani, Z.C. Stability and emissions control using air injection and H2 addition in premixed combustion. Proc. Combust. Inst. 2005, 30, 1765–1773. [Google Scholar] [CrossRef]
- Chen, Z.; Yong, J. Numerical investigation of the effects of H2/CO/syngas additions on laminar premixed combustion characteristics of NH3/air flame. Int. J. Hydrogen Energy 2021, 46, 12016–12030. [Google Scholar] [CrossRef]
- Kim, N.; Kim, V.; Jaafar, M.N.M.; Rahim, M.R.; Said, M. Effects of hydrogen addition on structure and NO formation of highly CO-Rich syngas counterflow nonpremixed flames under MILD combustion regime. Int. J. Hydrogen Energy 2021, 46, 10518–10534. [Google Scholar] [CrossRef]
- Semenikhin, A.S.; Matveev, S.S.; Chechet, I.V.; Matveev, S.G.; Idrisov, D.V.; Gurakov, N.I.; Radin, D.V.; Novichkova, S.S.; Fokin, N.I.; Simin, N.O.; et al. Kinetic Models of Methane-Hydrogen Mixture Combustion: Brief Review and Validation. Therm. Eng. 2022, 69, 792–801. [Google Scholar] [CrossRef]
- Papanikolaou, N.; Wierzba, I. The effects of burner geometry and fuel composition on the stability of a jet diffusion flame. J. Energy Resour. Technol. Trans. ASME 1997, 119, 265–270. [Google Scholar] [CrossRef]
- Karbasi, M.; Wierzba, I. The effects of hydrogen addition on the stability limits of methane jet diffusion flames. Int. J. Hydrogen Energy 1998, 23, 123–129. [Google Scholar] [CrossRef]
- Schefer, R.W.; Wicksall, D.M.; Agrawal, A.K. Combustion of hydrogen-enriched methane in a lean premixed swirl-stabilized burner. Proc. Combust. Inst. 2002, 29, 843–851. [Google Scholar] [CrossRef]
- Zhu, S.; Acharya, S. An experimental study of lean blowout with hydrogen-enriched fuels. J. Eng. Gas Turbines Power 2012, 134, 041507. [Google Scholar] [CrossRef]
- Schefer, R.W. Hydrogen enrichment for improved lean flame stability. Int. J. Hydrogen Energy 2003, 28, 1131–1141. [Google Scholar] [CrossRef]
- Griebel, P.; Boschek, E.; Jansohn, P. Lean Blowout Limits and NOx Emissions of Turbulent, Lean Premixed, Hydrogen-Enriched Methane/Air Flames at High Pressure. ASME 2006, 1, 405–412. [Google Scholar]
- Choi, J.; Rajasegar, R.; Lee, W.; Lee, T.; Yoo, J. Hydrogen enhancement on a mesoscale swirl stabilized burner array. Int. J. Hydrogen Energy 2021, 46, 23906–23915. [Google Scholar] [CrossRef]
- Zhang, J.; Ratner, A. Experimental study of the effects of hydrogen addition on the thermoacoustic instability in a variable-length combustor. Int. J. Hydrogen Energy 2021, 46, 16086–16100. [Google Scholar] [CrossRef]
- Davis, D.W.; Therkelsen, P.L.; Littlejohn, D.; Cheng, R.K. Effects of hydrogen on the thermo-acoustics coupling mechanisms of low-swirl injector flames in a model gas turbine combustor. Proc. Combust. Inst. 2013, 34, 3135–3143. [Google Scholar] [CrossRef]
- Shanbhogue, S.J.; Sanusi, Y.S.; Taamallah, S.; Habib, M.A.; Mokheimer, E.M.A.; Ghoniem, A.F. Flame macrostructures, combustion instability and extinction strain scaling in swirl-stabilized premixed CH4/H2 combustion. Combust. Flame 2016, 167, 497. [Google Scholar] [CrossRef]
- Zhang, J.; Ratner, A. Experimental study on the excitation of thermoacoustic instability of hydrogen-methane/air premixed flames under atmospheric and elevated pressure conditions. Int. J. Hydrogen Energy 2019, 44, 21324–21335. [Google Scholar] [CrossRef]
- Yilmaz, I.; Ratner, A.; Ilbas, M.; Huang, Y. Experimental investigation of thermoacoustic coupling using blended hydrogen-methane fuels in a low swirl burner. Int. J. Hydrogen Energy 2010, 35, 329–336. [Google Scholar] [CrossRef]
- El-Ghafour, S.A.A.; El-dein, A.H.E.; Aref, A.A.R. Combustion characteristics of natural gas-hydrogen hybrid fuel turbulent diffusion flame. Int. J. Hydrogen Energy 2010, 35, 2556–2565. [Google Scholar] [CrossRef]
- Imteyaz, B.A.; Nemitallah, M.A.; Abdelhafez, A.A.; Habib, M.A. Combustion behavior and stability map of hydrogen-enriched oxy-methane premixed flames in a model gas turbine combustor. Int. J. Hydrogen Energy 2018, 43, 16652–16666. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Lin, W.; Mao, R.; Xia, H.; Zhang, M.; Huang, Z. Effect of differential diffusion on turbulent lean premixed hydrogen enriched flames through structure analysis. Int. J. Hydrogen Energy 2020, 45, 10920–10931. [Google Scholar] [CrossRef]
- Lee, T.W.; North, G.L.; Santavicca, D.A. Surface properties of turbulent premixed propane/air flames at various Lewis numbers. Combust. Flame 1993, 93, 445–456. [Google Scholar] [CrossRef]
- Aspden, A.J.; Day, M.S.; Bell, J.B. Lewis number effects in distributed flames. Proc. Combust. Inst. 2011, 33, 1473–1480. [Google Scholar] [CrossRef]
- Burbano, H.J.; Pareja, J.; Amell, A.A. Laminar burning velocities and flame stability analysis of H2/CO/air mixtures with dilution of N2 and CO2. Int. J. Hydrogen Energy 2011, 36, 3232–3242. [Google Scholar] [CrossRef]
- Dinkelacker, F.; Manickam, B.; Muppala, S.P.R. Modelling and simulation of lean premixed turbulent methane/hydrogen/air flames with an effective Lewis number approach. Combust. Flame 2011, 158, 1742–1749. [Google Scholar] [CrossRef]
- Bell, J.B.; Cheng, R.K.; Day, M.S.; Shepherd, I.G. Numerical simulation of Lewis number effects on lean premixed turbulent flames. Proc. Combust. Inst. 2007, 31, 1309–1317. [Google Scholar] [CrossRef]
- Lee, H.C.; Dai, P.; Wan, M.; Lipatnikov, A.N. Lewis number and preferential diffusion effects in lean hydrogen–air highly turbulent flames. Phys. Fluids 2022, 34, 035131. [Google Scholar] [CrossRef]
- Aspden, A.J.; Bell, J.B.; Woosley, S.E.; Zingale, M. Turbulence-Flame Interactions in Type Ia Supernovae. Astrophys. J. 2008, 2, 1173–1185. [Google Scholar] [CrossRef]






| Hydrogen | Natural Gas | |
|---|---|---|
| Density at 273 K (kg/m3) | 0.09 | 0.72 |
| Molar mass (kg/kmol) | 2.016 | 16.04 |
| Ignition limits (Φ) | 0.1–7.1 | 0.4–1.6 |
| Low calorific value (MJ/kg) | 120 | 50 |
| Low calorific value (MJ/m3) | 11 | 36 |
| Maximum laminar flame speed (m/s) | 3.25 | 0.45 |
| Adiabatic flame temperature at Φ = 1 (K) | 2402 | 2216 |
| Stoichiometric coefficient | 34.2 | 17.2 |
| Melting temperature (K) | 14 | 91 |
| Boiling temperature (K) | 21 | 112 |
| Critical temperature (K) | 33 | 191 |
| Critical pressure (amt) | 12.8 | 45.2 |
| Autoignition temperature (K) | 850 | 810 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shchepakina, E.A.; Zubrilin, I.A.; Kuznetsov, A.Y.; Tsapenkov, K.D.; Antonov, D.V.; Strizhak, P.A.; Yakushkin, D.V.; Ulitichev, A.G.; Dolinskiy, V.A.; Hernandez Morales, M. Physical and Chemical Features of Hydrogen Combustion and Their Influence on the Characteristics of Gas Turbine Combustion Chambers. Appl. Sci. 2023, 13, 3754. https://doi.org/10.3390/app13063754
Shchepakina EA, Zubrilin IA, Kuznetsov AY, Tsapenkov KD, Antonov DV, Strizhak PA, Yakushkin DV, Ulitichev AG, Dolinskiy VA, Hernandez Morales M. Physical and Chemical Features of Hydrogen Combustion and Their Influence on the Characteristics of Gas Turbine Combustion Chambers. Applied Sciences. 2023; 13(6):3754. https://doi.org/10.3390/app13063754
Chicago/Turabian StyleShchepakina, Elena Anatolievna, Ivan Alexandrovich Zubrilin, Alexey Yurievich Kuznetsov, Konstantin Dmitrievich Tsapenkov, Dmitry Vladimirovich Antonov, Pavel Alexandrovich Strizhak, Denis Vladimirovich Yakushkin, Alexander Gennadievich Ulitichev, Vladimir Alexandrovich Dolinskiy, and Mario Hernandez Morales. 2023. "Physical and Chemical Features of Hydrogen Combustion and Their Influence on the Characteristics of Gas Turbine Combustion Chambers" Applied Sciences 13, no. 6: 3754. https://doi.org/10.3390/app13063754
APA StyleShchepakina, E. A., Zubrilin, I. A., Kuznetsov, A. Y., Tsapenkov, K. D., Antonov, D. V., Strizhak, P. A., Yakushkin, D. V., Ulitichev, A. G., Dolinskiy, V. A., & Hernandez Morales, M. (2023). Physical and Chemical Features of Hydrogen Combustion and Their Influence on the Characteristics of Gas Turbine Combustion Chambers. Applied Sciences, 13(6), 3754. https://doi.org/10.3390/app13063754









